* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Submembraneous microtubule cytoskeleton: biochemical and
G protein–coupled receptor wikipedia , lookup
Cell growth wikipedia , lookup
Cell encapsulation wikipedia , lookup
Membrane potential wikipedia , lookup
Cell culture wikipedia , lookup
Node of Ranvier wikipedia , lookup
Cyclic nucleotide–gated ion channel wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Cellular differentiation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell membrane wikipedia , lookup
Endomembrane system wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Signal transduction wikipedia , lookup
Cytokinesis wikipedia , lookup
List of types of proteins wikipedia , lookup
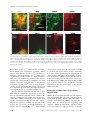
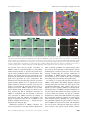
MINIREVIEW Submembraneous microtubule cytoskeleton: biochemical and functional interplay of TRP channels with the cytoskeleton Chandan Goswami and Tim Hucho Department for Molecular Human Genetics, Max Planck Institute for Molecular Genetics, Berlin, Germany Keywords actin; axonal guidence; cytoskeleton; growth cone; myosin; pain; signalling complex; transient receptor potential channels; tubulin; varicosity Correspondence C. Goswami, Department for Molecular Human Genetics, Max Planck Institute for Molecular Genetics, Ihnestrasse 73, 14195 Berlin, Germany Fax: +49 30 8413 1383 Tel: +49 30 8413 1243 E-mail: [email protected] Much work has focused on the electrophysiological properties of transient receptor potential channels. Recently, a novel aspect of importance emerged: the interplay of transient receptor potential channels with the cytoskeleton. Recent data suggest a direct interaction and functional repercussion for both binding partners. The bi-directionality of physical and functional interaction renders therefore, the cytoskeleton a potent integration point of complex biological signalling events, from both the cytoplasm and the extracellular space. In this minireview, we focus mostly on the interaction of the cytoskeleton with transient receptor potential vanilloid channels. Thereby, we point out the functional importance of cytoskeleton components both as modulator and as modulated downstream effector. The resulting implications for patho-biological situations are discussed. (Received 15 April 2008, revised 23 June 2008, accepted 30 July 2008) doi:10.1111/j.1742-4658.2008.06617.x The microtubule cytoskeleton plays a role in a variety of cellular aspects such as division, morphology and motility, as well as the transport of molecules and organelles toward and from the cell membrane. Although all these phenomena affect the plasma membrane, however, most of the microtubule filaments do not reach to the lipid membrane region, partially due to a thick hindering cortical actin network. However, recent studies indicate that a small number of dynamic microtubules can extend rapidly to the cell membrane. Although most contacts are established only transiently, there are membranous regions in which the plus end of these pioneering microtubules is stabilized. Stabilization appears to be mediated by the interaction with various membrane proteins, which often are part of large protein complexes. The dynamic properties and the complexity of tubulin as an interacting protein in large complexes at the membrane just are beginning to be unravelled. One apparent function is to serve as a scaffold protein and modulator of transmembrane signalling. Cytoskeletal components in signalling complexes at membranes The cytoplasmic domains of transient receptor potential (TRP) channels recruit large complexes of proteins, lipids and small molecules. Depending on the Abbreviations FHIT, fragile histidine triad protein; MAP, microtubule-associated protein; RTX, resinferatoxin; TRP, transient receptor potential; TRPV, transient receptor potential vanilloid; TRPV1, transient receptor potential vanilloid subtype 1; TRPV1-Ct, C-terminal of transient receptor potential vanilloid subtype 1; TRPV1-Nt, N-terminal of transient receptor potential vanilloid subtype 1; TRPV2, transient receptor potential vanilloid subtype 2; TRPV4, transient receptor potential vanilloid subtype 4. 4684 FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho preparation method, these structures have been referred to as ‘signalplex’, i.e. complexes involved in signalling events [1], or ‘channelosome’, i.e. complexes formed around functional ion channels [2], and ⁄ or as ‘lipid raft complexes’, i.e. complexes localized to this membranous subdomain [3]. Proteomic studies of ‘signalplexes’ or ‘channelosomes’ purified from cell lines, as well as from brain, give both direct and indirect evidence for the presence of the cytoskeleton as well as ion channels. Scaffolding adaptors like inactivation-no-afterpotential D [4–6], Na+ ⁄ H+ exchanger regulatory factor [7] and ezrin ⁄ moesin ⁄ radixin-binding phosphoprotein 50 [8] are also found, some of which interact directly with ion channels, e.g. TRP channels [9], but also contain binding motifs for cytoskeletal proteins [8,10]. Accordingly, cytoskeletal proteins such as spectrin, myosin, drebrin and neurabin, as well as tubulin and actin [1,2,6,11] are confirmed components in signalplexes and channelosomes. Complementary proteomic studies of purified lipid rafts reveal the presence of several cytoskeletal proteins [12,13] such as a- and b-tubulin, tubulin-specific chaperone A (a folding protein involved in tubulin dimer assembly), KIF13 (a kinesin) [12], actin, nonconventional myosin II and nonconventional myosin V [14]. Similarly, proteomics studies of the ‘membrane cytoskeleton’, a submembranous fraction, which is attached to the cytoskeleton [15], and of cytoskeleton-associated proteins in general [16], indicate the presence of lipid raft membrane proteins as well as cytoskeletal proteins. Together, these varying studies give strong evidence that cytoskeletal proteins are part of signalling complexes including transmembrane proteins and are involved in their organization at membrane. Structural features of TRP channels The TRP family of ion channels is named after the Drosophila melanogaster trp mutant, which is characterized by a transient receptor potential in the photoreceptors in response to light [17]. In the meantime, orthologues and paralogues of TRP channels have been described in organisms ranging from simple eukaryotes to human. They share a high degree of homology in their amino acid sequence. TRP channels are formed by monomers with six transmembrane regions that assemble into tetramers, which form the functional cation-permeable pore. The most conserved region is the sixth transmembrane domain, which constitutes most of the inner lining of the ion channel pore. The N- and C-termini of TRP channels are located in the cytoplasm and, depending on the respective TRP channel, consist of various functional TRP channels and cytoskeleton regulate each other domains like ankyrin repeats, Ca2+-sensing EF hands, phosphorylation sites, calmodulin-binding sites and a so-called ‘TRP box’. Based on their sequence, the mammalian TRP family is differentiated into six subfamilies, namely TRP canonical (TRPC), TRP vanilloid (TRPV), TRP melastatin (TRPM), TRP polycystin (TRPP), TRP mucolipin (TRPML) and TRP ankayrin (TRPA) ion channels [18]. All TRP channels investigated to date are involved in the detection and ⁄ or transduction of physical and chemical stimuli. TRPV1 and the cytoskeleton Physical interaction of TRPV1 with the cytoskeleton TRPV1 is the founding member of the vanilloid subfamily of TRP channels and detects several endogenous agonists (e.g. N-arachidonoyl-dopamine) and noxious exogenous stimuli, such as capsaicin (the main pungent ingredient of hot chilly) and high temperature (> 42 C) [19,20]. TRPV1 is a nonselective cation channel with high permeability for Ca2+. In recent years, TRPV1 has gained extensive attention for its involvement in signalling events in the context of pain and other pathophysiological conditions including cancer [21–27]. The interaction of TRPV1 with tubulin was first identified through a proteomic analysis of endogenous interactors enriched from neuronal tissue [28]. The interaction was then confirmed by biochemical approaches including co-immunoprecipitation, microtubule co-sedimentation, pull-down and cross-linking experiments. In contrast to the tubulin cytoskeleton, the physical interaction of TRPV1 with actin or neurofilament cytoskeleton has not been observed to date [28,29]. The C-terminus of TRPV1 (TRPV1-Ct) is sufficient for the interaction with tubulin while the N-terminus of TRPV1 (TRPV1-Nt) apparently does not interact [28]. Using deletion constructs and biotinylated peptides, the tubulin-binding region located within TRPV1-Ct was mapped to two short, highly basic regions (amino acids 710–730 and 770–797) [29]. If