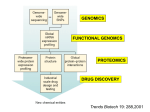
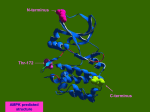
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Nap, a Novel Member of the Pentraxin Family, Promotes Neurite
Molecular neuroscience wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Long-term potentiation wikipedia , lookup
Aging brain wikipedia , lookup
Neuroregeneration wikipedia , lookup
Subventricular zone wikipedia , lookup
Development of the nervous system wikipedia , lookup
Synaptic gating wikipedia , lookup
Synaptogenesis wikipedia , lookup
Neuroplasticity wikipedia , lookup
Haemodynamic response wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Activity-dependent plasticity wikipedia , lookup
Optogenetics wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Metastability in the brain wikipedia , lookup
Biochemistry of Alzheimer's disease wikipedia , lookup
Neuroanatomy wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
De novo protein synthesis theory of memory formation wikipedia , lookup
The Journal of Neuroscience, April 15, 1996, 16(8):2483-2478 Nap, a Novel Member of the Pentraxin Family, Promotes Neurite Outgrowth and Is Dynamically Regulated by Neuronal Activity Cynthia C. Tsui,’ Neal G. Copeland, and Paul F. Worleyls* Debra J. Gilbert,4 Nancy A. Jenkins,4 Carol Barnes,3 Departments of 1Neuroscience and *Neurology, The Johns Hopkins University School of Medicine, Baltimore, Maryland 21205, 3Department of Psychology, Neurology, and Division of Neuronal Systems, Memory, and Aging, University of Arizona, Tucson, Arizona 84724, and 4Mammalian Genetic Laboratory, ABL-Basic Research Program, NC/-Frederick Cancer Research and Development Center, Frederick, Maryland 2 1702 Stimulus-linked RNA and protein synthesis is required for establishment of long-term neuroplasticity. To identify molecular mechanisms underlying long-term neuroplasticity, we have used differential cDNA techniques to clone a novel immediateearly gene (IEG) that is rapidly induced in neurons of the hippocampus and cortex by physiological synaptic activity. Analysis of the deduced amino acid sequence indicates homology to members of the pentraxin family of secreted lectins that include C-reactive protein and serum amyloid P component. Regions of homology include an 8 amino acid “pentraxin signature” sequence and a characteristic pentraxin calciumbinding domain. We have termed this gene and the encoded protein Narp (from neuronal activity-regulated pentraxin). Biochemical analyses confirm the presence of a functional signal sequence, and Narp is secreted by transfected COS-1 cells in culture. Additionally, Narp binds to agar matrix in a calciumdependent manner consistent with the lectin properties of the pentraxin family. When cocultured with Narp-secreting COS-1 cells, neurons of cortical explants exhibit enhanced growth of neuronal dendritic processes. Neurite outgrowth-promoting activity is also observed using partially purified Narp and can be specifically immunodepleted, demonstrating that Narp is the active principle. Narp is fully active at a concentration of -40 rig/ml, indicating a potency similar to known peptide growth factors. Because Narp is rapidly regulated by neuronal activity, its lectin and growth-promoting activities are likely to play role in the modification of cellular properties that underlie long-term plasticity. The mature CNS exhibits the capacity to alter cellular interactions as a function of the activity of specific neuronal circuits. This capacity is believed to underlie learning and memory as well as aspects of postnatal development of the brain (Shatz, 1990). Cellular mechanisms underlying activity-dependent plasticity are known to be initiated by rapid, transmitter-induced changes in membrane conductance properties and activation of intracellular signaling pathways (Bliss and Collingridge, 1993). Several lines of evidence also indicate a role for rapid synthesis of mRNA and protein in long-term neuroplasticity. For example, classical studies of learning and memory demonstrate a requirement for protein synthesis in long-term, but not short-term, memory (Flexner et al., 1963; Agranoff, 1981; Davis and Squire, 1984) and long-term enhancement of synaptic connectivity, studied in cultured invertebrate neurons (Montarolo et al., 1986; Bailey et al., 1992) or in the rodent hippocampus (Frey et al., 1993; Nguyen et al., 1994) is blocked by inhibitors of either RNA or protein synthesis. Impor- tantly, inhibitors of macromolecular synthesis are most effective when administered during a brief time window surrounding the conditioning stimulus, indicating a special requirement for molecules that are rapidly induced (Goelet et al., 1986). Immediate-early genes (IEGs) are rapidly induced in neurons by neurotransmitter stimulation and synaptic activity and are hypothesized to be part of the macromolecular response required for long-term plasticity (Goelet et al., 1986; Sheng and