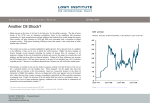
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Spatial organization of thalamocortical and corticothalamic
Survey
Document related concepts
Neuroeconomics wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Neuroplasticity wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Synaptic gating wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Development of the nervous system wikipedia , lookup
Neuroanatomy wikipedia , lookup
Optogenetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Transcript
THE JOURNAL OF COMPARATlVE NEUROLOGY 285325-338 (1989)
Spatial Organization of Thalamocortical
and Corticothalamic Projection Systems in
the Rat SmI Barrel Cortex
JOJANTA CHMIELOWSKA, GEORGE E. CARVELL, AND DANIEL J. SIMONS
Department of Neurophysiology, The Nencki Institute of Experimental Biology, Warsaw,
Poland (J.C.); Departments of Physical Therapy, School of Health Related Professions
(G.E.C.), and of Physiology, School of Medicine (D.J.S.), University of Pittsburgh, Pittsburgh,
Pennsylvania 15261
ABSTRACT
Axonal tracing techniques were used to examine the distribution of corticothalamic projection neurons in relation to the organization of the thalamocortical recipient zones in the whisker representation of the rat first somatic
sensory cortex. Following injection of horseradish peroxidase into the physiologically defined vibrissa area in the ventrobasal complex of the thalamus,
labeling in the cortex had a columnar appearance. Dense patches of anterograde labeling were located within the centers of the layer IV barrels and
extended superficially through lamina 111; the septa between barrels contained considerably less reaction product. Retrogradely labeled neurons were
observed in lower layer V and layer VI where they were concentrated preferentially deep to the barrel centers. Regions deep to the septa displayed less overall labeling and a lower relative number of thalamic projecting neurons. Zones
having the larger numbers of retrogradely labeled cells also contained terminallike labeling of either corticothalamic or thalamocortical origin. Following
an injection that included the posterior group medial to the ventrobasal complex, anterograde labeling in layer IV was located largely in the septa. In conjunction with previous findings concerning the origin and termination of other
projection systems in the barrel cortex, these results suggest that a vibrissal
column contains a central core zone intimately linked with the ventrobasal
thalamus that is bounded by narrower regions of more diverse inputs and outputs that form an interface between adjacent cortical columns.
Key words: cortical columns, vibrissae, trigeminal, HRP
The first somatic sensory cortex of many rodent species is
characterized by a cytoarchitectonically distinct modular
organization. Within layer IV of the face area, there is a
clear one-to-one relationship between the physiological representation of individual vibrissae on the contralateral mystacial pad and consistently identifiable aggregates of neurons called barrels (Woolsey and Van der Loos, '70; Welker,
'71, '76). Electrophysiological (Simons and Woolsey, '79;
Armstrong-James and Fox, '87) and 2-deoxyglucose (Durham and Woolsey, '77; Chmielowska et al., '86) studies have
provided evidence that each barrel is the morphological correlate of a single functional column that extends throughout
the thickness of the cortex (Woolsey and Van der Loos, '70).
One function of a cortical column is the integration of information arising from the array of whiskers on the face (see
Simons et al., '88). For example, though all driveable neurons within a column are activated by a particular whisker,
0 1989 ALAN R. LISS, INC.
cells at different depths respond differentially to movements of adjacent vibrissae, deflected alone or in combination with the columnar whisker (Simons, '78, '85). These
properties are thought to reflect in part connections both
among neurons located within an individual column and
among neurons in neighboring columns.
Recently we have demonstrated a substantial transformation within the layer IV barrel of afferent information from
the ventrobasal thalamus (Simons and Carvell, '89). Response properties of barrel neurons are characterized by single-whisker receptive fields having strong inhibitory sur-
Accepted March 21,1989.
Address reprint requests to Jolanta Chmielowska, Laboratory of Neuropsychology, National Institute of Mental Health, Building 9, Room 1N107,
Bethesda, MD 20892.
326
J. CHMIELOWSKA ET AL.
rounds; these properties contrast with the less uniformly
organized receptive fields of cells in the ventrobasal thalamus (VB) that provide the major source of extrinsic input to
the barrels. This transformation can be understood in terms
of local interactions among neurons within an individual
barrel, suggesting that information processing within a vibrissal column reflects operations of experimentally identifiable networks of cortical neurons. In this regard it may be
significant that cytochrome oxidase (CO) staining in the
barrel cortex reveals both a vertical and a tangential organization, suggestive of an intracolumnar organization (Land
and Simons, ’85a). Thus regions of high CO reactivity are
observed not only in the barrel centers in layer IV but also in
the regions deep to individual barrels in lower layer V and
layer VI. Some cells in this deeper aspect of the cortex project axons to the thalamus and send recurrent collaterals to
terminate on neurons in layer IV; also, the apical dendrites
of corticothalamic cells extend into the barrel centers where
they receive thalamocortical synapses (White and Hersch,
’82; White and Keller, ’87). Taken together, these findings
suggest the existence of two linked networks of cortical neurons, each associated with the VB thalamus, that are located
a t different depths within the cortical column.
Corticofugal neurons in a number of species and cortical
areas have been described as having a patchy distribution
(see Jones, ’84). Wise and Jones (’77) observed that corticothalamic cells in rat somatic sensory cortex are concentrated
more heavily in regions containing barrel fields in layer IV
than in the relatively agranular zones. On the basis of these
and the above findings, we hypothesized that cortical neurons which project to VB have a nonuniform distribution
that can be correlated with the modular organization of the
vibrissa/barrel cortex. We wanted to determine specifically
if such cells are preferentially located in register with the
barrel centers in layer IV which are known to receive thalamocortical inputs from VB. Consequently, horseradish peroxidase (HRP) was used to visualize the patterns of anterograde and retrograde labeling in the first somatic sensory
cortex following small injections of the enzyme into the
physiologically defined whisker representation in VB. Results demonstrate a columnar organization of the thalamocortical and corticothalamic projection systems.
MATERIALS AND METHODS
HRP injections
Data were obtained from 14 adult male and female rats
(200-300 g, Sprague-Dawley strain). Animals were initially
anesthetized by intraperitoneal injection of sodium pentobarbital (Nembutal, 50 mg/kg). A Silastic catheter was
inserted into the external jugular vein (Harms and Ojeda,
’74) for supplemental administration of Nembutal during
the remainder of the experiment. A steel post for holding
the rat’s head was fixed to the exposed skull by using dental
acrylic, and a small craniotomy was made dorsal to the right
ventrobasal complex according to skull coordinates derived
from the atlas of Paxinos and Watson (’82). The location of
the vibrissa representation in VB was then identified electrophysiologically by using tungsten microelectrodes for
multiunit recordings.