an a-helical conformation is assumed, these two regions project all their basic amino acids to one side, thus potentially enabling interactions with negatively charged residues (Fig. 1). Indeed, correspondingly, the C-terminal over-hanging region of tubulin contains a large number of negatively charged glutamate (E) residues in a stretch characterized as unstructured region of the tubulin and referred as E-hook. These E-hooks are known to be essential for the interaction of tubulin FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4685 TRP channels and cytoskeleton regulate each other C. Goswami and T. Hucho A B Fig. 1. Characteristic of the tubulin-binding motifs located at the C-terminus of TRPV1. (A) The extreme C-terminus of both a- and b-tubulin contains highly negatively charged amino acids (indicated in red) and is mostly unstructured. (B) The basic amino acids (indicated in blue) that are located within the tubulin-binding regions of TRPV1 are located at one side of the putative helical wheel, where it can interact with the acidic C-terminus of tubulin. with various microtubule-associated proteins such as MAPs, Tau, as well as others. Indeed, binding of TRPV1-Ct with tubulin was abolished when the E-hooks containing over-hangs were removed by protease treatment [29]. The tubulin-binding region of TRPV1 apparently is under high evolutionary pressure as its sequence is highly conserved in all TRPV1 orthologues [29]. Also between homologues, the distribution of basic amino acids composing the tubulin-binding regions is conserved even though the overall amino acid conservation is rather limited. Based on these data an interaction of tubulin with TRPV2, TRPV3 and TRPV4 (Fig. 2) can also be predicted. These TRPV1 homologues have the highest conservation of basic charge distribution within the tubulin-binding sequences. Indeed, in the meantime we could confirm this for TRPV2 and TRPV4 (unpublished observation). TRPV1 preferably interacts through its C-terminal domain with b-tubulin and to a lesser extend also with a-tubulin thereby forming a high-molecular weight complex [29]. This suggests stronger binding of TRPV1 to the plus end rather than the minus end of A B Fig. 2. Conservation of the tubulin-binding regions in TRPV1 orthologues and homologues. (A) The tubulin-binding region is conserved in mammals. The conserved basic amino acids are shown in blue and are indicated by an asterisk (*). NCBI accession numbers: rat (NP-114188), mouse (CAF05661), dog (AAT71314), human (NP_542437), guinea pig (AAU43730), rabbit (AAR34458), chicken (NP_989903) and pig (CAD37814). (B) TRPV1 homologues (based on sequences from rat species only) were aligned using CLUSTAL. The distribution of basic amino acids (in blue) located within the first tubulin-binding motif is partially conserved. NCBI accession numbers: TRPV1 (NP-114188), TRPV2 (AAH89215), TRPV3 (NP-001020928), TRPV4 (NP-076460), TRPV5 (AAV31121) and TRPV6 (Q9R186). 4686 FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho microtubules as the plus ends of microtubule protofilaments are decorated with b-tubulin. It is therefore tempting to speculate that TRPV1 may act as a microtubule plus-end-tracking protein (+TIP) [30]. This speculation is corroborated by the recent observation that despite their differences in primary amino acid sequences, the crystal structures of microtubule-binding regions of different classes of +TIP proteins such as Stu2p, EB1 and Bim1p contain a common motif of at least two a helices with positively charged residues at the surface [31]. The tubulin-binding ability of TRPV1-Ct is supported by the predicted structural models also [32,33]. This is particularly due to the fact that the tubulin-binding regions are predicted to contain a helices. Fragile histidine triad protein (FHIT), a tumour suppressor gene product has high sequence homology with TRPV1-Ct and the crystal structure of FHIT was used as a template for predicting the structure of TRPV1-Ct [32]. Remarkably, FHIT also binds to tubulin [34]. Different post-translationally modified tubulin, like tyrosinated tubulin (a marker for dynamic microtubules), detyrosinated tubulin, acetylated tubulin, polyglutamylated tubulin, phospho (serine) tubulin and neurone-specific b-III tubulin (all markers for stable microtubules) interact with TRPV1-Ct [29]. This implies that TRPV1 interacts not only with soluble tubulin, but also with assembled microtubules in various dynamic states. And indeed, the interaction of TRPV1-Ct also with polymerized microtubules could experimentally been proven [28]. In addition to sole binding, TRPV1Ct exerts a strong stabilization effect on microtubules, which becomes especially apparent under microtubules depolymerising conditions such as presence of nocodazol or increased Ca2+ concentrations [28]. TRPV1 channels are nonselective cation channels. Therefore, the role of increased concentration of Ca2+ on the properties of TRPV1–tubulin and ⁄ or TRPV1– microtubule complex is of special interest. Tubulin binding to TRPV1-Ct is increased by increased Ca2+ concentrations [28]. Interestingly, the microtubules formed with TRPV1-Ct in the presence of Ca2+ become ‘cold stable’ as these microtubules do not depolymerise further at low temperature [28]. The exact mechanism how Ca2+ modulates these physicochemical properties in vitro are not clear. In this regard, it is important to mention that tubulin has been shown to bind two Ca2+ ions to its C-terminal sequence [35–38] and thus Ca2+-dependent conformational changes of tubulin [39] may underlie the observed effects of Ca2+. The biochemical data of direct interaction as well as microtubule stabilization find their correlates in cell biological studies. Transfection of TRPV1 in dorsal TRP channels and cytoskeleton regulate each other root ganglia-derived F11 cells results in co-localization of TRPV1 and microtubules and accumulation of endogenous tyrosinated tubulin (a marker for dynamic microtubules) in close vicinity to the plasma membrane [28] (Fig. 3). As suggested by its preference to bind to the plus-end-exposed b-tubulin, TRPV1 apparently stabilizes microtubules reaching the plasma membrane and thereby increases the number of pioneering microtubules within the actin cortex (Fig. 4). But stabilization induces even stronger changes. The overall cellular morphology is altered dramatically by massive induction of filopodial structures in neuronal as well as in non-neuronal cells [40] (Fig. 4). The mechanism for this is currently under investigation and apparently also includes alterations in the actin cytoskeleton. But, co-localization of TRPV1 with tubulin was observed all along the filopodial stalk and, of note, including the filopodial tips [40]. Tubulin and components attributed to stable microtubules (like acetylated tubulin and MAP2ab) were also observed within these thin filopodial structures [40]. TRPV1-activation induced microtubule disassembly In contrast to the stabilization of microtubules at resting state, activation of TRPV1 results in rapid disassembly of microtubules irrespective of the investigated cellular system (Fig. 3) [41,42]. Again, the underlying mechanism of TRPV1 activation-mediated cytoskeleton remodelling is largely unknown. In F11 cells, TRPV1 activation leads to an almost complete destruction of peripheral microtubules, whereas microtubules close to the microtubule-organizing centre, a structure composed of c-tubulin and stable microtubules at the perinuclear region, remain intact (Fig. 3). Also, the integrity of other cytoskeletal filaments like actin and neurofilaments is not affected by activation of TRPV1 [41]. Potentially, TRPV1 activation may even increase the amount of polymerized actin [43]. Effects caused by the activation of a nonselective cation channel are suggestive of mediation by the influx of, for example, Ca2+. Indeed, high Ca2+ concentrations have the potential to depolymerize microtubules in vitro and in vivo [44,45] through either ‘dynamic destabilization’, i.e. a direct effect of Ca2+ on microtubules, or indirectly by a calcium-induced but signal-cascade-dependent depolymerization [46]. Also, chelating extracellular Ca2+ with EGTA and depletion of intracellular Ca2+ stores with thapsigargin cannot prevent TRPV1-activation-mediated microtubule disassembly [41,47]. Thus, TRPV1-activationinduced microtubule disassembly is apparently not a FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4687 TRP channels and cytoskeleton regulate each other C. Goswami and T. Hucho A 5 µm 10 µm B C 10 µm Fig. 3. TRPV1 regulates microtubule dynamics by two opposing manners. (A) In the absence of activation, TRPV1 co-localizes and stabilizes microtubules at the cell membrane. Confocal immunofluorescence images of a F11 cell and an enlarged area reveals the accumulation of tubulin (red) at the plasma