Greenberg, 1990; Silva and Giese, 1994). To identify cellular mechanisms that may contribute to long-term plasticity in the vertebrate brain, we and others have used differential cloning techniques to identify genes that are rapidly induced by depolarizing stimuli (Nedivi et al., 1993; Qian et al., 1993; Yamagata et al., 1993, 1994a,b; Andreasson and Worley, 1995; Lyford et al., 1995). In contrast to the earlier focus on transcription factors, many of the newly characterized IEGs represent molecules that can directly modify the function of cells and include growth factors (Nedivi et al., 1993; Andreasson and Worley, 1995) secreted enzymes that can modify the extracellular matrix, such as tissue plasminogen activator (Qian et al., 1993), enzymes involved in intracellular signaling, such as prostaglandin synthase (Kaufmann et al., 1996; Yamagata et al., 1993) and a novel homolog of H-Ras, termed Rheb (Yamagata et al., 1994b), as well as a novel cytoskeletonassociated protein, termed Arc (Lyford et al., 1995). The remarkable functional diversity of this set of rapid response genes is representative of the repertoire of cellular mechanisms that are likely to contribute to activity-dependent neuronal plasticity. Here we describe a novel IEG that is homologous to the Received July 3, 1995; revised Jan. 19, 1996; accepted Jan. 24, 1996. This work was supported by National Institutes of Health Grants MHS3608 (P.F.W.), AGO9219 (C.A.B., P.F.W.), a grant from the W. M. Keck Foundation, the DeVelbliss Fund, and the Krieger Mind/Brain Institute (P.F.W.), and by the National Cancer Institute, Department of Health and Human Services, under contract N01-CO-74101 with ABL. We thank Anthonv Lanahan for help in construction of cDNA libraries and Gopal Thinakaran, Maha Papapavlou, and D. Barnhart for excellent technical assistance. We also thank Daniel Nathans for support in the initial cloning of Narp. Corresoondence should be addressed to Paul F. Worley, Departments of Neuroscience and Neurology, The Johns Hopkins University School of Medicine, 725 North Wolfe Street, Baltimore, MD 21205. Copyright 0 1996 Society for Neuroscience 0270-6474/96/162463-16$05.00/O Key words: growth factors; immediate-early genes; lectins; long-term potentiation; neurite outgrowth: pentraxins 2464 J. Neurosci., April 15, 1996, 76(8):2463-2478 pentraxin family of secreted, calcium-dependent lectins, which includes C-reactive protein (CRP) and serum amyloid P component (SAP). We have termed this gene and the encoded protein Nulp (from neuronal activity-regulated pentraxin). Our studies demonstrate that Nurp is a secreted protein with biochemical properties of a calcium-dependent lectin. NUT mRNA is abundantly expressed in neurons of the developing and adult brain and spinal cord and is rapidly regulated by physiological synaptic activity. Functional studies indicate that Narp promotes neuronal migration and dendritic neurite outgrowth of neurons using cortical explant cultures, and it does so with a potency that is comparable to known neurotrophins and growth factors. Because Narp is rapidly regulated by physiological synaptic activity, our observations suggest a role for Nurp in neural development and activity-dependent neuronal plasticity. MATERIALS AND METHODS Animals und supplies. Adult male Sprague-Dawley or Fischer-344 [longterm potentiation (LTP) studies] rats were used in studies of Nurp regulation. Developmental studies used male and female Sprague-Dawley pups of the indicated age. Radiochemicals were obtained from DuPont NEN (Boston, NEN). AlI other reagents were from Fisher (Orangcburg. NY) and Sigma (St. Louis. MO) unless snecificallv noted. &nst&ion anYd scrkening of the sudtructed ci)NA lihr&y. A subtracted cDNA library was constructed as described previously (Yamagata ct al., 1993). The subtracted library was screened with [“P]cDNA prepared by reverse transcription of poly(A+) RNA prepared from hippocampus of control or seizure-stimulated rats pretreated with cycloheximide (20 mg/kg, i.p.) as described previously (Yamagata et al., 1993). Near fulllength cDNAs of rat Nurp were isolated by iterative screening of an unsubtracted, oligo(dT)-primed cDNA library prepared from hippocampus 4 hr after a maximal electroconvulsive seizure (MECS). DNA sequencing. Both strands of three independent, near full-length Nurp cDNAs were sequenced as double-stranded plasmids with synthetic, specific primers using the dideoxynucleotide chain termination method with deoxyadenosine 5’-[a-“Slthiotriphosphate and Sequenase (United States Biochemicals, Cleveland, OH). Northern analysis. This procedure was performed as described previously (Linzer and Nathans, 1983) with 10 pg of total RNA per lane. RNA was isolated by standard CsCl density centrifugation and assayed for yield and purity by ultraviolet spectroscopy. Gels were stained with ethidium bromide, and the ribosomal bands were visualized to assess equal loadings