* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Transcription - HCC Learning Web
Protein moonlighting wikipedia , lookup
Minimal genome wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Messenger RNA wikipedia , lookup
Transcription factor wikipedia , lookup
Genome (book) wikipedia , lookup
Non-coding DNA wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Oncogenomics wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Microevolution wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Non-coding RNA wikipedia , lookup
History of genetic engineering wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Epitranscriptome wikipedia , lookup
Gene expression profiling wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Designer baby wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Point mutation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
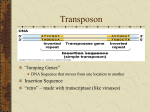
Therapeutic gene modulation wikipedia , lookup
CAMPBELL BIOLOGY TENTH EDITION Reece • Urry • Cain • Wasserman • Minorsky • Jackson 18 Regulation of Gene Expression Lecture Presentation by Nicole Tunbridge and Kathleen Fitzpatrick © 2014 Pearson Education, Inc. Overview: Conducting the Genetic Orchestra • Prokaryotes and eukaryotes alter gene expression in response to their changing environment • $ --- In multicellular eukaryotes, gene expression regulates development and is responsible for differences in cell types • RNA molecules play many roles in regulating gene expression in eukaryotes Figure 18.1 How can this fish’s eyes see equally well in both air and water? Bacteria often respond to environmental change by regulating transcription • Natural selection has favored bacteria that produce only the products needed by that cell F i g – a cell can regulate the production of an existing enzyme by feedback inhibition – or it can regulate the production of the enzyme by gene regulation • Gene regulation in bacteria uses an operon model Fig. 18.2 Operons: The Basic Concept • A cluster of functionally related genes can be under coordinated control by a single on-off “switch” • The regulatory “switch” is a segment of DNA called an operator usually positioned within the promoter • An operon is the entire stretch of DNA that includes the operator, the promoter, and the genes that they control • The operon can be switched off by a protein repressor • The repressor prevents gene transcription by binding to the operator and blocking RNA polymerase • The repressor is the product of a separate regulatory gene • The repressor can be in an active or inactive form, depending on the presence of other molecules • A corepressor is a molecule that cooperates with a repressor protein to switch an operon off • For example, E. coli can synthesize the amino acid tryptophan By default the trp operon is on and the genes for tryptophan synthesis are transcribed • When tryptophan is present, it binds to the trp repressor protein, which turns the operon off • The repressor is active only in the presence of its corepressor tryptophan; thus the trp operon is turned off (repressed) if tryptophan levels are high Figure 18.3 trp operon DNA Promoter Regulatory gene Promoter Genes of operon trpE trpR RNA polymerase Operator Start codon trpD trpC trpB trpA Stop codon 32 mRNA 52 mRNA 52 Inactive repressor Protein E DNA mRNA 52 trpE No RNA made 32 Protein Active repressor Tryptophan (corepressor) (b) Tryptophan present, repressor active, operon off C B Polypeptide subunits that make up enzymes for tryptophan synthesis (a) Tryptophan absent, repressor inactive, operon on trpR D A Repressible and Inducible Operons: Two Types of Negative Gene Regulation • A repressible operon is one that is usually on; binding of a repressor to the operator shuts off transcription • The trp operon is a repressible operon • An inducible operon is one that is usually off; a molecule called an inducer inactivates the repressor and turns on transcription • The lac operon is an inducible operon and contains genes that code for enzymes used in the hydrolysis and metabolism of lactose • By itself, the lac repressor is active and switches the lac operon off • A molecule called an inducer inactivates the repressor to turn the lac operon on Figure 18.4 DNA Promoter Regulatory gene Operator l a Ic IacZ No RNA made 3′ mRNA 5′ RNA polymerase Active repressor Protein (a) Lactose absent, repressor active, operon off lac operon DNA l a Ic lacZ RNA polymerase mRNA 5′ 32 3′ lacA Stop codon mRNA 5′ Protein Allolactose (inducer) Start codon lacY β-Galactosidase Inactive repressor (b) Lactose present, repressor inactive, operon on Permease Transacetylase Inducible Operons In the absence of lactose, this operon is off because an