Horseradish peroxidase (HRP) was deposited within VB
by one of two methods. In the first six experiments the
recording electrode was replaced with a microliter syringe
containing 30% w/v HRP in 0.5 M Tris HC1. A glass micro-
pipette having a tip diameter of -70 pm was sealed on the
end of the syringe needle, and 0.5-1.0 p1 of the H R P solution
was ejected from it during a period of several minutes by
manually depressing the syringe plunger. This procedure
produced relatively large injections and in some cases apparent leakage of H R P up the electrode track. In order to
control the size and location of the H R P injection more
carefully, eight rats were injected though double-barreled
glass micropipettes having combined tip diameters of 15-30
pm (see Simons and Land, ’87). One barrel was filled with 3
M NaCl for unit recordings and the other was filled with the
H R P solution. The location and deepest extent of the mystacial vibrissae representation were identified by multiunit
recordings and whisker stimulation. Subsequently the H R P
was iontophoretically ejected from the other barrel with 1
pA of continuous positive current. During iontophoresis, the
electrode was withdrawn in 3 pm steps/5 seconds by means
of a stepping microdrive. H R P injection was terminated a t
the depth a t which the dorsal border of VB had been identified by using the recording barrel during the initial advance
of the electrode. Constant negative current was applied during further withdrawal from the brain. In order to label corticothalamic cells corresponding to several rows of whiskers,
up to ten such injections were made a t 75 pm rostral-caudal
intervals.
Following H R P deposition the scalp wound margins were
sutured closed, Neosporin ointment was applied to the incision sites, and the animal was allowed to recover from the
barbiturate under observation. After a 36-48-hour survival,
the rats were anesthetized with a lethal dose of Nembutal,
the descending aorta was clamped, and the animal was perfused transcardially with 150 ml of warm, heparinized saline
(1ml heparid50 ml saline) followed by 300 ml of cold fixative. The fixative consisted of 2% paraformaldehyde and
1.25% glutaraldehyde in 0.1 M phosphate buffer, ph 7.4.
The brains were extracted from the skull, postfixed for 3-5
hours, and then sunk in 30% sucrose phosphate buffer at
4°C.
Histochemistry
Brains were sectioned on a freezing microtome. Prior to
cutting, the right cerebral hemisphere was separated from
the diencephalon. Thalami were sectioned a t 60 pm in the
coronal plane. Cortices were cut in one of three planes at 60
pm. In six cases the cortex was flattened on the microtome
stage during freezing and cut in a plane tangential to the pial
surface overlying t h e posteromedial barrel subfield
(PMBSF). Cortices from the other specimens were sectioned in oblique coronal planes perpendicular to the pial
surface (Fig. 1).In seven cases the brain was oriented so that
the microtome knife cut orthogonally to the alignment of
the barrel rows, which extend posteromedial to anterolateral; i.e., the sections were “across” the barrel rows. In the
remaining hemisphere the microtome knife was oriented
posteromedial to anterolateral in order to produce sections
that were parallel to or “along” the barrel rows. In all cases
serial sections were collected in 0.1 M phosphate buffer and
the free-floating tissue was processed for H R P histochemistry with either diaminobenzidine (DAB) and cobalt intensification (Adams, ’80) or tetramethyl-benzidine (TMB) as
the chromagen (Mesulam, ’81). In a few brains, alternate
sections were treated according to the two protocols. All but
one of the thalami were processed for DAB/cobalt. Incubated sections were mounted on chrome-alum-coated slides,
air dried, and counterstained with 0.1 % thionin.
327
THALAMOCORTICALAND CORTICOTHALAMIC PROJECTIONS
In 77 sections from nine specimens, the locations of all
retrogradely labeled cells were plotted on acetate transparencies with the aid of a camera lucida. As described above,
the boundaries of the layer IV barrels were projected deep
through the infragranular layers where the retrogradely
labeled cell bodies were observed. In five specimens, areal
measures were made of regions in register with the layer IV
barrels and of regions in register with the septa between
them, and the numbers of retrogradely labeled cells per
10,000 fim2were determined. The upper and lower borders
of the measured infragranular zones were defined by the
most superficial location of retrogradely labeled cell bodies
and the white matter. Selected sections were photographed
with a Leitz-Dialux photomicrography system.
RESULTS
Injection sites in the thalamus
Fig. 1. Highly schematic drawings showing the plane of oblique coronal sections relative to the organization of the posteromedial barrel
subfield (PMBSF) in the right hemisphere of the rat brain. Barrels are
arranged in five rows, A-E, oriented posteromedial to anterolateral in
the first somatic sensory cortex. These correspond to the five rows of
mystacial vibrissae on the contralateral face; row A whiskers are located
dorsally on the mystacial pad and within a row whiskers are denoted by
arcs, 1through 4-7 from caudal to rostral. In the cortex, lower-order arcs
are represented by the more posteromedially located barrels. Thus, letters A and E in the figures denote the location of barrels corresponding
to the rostral whiskers on the face, i.e., the higher-order arcs. Panel A
The orientation denoted by the solid line is “across” the barrel rows;
such sections would contain barrels in the same arc from different rows
(see Fig. 6A-C). Panel B: The orientation shown by the solid line is
“along” the barrel row, and such sections would contain barrels in different arcs from the same row (see Fig. 6D).
Data collation and analysis
For tangentially sectioned specimens, individual layer IV
barrels were identified by the cytoarchitecture and by anterograde labeling, which was located in the barrel centers.
Adjacent sections from layer IV through layer VI were
aligned by reference to prominent blood vessels. In oblique
coronal sections, barrel boundaries were projected radially
through layer VI (see Fig. 8 below).
Sections were examined with brightfield optics under
low- and high-power magnification. Neuronal somata with
clearly identifiable reaction product were considered to be
retrogradely labeled cells; in most cases their dendrites also
contained reaction product. Retrogradely labeled cells were
observed only in the deeper aspects of the cortex. Labeling
in granular and supragranular laminae was therefore attributed primarily to anterograde labeling of thalamocortical
axons and/or axon collaterals of the deep retrogradely
labeled cells; some of the labeling may also reflect terminal
arbors of the latter’s apical dendrites.