of RNA. The probe used for Northern analysis was a 1.8 kb 3’-end fragment of Nurp cDNA. The cDNA fragment was labeled by the random priming technique (Pharmacia, Uppsala, Sweden) using [olla’P]dCTP. In situ hvbridizution. Freshlv dissected brain tissue was raoidlv frozen in plastic molds placed on a dry ice/ethanol slurry as described previously (Cole et al., 1990). Control and experimental tissues were frozen in the same tissue block to ensure identical conditions during tissue sectioning, subsequent storage, and in situhybridization. “S-labeled Nurp antisense riboprobe was prepared from an appropriately restricted pBluescript plasmid containing the near full-length cDNA. In situ hybridization was performed as described previously (Saffen et al., 1988). Selected slides were treated with photographic emulsion (Kodak NTB2, Rochester, NY) and counterstained as previously described (Jordan, 1990). Semiquantitative analysis of autoradiographic images was performed using a computerized densitometer (Loats Associates, Westminster, MD). Electrophysiology. Seizures were induced in adult male Sprague-Dawley rats by MECS using a constant-current signal generator (ECT unit, Ugo, Basil, Switzerland) as described previously (Cole et al., 1990). For LTP studies, Fischer-344 rats were implanted bilaterally with stimulating and recording electrodes in the perforant path and hilus of the dentate gyrus as described previously (Worley et al., 1993). Rats were allowed to recover for at least 2 weeks before any recordings were performed. Twelve chronically implanted rats received high-frequency (HF) stimulation in one hemisphere and low-frequency (LF) stimulation in the other hemisphere. Electrical stimuli consisted of 200 msec diphasic, constantcurrent pulses given at a stimulus intensity of 500 FA. The LF test stimulation was delivered at 0.1 Hz, and the HF stimulation parameters consisted of 50 repetitions of a 20 msec train (i.e., 8 pulses) delivered at 400 Hz (400 total pulses). All response data were digitized by computer at 20 kHz and stored on disk for subsequent off-line analysis. The field Tsui et al. l Nafp Expression and Regulation in Brain EPSP amplitude was measured as the voltage difference between two cursors set at the EPSP onset and 1 msec later. The HF parameters reliably induce LTP (Worley et al., 1993) and increases in EPSP amplitude, assayed 25 min after the stimulus, ranged from 20 to 30%. After this treatment, the rats were killed at 30 min (n = 6), 1 hr (n = 2) 2 hr (n = 2) 4 hr (n = 2) or 24 hr (n = 2). Three additional animals were pretreated with MK-801 (1 mgikg, i.p.) 1 hr before the delivery of the HF stimulus. The intensity of the stimulus was adjusted such that the postsynaptic population response was identical to that before MK-801 administration. The MK-801 administration blocked LTP. Animals were killed 2 hr after the HF stimulus and processed for in situ hybridization. Monocular deprivation. Monocular deprivation was performed in adult Sprague-Dawley rats as described previously (Worley et al., 1991) usingan intravitreal injection of the sodium channel antagonist tetrodotoxin (TTX; 10 kl of 200 &M solution of TTX in PBS). To assess the effectiveness of TT’X injections, its anticipated blockade of the consensual pupillary light reflex was monitored immediately after injection and before killing. Identical treatment in the absence of TTX was performed in control rats. In vitro transcription and translation. Nurp protein was synthesized using in vitro transcription and translation (TNT) in a coupled reticulocyte lysate system (Promega, Madison, WI). Template DNA (I pg) was used with rabbit reticulocyte lysate, TNT reaction buffer, amino acid mix (1 mM) minus methionine, [3”S]methionine (1000 Ciimmol) at 10 mCi/ml, RNasin ribonuclease inhibitor (40 U/ml), and T3 RNA polymerase (2 U/ml). &$I and &-zI were used to linearize the DNA plasmids. TNT reaction products were analyzed by SDS-PAGE. The polyacrylamide gels were first fixed in 10% (w/v) trichloroacctic acid, 10% (v/v) glacial acetic acid, and 30% (v/v) methanol followed by soaking in autoradiography enhancer (Fluoro-Hance, Research Products International, Mt. Prospect, IL) before the gels were dried and exposed to autoradiography. Analysis ofpost-trunslutionul modification of Narp. TNT reactions were performed as described above except with the addition of 2.5 pg of dog microsomes (Promega). For the microsomal proteolytic protection assays, circular full-length Nurp plasmid DNA (1 pg) or 5’-truncated Nurp plasmid DNA (1 Fg) was used as template to synthesize full-length Narp protein (in the presence or absence of microsomes) or a 5’-truncated Nurp lacking the first 46 amino acids, respectively. Proteins were exposed to proteolytic digestion by trypsin (0.1 mg/ml), with or without Triton X-100 (0.1%). Reactions were analyzed by SDS-PAGE. Deglycosylation of Nurp was performed by first adding SDS (0.5%) to