active repressor binds to the operator and prevents transcription. An inducer inactivates the repressor. When lactose is present in the cell, allolactose, an isomer of lactose, binds to the repressor. This inactivates the repressor (it is released from the operator sequence), and the lac operon can be transcribed. Repressible and Inducible Operons • Repressible enzymes generally function in anabolic pathways, synthesizing end products from raw materials. – When the end product is present in sufficient quantities, the cell can allocate its resources to other uses. • Inducible enzymes usually function in catabolic pathways, digesting nutrients to simpler molecules. – By producing the appropriate enzymes only when the nutrient is available, the cell avoids making proteins that have nothing to do. • Both repressible and inducible operons demonstrate negative control of genes because active repressors switch off the active form of the repressor protein. Positive Gene Regulation • Positive gene control occurs when a protein molecule interacts directly with the genome to switch transcription on. – The lac operon is an example of positive gene regulation. • When glucose and lactose are both present, E. coli preferentially uses glucose. • The enzymes for glucose breakdown in glycolysis are always present in the cell. • Only when lactose is present and glucose is in short supply does E. coli use lactose as an energy source and synthesize the enzymes for lactose breakdown. If glucose is low→↑cAMP→ cAMP binds to CAP→↑lac transcription Fig. 18.5 • When glucose levels are low, cyclic AMP (cAMP) is high. • The regulatory protein catabolite activator protein (CAP) is an activator of transcription. • When cAMP is abundant, it binds to CAP, and the regulatory protein assumes its active shape and can bind to a specific site at the upstream end of the lac promoter. • The attachment of CAP to the promoter increases the affinity of RNA polymerase for the promoter, directly increasing the rate of transcription. • Thus, this mechanism qualifies as positive regulation. If glucose is high→↓cAMP→ cAMP does not bind to CAP→↓lac transcription Fig. 18.5 • • • • If glucose levels in the cell rise, cAMP levels fall. Without cAMP, CAP detaches from the operon. The lac operon is transcribed but at a low level. CAP helps regulate other operons that encode enzymes used in catabolic pathways The lac operon is under dual control • Negative control by the lac repressor • Positive control by CAP • The state of the lac repressor determines whether or not the lac operon’s genes are transcribed. • The state of CAP (with or without bound cAMP) controls the rate of transcription if the operon is repressor-free. Eukaryotic gene expression can be regulated at any stage • All organisms must regulate which genes are expressed at any given time. Cells have to respond to their environments, energy demands, etc. • In multicellular organisms gene expression is also essential for cell specialization • Although all the cells in an organism contain an identical genome, the genes expressed in the cells of each type is unique. • Differences between cell types result from differential gene expression- the expression of different genes by cells with the same genome • Errors in gene expression can lead to diseases including cancer • Gene expression is regulated at many stages • The expression of specific genes is most commonly regulated at transcription, often in response to signals coming from outside the cell. • For this reason, the term gene expression is often equated with gene transcription. Gene Regulation in Eukaryotes • $ --- Eukaryotic gene expression can be regulated at any stage: • Chromatin modifications – Acetylation and DNA Methylation • Transcription – Inhibition or Activation • Post-Transcriptional Regulation – RNA Splicing, Degradation • Translation – Inhibition or Activation • Protein Processing – Modifications, Degradation, Cellular Location Fig. 18.6 Regulation of Chromatin Structure • Genes within highly packed heterochromatin are usually not expressed • Chemical modifications to histones and DNA of chromatin influence chromatin structure and gene expression Fig. 18.6 Histone Modifications: Histone Acetylation increases transcription • In histone acetylation, acetyl groups (COCH3) are attached to positively charged lysines in histone tails • Histone acetylation loosens chromatin structure, thereby promoting the initiation of transcription Fig. 18.7 Histone Modifications: Histone Methylation decreases transcription • The addition of methyl groups (methylation) to histone tails condenses chromatin and decreases transcription. • Some enzymes methylate certain bases in DNA itself. • Inactive DNA