membrane due to the presence of TRPV1 (green). (B) Activation of TRPV1 by RTX results in rapid the disassembly of polymerized microtubules. Filamentous microtubules disappear in the TRPV1 expressing cells but not in the nontransfected cells. (C) Detergent extraction after RTX treatment of TRPV1 expressing cells reveals loss of peripheral microtubules from majority of the cell body. The presence of microtubules is restricted only to the microtubule organizing centre region. Some fragmented microtubules near perinuclear region are also visible. direct effect of high Ca2+ concentrations. Even combined EGTA and thapsigargin, treatment cannot exclude small changes in local Ca2+ concentration. Therefore, these small changes in Ca2+ might trigger an enzymatic cascade leading to depolymerization. This view is also supported by previous studies demonstrating that a small amount of calmodulin can cause massive microtubule depolymerization in the presence of catalytic amounts of Ca2+, but not in the complete absence of Ca2+ [45,48–50]. Subsequent activation of Ca2+-dependent proteases may also trigger proteolysis of structural proteins as a downstream effect [51]. Another potential mechanism that can lead to rapid disassembly of microtubules might be the phosphorylation of microtubule-associated proteins (MAPs). We observed fragmented microtubules all over the cytoplasm after TRPV1 activation, which suggest that specific microtubule-severing proteins like katanin, fidgetin and spastin are probably also involved in this process (Fig. 3) [52–54]. Prolonged stimulation of TRPV1 activates through high Ca2+ concentrations 4688 among others caspase 3 and 8, which leads eventually to cell death [55–59]. In general, extensive fragmentation of the cellular cytoskeleton and programmed cell death correlate well. However, in response to shortterm stimulation of TRPV1 we have not observed any fragmented tubulin bands in western blot analysis [41]. Last, but not least, TRPV1 activation-mediated inhibition of protein synthesis and endoplasmic reticulum fragmentation may also have impact on the microtubule integrity [42]. Implications of TRPV1-induced cytoskeleton destabilization TRPV1 affects biological functions, like cell migration and neuritogenesis, that are largely dependent on the cytoskeleton [42,60,61]. Indeed, rapid disassembly of dynamic microtubules by TRPV1 activation has a strong effect on axonal growth, morphology and migration. TRPV1 is endogenously expressed already at an early embryonic stage and localizes to neurites FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho TRP channels and cytoskeleton regulate each other A i B ii iii C D Fig. 4. Effect of TRPV1–cytoskeletal cross-talk on neuritis and growth cones. (A) At growth cones, TRPV1-enriched plasma membranes stabilize pioneer microtubules within the filopodial structures. (B) Such stabilization of the microtubule results in the induction of neuritogenesis and the formation of elongated cells. (C) Time series of a growth cone developed from F11 cell expressing TRPV1–GFP. Application of RTX results in rapid collapse and retraction of the growth cone. (D) Longer neurites develop multiple varicosities (arrow heads) after RTX application due to disassembly of microtubules. Such varicosities are not visible in TRPV1 expressing cells in absence of activation. Even in case of neurites developed from non-TRPV1 expressing cells, RTX application remains ineffective and do not produce such varicosities. and growth cones (Fig. 4) [47,62]. Activation of TRPV1 results in rapid disassembly of microtubules within neurites (and also at growth cones) while keeping the actin cytoskeleton intact and functional. This destroys the balance between the anterograde force (generated by microtubule cytoskeleton) and the retrograde force (generated by actin cytoskeleton) that determines the axonal morphology and the net neurite growth [63,64]. Sudden loss of polymerized microtubules results in retraction of growth cones and formation of varicosities all along the neurites (Fig. 5). Long-term low-level TRPV1 activation by an endogenous ligand results in shortening of neurites in primary neurons [47]. But as endogenous expression of TRPV1 is widespread and not restricted to neuronal cells, activation of TRPV1 increases the motility of non-neuronal cells like HepG2 and dendritic cells [42,65]. In agreement with the role of TRPV1 in cell motility, dendritic cells from trpv1 - ⁄ - animal show less migration than wild-type [65]. Differential activation of TRPV1 complexes can create an asymmetry in the microtubular organization. Thus, activation of TRPV1 in a specific cellular region may result in the disassembly of microtubules, thereby facilitating the retraction of that part of the cell, thus creating a trailing edge. By contrast, stabilization of microtubules at TRPV1-enriched plasma membranes may facilitate a cell to extend at this region, marking the leading edge and initiating cell migration [66]. In contrast to a strong and long-term activation of TRPV1, which affects microtubules globally, mild and localized short-term activation may affect parts of the cytoskeleton differently. Thus, growth cones may be helped to avoid a repulsive guidance cue. Reciprocally, stabilization effect of TRPV1-enriched membranes on the plus ends of microtubules may help a growth cone to steer towards an attractive cue (Fig. 4). A similar mechanism by which other TRP channels can regulate the growth cone attraction, repulsion or retraction has been described [67,68]. Although not tested, TRPV1 may potentially regulate the sperm motility as the presence of TRPV1 at the sperm acrosome and throughout the tail has been reported [69]. Short-term and lowlevel activation may increase sperm motility whereas FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4689 TRP channels and cytoskeleton regulate each other A C. Goswami and T. Hucho B C Fig. 5. Schematic model depicting how TRPV1 regulates growth cone and neurite movement via cytoskeletal reorganization. (A) Presence of microtubule cytoskeleton (Mt, red) and actin cytoskeleton (blue) at the neurite and at the growth cones are shown. Both an anterograde force from microtubule cytoskeleton (up arrow) and a retrograde force provided by actin cytoskeleton (down arrow) determine the net axonal growth and movement. (B) Most of the axonal microtubules is restricted to the central zone (C-zone) of the growth cone. Few dynamic pioneer microtubules at the peripheral zone (P-zone) are selectively stabilized by TRPV1-enriched membrane patches (green stars). This may have implications for the turning of the growth cone in response to a signal (green asterisk). (C) TRPV1 activation-mediated growth cone retraction and varicosity formation is dependent on the degree of microtubule disassembly. (Stage 1) Activation of TRPV1 (indicated by arrow) results in the partial disassembly of microtubules, leading to the retraction of growth cone. (Stage 2) Further disassembly leads to more retraction and initiates varicosity formation. (Stage 3) Complete disassembly of microtubules results in a stage where further retraction is no more possible. (Stage 4) The force from the functional actin cytoskeleton and complete disassembled microtubules results in the varicosity formation. Strong agonists like RTX result in a quick and irreversible shift to stage 3 and 4. By contrast, transient and mild activation by endogenous ligands like N-arachidonoyl-dopamine results in retraction for a longer time but rarely forms varicosities, indicating that N-arachidonoyl-dopamine most likely results in slow and reversible shifting at stage 1 and 2. robust activation may cause a non-motile sperm due to complete disassembly of microtubules at the sperm tail. TRPV4 and the cytoskeleton TRPV4 is a member of the TRPV subfamily and a close homologue of TRPV1. It is activated by endogenous endovanilloids, by temperatures of > 37 C, and by both hypo- and hyperosmotic 4690 stimuli. In many studies, the synthetic ligand 4a-PDD is alternatively employed [70]. TRPV4 is involved in mechanosensation of the normal and the sensitized neuron [71]. To date, the evidence for the functional, as well as physical, interaction of TRPV4 with cytoskeletal components is mostly indirect. For example, TRPV4 has been shown to be important for the development of taxol-induced mechanical hyperalgesia suggesting a functional link of TRPV4 with microtubule cytoskeleton [72]. Often, activation of TRPV4 is correlated to cellular changes, which in turn are known to involve cytoskeletal rearrangement such as cell volume in regulatory volume decrease [72–74] and cell motility due to changes in lamellipodia dynamics [60]. However, the extent to which the changes in the cytoskeleton are induced by TRPV4 directly is mostly unknown. Biochemical and cell biological data