the reticulocyte lysate and boiling for 2 min to lyse the microsomes. Glycosidase reactions were carried out at 37°C overnight with endoglycosidase H (6 mu; Boehringer Mannheim, Indianapolis, IN) in reaction buffer (150 ItIM sodium acetate, pH 5.5). Generation of Narp polyclonal antisera. The full-length Nurp cDNA sequence, exclusive of the putative signal sequence, was generated using PCR primers that encoded flanking restriction enzime sequences (EcoRI, 5’-primer; BumHI, 3’-primer). The amnhfied Naro insert was subcloned in frame into pTrcHisA prokaryotic expression vector containing an N-terminal hexahistidine (Invitrogen, San Diego, CA). Full-length Nurp protein was isolated and purified using the Niz+ agarose purification system (Qiagen, Hilden, Germany). Eluted fractions were separated on preparative SDS-PAGE, and the specific band was excised from the gel. Approximately 1 mg of the recombinant Nurp protein was used to immunize each rabbit (HRP, Denver, PA). The crude antisera specifically recognized the bacterial recombinant protein and the in vitro TNT product. Metabolic labeling of COS-I cells und immunoprecipitation of’ Narp. Full-length Nurr, cDNA insert was subcloned into a CMV-uromoter I containing mammalian expression construct (pRK5; Genentech, San Francisco, CA). COS-1 cells were transiently transfected with 10 Fg of plasmid DNA (vector alone and Nurp-expressing construct) using the calcium phosphate method (Chen and Okayamam, 1987). For metabolic labeling of COS-1 cells, 250 mCi/ml [truns-35S]methionine (ICN Biochemicals, Costa Mesa, CA) was added with a media change 40 hr after the initial transfection in DMEM (Gibco, Gaithersburg, MD) lacking methionine and supplemented with 1% fetal bovine serum (HyClone, Logan, UT) for 3 hr. Conditioned media were analyzed by SDS-PAGE and autoradiography. Immunoprecipitation of Nurp from the COS-1 cell conditioned media was performed as follows. Conditioned media were first cleared of cellular debris by centrifugation for 15 min at 1500 X g. Samples were adjusted to 1X immunoprecipitation buffer (150 mM NaCI, 50 mM Tris_ a , Tsui et al. . Narp Expression and Regulation in Brain HCl, pH 7.4, 0.5% NP-40, 0.5% sodium deoxycholate, 5 mM EDTA, 50 Fg/rnl pepstatin, 50 pg/ml leupeptin, 10 &ml aprotinin, and 0.25 mM phenylmethylsulfonyl fluoride) and precleared by mixing with 50 ~1 of protein A agarose (Pierce, Rockford, IL) for 30 min at 4°C (Sisodia et al., 1990). Secreted Nurp was immunoprecipitated by mixing 3 ~1 of polyclonal Nurp antisera with the conditioned media at 4°C for 6 hr. The immune complexes were collected by adding 50 ~1 of protein A agarose (Pierce). Samules were resusoended in SDS eel loading buffer. boiled for 5 min before ‘SDS-PAGE, and analyzed by &toradio&aphy. Calcium-dependent binding of Narp to agar and aj2ypurification. Narp was harvested from conditioned media of transiently transfected COS-1 cells as described above. For initial studies examining the substratebinding properties of Nurp, columns (0.5 ml of gel matrix) were prepared with Sepharose-phosphatidylcholine (Pharmacia), Sepharosephosphatidylethanolamine (Sigma), pulverized 4% agarose (Sigma), or pulverized 4% agar (Sigma). Columns were washed in a calciumchelating elution buffer (0.15 M NaCl, 20 ITIM EDTA, 0.05 M Tris, pH 7.4) and equilibrated in Narp-binding buffer (0.15 M NaCl, 10 mM CaCl,, 0.05 M Tris, pH 7.4). Conditioned media from ten 10 cm plates of COS-1 cells transfected with either the Nurp expression vector or the same vector without insert were applied to the columns and were washed with 2 column volumes of the binding buffer. Proteins that bound to the column were eluted in calcium-chelating elution buffer. Proteins that remained adherent ,to the columns were solubilized in SDS-loading buffer [6% SDS, 15% (v/v) P-mercaptoethanol, 30% (v/v) glycerol, 0.350 M Tris, pH 6.81. All fractions were analyzed by SDS-PAGE and visualized by autoradiography. In experiments that examined the neurite outgrowth-promoting effects of Nurp, we used agar column chromatography to partially purify Nurp or myc-tagged Narp. The c-myc epitope tag was subcloned into the C terminus of Narp-pRK5 construct. c-myc epitope (MEQKLISEEDLN) was designed with a 5’-EcoRI and a 3’-BamHI restriction sequence site. Two 27-mer PCR primers containing flanking Nurp sequence were designed with an EcoRI site in the 5’-PCR primer and a BarnHI site in the 3’-PCR primer along with a stop codon. A total of 100 ml of COS-1 cell conditioned media was incubated with 20 ml of pulverized agar suspension in binding buffer (same as above) at 4°C for 4 hr followed by subsequent washes with binding buffer. Nurp was eluted in four 1 ml fractions with elution buffer. The yield of myc-tagged Nurp was determined by direct competitive ELISA (described below) and was typically -250 ng/lO cm dish. Microexplant cultures of the cerebral cortex. Cortices were dissected from postnatal day 1 (Pl) rat pups and dissociated into cortical explants by passing the tissue through a 24-gauge needle. Explants were washed twice with minimum essential medium (MEM; Gibco) supplemented with glucose (6 gm/l) by sedimentation and resuspension in fresh media. The explants were then plated in 35 mm tissue culture dishes coated with poly+ornithine (Sigma). The tissue culture dishes were coated with 1 ml of poly-L-ornithine (0.1 mg/ml) overnight at room temperature and rinsed twice with d-H,0 before culturing explants in MEM with -7-8 explants per dish in a 5% CO,-humidified incubator at 37°C. Transiently transfected COS-1 cells (with pRK5 vector alone, pRK5 containing the full-length Nurp, or pRK containing a C-terminal myctagged Nurp construct) were trypsinized and harvested 24 hr after transfection. Aggregates of COS-1 cells (5 X 10’ cells) were formed by inverted hanging drop cell culture (Kennedy et al., 1994) for 16-18 hr and transferred to cortical explant cultures. A single aggregate was added to each 35 mm dish. Cocultures were maintained for 24 or 48 hr, and neurite outgrowth from each explant was assessed by light microscopy (magnification 150x) and scored in triplicate dishes by an observer blind to the protocol. The criteria for scoring an explant as “positive” for neurite outgrowth were the presence of neurites that surrounded the periphery of the explant and extended radially over the substrate at least lo-15 cell body lengths. In other control experiments, we examined the effect of coculturing cortical explants with COS-1 cells expressing amyloid precursor protein-like protein (Slunt et al., 1994) from the mammalian expression vector CB6. In other experiments, myc-tagged Narp was partially purified from COS-1 cell supernatant using agar column chromatography as described above and examined for neurite outgrowth activity. The concentration of myc-Narp was quantitated using a direct competitive ELISA described below. Differing concentrations of myc-Narp were added to culture dishes, and the effects on neurite outgrowth were examined. Immunodepletion of myc-tagged Nurp was performed by incubating partially purified myc-tagged Nurp prepared from COS-1 cell-conditioned J. Neurosci., April 15, 1996, 76(8):2463-2478 2465 media (-60 ng of Nurp) with mouse anti-myc monoclonal antibody (mAb; 0.5 pg, Oncogene Research Products, Cambridge, MD) previously conjugated with G-protein-agarose beads. The anti-myc-G-protein conjugate was prepared by incubating the Ab with 1 ml of gel slurry, (Pierce) for 2 hr in IgG-binding buffer (0.1 M sodium acetate, pH 5.0) at 4°C followed by centrifugation. The anti-myc G-protein conjugate was then resuspended in buffer (0.15 M NaCl, 0.05 M Tris, pH 7.4) containing Nurp and incubated overnight at 4°C. Control experiments substituted mouse fluid ascites (0.5 pg, Sigma) for the anti-myc Ab. The control immunodepletion experiment procedure was identical to the anti-myc Ab experiment. Quantitation of myc-tagged Narp using direct competitive ELISA. myctagged Narp was quantitated using a standard antigen-inhibition curve generated with serial dilutions of a standard c-myc peptide (amino acids 412-418, Santa Cruz Biotechnology, Tebu, France). Partially purified myc-tagged Nurp was coated onto a 96-well, flat-bottom, high-binding plate (EWRIA, Costar, Cambridge, MA) for 1 hr at room temnerature. hells‘were rinsed three times with wash buffer (0.5% Surf&t-Amps Tween 20,1% Blocker BSA in PBS, and 1 pack of BupH Dulbecco’s PBS, Pierce) and blocked with blocking buffer (10% Blocker BSA in PBS, Pierce) for 30 min at room temperature. Primary mouse anti-myc Ab at 1000 rig/ml dilution (Oncogene Research Products) was preincubated with known amounts of c-myc peptide for 1 hr at room temperature before applying to wells coated with partially purified myc-tagged Nurp. After 2 hr incubation at room temperature, the wells were rinsed three times with wash buffer, and goat anti-mouse IgG peroxidase-conjugated Ab (l:lOOO, Pierce) was added for I hr at room temperature. 2,2’Azinobis(3-ethylbenzothiazoline)-6 sulfonic acid diammonium salt reagent (ABTS; Pierce) was used as the peroxidase substrate solution, and the hydrolysis was measured using an ELISA reader with a 410 nm filter. Concentration of Nurp was determined by calculating the amount of c-myc peptide necessary to competitively inhibit half-maximal binding of anti-myc Ab to myc-Nurp. Immunohistochemistry. Cortical explants cocultured with COS-1 cells from Pl rat pups were prepared as described above. Cultures were rinsed with HBSS (Gibco) at 37°C air-dried for 20 min, and fixed with 3.7% formaldehyde in 10 mM PBS, pH 7.4, for 30 min at room temperature. Explants were blocked and permeabilized with a solution containing 0.5% Triton X-100, 10% normal blocking serum (Vector Laboratories. Burlingame, CA) in PBS for 4 hr at 4°C and then incubated in primary Abs [anti-microtubule-associated protein-2 mAb (MAP-2) at 1:lOOO (SMI 52, Sternberger Monoclonals Incorporated), anti-tau mAb at 1:200 (Boehringer Mannheim), anti-glial fibrillary acidic protein mAb (GFAP) at 1:400 (Chemicon, Temecula, CA), or control mouse ascites fluid at 1:200 (Sigma)] overnight at 4°C. After rinsing off the primary Abs, endogenous peroxidase was quenched by incubating with 1% H,O, in PBS for 15 min at room temperature. Cultures were incubated further with biotinylated anti-mouse secondary Ab (50 Fliml, Vector) in PBS for 1 hr at room temperature, and immunoreactivity was visualized using the Vectastain Elite ABC and DAB substrate (Vector Laboratories, Burlingame, CA). Interspecific mouse backcross mapping. Interspecific backcross progeny were generated by mating (C57BU6J X M. spretus) F, females and C57BL/6J males as described previously (Copeland and Jenkins, 1991). A total of 205 N, mice were used to map the Nurp locus. DNA isolation, restriction enzyme digestion, agarose gel electrophoresis, Southern blot transfer, and hybridization were performed as described previously (Jenkins et al., 1982). All blots were prepared with Hybond-N+ nylon membrane (Amersham, Arlington Heights, IL). The probe, an -500 bp EcoRIiBamHI fragment of rat cDNA corresponding to the 3’-end of the open reading frame (ORF), was labeled with [a-32P]dCTP using a nick translation labeling kit (Boehringer Mannheim); washing was done to a final stringency of 0.8X SSCP, 0.1% SDS, 65°C. A major fragment of 6.4 kb was detected in SphI-digested C57BL/6J DNA, and a fragment of 7.6 kb was detected in SphI-digested M. spretus DNA. The presence or absence of the 7.6 kb M. spretus-specific 5phI fragment was followed in backcross mice. A description of the probes and RFLPs for the loci linked to Narp including erythropoitin (Epo) and platelet-derived growth factor-a (Pdgfu) has been reported previously (Singh et al., 1991). One locus has not been reported previously for our interspecific backcross: FMS-like tyrosine kinase 3 (Flt3). The probe was an -850 bp fragment of mouse cDNA that was kindly provided by Ihor Lemischka (Princeton University, Princeton, NJ). The probe detected 11.5,7.5, and 4.3 kb and 11.5,7.5,6.0, and -1.0 kb QhI fragments. The presence or absence of the 6.0 and -1.0 kb M. spretus-specific .SphI fragments, which cosegregated, was followed 2466 J. Neurosci., April 15, 1996, 76(8):2463-2478 in backcross mice. Recombination distances were calculated as described previously (Green, 1981) using the computer program SPRETUS MADNESS. Gene order was determined by minimizing the number of recombination events required to explain the allele distribution patterns. Sequence analysis. cDNA and amino acid sequences were analyzed using Geneworks (IntelliGenetics, Mountain View, CA), Strider (Commissariat al’Energie Atomique, Gif-Sur-Yvette Cedex, France), ProteinPredict (Rost and Sander, 1993, 1994), and SBASE (Pongor et al., 1994). RESULTS Narp cDNA sequence Anovel cDNA corresponding to the 3’-noncoding region of an -2.5 kb mRNA was identified by differential screening of a subtracted cDNA library prepared from seizure-stimulated hippocampus (Yamagata et al., 1993). A near full-length cDNA was identified by iterative screening of an unsubtracted cDNA library prepared from hippocampus. The size of the cDNA (2561) corresponds closely to the estimated size of the mRNA determined by Northern analysis. The longest ORF with an initiator methionine predicts a 432 amino acid protein. A second, independent clone (2460 bp that begins at nucleotide 101 of the longest clone) contains the entire ORF, and the nucleotide and the predicted amino acid sequences are identical to those of the longer clone. Figure 1 shows the complete cDNA and deduced protein sequences. The initiator methionine was selected to be the best Kozak consensus for translation initiation and occurs at nucleotide 128 of the longest cDNA (Kozak, 1987). The ORF continues to the 5’-end of the cDNA, and there is no in-frame stop 5’ to the predicted initiator methionine. Alternative initiator methionines are present at nucleotides 265, 271, and 466. The identified location of the translation start site is supported by analysis of the in vitro transcription/translation products (see below). The 3’noncoding region contains the ATTTA motif in positions 2362 and 2396, which may contribute to the rapid turnover of Narp mRNA (Shaw and Kamen, 1986) and is present in many IEGs. No classical polyadenylation signal sequence (AATAAA) was identified; however, the sequence ATTAAA is present in 5 independent clones 21 nucleotides from the 3’-terminal poly(A) tail. Predicted protein sequence of Narp: homology to the pentraxin family Nurp has a calculated molecular weight of 46.2 kDa and a p1 of 5.46. There is an N-terminal secretory signal peptide sequence (underlined in Fig. 1) predicted using methods from von Heigne (1986). The predicted cleavage site is between glycine and glutamine. Three