Figure 2A is a Nissl-stained section through the rat diencephalon showing the ventrobasal complex and its relationship to some other thalamic nuclei. Panels B-D illustrate
the size and location of three representative iontophoretic
injection sites. All injections were centered within the dorsal
and medial aspects of VB, corresponding to the representation of the large mystacial vibrissae on the contralateral
face. In these and other specimens there appeared to be relatively little effective spread of H R P into the nearby posterior group (PO) which also contains a representation of the
whiskers (see Carve11 and Simons, ’87). Even with injections
that appeared to fully encompass the VB whisker representation, the transport of H R P to the cortex was typically circumscribed in that anterograde labeling of barrels was
observed only in that part of the PMBSF that corresponded
to the vibrissae represented physiologically at the thalamic
injection sites. Indeed it was necessary to make multiple
injection penetrations spanning the representation of several whisker rows in order to obtain substantial labeling of
barrels in adjacent rows in the cortex. In addition a distinctly different pattern of cortical labeling was observed
when a large injection clearly included PO just medial to
VB. In no case was labeling observed in visual cortex even
though in some thalami the reaction product halo around
the injection site extended into the dorsal lateral geniculate
nucleus. We therefore assume that the zone of effective
uptake of the injected H R P was confined to VB in all cases
but one, which will be discussed below.
Appearance of cortical labeling in
tangential sections
H R P reaction product in the first somatic sensory cortex
had a patchy, discontinuous distribution that reflected the
spatial organization of barrels in layer IV. In tangential sections the densest anterograde labeling was observed in the
centers of individual layer IV barrels. The term “center”
here refers to the cytochrome-oxidase-rich component of
the barrel which in mice is relatively cell sparse and called
the barrel “hollow” (see Land and Simons, ’85a). Anterograde labeling extended superficially through layer 111. In
sections through lower layer V and layer VI barrellike
patches of labeling were also apparent. Areas of densest
labeling were located in register with the overlying layer IV
barrels and consisted of labeled somata, dendrites, and
axons. Retrogradely labeled cell bodies were distributed
over a broader expanse of cortex than the anterograde labeling that could be used to help define barrel centers.
328
J. CHMIELOWSKA ET AL.
Fig. 2. Photomicrographs of coronal sections of the rat diencephalon
through the ventrobasal complex (VB). Panel A A Nissl-stained section showing VB, the posterior group medial to VB (PO), and the thalamic reticular nucleus (R). The whisker representation is located in the
medial two-thirds of VB, with arc 1 vibrissae represented dorsally and
row A vihrissae caudally. Panels B - D The size and location of HRP
injections in three specimens. Tissue in panel B was processed by using
TMB as the chromagen; specimens in C and D were processed with
DAB/cobalt. Scale in A = 500 pm; also applies B-D.
Fig. 3. Appearance of cortical labeling in tangential sections following the thalamic injection shown in Figure 2B. Panel A Photomicrograph of a section through layer IV of the PMBSF. Note the high density
of labeling in the barrel centers, particularly those of row C and D, and
the relatively sparse labeling in the septa, especially between rows C and
D. Anterogradely labeled barrels in row C are Cl-C3 (upper left to lower
right). Panel B: Photomicrograph of a section from the same hemisphere through layer VI. Arrows denote the zone corresponding to the
septum between rows C and D that is shown at higher magnification in
panel C. All sections were reacted with DAB/cobalt. Scale in A = 200
pm, applies also to panel B. Scale in C = 100 pm. Orientation, mediakup;
anterior:right.
THALAMOCORTICALAND CORTICOTHALAMIC PROJECTIONS
Figure 3
329
330
Figure 3 shows cortical labeling following the thalamic
injection illustrated in Figure 2B. In this experiment H R P
was iontophoretically injected during seven closely spaced
penetrations (see Materials and Methods). Responses from
whiskers in row D, arcs 2-4, and/or in row C, arcs 1-3, were
recorded in all of the penetrations, and in one penetration
some responses from row B whiskers were observed. As
shown in Figure 3A the densest anterograde labeling was
found in the corresponding row C and D barrels, with considerably less dense labeling in row B. Labeling was concentrated within the barrel centers. There, reaction product
was not uniformly distributed; rather, the barrel center was
crisscrossed by narrow bands of very dense labeling that
appeared to segment the barrel neuropil into a latticelike
arrangement of different compartments. In contrast to the
barrel center, the septum contained far less reaction product. This was most apparent for septa between barrel rows.
Within these zones horizontally running axons were clearly
visible, and occassionally “bridges” of fine-grained reaction
product suggestive of axon terminals were observed. Such
labeling was much more commonly seen in the septa
between barrels in the same row. Thus, segmentation of
layer IV into barrellike patches of anterograde labeling was
more pronounced between barrel rows than within them.
Labeling in lower layer V/layer VI in this hemisphere is
shown a t the same magnification in Figure 3B. The pattern,
including the “bridge” between the rostra1 C and D barrels,
is similar to that seen in panel A. Segmentation of the overall labeling is less pronounced, particularly within the zones
corresponding to the whisker rows. In addition reaction
product here is located within somata and dendrites. As
shown at higher magnification in Figure 3C the retrogradely
labeled cell bodies are observed more frequently deep to the
barrel centers than deep to the between-row septum. Numerous horizontally running processes can be distinguished
in the sparsely labeled zone. These consist of dendrites of
retrogradely labeled corticothalamic cells and axons of
either these neurons and/or of thalamocortical cells. Even in
zones containing the highest concentration of labeled cells,
somata with and without reaction product were intermingled.
With respect to the distribution of labeled cell bodies
visual inspection of the specimens can be misleading since
attention is drawn to regions containing the most reaction
product. Consequently, the locations of retrogradely labeled
somata only were plotted with the aid of a camera lucida.
Figure 4 is a line drawing from a single section of a tangentially cut hemisphere different from that shown in Figure 3
illustrating the distribution of labeled cell bodies with
respect to the overlying barrels. Because of the difficulty in
precisely aligning many sections and because the cortex is
organized in radial, not parallel, lines, projecting the boundaries of the layer IV barrels deep into the cortex in this section plane is at best an approximation. Nevertheless, labeled
somata appear to be concentrated more densely beneath the
barrel centers than beneath the septa. Cell counts showed
that there were 20% more labeled cells bodies per unit area
deep t o the barrel centers than deep to the septa-that is,
the barrelseptum ratio was 1.20.