are sparse and patchy. The distance between actin and TRPV4 in a live cell was measured by FRET to be < 4 nm [75] assuring, that these two components have the potentiality to interact with each other. In addition, TRPV4 has been identified by yeast two-hybrid screen to interact with MAP7, an interaction confirmed by immunoprecipitation as well as pull-down experiments [76]. This interaction is dependent on the C-terminal amino acids 798–809. Interestingly, the C-terminal cytoplasmic domain of TRPV4 also contains a partially conserved putative tubulin-binding site [29]. Physical and functional interaction of other TRP channels with cytoskeletal components Physical links of several TRP channels other than TRPV1 and TRPV4 with the cytoskeleton have been established. For example, b-tubulin interacts directly with TRPC1 [77]. Two other members of the TRPC family, TRPC5 and TRPC6, are also interacting with cytoskeletal proteins [1]. These also include actin and tubulin as they are confirmed components of the purified ‘signalplex’ [1]. TRPC5 interacts with stathmin 2, a microtubule cytoskeleton-binding protein [78]. Direct physical and functional interplay with both the microtubule and actin cytoskeleton has also been described for TRPP channels (see minireview by Chan et al. in this series). Apart from the direct interaction, many of these TRP channels are localized to the microtubule and actin cytoskeleton-enriched structures like filopodia, cilia and growth cones, indicating a potential association and complex signalling with the cytoskeleton. Again, activation of these TRP channels correlates FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho with cytoskeleton-dependent morphological changes. For example, both rat and human pulmonary arterial endothelial cells express TRPP1, the activation of which leads to a change in cell shape due to reorganization of cortical actin network [79]. Likewise, Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner ear hair cells [80]. Almost all of the TRPC channels have been reported to localize to growth cones [67,77,81–84]. In all cases, activation of TRPC channels regulates growth cone morphology and motility in response to chemical guidance cues. TRPC1 modulates the actin cytoskeleton by modulating ADF ⁄ cofilin activity via LIM kinase [85]. It is involved in growth cone turning in response to Netrin 1 [82,83]. TRPC4 is upregulated after nerve injury and is important for neurite outgrowth [81]. TRPC3 and TRPC6 are important for growth cone turning in response to brain-derived neurotrophic factor [84]. Likewise, TRPC5 expression results in increased length of neurites and filopodia [78]. In addition to data on the interaction of TRP channels with the cytoskeleton, there are few examples suggesting that the activity of the TRP channel is influenced by the cytoskeleton. Mostly, alteration of the cytoskeleton results in inhibition of TRP channel opening. For example, the requirement for a functional cytoskeleton in the activation of TRP channels in store-mediated Ca2+ entry and ⁄ or store-operated Ca2+ entry has been reported [86–88]. In human platelets, physical coupling of hTRP1 with inositol 1,4,5-triphosphate receptor (IP3R), a prerequisite step for store-mediated Ca2+ entry, depends on the degree of polymerized actin [86,87]. Disruption of the actin cytoskeleton by cytochalasin D also prevents phosphatidylinositol 4,5-bisphosphate (PIP2)-mediated inhibition of TRPC4a [89]. Another example has been reported from primary human polymorphonuclear neutrophil cells, in which reorganization of actin results in the internalization of endogenous TRPC1, TRPC3 and TRPC4 from plasma membrane to the cytosol, which correlates well with the loss of store-operated calcium entry [88]. Accordingly, pre-disruption of the actin cytoskeleton by cytochalasin D rescues the loss of store-operated calcium entry, indicating that the actin dynamics are important for this TRPC-mediated storeoperated calcium entry. Apparently, an intact actin cytoskeleton is also essential for strong agonist-mediated activation of TRPC7. Thus, pharmacological disruption of the actin cytoskeleton results in reduced agonist-induced activation [90]. All these examples suggest that the cytoskeleton can indeed act as modulators of TRP channel function. TRP channels and cytoskeleton regulate each other TRP channels and myosin motors Nonconventional myosin motors and TRP channels are often localized within specific subcellular regions such as filopodia or ciliary tips. These two groups of proteins also share a special genotype–phenotype correlation as abnormal expression ⁄ function of these myosins or TRPs gives rise to similar pathophysiological conditions like deafness, blindness, and syndromes affecting the function of other tissues and ⁄ or organs. For example, in case of deafness, several nonconventional myosin motors (myosin I, IIA, IIIA, VI, VIIA and XV) are important for either development of the stereocilia of hair cells in the inner ear or proper localization of TRP channels at the tip of these stereocilia, which is crucial for the activity of these cells [91,92]. Reciprocally, mutations and abnormal expression ⁄ function of several TRP channels (TRPML1, TRPML2, TRPML3, TRPV4, TRPV5 and TRPV6) also lead to deafness [93–97]. In a similar manner, both myosins and TRP channels are causally involved in blindness. Recently it has been reported that translocation of eGFP-tagged TRP-like channels to the rhabdomeral membrane in Drosophila photoreceptors is myosin III dependent [98]. Apart from the above genetic interactions, TRP channels interact directly with myosins. Using a proteomic screen, myosin was identified to bind to TRPC5 and TRPC6 [1]. Another study showed that myosin IIa is directly phosphorylated by TRPM7, a cation channel fused to an alpha-kinase [99]. This phosphorylation in turn regulates cell contractility and adhesion. Notably, TRPM7 phosphorylates positively charged coiled-coil domain of myosin II [100]. In some cases, similar cellular phenotypes also suggest a functional link between TRP channels and nonconventional myosin motors. For example, we observed that ectopic expression of TRPV1 induces extensive filopodial and neurite-like structures in neuron-derived F11 cells as well as in non-neuronal cells (e.g. HeLa, Cos and HEK 293 cells) [40]. Interestingly, TRPV1 expression induces club-shaped filopodia with a bulbous head structure that contains negligible amount of F-actin but accumulates TRPV1 [40]. This phenotype resembles the dominant negative effect of the expression of the non-conventional myosin II, III, V, X and XV [101–112]. This renders the observation that TRPV1 expression induces drastic upregulation of endogenous myosin IIa and IIIa temptingly suggestive [40]. In addition, the subcellular distribution of myosins is markedly changed from a uniformly cytoplasmic to a strongly clustered localization mostly at the cell periphery [40]. In another study, cardiac-specific overexpression of TRPC6 in transgenic mice resulted FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4691 TRP channels and cytoskeleton regulate each other C. Goswami and T. Hucho in an increase expression of beta-myosin heavy chain [113]. Such phenomena strongly suggest the cooperative role of myosins and TRP channels in development as well as proper function of ciliary and filopodial structures. The molecular mechanisms behind the increased expression of these myosins are poorly understood. In some cases, TRP channels apparently increase myosin expression by regulating transcription factors [113]. As myosins are also susceptible to protease-mediated degradation; a higher level of endogenous myosin might also imply less proteolytic degradation. It is therefore worth exploring how TRP channels affect the distribution, function and endogenous level of myosins. Modulation of TRP ion channels activities by the cytoskeleton TRP channels can be modulated by Ca2+-dependent or Ca2+-independent mechanisms. Desensitization can be initiated by the Ca2+-influx through the channel itself and is manifested through phosphorylation– dephosphorylation of the TRP channel and ⁄ or by the Ca2+-dependent interaction with calmodulin at the C-termini of, for example, TRPV1 and TRPV4 [114– 121]. As an example of Ca2+-independent mechanisms, the channel inactivation through physical interaction of the TRPC channel with the cytoplasmic protein homer has been described. TRPC mutants lacking the homer-binding site become constitutively active [9]. This latter example spurs one to hypothesize whether other scaffolding proteins than homer, such as actin and ⁄ or tubulin, can regulate TRP channel properties though to date the experimental evidence is only circumstantial. Modulation of TRPV4 by a cytosolic component is suggested, as the channel can be activated by heat