potential N-linked glycosylation sites were identified (Geneworks program) at amino acids 133, 174, and 378. Nurp is 24 and 26% identical to CRP and SAP, respectively, over a 210 amino acid span that includes the full-length sequence of mature CRP and SAP and the C-terminal 216 amino acids of NUT (Dowton and McGrew, 1990; Rassouli et al., 1992). Figure 2A shows the alignment of Narp with rat CRP and SAP. Islands of homology are clustered throughout the sequence. Eight amino acids have been identified that constitute the “pentraxin family signature” sequence (H-X-C-X-S/T-W-X-S/T), which is present in all pentraxin family members (Breviaro et al., 1992) and is also present in Narp. The recent resolution of the crystal structure of SAP provides detailed structure-function information for the pentraxin family and identifies several functional domains (Emsley et al., 1994). As isolated from ascites or pleural effusion fluids, SAP is a decameric molecule composed of identical subunits noncovalently associated in two pentameric rings that interact face to face. Each of the Tsui et al. l Narp Expression and Regulation in Brain monomers contains 15 antiparallel P-strands (designated strands A-O, Fig. 2A) that form two p sheets. Corresponding regions of NUT are predicted to form P-strands as predicted by computer modeling (Rost and Sander, 1993, 1994). The pentraxin signature sequence corresponds to strand H. The region of Narp with highest homology to SAPICRP spans the sequence between P-sheets K and L. This region of SAP is important in binding calcium (Emsley et al., 1994). SAP binds two calcium ions with different affinities. The higher-affinity binding site includes coordinating side chains of amino acids Asp-58, Asn-59, Glu-136, Asp-138, and the main-chain carbonyl of Gln-137. All of these residues are present at homologous positions in Narp, with the single exception that Narp encodes an Ala at homologous position 58 (Fig. 2A). Asp-58 is present in hamster SAP, human CRP, and Limulus CRP but varies in CRPs from other species (Emsley et al., 1994). The second calcium ion is coordinated by Glu-136, Asp-138, and Gln-148. Each of these residues is conserved in Narp. SAP binding to derivatized sugars involves the amide nitrogens of Gln-148 and Asn-59, which are oriented in the proper position to form hydrogen bonds with the sugar derivative via interactions of their amide oxygens with the calcium ions (Emsley et al., 1994). Cysteine residues Cys-36 and Cys-95 form a disulfide link between adjacent strands C and L and are present in most members of the pentraxin family (Emsley et al., 1994). Cysteines are present in the homologous positions in Nary. Based on these homologies, we conclude that Nurp is a member of the pentraxin family. Computer modeling of the N-terminal 200 amino acids of Narp predicts that this region possesses a high degree (61%) of a-helical secondary structure (Rost and Sander, 1993, 1994). Helical wheel analysis (Cohen and Parry, 1994) of two of these putative helical domains (amino acids 40-82 and 105-132) indicates that they are strongly amphipathic with heptad repeats of hydrophobic amino acids (data not shown). Four additional novel pentraxins have been reported recently that are similar to Narp in that they exhibit the pentraxin consensus sequences in the C-terminal half of the molecule but possess an extended N-terminal sequence that is novel. Information regarding the biochemistry and function of these molecules is limited and is based primarily on their sequence homology to SAP and CRP. TSG-14 is rapidly induced in endothelial cells by growth factor stimulation and is hypothesized to modify the extracellular matrix of the endothelium (Breviaro et al., 1992; Lee et al., 1993). Overall, TSG-14 is 22% identical to Nurp but is similar to Narp to the penin that it encodes -200 amino acids N-terminal traxin homology region, and this domain is predicted to possess a high degree of a-helical structure (Rost and Sander, 1993, 1994). Another pentraxin, termed NP (from neuronal pentraxin), was purified from brain membranes as a binding protein for the snake venom toxin taipoxin (Schlimgen et al., 1995). NP is similar in size to Narp and TSG-14, and its amino acid sequence is 45% identical to Narp. NP is expressed in discrete populations of neurons in the hippocampus, cortex, and brainstem, indicating a partially overlapping pattern of expression with Nurp. The regulation of NP, particularly whether it is an IEG, has not been examined. NP potentiates the toxicity of taipoxin on glia and is hypothesized to play a role in reuptake of extracellular proteins. The third novel pentraxin, termed Apexin, was purified and cloned independently by two groups from guinea pig sperm (Noland et al., 1994; Reid and Blobel, 1994). The Apexin amino acid sequence is 90% identical to Narp and, therefore, Apexin may represent the guinea Tsui et al. l Nap Expression and Regulation in