The pattern of anterograde labeling observed in tangential sections was distinctly different in the one experiment
in which a relatively large injection clearly included PO just
medial to VB. A photomicrograph of a section through layer
IV of this specimen is shown in Figure 5. Anterograde labeling in the anterolateral aspect of the barrel field is located
within both the barrel centers and the septa. More pos-
J. CHMIELOWSKA ET AL.
Fig. 4. Line drawing showing the location of retrogradely labeled cell
bodies in a tangential section from a different hemisphere from that
shown in Figure 3. One dot = 1 cell. Shaded areas indicate the overlying
layer IV barrels in rows B-D. Orientation, posteromedial: left. Scale =
200 pm.
teromedially, only the septa contain substantial amounts of
reaction product.
Appearance of labeling in oblique
coronal sections
In sections cut normal to the pial surface labeling in the
cortex displayed a distinctly vertical organization. This is
nicely illustrated in Figure 6A, where the HRP reaction
product is confined largely within a single vertical column.
In this experiment H R P was injected during five penetrations centered in the representation of the D1 and D2 vibrissae. The plane of this section is across the barrel rows (see
Fig. IA). The zone of anterograde labeling in layer IV is
highly circumscribed, and as in the tangential section of Figure 3A, labeling within the barrel center itself is heterogeneously distributed. Labeled axons extend superficially
through lamina 111. The densest and most superficially
located reaction product is present above the centralmost
aspect of the layer IV barrel. Immediately deep to the barrel, in upper and middle layer V, labeling consists primarily
of vertically oriented processes. These processes include
apical dendrites of retrogradely labeled corticothalamic
cells whose somata are in the deeper aspects of the cortex.
Labeled cell bodies are located in lower layer V and in layer
VI where they are observed more often in the upper half of
the lamina. Labeled cells tend to be clustered deep to the
layer IV barrel. Compared to the more superficial aspects of
layer V, the zone of retrogradely labeled cell bodies contains
considerably denser and more widely distributed reaction
product. This parallels the tangential extent of the labeling
in layer IV. Thus the overall pattern of labeling within this
section has a vertically oriented hourglass appearance.
The preferential distribution of labeled corticothalamic
cells deep to the barrel centers is best appreciated in sections where two or more adjacent columns of labeling are
visible. Figure 6B and C are photomicrographs of sections
from the same specimen shown in Figure 6A. Sections in
panels A-C are located in progressively more anterior and
331
THALAMOCORTICAL AND CORTICOTHALAMIC PROJECTIONS
Fig. 5. Anterograde labeling in the layer IV barrel field following an HRP injection that included both
VB and PO. Note that in the posteromedial aspect of the barrel field, reaction product is largely confined to
the septa. Letters B-D denote barrels B1, C1, and D1. Scale = 500 pn. Anterior: right; medial up.
lateral aspects of the barrel field and show increasing density of labeling in the adjacent row C and E barrels. Clustering of the retrogradely labeled neurons is most apparent
where the patches of label in layer IV are most widely separated-that is, between the row D and E barrels.
A similar columnar pattern was observed in oblique coronal planes oriented parallel to or along the whisker rows
(see Fig. lB). A section along row B is shown in Figure 6D.
Thalamic injections were centered in the physiological representation of row B whiskers, arcs 1-3. Anterograde labeling is densest in the B2 and B3 barrels. The septa are more
narrow than in panels A-C in part because within-row septa
are normally quite narrow and in part because the plane of
section was not exactly parallel to the whisker rows. Nevertheless, retrogradely labeled corticothalamic cells are
more common deep to the barrel centers. Regions underlying the narrow septa between barrels in TOW B contain comparatively fewer numbers of labeled somata per unit area
(see also below). Note that because of slight differences in
the section plane with respect to the pial surface, the relative thicknesses of supragranular and infragranular laminae
appear different in this specimen compared to that of Figure
6A-C. With the relative compression of the deepest aspects
of the cortex, the retrogradely labeled cells appear to be
closer to the white matter than in the other panels.
Apical dendrites of
labeled corticothalamic cells
HRP-containing apical dendrites were observed often.
These processes emanated from retrogradely labeled somata and ascended through layer V where there was little
evidence for extensive terminal branching. Because of the
dense anterograde labeling of thalamocortical axon terminals within layer IV, it was net possible to follow individual
dendrites within the labeled barrels. In columns containing
less anterograde labeling, apical dendrites of labeled corticothalamic cells could, however, be clearly seen to have terminal arbors within layer IV. Figure 7 is a photomicrograph
illustrating the morphology of some apical dendrites. A t the
right side of the figure, a pyramidal cell can be seen to project its apical dendrite into an overlying, anterogradely
labeled barrel. Because of the relative absence of reaction
product in the barrel centers at the left, the terminal apical
arbors of dendrites from two other neurons are visible in
layer IV. As shown here and in other figures, apical dendrites of cells lying deep to the barrel centers ascended
directly to the barrel in its parent column. Cells located
deep to the septa also had vertically directed apical dendrites; it is not clear from our material whether these terminated only within the overlying septum and/or whether
some of their arbors terminated within one or more of the
barrel centers belonging to neighboring columns.
Quantitative measures of
corticothalamic cells
Qualitatively the somata of labeled corticothalamic cells
appeared to be most densely clustered deep to the barrel
centers. To determine whether the relative numbers of
labeled cell bodies, as distinct from dendrites and axonal
processes, were in fact greater deep to the barrel center than
deep to the septa, cell counts/unit area were made. Figure 8
illustrates the method used to delineate the measured areas
(see Materials and Methods).
Data were obtained only from oblique coronal sections
containing at least two well-labeled adjacent barrels in layer
332
Fig. 6. Appearance of labeling in oblique coronal sections. Photomicrographs in panels A-C show sections from a single hemisphere cut in
a plane across the barrel rows. Labeling corresponding to barrel rows CE has a columnar appearance. Panel D: Photomicrograph of a section
from a specimen oriented along row B; columns B2 and B3 are indicated.
J. CHMIELOWSKA ET AL.
Laminar boundaries are shown in panel A and apply also to B and C.
The relative thicknesses of the laminae are somewhat different in panel
D because of the orientation of the section with respect to the pial surface. Scale in A = 200 pm applies also to B-D.
THALAMOCORTICALAND CORTICOTHALAMIC PROJECTIONS
333
Fig. 7. Photomicrograph of an oblique coronal section showing apical dendrites of retrogradely labeled
corticothalarnic neurons. Two of these (arrows) can be seen to emanate from their parent somata in lower
layer V. Scale = 200 prn.
IV. A total of 7,780 cells in 48 serial sections from four specimens were examined. Results are presented in Table 1,
which shows the mean number (i1 standard deviation) of
retrogradely labeled corticothalamic cells per 10,000 pm2for
the indicated number of sections from the four specimens.