only if analysed using whole-cell recordings and not in excised patches of cell-free membranes [122,123]. In turn, the involvement of cytoskeletal components in the regulation of TRPV4 channel activity has been demonstrated experimentally by the addition of cytoskeletal regulating drugs. The microtubule stabilizer taxol reduces TRPV4-dependent currents while the microtubule-disrupting agents colchicine and vincristine as well as actin cytoskeleton regulating drugs like phalloidin (a stabilizer) or cytochalasin B (a destabilizer) do not alter the TRPV4-mediated current [76]. In the same manner, mechanosensitive ion channel activity in cultured sensory neurons appears to depend largely on the status of the cytoskeleton. Thus, disruption of actin or microtubule cytoskeleton by pharmacological agents greatly reduces the activity of mechanosensitive channels [124]. However, if the 4692 modulation of TRP channels occurs through direct interaction with the cytoskeleton remains to be proven. In addition to purely circumstantial evidence, few studies attempted the establishment of a direct modulatory role of the cytoskeleton, the best of which was performed on TRPP channels [125,126]. Montalbetti and co-workers isolated syncytiotrophoblast apical membrane vesicles from human placenta, and performed single-channel electrophysiological experiments of polycystin channel 2 (PC2) on reconstituted lipid bilayers. This system eliminates all factors except the channel-associated complex. Biochemical analysis revealed the presence of actin, the actin-related components a-actinin and gelsolin, tubulin including acetylated a-tubulin, and the kinesin motor proteins KIF3A and KIF3B in these membranes [125,126]. PC2 channels interact directly with KIF3. Disruption of actin filaments with cytochalasin D or with the actin-severing protein gelsolin activates the channel. This activation can be inhibited by the addition of soluble monomeric G-actin with ATP, which induces actin polymerization. This indicates that actin filaments, but not soluble actin, are an endogenous negative regulator of PC2 channels. Also microtubules regulate PC2 channel function only in opposing manner. Depolymerization of microtubules with colchicine rapidly inhibits the basal level of PC2 channel activity, whereas polymerization and ⁄ or stabilization of microtubules from soluble tubulin with GTP and taxol stimulates the PC2 channel activity [125]. Involvement of the microtubule cytoskeleton in the regulation of PC2 channel has also been described in vivo in primary cilia of renal epithelial cells [127]. In that system, addition of microtubule destabilizer (colchicine) rapidly abolished channel activity, whereas the addition of microtubule stabilizers (taxol) increased channel activity [127]. Similar results were obtained using reconstituted lipid bilayer system, which reveals that both spontaneous activity and the opening probability of TRPP3 ion channels is increased by the addition of a-actinin, demonstrating that this channel can be indeed modulated by cytoskeleton [128]. TRPV1, TRPV4 and the cytoskeleton in pathophysiological conditions The importance of TRP channels in the development of disease becomes increasingly evident. In particular, TRPV channels are involved in various aspects of pain such as inflammatory pain, cancer pain and neuropathic pain, as well as other diseases including allergy, diabetes and cough [129]. Indeed, data tie TRPV1 and TRPV4 to the status of the cytoskeleton in models of pain. For FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho example, rapidly dividing cancer cells are pharmacologically targeted by modulators of the microtubule cytoskeleton such as taxol, vincristin and their derivatives. But in addition to the deleterious effect of these agents on the cancer, if applied systemically over the long-term they are highly potent inducers of strong neuropathic pain [130–132]. Systemic vincristine treatment strongly alters the cytoskeletal architecture [133,134]. On a shorter timescale, inflammatory signalling pathways leading to sensitization in a healthy animal are dependent on both the ‘integrity’ and the ‘dynamics’ of the microtubule cytoskeleton [135,136]. Short-term modulation of the cytoskeleton abolishes inflammatory mediator-induced sensitization [135]. The precise mechanism by which vinca-drugs and taxoid-group-containing molecules influence pain is not clear. Vincristine by itself is known to form tubulin paracrystals [137]. Taxol can also form crystals, which can masquerade as stabilized microtubules and can rapidly incorporate tubulin dimers [138]. In addition, a unique type of straight GDP–tubulin protofilament forms in the presence of taxol [139]. Whether these uncommon altered physical forms of tubulins ⁄ microtubules are important for pain development remains a central question. However, more subtle effects like differential binding properties to other proteins might play a role. In addition, the involvement of several TRP channels in the development of cancer and cancer pain is increasingly prominent [22,140–142]. Endogenous expressions of some TRP channels are either upregulated or downregulated in different tumours, cancerous tissue and also in different cancerous cell lines. For example, TRPV1 is overexpressed in bone cancer, prostate cancer and pancreatic cancer [143–146]. Along the same lines, TRPV4, which is involved in mechanosensation, has been shown to be essential for the development of chemotherapy-induced neuropathic pain in the rat [72]. TRP channels also share functional links with the cytoskeleton by other means, namely cytotoxicity and cell death. For example, activation of TRPV1 leads to the inhibition of protein synthesis and endoplasmic reticulum fragmentation [42]. Prolonged stimulation with capsaicin induces apoptosis in TRPV1 expressing neurons by activating different caspase pathways (mainly caspase 8, caspase 3) [55–58,147–153]. However, whether the cytotoxicity and cell death described above is due to disassembly of microtubule has not been tested. Loss of TRPV1-expressing neurons ⁄ cells from specific tissues are functionally linked with the development of patho-physiological conditions. For example, loss of TRPV1 positive neurons in liver is linked with diabetes [154]. TRP channels and cytoskeleton regulate each other However, the deleterious effect of TRP channels on the specific subtype of neurons or cells has some clinical advantages. In fact, retraction and degeneration of a subset of sensory neurons (specifically TRPV1expressing neurons), which involve events that affect the integrity of the cytoskeleton, forms the basis for the analgesic effect of topical capsaicin-cream treatment [155]. Resinferatoxin (RTX), a potent agonist of TRPV1 has been used successfully to eliminate pancreatic cancerous cells because TRPV1 is highly expressed in this pancreatic cancer conditions [143–146]. Such clinical application of agonists specific for different TRP channels may, therefore, turn out to be effective and has full potential as chemotherapeutics. Based on this approach, recently use of TRP agonists as therapeutics is becoming popular in ‘TRPpathies’ or ‘channelopathies’ [156]. Concluding remarks The last few years have seen rapid progress in the study of TRP channels as well as other ion channels in the context of both actin and the microtubule cytoskeleton. The presence of the microtubule cytoskeleton at the membrane is now beyond doubt [157]. The role of microtubule plus-end-binding proteins for specific sorting and targeting of different ion channels, receptors to specific regions of the membrane is well established [158]. However, the functional implications of this remain one of the current challenges. We find the role of the cytoskeleton to be both direct and indirect. In particular, the inside-out modulation of ion channels emerges as a peculiarly novel aspect with wide ranging consequences for both the pathological and the general homeostatic state. Because the large extended structure of the cytoskeleton is able to potentially integrate signalling events from very distant sides, single signals as well as associative stimuli will have to be investigated. In addition, the cytoskeleton has been proven to be both a target of signalling cascades and to initiate them itself. Thus it has the potential to recruit feedback and feed-forward regulation of a number of cellular effects. This renders the cytoskeleton as an interesting target for therapeutic approaches with respect to the TRP channels with much to discover. Acknowledgements We thank Julia Kuhn for critically reading this minireview and for her suggestions. We thank Prof. H. H. Ropers for supporting this work. Funding from Max Planck Institute for Molecular Genetics (Berlin, Germany) is gratefully acknowledged. We regret for FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4693 TRP channels and cytoskeleton regulate each other C. Goswami and T. Hucho not been able to mention many of the relevant citations due to space limitation. 