is generally highly methylated compared to DNA that is actively transcribed. – The inactivated mammalian X chromosome in females is heavily methylated. – Genes are usually more heavily methylated in cells where they are not expressed. • Demethylating certain inactive genes turns them on. • DNA methylation proteins recruit histone deacetylation enzymes, providing a mechanism by which DNA methylation and histone deacetylation cooperate to repress transcription. • In some species, DNA methylation is responsible for the long-term inactivation of gene DNA Methylation results in Genomic Imprinting • Methylation enzymes recognize sites on one strand that are already methylated and correctly methylate the daughter strand after each round of DNA replication. • This methylation pattern accounts for genomic imprinting: methylation turns off either the maternal or paternal alleles of certain genes at the start of development. • The chromatin modifications just discussed do not alter the DNA sequence, and yet they may be passed along to future generations of cells. • Inheritance of traits by mechanisms not directly involving the nucleotide sequence is called epigenetic inheritance. Transcription initiation is controlled by proteins that interact with DNA • Multiple control elements are associated with eukaryotic genes. • Control elements are noncoding DNA segments located near the promoter that regulate transcription by binding certain proteins. • Control elements and the transcription factors they bind are critical to the precise regulation of gene expression in different cell types Figure 18.8 Enhancer (group of distal control elements) Proximal control elements DNA Exon Upstream Poly-A signal sequence Transcription start site Intron Promoter Primary RNA transcript (pre-mRNA) Exon Intron Transcription Exon Intron Exon Intron 5′ Exon Transcription termination region Downstream Poly-A signal Cleaved 3′ end Exon of primary transcript RNA processing Intron RNA Coding segment mRNA G P P 5′ Cap P AAA⋯AAA 5′ UTR Start codon Stop codon 3′ UTR Poly-A tail 3′ Transcription initiation is controlled by proteins that interact with DNA • RNA polymerase requires the assistance of proteins called transcription factors to initiate transcription. • Transcription factors are essential for the transcription of all protein-coding genes. • Only a few transcription factors bind a DNA sequence such as the TATA box within the promoter. • Others are involved in protein-protein interactions, binding each other and RNA polymerase II. • Only when the complete initiation complex has been assembled can the polymerase begin to move along the DNA template strand to produce a complementary strand of RNA. • Interactions between enhancers and specific transcription factors (activators or repressors) are important in controlling gene expression. Enhancers and Specific Transcription Factors • Proximal control elements are located close to the promoter • Distal control elements, groupings of which are called enhancers, may be far away from a gene or even located in an intronAn activator is a protein that binds to an enhancer and stimulates transcription of a gene • Activators have two domains, one that binds DNA and a second that activates transcription • Bound activators facilitate a sequence of protein-protein interactions that result in transcription of a given gene © 2014 Pearson Education, Inc. Figure 18.9 The structure of MyoD, a specific transcription factor that acts as an activator. Activation domain DNA-binding domain DNA Enhancers and Transcription Activators Increase the Rate of Transcription 1. 2. 3. Activator proteins, containing a DNA-binding domain and one or more activation domains, bind to the 3 enhancer sites. A DNA bending protein brings the bound activators close to the promoter. The activators bind to mediators and transcription factors, helping them for an active transcription initiation complex on the promoter. Fig. 18.10 Activators and Repressors • Some transcription factors function as repressors, inhibiting expression of a particular gene • Some activators and repressors act indirectly by influencing chromatin structure to promote or silence transcription – – – Some activators recruit proteins that acetylate histones near the promoters of specific genes, promoting transcription. Some repressors recruit proteins that deacetylate histones, reducing transcription or silencing the gene. Recruitment of chromatin-modifying proteins seems to be the most common mechanism of repression in eukaryotes. The control of transcription in eukaryotes depends on the binding of activators to DNA control elements Fig. 18.11 Nuclear Architecture and Gene Expression • Loops of chromatin extend from