“Barre1:septum” ratios were obtained by dividing the mean
for the zone deep to the barrel centers by the mean for the
zone deep to the septa. The observed ratios were 1.22-1.45,
indicating that regions deep to the barrels contained approximately 20-40 % more labeled corticothalamic cells per
unit area than regions deep to the septa. Note that a similar
ratio (1.20) was observed in the tangentially sectioned specimen of Figure 4. Paired t-tests for the cases of Table 1
showed that differences between the barrel and septum
means were statistically significant in three specimens
(P‘s < .02, two tail). For the fourth case, the probability
associated with the observed t value was .055.
Within individual specimens there was variability in the
overall number of labeled cells and in the relative numbers
of labeled cell cells deep to the barrel vs. those deep to the
septa. This is illustrated by the line drawings of Figure 9.
Each panel shows data from a single section. Panels A and C
and panels B and D are from cases 4 and 2, respectively, in
Table 1.For each hemisphere, the two sections were selected
to illustrate the range of barrekseptum ratios; these sections
were not adjacent to one another. For each specimen sections in panels A and B contain the highest relative numbers
of labeled somata in the zones deep to the barrel; those of
panels C and D had the lowest. Thus, in panel A the zone
deep to the barrel centers contained 8.54 cells/10,000 pm2
whereas the zone deep to the septum contained no labeled
cells. In panel C there were 4.17 cells/10,000 pm2 in the
regions deep to the barrels and 5.50 cells/10,000 pm2deep to
the septum. In panel B, the barrekseptum ratio was 1.86
(4.18/2.24); that of panel D was 0.81 (7.47/9.25). Through
the series of adjacent sections there were no clear periodic
fluctuations in barre1:septum ratios suggestive of a greater
concentration of labeled cells deep to the geometric center
of a barrel or to its outer margins. Similarly, in tangential
specimens, such as that of Figure 4, there was no apparent
preferential distribution of labeled cells with respect to specific aspects of the barrel centers.
Table 1 reveals substantial differences among the four
specimens in terms of the overall numbers of labeled cells.
These are likely due to differential tissue shrinkage, to
vagaries of the HRP technique, and/or to plane of section
relative to the pial surface. With respect to the latter, for
example, the comparatively large number of labeled cells in
case 1 may reflect the fact that the lower layers, e.g., layer
VI, appeared compressed relative to the more superficial
laminae (see Fig. 6). Importantly, however, differences
among the specimens in overall cell numbers were not
reflected in differences in the barrekseptum ratios. Specifically, a one-way analysis of variance comparing these ratios
J. CHMIELOWSKA ET AL.
334
Figure 8
335
THALAMOCORTICALAND CORTICOTHALAMIC PROJECTIONS
Fig. 9. Line drawings illustrating the spatial distribution of labeled
corticothalamic cells from each of four sections. Shading denotes the
zone deep to the overlying septa, determined as illustrated in Figure 8.
Panels A and C and panels B and D illustrate locations of labeled
somata in nonadjacent sections from two specimens, respectively. Panels A and B are from sections containing the greatest barrekseptum
ratios; those of C and D contain the lowest; see text. Bar in A = 50 .urn,
applies also to B-D.
TABLE 1. Numbers of Corticothalamic Cells/10,000 pm’ Deep to the
Barrels and Septa
made across the greatest width of the soma in a plane perpendicular to the apex of the neuron. Data were obtained
from all labeled cells in single sections from two different
specimens. Mean soma1 diameters were 10.30 ym k 1.94 s.d.
(N= 106) and 10.17 ym k 1.86 (N = 329). Though the distributions were unimodal, the larger cells tended to be
located most often in layer V.
No. of cells/10,000 pm2
(mean S.D.)
Case
Plane
N’
“Barrel”
1
Along
Across
Across
Across
11
10.98 t 2.56
5.20 t 1.19
3.26 + 0.78
6.73 t 2.04
2
3
4
14
12
11
“Septum”
8.97
4.19
2.30
t
t
3.38
1.68
t
454
t
0.59
2.51
Ratio
tvalue’
P3
1.22
1.24
1.42
2.75
3.84
3.56
2.17
.02
,002
1.45
.004
,055
‘Number of sections.
‘Paired t-test.
T w o tail probability.
DISCUSSION
for the 48 sections and four cases that compose the data of
Table 1 failed to reveal a significant difference among the
four cases (F = 1.86, P = .15). Thus the relative numbers of
corticothalamic cells deep to the barrels vs. the septa are
similar in these specimens regardless of whether the overlying septa are between barrels in the same row or between
barrels in different rows.
Soma1 diameters of labeled corticothalamic cells were
measured in oblique coronal sections. Measurements were
The present findings demonstrate a distinctive pattern of
anterograde and retrograde labeling in the first somatic sensory cortex of rats following HRP injections into the physiologically defined vibrissae representation in the ipsilateral
VB thalamus. The termination of thalamic afferents and the
origin of the reciprocal efferent projections are distributed
in a laminar-dependent fashion, and at different cortical
depths the tangential organization of both systems largely
parallels the known physiological and cytoarchitectonic representation of the whiskers on the contralateral face. The
close correspondence between these afferent and efferent
systems suggests that an individual column in the barrel
Fig. 8. Photomicrographs illustrating the method used to delineate
zones for determining numbers of corticothalamic cells per unit area.
Panel A: A section oriented across the barrel rows that contains three
well-labeled barrel columns. The same section is shown in panel B with
solid lines in layer IV denoting barrel boundaries which were projected
radially into infragranular laminae to delineate zones deep to the barrel
centers (boxes) from zones deep to the septa; see text. Scale in A = 200
.urn,applies also to B.
336
J. CHMIELOWSKA ET AL.
cortex contains functionally related neuronal networks that
are intimately associated with the major source of discriminative tactile information to this cortical area.