13 References 1 Goel M, Sinkins W, Keightley A, Kinter M & Schilling WP (2005) Proteomic analysis of TRPC5- and TRPC6-binding partners reveals interaction with the plasmalemmal Na(+) ⁄ K(+)-ATPase. Pflugers Arch 451, 87–98. 2 Ambudkar IS & Ong HL (2007) Organization and function of TRPC channelosomes. Pflugers Arch 455, 187–200. 3 Sanxaridis PD, Cronin MA, Rawat SS, Waro G, Acharya U & Tsunoda S (2007) Light-induced recruitment of INAD-signaling complexes to detergent-resistant lipid rafts in Drosophila photoreceptors. Mol Cell Neurosci 36, 36–46. 4 Shieh BH & Zhu MY (1996) Regulation of the TRP Ca2+ channel by INAD in Drosophila photoreceptors. Neuron 16, 991–998. 5 Huber A, Sander P, Gobert A, Bähner M, Hermann R & Paulsen R (1996) The transient receptor potential protein (Trp), a putative store-operated Ca2+ channel essential for phosphoinositide-mediated photoreception, forms a signaling complex with NorpA, InaC and InaD. EMBO J 15, 7036–7045. 6 Li HS & Montell C (2000) TRP and the PDZ protein, INAD, form the core complex required for retention of the signalplex in Drosophila photoreceptor cells. J Cell Biol 150, 1411–1422. 7 Voltz JW, Weinman EJ & Shenolikar S (2001) Expanding the role of NHERF, a PDZ-domain containing protein adapter, to growth regulation. Oncogene 20, 6309–6314. 8 Mery L, Strauss B, Dufour JF, Krause KH & Hoth M (2002) The PDZ-interacting domain of TRPC4 controls its localization and surface expression in HEK293 cells. J Cell Sci 115, 3497–3508. 9 Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S et al. (2003) Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 114, 777–789. 10 Tang Y, Tang J, Chen Z, Trost C, Flockerzi V, Li M, Ramesh V & Zhu MX (2000) Association of mammalian trp4 and phospholipase C isozymes with a PDZ domain-containing protein, NHERF. J Biol Chem 275, 37559–37564. 11 Sinkins WG, Goel M, Estacion M & Schilling WP (2004) Association of immunophilins with mammalian TRPC channels. J Biol Chem 279, 34521–34529. 12 Li N, Shaw AR, Zhang N, Mak A & Li L (2004) Lipid raft proteomics: analysis of in solution digest of sodium dodecyl sulfate-solubilized lipid raft proteins 4694 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 by liquid chromatography-matrix-assisted laser desorption ⁄ ionization tandem mass spectrometry. Proteomics 4, 3156–3166. Foster LJ, De Hoog CL & Mann M (2003) Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA 100, 5813–5818. Ishmael JE, Safic M, Amparan D, Vogel WK, Pham T, Marley K, Filtz TM & Maier CS (2007) Nonmuscle myosins II-B and Va are components of detergentresistant membrane skeletons derived from mouse forebrain. Brain Res 1143, 46–59. Nebl T, Pestonjamasp KN, Leszyk JD, Crowley JL, Oh SW & Luna EJ (2002) Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J Biol Chem 277, 43399–43409. Meng X & Wilkins JA (2005) Compositional characterization of the cytoskeleton of NK-like cells. J Proteome Res 4, 2081–2087. Minke B (1977) Drosophila mutant with a transducer defect. Biophys Struct Mech 3, 59–64. Clapham DE (2003) TRP channels as cellular sensors. Nature 426, 517–524. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD & Julius D (1997) The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ et al. (2002) An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci USA 99, 8400–8405. Jordt SE & Ehrlich BE (2007) TRP channels in disease. Subcell Biochem 45, 253–271. Prevarskaya N, Zhang L & Barritt G (2007) TRP channels in cancer. Biochim Biophys Acta 1772, 937– 946. Birder LA (2007) TRPs in bladder diseases. Biochim Biophys Acta 1772, 879–884. Szallasi A, Cortright DN, Blum CA & Eid SR (2007) The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 6, 357–372. Liddle RA (2007) The role of transient receptor potential vanilloid 1 (TRPV1) channels in pancreatitis. Biochim Biophys Acta 1772, 869–878. Levine JD & Alessandri-Haber N (2007) TRP channels: targets for the relief of pain. Biochim Biophys Acta 1772, 989–1003. Immke DC & Gavva NR (2006) The TRPV1 receptor and nociception. Semin Cell Dev Biol 17, 582–591. Goswami C, Dreger M, Jahnel R, Bogen O, Gillen C & Hucho F (2004) Identification and characterization of a Ca2+ -sensitive interaction of the vanilloid FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 receptor TRPV1 with tubulin. J Neurochem 91, 1092–1103. Goswami C, Hucho TB & Hucho F (2007) Identification and characterisation of novel tubulin-binding motifs located within the C-terminus of TRPV1. J Neurochem 101, 250–262. Lansbergen G & Akhmanova A (2006) Microtubule plus end: a hub of cellular activities. Traffic 7, 499– 507. Slep KC & Vale RD (2007) Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol Cell 27, 976–991. Vlachová V, Teisinger J, Susánková K, Lyfenko A, Ettrich R & Vyklický L (2003) Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci 23, 1340–1350. Garcı́a-Sanz N, Fernández-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sánchez E, FernándezBallester G & Ferrer-Montiel A (2004) Identification of a tetramerization domain in the C-terminus of the vanilloid receptor. J Neurosci 24, 5307–5314. Chaudhuri AR, Khan IA, Prasad V, Robinson AK, Ludueña RF & Barnes LD (1999) The tumor suppressor protein Fhit. A novel interaction with tubulin. J Biol Chem 274, 24378–82432. Hayashi M & Matsumura F (1975) Calcium binding to bovine tubulin. FEBS Lett 58, 222–225. Solomon F (1977) Binding sites for calcium on tubulin. Biochemistry 16, 358–363. Serrano L, Valencia A, Caballero R & Avila J (1986) Localization of the high affinity calcium-binding site on tubulin molecule. J Biol Chem 261, 7076–7081. Fong KC, Babitch JA & Anthony FA (1988) Calcium binding to tubulin. Biochim Biophys Acta 952, 13–19. Soto C, Rodriguez PH & Monasterio O (1996) Calcium and gadolinium ions stimulate the GTPase activity of purified chicken brain tubulin through a conformational change. Biochemistry 35, 6337–6344. Goswami C & Hucho T (2007) TRPV1 expressiondependent initiation and regulation of filopodia. J Neurochem 103, 1319–1333. Goswami C, Dreger M, Otto H, Schwappach B & Hucho F (2006) Rapid disassembly of dynamic microtubules upon activation of the capsaicin receptor TRPV1. J Neurochem 96, 254–266. Han P, McDonald HA, Bianchi BR, Kouhen RE, Vos MH, Jarvis MF, Faltynek CR & Moreland RB (2007) Capsaicin causes protein synthesis inhibition and microtubule disassembly through TRPV1 activities both on the plasma membrane and intracellular membranes. Biochem Pharmacol 73, 1635–1645. Wang JP, Tseng CS, Sun SP, Chen YS, Tsai CR & Hsu MF (2005) Capsaicin stimulates the non-storeoperated Ca2+ entry but inhibits the store-operated TRP channels and cytoskeleton regulate each other 44 45 46 47 48 49 50 51 52 53 54 55 56 57 Ca2+ entry in neutrophils. Toxicol Appl Pharmacol 209, 134–144. Karr TL, Kristofferson D & Purich DL (1980) Mechanism of microtubule depolymerization. Correlation of rapid induced disassembly experiments with a kinetic model for endwise depolymerization. J Biol Chem 255, 8560–8566. Job D, Fischer EH & Margolis RL (1981) Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci USA 78, 4679–4682. Lieuvin A, Labbé JC, Dorée M & Job D (1994) Intrinsic microtubule stability in interphase cells. J Cell Biol 124, 985–996. Goswami C, Schmidt H & Hucho F (2007) TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J 274, 760–772. Yamamoto H, Fukunaga K, Tanaka E & Miyamoto E (1983) Ca2+ - and calmodulin-dependent phosphorylation of microtubule-associated protein 2 and tau factor, and inhibition of microtubule assembly. J Neurochem 41, 1119–1125. Keith C, DiPaola M, Maxfield FR & Shelanski ML (1983) Microinjection of Ca++-calmodulin causes a localized depolymerization of microtubules. J Cell Biol 97, 1918–1924. Lee YC & Wolff J (1982) Two opposing effects of calmodulin on microtubule assembly depend on the presence of microtubule-associated proteins. J BiolChem 257, 6306–6310. Chard PS, Bleakman D, Savidge JR & Miller RJ (1995) Capsaicin-induced neurotoxicity in cultured dorsal root ganglion neurons: involvement of calcium-activated proteases. Neuroscience 65, 1099–1108. McNally FJ & Vale RD (1993) Identification of katanin, an ATPase that severs and disassembles stable microtubules. Cell 75, 419–429. Baas PW, Karabay A & Qiang L (2005) Microtubules cut and run. Trends Cell Biol 15, 518–524. Ahmad FJ, Yu W, McNally FJ & Baas PW (1999) An essential role for katanin in severing microtubules in the neuron. J Cell Biol 145, 305–315. Sancho R, de la Vega L, Appendino G, Di Marzo V, Macho A & Munoz E (2003) The CB1 ⁄ VR1 agonist arvanil induces apoptosis through an FADD ⁄ caspase-8-dependent pathway. Br J Pharmacol 140, 1035–1044. Shin CY, Shin J, Kim BM, Wang MH, Jang JH, Surh YJ & Oh U (2003) Essential role of mitochondrial permeability transition in vanilloid receptor 1-dependent cell death of sensory neurons. Mol Cell Neurosci 24, 57–68. Kim SR, Kim SU, Oh U & Jin BK (2006) Transient receptor potential vanilloid subtype 1 mediates microglial cell death in vivo and in vitro via Ca2+ -mediated FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4695 TRP channels and cytoskeleton regulate each other 58 59 60 61 62 63 64 65 66 67 68 69 70 C. Goswami and T. Hucho mitochondrial damage and cytochrome c release. J Immunol 177, 4322–4329. Sánchez AM, Sánchez MG, Malagarie-Cazenave S, Olea N & Dı́az-Laviada I (2006) Induction of apoptosis in prostate tumor PC-3 cells and inhibition of xenograft prostate tumor growth by the vanilloid capsaicin. Apoptosis 11, 89–99. Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, Giangaspero F & Santoni G (2007) Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem 102, 977–990. Waning J, Vriens J, Owsianik G, Stuwe L, Mally S, Fabian A, Frippiat C, Nilius B & Schwab A (2007) A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium 42, 17–25. Jin K, Xie L, Kim SH, Parmentier-Batteur S, Sun Y, Mao XO, Childs J & Greenberg DA (2004) Defective adult neurogenesis in CB1 cannabinoid receptor knockout mice. Mol Pharmacol 66, 204–208. Funakoshi K, Nakano M, Atobe Y, Goris RC, Kadota T & Yazama F (2006) Differential development of TRPV1-expressing sensory nerves in peripheral organs. Cell Tissue Res 323, 27–41. Ahmad FJ, Hughey J, Wittmann T, Hyman A, Greaser M & Baas PW (2000) Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat Cell Biol 2, 276–280. Ahmad FJ & Baas PW (2001) Force generation by cytoskeletal motor proteins as a regulator of axonal elongation and retraction. Trends Cell Biol 11, 244– 249. Basu S & Srivastava P (2005) Immunological role of neuronal receptor vanilloid receptor 1 expressed on dendritic cells. Proc Natl Acad Sci USA 102, 5120– 5125. Schwab A, Nechyporuk-Zloy V, Fabian A & Stock C (2007) Cells move when ions and water flow. Pflugers Arch 453, 421–432. Gomez T (2005) Neurobiology: channels for pathfinding. Nature 434, 835–838. Ramsey IS, Delling M & Clapham DE (2006) An introduction to TRP channels. Annu Rev Physiol 68, 619–647. Maccarrone M, Barboni B, Paradisi A, Bernabò N, Gasperi V, Pistilli MG, Fezza F, Lucidi P & Mattioli M (2005) Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. J Cell Sci 118, 4393–4404. Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J et al. (2002) Activation of TRPV4 channels (hVRL-2 ⁄ mTRP12) by phorbol derivatives. J Biol Chem 277, 13569–13577. 4696 71 Alessandri-Haber N, Yeh JJ, Boyd AE, Parada CA, Chen X, Reichling DB & Levine JD (2003) Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 39, 497–511. 72 Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB & Levine JD (2004) Transient receptor potential vanilloid 4 is essential in chemotherapyinduced neuropathic pain in the rat. J Neurosci 24, 4444–4452. 73 Becker D, Blase C, Bereiter-Hahn J & Jendrach M (2005) TRPV4 exhibits a functional role in cell-volume regulation. J Cell Sci 118, 2435–2440. 74 Liu X, Bandyopadhyay B, Nakamoto T, Singh B, Liedtke W, Melvin JE & Ambudkar I (2006) A role for AQP5 in activation of TRPV4 by hypotonicity: concerted involvement of AQP5 and TRPV4 in regulation of cell volume recovery. J Biol Chem 281, 15485–15495. 75 Ramadass R, Becker D, Jendrach M & Bereiter-Hahn J (2007) Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch Biochem Biophy 463, 27–36. 76 Suzuki M, Hirao A & Mizuno A (2003) Microtubuleassociated protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4). J Biol Chem 278, 51448–51453. 77 Bollimuntha S, Cornatzer E & Singh BB (2005) Plasma membrane localization and function of TRPC1 is dependent on its interaction with beta-tubulin in retinal epithelium cells. Vis Neurosci 22, 163–170. 78 Greka A, Navarro B, Oancea E, Duggan A & Clapham DE (2003) TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nat Neurosci 6, 837–845. 79 Moore TM, Brough GH, Babal P, Kelly JJ, Li M & Stevens T (1998) Store-operated calcium entry promotes shape change in pulmonary endothelial cells expressing Trp1. Am J Physiol 275, L574–L582. 80 Shin JB, Adams D, Paukert M, Siba M, Sidi S, Levin M, Gillespie PG & Gründer S (2005) Xenopus TRPN1 (NOMPC) localizes to microtubule-based cilia in epithelial cells, including inner-ear hair cells. Proc Natl Acad Sci USA 102, 12572–12577. 81 Wu D, Huang W, Richardson PM, Priestley JV & Liu M (2008) TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J Biol Chem 283, 416–426. 82 Shim S, Goh EL, Ge S, Sailor K, Yuan JP, Roderick HL, Bootman MD, Worley PF, Song H & Ming GL (2005) XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nat Neurosci 8, 730–735. 83 Wang GX & Poo MM (2005) Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434, 898–904. 84 Li Y, Jia YC, Cui K, Li N, Zheng ZY, Wang YZ & Yuan XB (2005) Essential role of TRPC channels in FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho 85 86 87 88 89 90 91 92 93 94 95 96 97 98 the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898. Wen Z, Han L, Bamburg JR, Shim S, Ming GL & Zheng JQ (2007) BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF ⁄ cofilin. J Cell Biol 178, 107–119. Rosado JA & Sage SO (2001) Activation of store-mediated calcium entry by secretion-like coupling between the inositol 1,4,5-trisphosphate receptor type II and human transient receptor potential (hTrp1) channels in human platelets. Biochem J 356, 191–198. Rosado JA & Sage SO (2000) A role for the actin cytoskeleton in the initiation and maintenance of storemediated calcium entry in human platelets. Trends Cardiovasc Med 10, 327–332. Itagaki K, Kannan KB, Singh BB & Hauser CJ (2004) Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol 172, 601–607. Otsuguro KI, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX & Zholos AV (2008) Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem 283, 10026–10036. Lemonnier L, Trebak M, Lievremont JP, Bird GS & Putney JW Jr (2006) Protection of TRPC7 cation channels from calcium inhibition by closely associated SERCA pumps. FASEB J 20, 503–505. Redowicz MJ (2002) Myosins and pathology: genetics and biology. Acta Biochim Pol 49, 789–804. Libby RT & Steel KP (2000) The roles of unconventional myosins in hearing and deafness. Essays Biochem 35, 159–174. Grimm C, Cuajungco MP, van Aken AF, Schnee M, Jörs S, Kros CJ, Ricci AJ & Heller S (2007) A helix-breaking mutation in TRPML3 leads to constitutive activity underlying deafness in the varitintwaddler mouse. Proc Natl Acad Sci USA 104, 19583–19588. Tabuchi K, Suzuki M, Mizuno A & Hara A (2005) Hearing impairment in TRPV4 knockout mice. Neurosci Lett 382, 304–308. Sidi S, Friedrich RW & Nicolson T (2003) NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301, 96–99. Di Palma F, Belyantseva IA, Kim HJ, Vogt TF, Kachar B & Noben-Trauth K (2002) Mutations in Mcoln3 associated with deafness and pigmentation defects in varitint-waddler (Va) mice. Proc Natl Acad Sci USA 99, 14994–14999. Nilius B, Voets T & Peters J (2005) TRP channels in disease. Sci STKE 295, re8. Meyer NE, Joel-Almagor T, Frechter S, Minke B & Huber A (2006) Subcellular translocation of the eGFPtagged TRPL channel in Drosophila photoreceptors TRP channels and cytoskeleton regulate each other 99 100 101 102 103 104 105 106 107 108 109 110 111 112 requires activation of the phototransduction cascade. J Cell Sci 119, 2592–2603. Clark K, Langeslag M, van Leeuwen B, Ran L, Ryazanov AG, Figdor CG, Moolenaar WH, Jalink K & van Leeuwen FN (2006) TRPM7, a novel regulator of actomyosin contractility and cell adhesion. EMBO J 25, 290–301. Clark K, Middelbeek J, Morrice NA, Figdor CG, Lasonder E & van Leeuwen FN (2008) Massive autophosphorylation of the Ser ⁄ Thr-rich domain controls protein kinase activity of TRPM6 and TRPM7. PLoS ONE 3, e1876. Loudon RP, Silver LD, Yee HF Jr & Gallo G (2006) RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol 66, 847–867. Medeiros NA, Burnette DT & Forscher P (2006) Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol 8, 215–226. Les Erickson F, Corsa AC, Dose AC & Burnside B (2003) Localization of a class III myosin to filopodia tips in transfected HeLa cells requires an actin-binding site in its tail domain. Mol Biol Cell 14, 4173–4180. Bridgman PC, Dave S, Asnes CF, Tullio AN & Adelstein RS (2001) Myosin IIB is required for growth cone motility. J Neurosci 21, 6159–6169. Bridgman PC & Elkin LL (2000) Axonal myosins. J Neurocytol 29, 831–841. Berg JS, Derfler BH, Pennisi CM, Corey DP & Cheney RE (2000) Myosin-X, a novel myosin with pleckstrin homology domains, associates with regions of dynamic actin. J Cell Sci 113, 3439–3451. Berg JS & Cheney RE (2002) Myosin-X is an unconventional myosin that undergoes intrafilopodial motility. Nat Cell Biol 4, 246–250. Sousa AD, Berg JS, Robertson BW, Meeker RB & Cheney RE (2006) Myo10 in brain: developmental regulation, identification of a headless isoform and dynamics in neurons. J Cell Sci 119, 184–194. Sousa AD & Cheney RE (2005) Myosin-X: a molecular motor at the cell’s fingertips. Trends Cell Biol 15, 533–539. Bohil AB, Robertson BW & Cheney RE (2006) Myosin-X is a molecular motor that functions in filopodia formation. Proc Natl Acad Sci USA 103, 12411–12416. Bennett RD, Mauer AS & Strehler EE (2007) Calmodulin-like protein increases filopodia-dependent cell motility via up-regulation of myosin-10. J Biol Chem 282, 3205–3212. Belyantseva IA, Boger ET & Friedman TB (2003) Myosin XVa localizes to the tips of inner ear sensory cell stereocilia and is essential for staircase formation of the hair bundle. Proc Natl Acad Sci USA 100, 13958–13963. FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4697 TRP channels and cytoskeleton regulate each other C. Goswami and T. Hucho 113 Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA & Olson EN (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116, 3114–31126. 