individual chromosomes into specific sites in the nucleus • Loops from different chromosomes may congregate at particular sites, some of which are rich in transcription factors and RNA polymerases • These may be areas specialized for a common function © 2014 Pearson Education, Inc. Figure 18.12 Chromosomes in the interphase nucleus Chromosome territory 10 µm Chromatin loop Transcription factory Coordinately Controlled Genes in Eukaryotes • Unlike the genes of a prokaryotic operon, each of the coordinately controlled eukaryotic genes has a promoter and control elements • These genes can be scattered over different chromosomes, but each has the same combination of specific control elements • Activators recognize these specific control elements and promote simultaneous transcription of the genes that contain them Post-transcriptional Regulation Fig. 18.13 • • • • • A cell can rapidly fine-tune gene expression in response to environmental changes without altering its transcriptional patterns. RNA processing in the nucleus and the export of mRNA to the cytoplasm provide opportunities for gene regulation that are not available in prokaryotes. In alternative RNA splicing, different mRNA molecules are produced from the same primary transcript, depending on which RNA segments are treated as exons and which as introns. Regulatory proteins specific to a cell type control intron-exon choices by binding to regulatory sequences within the primary transcript. Alternative RNA splicing significantly expands the types of proteins produced by a set of genes. mRNA Degradation • The life span of mRNA molecules in the cytoplasm is a key to determining protein synthesis • Eukaryotic mRNA is more stable than prokaryotic mRNA • Nucleotide sequences that influence the lifespan of mRNA in eukaryotes reside in the untranslated region (UTR) at the 3′ end of the molecule • The mRNA life span is determined in part by sequences in the leader and trailer regions – A common pathway of mRNA breakdown begins with enzymatic shortening of the poly-A tail. – This triggers the enzymatic removal of the 5′ cap and degradation of the mRNA. Initiation of Protein Translation • The initiation of translation of selected mRNAs can be blocked by regulatory proteins that bind to the mRNA and block ribosome attachment • Alternatively, translation of all mRNAs in a cell may be regulated simultaneously – Translation initiation factors are simultaneously activated in an egg following fertilization Protein Processing and Degradation • After translation, various types of protein processing can occur – Cleavage – Degradation – The addition of chemical groups • Phosphate groups, carbohydrate groups, etc Protein Degradation Fig. 18.14 • After translation, various types of protein processing, including cleavage and the addition of chemical groups, are subject to control • Several molecules of a small protein, ubiquitin, are added to the protein that will be degraded. • Proteasomes are giant protein complexes that bind to ubiquitin tagged proteins then unfold and degrade them with enzymes. Noncoding RNAs play multiple roles in controlling gene expression • Only a small fraction of DNA codes for proteins, rRNA, and tRNA • A significant amount of the genome may be transcribed into noncoding RNAs • Noncoding RNAs regulate gene expression at two points: mRNA translation into protein and chromatin configuration Effects on mRNA by MicroRNA • MicroRNAs (miRNAs) are small single-stranded RNA molecules that can bind to mRNA, making it doublestranded instead of singlestranded. • miRNAs are formed from longer RNA precursors that fold back on themselves to form one or more short, double-stranded hairpin structures stabilized by hydrogen bonding. • When miRNA binds to mRNA it can degrade mRNA or block its translation • Inhibition of gene expression by RNA molecules is called RNA interference (RNAi). • siRNAs and miRNAs are similar but form from different RNA precursors Effects on mRNA by MicroRNA 1. 2. 3. 4. An enzyme cuts the hairpins from the mRNA. A second enzyme, Dicer, cuts each hairpin into a short, double-stranded fragment of about 20 nucleotide pairs. One of the two strands is degraded. The other strand (miRNA) associates with a protein complex and directs the complex to any mRNA molecules that have a complementary sequence. The miRNA–protein complex either degrades the target mRNA or blocks its translation. Fig. 18.15 Chromatin Remodeling and Silencing of Transcription by Small RNAs • RNA interference can also be caused by small interfering RNAs (siRNAs) • siRNAs and miRNAs are similar but form from different RNA precursors • In addition to their inhibitory effects on mRNA, siRNAs play a role in heterochromatin