Thalamocortical projections
Numerous studies using a variety of axonal tracing techniques have shown that projections from VB to the barrel
field in layer IV are organized into discrete patches that correspond to the cytoarchitectural organization of the barrels
(Killackey, '73; Killackey and Leshin, '75; Wise and Jones,
'78; Woolsey and Dierker, '78). Axons ramify largely though
not exclusively in the barrel centers, which are also distinctive for their high levels of metabolic activity (Wong-Riley
and Welt, '80; Land and Simons, '85a; Chmielowska et al.,
'86). Recently, Jensen and Killackey ('87) reported that
arbors of individual thalamocortical axons in the rat barrel
field extend superficially into layer I11 and in some cases to
lamina I1 (see also Lorente de Nb, '49). In addition, for some
axons less extensive arborizations were observed in lower
layer V and upper layer VI. Similar observations have been
made in mice (Bernardo and Woolsey, '87). Our results are
entirely consistent with these observations. An interesting
finding to emerge from our material is an apparent nonuniformity in the density of reaction product in the barrel centers. Similarly, using computer-assisted analyses of silver
grain densities following thalamocortical transport of tritiated amino acids in mice, Woolsey and Dierker ('78)
observed clustering of presumed axon terminals within the
barrel. Moreover, within the barrel centers of rats cytochrome oxidase reactivity is distributed in a nonuniform
fashion (Land and Simons, '85a), and there is some physiological evidence suggestive of functional heterogeneity
within individual cortical barrels (Simons and Land, '85;
Chmielowska et al., '86; McCasland and Woolsey, '89).
Characteristics of corticothalamic neurons
Corticothalamic neurons were located in lower layer V
and in layer VI where the largest concentration was in the
upper half of the lamina. These findings accord with others
in the first somatic sensory cortex of mice (Hersch and
White, '81; White and Hersch, '82) and rats (Wise, '75; Wise
and Jones, '77; for a review see Jones, '84). Wise and Jones
observed additionally some labeled cells in upper layer V
following large pressure injections of H R P into the diencephalon. White and Hersch ('82), who also employed pressure injections, suggested that the presence of retrogradely
labeled layer V neurons might be due to the spread of H R P
into PO. In the present study, labeling in lower layer V was
observed even with small iontophoretic injections that appeared to be restricted to VB. Although we cannot rule out
the possibility of H R P uptake by fibers of passage from PO,
the different patterns of anterograde labeling observed with
VB vs. VB plus PO injections (see also Koralek et al., '88)
suggest that relatively few, if any, of the retrogradely
labeled corticothalamic cells studied here represent cells
projecting only to PO that were labeled because their axons
pass through the VB injection site.
Retrogradely labeled neurons had apical dendrites and
were of the pyramidal or the modified pyramidal variety.
Mean somal diameters, which were approximately 10 pm,
are similar to those in mice (White and Hersch, '82), but
smaller than those reported in rats by Wise and Jones, even
in layer VI. This discrepancy may reflect differences in tissue shrinkage and/or in the method of measurement. For
example, our measures were based on cell diameter perpen-
dicular to the apex of the soma whereas data in the study by
Wise and Jones appear to have been derived secondarily
from somal areal measures obtained with a computer microscope. Labeled apical dendrites ascended to layer 1V and,
when visible, were observed to terminate there. Similar
findings have been reported for corticothalamic cells in mice
(White and Hersch, '82) and are consistent with the more
general observation that apical dendrites of layer VI pyramidal cells terminate largely within the thalamic recipient
zones in the middle cortical depths (Hendry and Jones, '83;
Escobar et al., '86). In mice corticothalamic apical dendrites
in layer IV receive synapses from thalamocortical axons
(White and Hersch, '82).
Columnar organization
Afferent input from VB to the face region of the first
somatic sensory cortex is organized into discrete zones corresponding to barrel centers within layer IV. Input to an
individual barrel arises largely from its corresponding "barreloid" in the VB thalamus (Land et al., '86; Land and
Simons, '85b). Anterograde labeling of presumed thalamocortical origin is observed also in the deeper layers as
patches that are in register with the overlying barrels (see
also Bernardo and Woolsey, '87).Individual thalamocortical
axons in rodents (Bernardo and Woolsey, '87; Jensen and
Killackey, '87) and cats (Landry and Deschgnes, '81) traverse the cortical gray matter by a roughly radial trajectory
and distribute terminal arbors to granular and deep infragranular zones that are vertically aligned.
The present findings show that corticothalamic neurons
projecting back to VB are distributed in the deeper layers in
a similarly patchy fashion. Cell counts per unit area showed
a larger relative number of these cells deep to the barrel centers than deep to the septa. This does not seem to be correlated with an overall cytoarchitecture in the deeper layers
suggestive of barrellike aggregations of neurons. Rather, it
appears that corticothalamic cells are most likely to be
located within regions traversed by the incoming thalamocortical fibers. This is supported by the observation that the
clustering of retrogradely labeled somata is most readily
apparent where the separation of barrels in layer IV is
greatest, i.e., between barrels in different rows. This is supported also by the data of Table 1;excluding case 4, t values
for the two "across"-row cases are larger than for the
''along''-row case. Nevertheless, data obtained in sections
cut along the rows must be interpreted with caution
because, unless the section plane is optimal, the narrow
within-row septa are difficult to visualize. A patchy pattern
of corticothalamic cells might be even less readily apparent
in mice, which lack wide septa between barrels in the same
or in different rows.
The present findings might imply a reciprocal one-to-one
correspondence between a single cortical column and an
individual thalamic barreloid. A recent study of corticothalamic projection patterns, however, indicates that this is not
entirely the case because corticothalamic projections from a
single cortical column diverge to terminate within many
barreloids (Hoogland et al., '87). Divergence in corticothalamic feedback may provide a capacity for sensory systems to
selectively enhance activity within a topographically organized projection system on the basis of predictions about the
trajectory of a stimulus across the receptor array (see, for
example, Anderson and Van Essen, '87; Koch, '87).
The pattern of anterograde and retrograde labeling observed following H R P injections into VB is highly reminis-
337
THALAMOCORTICALAND CORTICOTHALAMIC PROJECTIONS
cent of the pattern of cytochrome oxidase (CO) reactivity in
this cortex (Land and Simons, '85a). Specifically, CO reactivity is greatest in the barrel centers, extending superficially into supragranular laminae, and is heightened also in
lower layer V and layer VI deep to the barrel centers. The
CO-rich infragranular zones thus appear to be coextensive
with regions containing the largest population of corticothalamic projection neurons. Our observation of substantial,
terminallike labeling in these zones is consistent with electron microscopic evidence of thalamocortical synapses here,
and in fact some corticothalamic neurons receive such
inputs in the deeper layers (White and Hersch, '82). Extracellular unit recordings have demonstrated that some cells
in the deeper aspect of the rat barrel cortex are particularly
well-driven by whisker vibrations (Simons, '78), and latencies of excitatory postsynaptic potentials evoked by whisker
deflections are among the shortest observed in this cortex
(Carvell and Simons, '88).