114 Lishko PV, Procko E, Jin X, Phelps CB & Gaudet R (2007) The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron 54, 905–918. 115 Mandadi S, Numazaki M, Tominaga M, Bhat MB, Armati PJ & Roufogalis BD (2004) Activation of protein kinase C reverses capsaicin-induced calcium-dependent desensitization of TRPV1 ion channels. Cell Calcium 35, 471–478. 116 Mohapatra DP & Nau C (2005) Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J Biol Chem 280, 13424–13432. 117 Mohapatra DP & Nau C (2003) Desensitization of capsaicin-activated currents in the vanilloid receptor TRPV1 is decreased by the cyclic AMP-dependent protein kinase pathway. J Biol Chem 278, 50080–50090. 118 Zhu MX (2005) Multiple roles of calmodulin and other Ca2+ -binding proteins in the functional regulation of TRP channels. Pflugers Arch 451, 105–115. 119 Lambers TT, Weidema AF, Nilius B, Hoenderop JG & Bindels RJ (2004) Regulation of the mouse epithelial Ca2+ channel TRPV6 by the Ca2+ -sensor calmodulin. J Biol Chem 279, 28855–28861. 120 Rosenbaum T, Gordon-Shaag A, Munari M & Gordon SE (2004) Ca2+ ⁄ calmodulin modulates TRPV1 activation by capsaicin. J Gen Physiol 123, 53–62. 121 Strotmann R, Schultz G & Plant TD (2003) Ca2+-dependent potentiation of the nonselective cation channel TRPV4 is mediated by a C-terminal calmodulin binding site. J Biol Chem 278, 26541–26549. 122 Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G & Nilius B (2002) Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 277, 47044–47051. 123 Chung MK, Lee H & Caterina MJ (2003) Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem 278, 32037–32046. 124 Cho H, Shin J, Shin CY, Lee SY & Oh U (2002) Mechanosensitive ion channels in cultured sensory neurons of neonatal rats. J Neurosci 22, 1238–1247. 125 Montalbetti N, Li Q, Timpanaro GA, González-Perrett S, Dai XQ, Chen XZ & Cantiello HF (2005) Cytoskeletal regulation of calcium-permeable cation channels in the human syncytiotrophoblast: role of gelsolin. J Physiol 566, 309–325. 126 Montalbetti N, Li Q, Wu Y, Chen XZ & Cantiello HF (2007) Polycystin-2 cation channel function in the human syncytiotrophoblast is regulated by microtubular structures. J Physiol 579, 717–728. 4698 127 Li Q, Montalbetti N, Wu Y, Ramos A, Raychowdhury MK, Chen XZ & Cantiello HF (2006) Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. J Biol Chem 281, 37566–37575. 128 Li Q, Dai XQ, Shen PY, Wu Y, Long W, Chen CX, Hussain Z, Wang S & Chen XZ (2007) Direct binding of alpha-actinin enhances TRPP3 channel activity. J Neurochem 103, 2391–2400. 129 Nilius B, Owsianik G, Voets T & Peters JA (2007) Transient receptor potential cation channels in disease. Physiol Rev 87, 165–217. 130 Polomano RC & Bennett GJ (2001) Chemotherapyevoked painful peripheral neuropathy. Pain Med 2, 8–14. 131 Quasthoff S & Hartung HP (2002) Chemotherapyinduced peripheral neuropathy. J Neurol 249, 9–17. 132 Hudes GR, Greenberg R, Krigel RL, Fox S, Scher R, Litwin S, Watts P, Speicher L, Tew K & Comis R (1992) Phase II study of estramustine and vinblastine, two microtubule inhibitors, in hormone-refractory prostate cancer. J Clin Oncol 10, 1754–1761. 133 Topp KS, Tanner KD & Levine JD (2000) Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol 424, 563–576. 134 Tanner KD, Levine JD & Topp KS (1998) Microtubule disorientation and axonal swelling in unmyelinated sensory axons during vincristine-induced painful neuropathy in rat. J Comp Neurol 395, 481–492. 135 Dina OA, McCarter GC, de Coupade C & Levine JD (2003) Role of the sensory neuron cytoskeleton in second messenger signaling for inflammatory pain. Neuron 39, 613–624. 136 Bhave G & Gereau RW 4th (2003) Growing pains: the cytoskeleton as a critical regulator of pain plasticity. Neuron 39, 577–579. 137 Takanari H, Yosida T, Morita J, Izutsu K & Ito T (1990) Instability of pleomorphic tubulin paracrystals artificially induced by Vinca alkaloids in tissue-cultured cells. Biol Cell 70, 83–90. 138 Foss M, Wilcox BW, Alsop GB & Zhang D (2008) Taxol crystals can masquerade as stabilized microtubules. PLoS ONE 3, e1476. 139 Elie-Caille C, Severin F, Helenius J, Howard J, Muller DJ & Hyman AA (2007) Straight GDP-tubulin protofilaments form in the presence of taxol. Curr Biol 17, 1765–1770. 140 Asai H, Ozaki N, Shinoda M, Nagamine K, Tohnai I, Ueda M & Sugiura Y (2005) Heat and mechanical hyperalgesia in mice model of cancer pain. Pain 117, 19–29. 141 Prevarskaya N, Flourakis M, Bidaux G, Thebault S & Skryma R (2007) Differential role of TRP channels in prostate cancer. Biochem Soc Trans 35, 133–135. FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS C. Goswami and T. Hucho 142 Bödding M (2007) TRP proteins and cancer. Cell Signal 19, 617–624. 143 Hartel M, Di Mola FF, Salvaggi F, Mascetta G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Buchler MW et al. (2006) Vanilloids in pancreas cancer: potential for chemotherapy and pain management. Gut 55, 519–528. 144 Ghilardi JR, Rohrich H, Lindsay TH, Sevcik MA, Schwei MJ, Kubota K, Halvorson KG, Poblete J, Chaplan SR, Dubin AE et al. (2005) Selective blockade of the capsaicin receptor TRPV1 attenuates bone cancer pain. J Neurosci 25, 3126–3131. 145 Vriens J, Janssens A, Prenen J, Nilius B & Wondergem R (2004) TRPV channels and modulation by hepatocyte growth factor ⁄ scatter factor in human hepatoblastoma (HepG2) cells. Cell Calcium 36, 19–28. 146 Sanchez MG, Sanchez AM, Collado B, MalagarieCazenave S, Olea N, Carmena MJ, Prieto JC & DiazLaviada II (2005) Expression of the transient receptor potential vanilloid 1 (TRPV1) in LNCaP and PC-3 prostate cancer cells and in human prostate tissue. Eur J Pharmacol 515, 20–27. 147 Jancsó G, Karcsú S, Király E, Szebeni A, Tóth L, Bácsy E, Joó F & Párducz A (1984) Neurotoxin induced nerve cell degeneration: possible involvement of calcium. Brain Res 295, 211–216. 148 Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z & Iadarola MJ (2004) Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J Clin Invest 113, 1344–1352. 149 Kim SR, Lee daY, Chung ES, Oh UT, Kim SU & Jin BK (2005) Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J Neurosci 25, 662–671. TRP channels and cytoskeleton regulate each other 150 Puntambekar P, Van Buren J, Raisinghani M, Premkumar LS & Ramkumar V (2004) Direct interaction of adenosine with the TRPV1 channel protein. J Neurosci 24, 3663–3671. 151 Reilly CA, Johansen ME, Lanza DL, Lee J, Lim JO & Yost GS (2005) Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J Biochem Mol Toxicol 19, 266–275. 152 Contassot E, Wilmotte R, Tenan M, Belkouch MC, Schnuriger V, de Tribolet N, Burkhardt K & Dietrich PY (2004) Arachidonylethanolamide induces apoptosis of human glioma cells through vanilloid receptor-1. J Neuropathol Exp Neurol 63, 956–963. 153 Movsesyan VA, Stoica BA, Yakovlev AG, Knoblach SM, Lea PM 4th, Cernak I, Vink R & Faden AI (2004) Anandamide-induced cell death in primary neuronal cultures: role of calpain and caspase pathways. Cell Death Differ 11, 1121–1132. 154 Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, Tsui H, Tang L, Tsai S, Santamaria P et al. (2006) TRPV1+ sensory neurons control beta cell stress and islet inflammation in autoimmune diabetes. Cell 127, 1123–1135. 155 McMahon SB, Lewin G & Bloom SR (1991) The consequences of long-term topical capsaicin application in the rat. Pain 44, 301–310. 156 Kiselyov K, Soyombo A & Muallem S (2007) TRPpathies. J Physiol 578, 641–653. 157 Stephens RE (1986) Membrane tubulin. Biol Cell 57, 95–109. 158 Gu C, Zhou W, Puthenveedu MA, Xu M, Jan YN & Jan LY (2006) The microtubule plus-end tracking protein EB1 is required for Kv1 voltage-gated K+ channel axonal targeting. Neuron 52, 803–816. FEBS Journal 275 (2008) 4684–4699 ª 2008 The Authors Journal compilation ª 2008 FEBS 4699