formation and can block large regions of the chromosome Small RNAs can remodel chromatin and silence transcription • In yeast an RNA transcript produced from DNA in the centromeric region of the chromosome is copied into double-stranded RNA by a yeast enzyme and then processed into siRNAs. • The siRNAs associate with a protein complex, targeting the complex back to the centromeric sequences of DNA. • The proteins in the complex recruit enzymes to modify the chromatin, turning it into the highly condensed centromeric heterochromatin. Chromatin Remodeling and Effects on Transcription by ncRNAs • In some yeasts siRNAs play a role in heterochromatin formation and can block large regions of the chromosome • Small ncRNAs called piwi-associated RNAs (piRNAs) induce heterochromatin, blocking the expression of parasitic DNA elements in the genome, known as transposons • RNA-based mechanisms may also block transcription of single genes © 2011 Pearson Education, Inc. The Evolutionary Significance of Small ncRNAs • Small ncRNAs can regulate gene expression at multiple steps • An increase in the number of miRNAs in a species may have allowed morphological complexity to increase over evolutionary time • siRNAs may have evolved first, followed by miRNAs and later piRNAs © 2011 Pearson Education, Inc. A program of differential gene expression leads to the different cell types in a multicellular organism • During embryonic development, a single fertilized egg (zygote) gives rise to many different cell types. – With repeated cycles of mitotic cell divisions, the zygote gives rise to a large number of cells. – Cell division alone would produce only a great ball of identical cells. How do different cell types arise from a single-celled zygote? • Gene expression orchestrates the developmental program of cell division, cell differentiation, and morphogenesis. – Creating specialized cell types that are organized into tissues, organs, organ systems, and the whole organism Cell Differentiation Fig. 18.16 • During development, cells become specialized in structure and function, undergoing cell differentiation. • Different kinds of cells are organized into tissues and organs. • The physical processes that give an organism its shape constitute morphogenesis, the “creation of form.” Morphogenesis can be traced back to changes in the shape and motility of cells in different regions of the embryo. • Almost all cells in an organism have the same genome, differential gene expression results from differential gene regulation in different cell types. • What influences early embryonic development? Cytoplasmic Determinants and Inductive Signals • Maternal substances that influence the course of early development are called cytoplasmic determinants. • These substances regulate the expression of genes that affect the developmental fate of the cell. • After fertilization, the cell nuclei resulting from mitotic division of the zygote are exposed to different cytoplasmic environments. • The set of cytoplasmic determinants a particular cell receives helps determine its developmental fate by regulating expression of the cell’s genes during cell differentiation. • One important source of information early in development is the egg’s cytoplasm, which contains both RNA and proteins encoded by the mother’s DNA. Cytoplasmic materials are distributed unevenly in the unfertilized egg. Fig. 18-17 Unfertilized egg cell Sperm Fertilization Nucleus Two different cytoplasmic determinants Zygote Mitotic cell division Two-celled embryo (a) Cytoplasmic determinants in the egg Early embryo (32 cells) Signal transduction pathway Signal receptor Signal molecule (inducer) (b) Induction by nearby cells NUCLEUS Signals produced by neighboring cells are important • These signals cause changes in the target cells, a process called induction. • The molecules conveying these signals within the target cells are cell-surface receptors and other proteins expressed by the embryo’s own genes. • The signal molecules send a cell down a specific developmental path by causing a change in its gene expression that eventually results in observable cellular changes. Cell differentiation is due to the sequential regulation of gene expression • During embryonic development, cells become visibly different in structure and function as they differentiate. • The earliest changes that set a cell on a path to specialization show up only at the molecular level (determination) • Determination- Molecular changes in the embryo that drive the specialization pathways leading to the observable differentiation of cells. – Once it has undergone determination, an embryonic cell is irreversibly committed to its final fate. – If a determined cell is