Barrels in layer IV and the CO-rich, corticothalamic
infragranular zones are linked by axon collaterals of corticothalamic neurons which synapse preferentially on smooth,
presumably GABAergic and inhibitory, barrel cells (White
and Keller, '87). Also, though both the granular and infragranular zones contain synapses of thalamocortical and of
corticothalamic origin, the relative proportions of such synapses are somewhat reciprocal. The barrel neuropil contains
a greater incidence of thalamocortical synapses and the
infragranular zones have a greater incidence of corticothalamic ones. Thus, a functional column in this cortex may be
characterized by a central core zone containing a t least two
linked neural networks each of which is intimately associated with the VB thalamus.
There is increasing evidence that some afferent and efferent projection systems other than those associated with VB
are organized in a fashion complementary to the VB thalamocortical/corticothalamic system. For example, diencephalic inputs from PO tend to avoid the barrel centers,
being concentrated most heavily in the layer IV septa and in
layer Va deep to the barrel (Lu and Lin, '86; Lin et al., '87;
Koralek et al., '88; see also Herkenham, '80). Axonal terminations of some corticocortical systems within the somatic
sensory cortex itself largely avoid barrel centers (Chapin et
al., '87). Callosal inputs are likewise largely excluded from
the barrels and are distributed more to the septa and the
regions immediately superficial and deep to them; these
regions also contain somata of callosally projecting neurons
(Olavarria et al., '84). In mice efferent neurons of layer V
that project to ipsilateral motor cortex or to the brainstem
tend to be located more often deep to the outer margins of
the barrel centers and deep to the septa (Crandall et al., '86).
The targets of neurons in layer VI that were not labeled by
our VB injections are not yet known.
An unresolved issue concerns the targets of projection
neurons situated superficial to the barrel centers in layer I11
and deep to them in middle and upper layer V. For example,
though corticotrigeminal projecting pyramidal cells in layer
V are located deep to the barrel fields, it is not clear how
they are distributed relative to the overlying centers of individual barrels (see Wise et al., '79). In addition, patterns of
local corticocortical connections within and among barrel
columns have not been extensively studied. In spite of the
absence of these important data, available anatomical evidence is consistent with the idea that an individual cortical
column in the rodent barrel cortex contains a central "core"
zone closely associated with a specific extrinsic source, i.e.,
VB, that is surrounded by a narrower region of more diverse
inputs and outputs that forms the interface between adjacent columns. There is some evidence that the receptive
field properties of neurons in these different compartments
are functionally distinctive. Response properties of cells in
the barrel cortex differ in a laminar-dependent fashion (Simons, '78; Chapin, '86; Armstrong-James and Fox, '87), and
where examined, in tangential fashion as well. For example,
in layer IV barrel neurons are known to have smaller receptive fields than those in the septa (Armstrong-James and
Fox, '87). Thus, information processing within a vibrissal
column may be understandable in terms of functionally and
anatomically identifiable subsets of neurons that are differentially engaged by peripheral stimuli and the behavioral
context in which they occur.
ACKNOWLEDGMENTS
We thank Seth Lichtenstein for technical assistance and
Dr. Peter W. Land for valuable advice and suggestions. Dr.
Chmielowska was a visiting research fellow in the Department of Physiology, University of Pittsburgh School of
Medicine. This work was supported by NIH grant NS
19950.
LITERATURE CITED
Adams, J.C. (1980) Stabilizing and rapid thionin staining of TMB-based
HRP reaction product. Neurosci. Lett. 17:7-9.
Anderson, C.H., and D.C. Van Essen (1987) Shifter circuits: A computational
strategy for dynamic aspects of visual processing. Proc. Natl. Acad. Sci.
USA 84:6297-6301.
Armstrong-James, M., and K. Fox (1987) Spatiotemporal convergence and
divergence in the rat SI "barrel" cortex. J. Comp. Neurol. 263:265-281.
Bernardo, K.L., and T.A. Woolsey (1987) Axonal trajectories between mouse
somatosensory thalamus and cortex. J. Comp. Neurol. 258542-564.
Carvell, G.E., and D.J. Simons (1987) Thalamic and corticocortical connections of the second somatic sensory area of the mouse. J. Comp. Neurol.
265:409-427.
Carvell, G.E., and D.J. Simons (1988) Membrane potential changes in rat SmI
cortical neurons evoked by controlled stimulation of mystacial vibrissae.
Brain Res. 448:186-191.
Chapin, J.K. (1986) Laminar differences in sizes, shapes, and response profiles of cutaneous receptive fields in the rat SI cortex. Exp. Brain Res.
62549-559.
Chapin, J.K., M. Sadeq, and J.L.U. Guise (1987) Corticocortical connections
within the primary somatosensory cortex of the rat. J. Comp. Neurol.
263:326-346.
Chmielowska, J., M. Kossut, and M. Chmielowski (1986) Single vibrissal cortical column in the mouse labeled with 2-deoxyglucose. Exp. Brain Res.
63:607419.
Crandall, J.E., M. Korde, and V.S. Caviness, Jr. (1986) Somata of layer V projection neurons in the mouse barrelfield cortex are in preferential register
with the sides and septa of the barrels. Neurosci. Lett. 67t19-24.
Durham, D., and T.A. Woolsey (1977) Barrels and columnar organization:
Evidence from 2-deoxyglucose (2-DG) experiments. Brain Res. 137:169174.
Escobar, M.I., H. Pimienta, V.S. Caviness, Jr., M. Jacobson, J.E. Crandall,
and K.S. Kosik (1986) Architecture of apical dendrites in the murine neocortex: Dual apical dendritic systems. Neuroscience 17(4):975-989.
Harms, P.G., and S.R. Ojeda (1974) A rapid and simple procedure for chronic
cannulation of the rat jugular vein. J. Appl. Physiol. 36:391-392.
Hendry, S.H.C., and E.G. Jones (1983) The organization of pyramidal and
non-pyramidal cell dendrites in relation to thalamic afferent terminations in the monkey somatic sensory cortex. J. Neurocytol. 12277-298.
Herkenham, M. (1980) Laminar organization of thalamic projections to the
rat neocortex. Science 207532-534.
Hersch, S.M., and E.L. White (1981) Thalamocortical synapses with corticothalamic projection neurons in mouse SmI cortex: Electron microscopic
demonstration of a monosynaptic feedback loop. Neurosci. Lett. 24207210.
338
Hoogland, P.V., E. Welker, and H. Van der Loos (1987) Organization of the
projections from barrel cortex to thalamus in mice studied with Phaseolus
vulgaris-leucoagglutininand HRP. Exp. Brain Res. 68:73-87.
Jensen, K.F., and H.P. Killackey (1987) Terminal arbors of axons projecting
to the somatosensory cortex of the adult rat. I. The normal morphology of
specific thalamocortical afferents. J. Neurosci. 7(11):3529-3543.
Jones, E.G. (1984) Laminar distribution of cortical efferent cells. In A. Peters
and E.G. Jones (eds): Cerebral Cortex, Vol. 1,Cellular Components of the
Cerebral Cortex. New York Plenum Press, pp. 521-553.
Killackey, H.P. (1973) Anatomical evidence for cortical subdivisions based on
vertically discrete thalamic projections from the ventral posterior nucleus
to cortical barrels in the rat. Brain Res. 51:326-331.
Killackey, H.P., and S. Leshin (1975) The organization of specific thalamocortical projections to the posteromedial barrel subfield of the rat somatic
sensory cortex. Brain Res. 86:469-472.
Koch, C. (1987) The action of the corticofugal pathway on sensory thalamic
nuclei: A hypothesis. Neuroscience 23(2):399-406.
Koralek, K.A., K.F. Jensen, and H.P. Killackey (1988) Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex.
Brain Res. 463:346-351.
Land, P.W., and D.J. Simons (1985a) Cytochrome oxidase staining in the rat
SmI barrel cortex. J. Comp. Neurol. 238:225-235.
Land, P.W., and D.J. Simons (1985b) Metabolic and structural correlates of
the vihrissae representation in the thalamus of the adult rat. Neurosci.
Lett. 60:319-324.
Land, P.W., D.J. Simons, and S.A. Buffer, Jr. (1986) Specificity of thalamocortical connections in the rat somatosensory system. Neurosci. Abstr.
12:1434.
Landry, P., and M. Deschhes (1981) Intracortical arborizations and receptive fields of identified ventrobasal thalamocortical afferents to the primary somatic sensory cortex in the cat. J. Comp. Neurol. 199:345-371.
Lin, C.-S., S.M. Lu, and R.M. Tamawaki (1987) Laminar and synaptic organization of terminals from the ventrobasal and posterior thalamic nuclei in
rat barrel cortex. Neurosci. Abstr. 13:248.
Lorente de N6, R. (1949) Cerebral cortex: Architecture, intracortical connections, motor projections. In J.F. Fulton (ed): Physiology of the Nervous
System. New York Oxford University Press, pp. 288-330.
Lu, S.M., and C.-S. Lin (1986) Cortical projection patterns of the medial division of the nucleus posterior thalami in the rat. Neurosci. Ahstr. 12:1434.
McCasland, J.S., and T.A. Woolsey (1988) High-resolution 2-deoxyglucose
mapping of functional cortical columns in mouse barrel cortex. J. Comp.
Neurol. 278:555-569.
Mesulam, M.M. (1981) Enzyme histochemistry of horseradish peroxidase for
tracing neural connections with the light microscope. In J.E. Johnson
(ed): Current Trends in Morphological Techniques. Boca Raton, FL:
CRC Press, pp. 1-54.
Olavarria, J., R.C. Van Sluyters, and H.P. Killackey (1984) Evidence for the
complementary organization of callosal and thalamic connections within
rat somatosensory cortex. Brain Res. 291:364-368.
J. CHMIELOWSKA ET AL.
Paxinos, G., and C. Watson (1982) The Rat Brain in Stereotaxic Coordinates.
Sydney: Academic Press.
Simons, D.J. (1978) Response properties of vibrissa units in the rat SI somatosensory neocortex. J. Neurophysiol. 41:79&820.
Simons, D.J. (1985) Temporal and spatial integration in the rat SI vihrissa
cortex. J. Neurophysiol. 54(3):615-635.
Simons, D.J., and G.E. Carvell (1989) Thalamocortical response transformation in the rat vihrissaharrel system. J. Neurophysiol. 61:311-330.
Simons, D.d., G.E. Carvell, and P.W. Land (1988) The vihrissalbarrel cortex
as a model of sensory information processing. In J.S. Lund (ed): Sensory
Processing in the Mammalian Brain: Neural Substrates and Experimental Strategies. New York Oxford University Press, pp. 67-83.
Simons, D.J., and P.W. Land (1985) Angular sensitivities of SmI barrel neurons and the effects of fentanyl anesthesia. Neurosci. Ahstr. 11:751.
Simons, D.J., and P.W. Land (1987) A reliable technique for marking the
location of extracellular recording sites using glass micropipettes. Neurosci. Lett. 81:lOO-104.
Simons, D.J., and T.A. Woolsey (1979) Functional organization in mouse harre1 cortex. Brain Res. 165:327-332.
Welker, C. (1971) Microelectrode delineation of fine grain somatotopic organization of SmI cerebral neocortex in albino rat. Brain Res. 26:259-275.
Welker, C. (1976) Receptive fields of barrels in the somatosensory neocortex
of the rat. J. Comp. Neurol. 166:173-190.
White, E.L., and S.M. Hersch (1982) A quantitative study of thalamocortical
and other synapses involving the apical dendrites of corticothalamic projection cells in mouse SmI cortex. J. Neurocytol. 11:137-157.
White, E.L., and A. Keller (1987) Intrinsic circuitry involving the local axon
collaterals of corticothalamic projection cells in mouse SmI cortex. J.
Comp. Neurol. 262:13-26.
Wise, S.P. (1975) The laminar organization of certain afferent and efferent
fiber systems in the rat somatosensory cortex. Brain Res. 90:139-142.
Wise, S.P., and E.G. Jones (1977) Cells of origin and terminal distribution of
descending projections of the rat somatic sensory cortex. J. Comp. Neurol. 175:129-158.
Wise, S.P., and E.G. Jones (1978) Developmental studies of thalamocortical
and commissural connections in the rat somatic sensory cortex. J. Comp.
Neurol. 178:187-208.
Wise, S.P., E.A. Murray, and J.D. Coulter (1979) Somatotopic organization of
corticospinal and corticotrigeminal neurons in the rat. Neuroscience
4:65-78.
Wong-Riley, M.T.T., and C. Welt (1980) Histochemical changes in cytochrome oxidase of cortical barrels after vibrissal removal in neonatal and
adult mice. Proc. Natl. Acad. Sci. USA 77r2333-2337.
Woolsey, T.A., and M.L. Dierker (1978) Computer-assisted recording of neuroanatomical data. In R.T. Robertson (ed): Neuroanatomical Research
Techniques. New York: Academic Press, pp. 47-85.
Woolsey, T.A., and H. Van der Loos (1970) The structural organization of
layer IV in the somatosensory region (SI) of mouse cerebral cortex. Brain
Res. I7:205-242.