experimentally placed in another location in the embryo, it will differentiate as if it were in its original position. • The outcome of determination—observable cell differentiation—is caused by the expression of genes that encode tissue-specific proteins. • These proteins give a cell its characteristic structure and function. • Determination leads to Differentiation Differentiation • Differentiation is controlled by the transcription of cell-specific mRNAs and is eventually observable in the microscope as changes in cellular structure. – Cells produce the proteins that allow them to carry out their specialized roles in the organism. – Example: Muscle cells develop from embryonic precursors that have the potential to develop into a number of alternative cell types, including cartilage cells, fat cells, or muscle cells. Determination and Differentiation of muscle cells Determination: Signals from neighboring embryonic cells turn on the transcription of a master regulatory gene, MyoD. The cell is now a myoblast. Differentiation: MyoD protein is a transcription factor that activates the transcription (and subsequent translation) of MyoD as well as other muscle-specific proteins. MyoD also activates genes that block the transcripton and translation of cell cycle protiens, so the muscle cells cannot divide. The non-dividing myoblasts fuse together, forming muscle fibers. Fig. 18.18 Pattern formation sets up the embryo’s body plan • Cytoplasmic determinants (maternal) and inductive signals (neighboring cells) contribute to pattern formation- the development of spatial organization in which the tissues and organs of an organism are all in their characteristic places. • Pattern formation begins in the early embryo, when the major axes of an animal are established. – Axes: head/tail, left/right sides, and back/front • Before specialized tissues and organs form, the relative positions of a bilaterally symmetrical animal’s axes (head and tail, right and left sides, and back and front) are established. Pattern Formation is controlled by Signals • The molecular cues that control pattern formation (positional information) are provided by cytoplasmic determinants and inductive signals. • These signals tell a cell its location relative to the body axes and to neighboring cells. • The signals also determine how the cell and its progeny will respond to future molecular signals. • Pattern formation has been most extensively studied in fruit flies Drosophila melanogaster, where genetic approaches have had spectacular success. • Combining anatomical, genetic, and biochemical approaches, researchers have discovered developmental principles common to many other species, including humans The Life Cycle of Drosophila • In Drosophila, cytoplasmic determinants in the unfertilized egg determine the axes before fertilization • After fertilization, the embryo develops into a segmented larva with three larval stages The Life Cycle of Drosophila • Fruit flies have a modular construction, an ordered series of segments. – These segments make up the three major body parts: the head, thorax (with wings and legs), and abdomen. • Like other bilaterally symmetrical animals, Drosophila has an anteriorposterior axis, a dorsal-ventral axis, and a right-left axis. • Cytoplasmic determinants in the unfertilized egg provide positional information for two developmental axes (anterior-posterior and dorsalventral axis) before fertilization. • The Drosophila egg develops in the female’s ovary, surrounded by ovarian cells called nurse cells and follicle cells that supply the egg cell with nutrients, mRNAs, and other substances needed for development. • Development of the fruit fly from egg cell to adult fly occurs in a series of discrete stages. The Life Cycle of Drosophila 1. The egg cell is surrounded by nurse cells in the follicle, within one of the ovaries. 2. The nurse cells provide nutrients and mRNA to the maturing egg cell. The follicle cells secrete proteins that form the egg shell. Eventually, the nurse cells disappear and the egg completely fills the shell. 3. The egg is fertilized within the mother and is laid. 4. The embryo develops in 3 stages: Larva Larva in a cocoon Larva metamorphesis into fly Fig. 18.19 Genetic Analysis of Early Development: Scientific Inquiry • Edward B. Lewis, Christiane Nüsslein-Volhard, and Eric Wieschaus won a Nobel 1995 Prize for decoding pattern formation in Drosophila • Lewis discovered the homeotic genes, which control pattern formation in late embryo, larva, and adult stages • Lewis demonstrated that genes direct the developmental process • Nüsslein-Volhard and Wieschaus studied segment formation • They created mutants, conducted breeding experiments, and looked for corresponding genes • Breeding experiments were complicated by embryonic lethals, embryos with lethal mutations • They found 120 genes essential for normal segmentation Fig. 18-20 Eye Leg Antenna Wild type Mutant Gradients of maternal molecules in the early Drosophila embryo control axis formation • Cytoplasmic determinants, maternal genes that are deposited in the unfertilized egg, establish the axes of the Drosophila body. • A maternal effect gene is a gene that, when mutant in the mother, results in a mutant phenotype in the offspring, regardless of the offspring’s own genotype. – In fruit fly development, maternal effect genes encode proteins or mRNA that are placed in the egg while it is still in the ovary. – When the mother has a mutation in a maternal effect gene, she makes a defective gene product (or none at all) and her eggs will not develop properly when fertilized. • Maternal effect genes are also called egg-polarity genes because they control the orientation of the egg and consequently the fly. – One group of genes sets up the anterior-posterior axis, while a second group establishes the dorsal-ventral axis. • One gene, bicoid, affects the front half of the body. Figure 18.21 Head Tail A8 T1 T2 T3 A1 A2 A3 A4 A5 A6 Wild-type larva A7 250 µm Tail Tail A8 A8 A7 A6 A7 Mutant larva (bicoid) Figure 18.22 100 µm RESULTS Anterior end Fertilization, translation of bicoid mRNA Bicoid mRNA in mature unfertilized egg Bicoid mRNA in mature unfertilized egg Bicoid protein in early embryo Bicoid protein in early embryo Bicoid Fig. 18.22 • An embryo whose mother has a mutant bicoid gene lacks the front half of its body and has duplicate posterior structures at both ends. – Bicoid mRNA is concentrated at the extreme anterior end of the egg cell. – Bicoid mRNA is produced in nurse cells, transferred to the egg and anchored to the cytoskeleton at the anterior end of the egg. – After the egg is fertilized, bicoid mRNA is transcribed into Bicoid protein, which diffuses from the anterior end toward the posterior, resulting in a gradient of proteins in the early embryo. • Gradients of specific proteins, morphogens, determine the posterior end as well as the anterior end and also are responsible for establishing the dorsal-ventral axis. Cancer results from genetic changes that affect cell cycle control • Cancer is a set of diseases in which cells escape the control mechanisms that normally regulate cell growth and division. – The genes that normally regulate cell growth and division during the cell cycle include genes for growth factors, their receptors, and the intracellular molecules of signaling pathways. – Mutations altering any of these genes in somatic cells can lead to cancer. • The gene regulation systems that go wrong during cancer are the very same systems that play important roles in embryonic development, the immune response, and other biological processes. • The agent of such changes can be random spontaneous mutations or environmental influences such as chemical carcinogens, X-rays, or tumor viruses. • Oncogenes are cancer-causing genes Proto-Oncogenes • Proto-oncogene products are proteins that stimulate normal cell growth and division and play essential functions in normal cells. • A proto-oncogene becomes a cancer promoting oncogene following genetic changes that lead to an increase in the protooncogene’s protein production or in the activity of each protein molecule. • Conversion of a proto-oncogene to an oncogene can lead to abnormal stimulation of the cell cycle Proto-Oncogene → Oncogene Fig. 18.23 • Translocation of DNA: – A fragment may be moved to a location near an active promoter or other control element, resulting in excess protein expression. • Gene Amplification: – Movement of transposable elements may also place multiple copies of the DNA segment near an active promoter, increasing protein expression. • Point Mutations within a control element or the coding sequence: – Increased protein production – Hyperactive protein, or one that is not degraded properly Mutations to tumor-suppressor genes may contribute to cancer • The normal products of tumor-suppressor genes inhibit cell division. • Any decrease in the normal activity of a tumorsuppressor protein may contribute to cancer. – Some tumor-suppressor proteins normally repair damaged DNA, preventing the accumulation of cancer-causing mutations. – Other tumor-suppressor proteins control the adhesion of cells to each other or to an extracellular matrix, which is crucial for normal tissues and often absent in cancers. – Still others are components of cell-signaling pathways that inhibit the cell cycle. Oncogene proteins and faulty tumorsuppressor proteins • • • Oncogene proteins and faulty tumor-suppressor proteins interfere with normal cell-signaling pathways. Mutations in the products of two key genes, the ras proto-oncogene and the p53 tumor-suppressor gene, occur in 30% and 50% of human cancers, respectively. Both the Ras protein and the p53 protein are components of signaltransduction pathways that convey extracellular signals from the plasma membrane to the DNA in the cell’s nucleus to effect expression of proteins. Figure 18.24 Normal and mutant cell cycle–stimulating pathway 1 Growth factor 3 G protein P P P P P P NUCLEUS Ras GTP 2 Receptor 5 Transcription factor (activator) 6 Protein that stimulates the cell cycle 4 Protein kinases MUTATION Ras GTP NUCLEUS Transcription factor (activator) Ras protein active with or without growth factor. Overexpression of protein Ras The Ras protein, the product of the ras gene, is a protein that relays a growth signal from a growth factor receptor on the plasma membrane to a cascade of protein kinases. At the end of the pathway is the synthesis of a protein that stimulates the cell cycle. Many ras oncogenes have a point mutation that leads to a hyperactive version of the Ras protein that can issue signals on its own, resulting in excessive cell division in the absence of growth factors. p53 • The p53 gene is a tumor-suppressor gene. • The p53 protein is a specific transcription factor for the synthesis of several cell cycle-inhibiting proteins. • Damage to the cell’s DNA acts as a signal that leads to expression of the p53 gene. • The p53 protein can activate the p21 gene, whose product halts the cell cycle by binding to cyclindependent kinases, allowing time for DNA repair. • The p53 protein can also turn on genes directly involved in DNA repair. • When DNA damage is irreparable, the p53 protein can activate “suicide genes” whose protein products cause cell death by apoptosis. • A mutation that knocks out the p53 gene can lead to excessive cell growth and cancer. Figure 18.25 2 Protein kinases 5 Protein that NUCLEUS inhibits the cell cycle UV light 1 DNA damage in genome 3 Active form 4 Transcription of p53 Inhibitory protein absent UV light MUTATION DNA damage in genome Defective or missing transcription factor. The Multistep Model of Cancer Development • Multiple DNA mutations are generally needed for full-fledged cancer thus the incidence increases with age. • If cancer results from an accumulation of mutations, and if mutations occur throughout life, then the longer we live, the more likely we are to develop cancer. – A cancerous cell is usually characterized by at least one active oncogene and the mutation of several tumor-suppressor genes Figure 18.26 Colon 1 Loss of tumor- 2 Activation of 4 Loss of suppressor gene APC (or other) ras oncogene tumor-suppressor gene p53 Colon wall Normal colon epithelial cells Small benign growth (polyp) 3 Loss of tumorsuppressor gene SMAD4 Larger benign growth (adenoma) 5 Additional mutations Malignant tumor (carcinoma) Cancer can run in families • An individual inheriting an oncogene or a mutant allele of a tumor-suppressor gene is one step closer to accumulating the necessary mutations for cancer to develop. • About 15% of colorectal cancers involve inherited mutations of the tumor-suppressor gene adenomatous polyposis coli, or APC. – Normal functions of the APC gene include regulation of cell migration and adhesion. – Even in patients with no family history of the disease, APC is mutated in about 60% of colorectal cancers. • Between 5% and 10% of breast cancer cases show an inherited predisposition for mutations in one of two tumor-suppressor genes, BRCA1 and BRCA2. – A woman who inherits one mutant BRCA1 allele has a 60% probability of developing breast cancer before age 50. Figure 18.27 MAKE CONNECTIONS: Genomics, Cell Signaling, and Cancer Normal Breast Cells in a Milk Duct • ERα+ • PR+ • HER2+ Breast Cancer Subtypes Luminal A Luminal B Duct Estrogen interior receptor alpha (ERα) Progesterone receptor (PR) HER2 (a receptor tyrosine kinase) Support cell Extracellular matrix • ERα+++ • PR++ • HER2− • 40% of breast cancers • Best prognosis HER2 • ERα− • PR− • HER2++ • 10–15% of breast cancers • Poorer prognosis than luminal A subtype • ERα++ • PR++ • HER2− (shown); some HER2++ • 15–20% of breast cancers • Poorer prognosis than luminal A subtype Basal-like • ERα− • PR− • HER2− • 15–20% of breast cancers • More aggressive; poorer prognosis than other subtypes The Role of Viruses in Cancer • A number of tumor viruses can also cause cancer in humans and animals • Viruses can interfere with normal gene regulation in several ways if they integrate into the DNA of a cell • Viruses are powerful biological agents Testing for mutations in BRCA1 and BRCA2.