Brain J. Neurosci., April 15, 1996, TGGTGCTGGCGTTTCCCTGCTTGCACGCGGTTCCCTCGAGCGCCGCTC CGACCCACGTAGCCGGCCGCCAAGGCGCCCAGACGGCAACGCGAG ATG CTG GCG CTG CTG ACC GCC GGC GTG GCG CTC GCC GTG GCC GCG GGA CAA GCC CAG GAT -16 M MC L A CCG ATA L L T A G CCT GGC AGT CGC TTC V A L GTG TGC ACC A V A A G GCG CTG CCC CCC GAA ij A Q 76(8):2463-2478 2467 48 127 206 D GCG GCG CGC GCC 266 CCT GAG GAG GAG CTG 326 5NPIPGSRFVCTALPPEAARA CCC TGC 25G C CCG CTG P L CCC GCG ATG CCC ATG CAG GGA GGC GCG CTG AGC P A M P M Q G G A L S P CGA GCC GCT GTG CTG CAC TGG CGC GAG ACC GTC GTG CAG CAG AAG 45R A A V L CAG CGA GAA GCC ATC 65Q R E A I H W CGA GM R GCT AAG GCG CGC GGC ACG E R E T V V Q Q K T S X L A R E E GAG ACG CTG E CTC ACC AGC AAG CTG GCC CCC TGI L E T L E G GGC GCT G GAG GGA Cl-A C L L CCC GGC A GGG GCC ACG GGC AAG GAC ACC ATG GGC GAC CTG CCG 386 A 446 G CGG GAC 506 GAC CGC TTG GAG 566 85GKARGTGATGKDTMGDLPRD CCG GGC CAC GTC GTG GAG CAG CTT AGC CGC TCG CTG CAG ACC CTC AAG 105 P AGC 125 S G E L V CTG GM 165 185 205 L E MC ACA N T MG TCA K S E I K GCC TCG 245 A L S V L L Q D E CTG MT L N CCA GAT P K D L P T D G CCC TGG 325 P W P I G G G Q L H R L E L F M W S A L GCA TTC A L I W F D R P G N H P R F V L I I CCT GTG GAG ACG 405 P V R S GCG TCT L Q MT T L G E L GM E I E T L Q AAA GI'G K E G T V L P GGT AAC MT G S N R GCA TTC A K F A R A S Y N F V L G TGC C W R Q GAG ACC TCG T P GCC TTC A S CCC ATA P K L Q R K E L E GGC MC R G F I Y L R ACC ATC TGC T I T Q V E C N CGA 626 GTG GCC GAG 686 G Q D R A E CTG TGG L D G A I N L AGT S L Y A V L L P G ATC AAC I N GCA TTC A 746 GGC AAG G L R Q A GAC MG D K N S A W H H I C I G E K L G T T W V F L I AAT N I L L G Q GTT GGA GAG CTT AGC V N G ATC I E L CCC MC GGG GAG AAC G E D D E Q CAG TTC Q F N T GAC ACT D T I 1166 A GTG 1226 V AAC ATA TGG N 1106 R CTG GCA L 1046 Q 1286 W TGC TCC ACG AAC ATG CCT 1346 CGA GGG AAG TGG 1406 A@CSTNMP GT'C GAT GTG TTT V S T 986 I GTC GCA CAG V 926 S GAG ATT E 866 K CTG CGG TCC AGC W 806 F TGG CAC CAT ATC TGC ATC ACC TGG ACC ACT CGA GAG ATC ATC MC E V CTA TAC Y V F G G GCT TCC A S K W 1472 CTACCTTCTCCCTGTCCCAGAGGCCAA L GAGCGGGCTmCTGGGCAGTTCAAGGCATCTATTCCCGAGTTCAACTAAAATffCTGGCCTtACTAGGAAACAACCAG AGCCCtTMGGCAGGCTGTGTGGCCTCCrrTGTCTTAGGCTCCTATG~CTTAffGC~TGTTCTTTGGTGGGAAGTtA CCGAAGCCCTGGGAAGAGTCCTCAGCCAClTCCTGCTGGG~~~A~~~~GTGAGC~~C~CCC~C~~ MATGffAGTGCMCCCAGCCCTGCCTGTCATmGGATCCTTAGTCTCTCGTGTGTGCrrCCCCTC TGGCTGTGTGGTCATCCTACCGGGGTGGCCTGGGTCCCTTGTG CACAGMGCTACCCGCCCCTGAMCAGGGTCTCTCCCTCA~~~T~G~~~G~~CTCC~~~GGG~CTG CAGCTGCTGGAGGGCCACGTGCCCAGACACTCCCCAGCATCCCCCAC AG~CCCCACCCTCTGTCCACCTCTCAGACCCCACGCTTCTAAGGACCATTGCTGGGFTGGCTTTCMAAGCTGCTGC TCTCATCTGGTGCCAAAAGTTCATTTGC4GCTTCTACACCGTTCTGGGTrrGGGGATTGACTTTATTCCCCCACAA AAGAGGAACAGCCATTAGAAGCCAtCCTCCCCTCCTTTTGGC C9CATTGmTCTGTGAGCATCTGACTCTCCCCCGTCCAGFA GCTACCTGCTTAGTGACTCCAGGCTGCATCATGTATCATA ITTTTAMGACAAAGTGATTCAGTGGGGAA ~TAAAGCTATMATATTATATATTTTATTTTTCATACATGTTTAAAGTGCGGATCCATGCATGTTCCATTTGTAGG ACCAGCTTCACGTGCCCATCCTGACATTGTATGCCACMCAGCTCTTGTGATGGMTTTTGATTMA~GCA~GG MGATGA, Figure E GAC TTC GCT GTG CCT GGG CAA GCC AAT GAG CTG CTT E T N CCA GGG GCT GTG CTC ATC CTG GGG CAG GAG GIG P L GCT CAC CGG CAG AAG ACA GAG GAG CTG GAG.AGA GAA GAG CGT CT-C CTG GAC TTG TAG E CTA CGC MG L CAG GAC GGG GAG AAG CTG GGC ACC CCA TGG GTG GAC MC P R GCC CCC CTG CCG AGC TCC CTC CCT CTC CGT ACA AAC TAC GAT GGC MG D D HBETSAHRQKTE GAT GCC ACA CAG CCC TTC D E CTG CAG AGG GTG ACT L K TBASNAGLPSDFR GGC ACG CCA TTC TCC TAC CAT CCC ATC MG GGA AAC ATC ATC 385 S CCC GAG CTG TAT GTC AGC V L GAC CGT GTC CTC CCC GCA CM 365 L CT'C CAC ACC MC GCA Cl-G GAG TGG GGA GGC AGA TTT 345 K CCA GGC ATC GAC GGC ATG TGG 305 R ACG TTG CTG CCC CTG TTT 285 Q E GAC GAG AAG TCC CTG CTC CAC AAT GFG CTG ATA 265 V CTC CAG CGG AGG CTG GGG GAG CTG GAG AGG CAG TTG ATC AAG MG 225 V CTC GAG CTC CM GAG GTG 145 H @ 1551 1630 1709 1788 1867 1946 2025 2104 2183 2262 2341 2417 2494 2555 2561 sequence of Nurp cDNA and its predicted amino acid sequence. The last nucleotide of each line is numbered to the right. The I. Nucleotide translated protein sequence is shown below corresponding nucleotide sequence and is numbered at the left. The putative signal peptide of 16 amino acids is underlined. A dot indicates the predicted first amino acid of the mature protein. Putative glycosylation sites are circled. Two putative ATITA mRNA instability motifs are present in the 3’untranslated region and are boxed. The putative polyadenylation signal (ATTAAA) is underlined. 2466 Rat J. Neurosci., April 15, 1996, 76(8):2463-2478 Tsui et al. l Narp Expression and Regulation in Brain Narp 234 81 81 Rat Narp 284 * G - J- - K Rat SAP Rat CPZ? Rat Narp Rat SAP Rat CRP 180 Rat Narp 384 Rat SAP 208 Rat CRP 211 Rat Narp 416 Figure 2. Comparison of rat Nurp amino acid sequence with rat CRP, rat SAP, guinea pig Apexin, and human NPII. A, The full-length amino acid sequences of rat CRP (Rassouli et al., 1992) and SAP (Dowton and McGrew, 1990) are shown with the corresponding homologous region of Nap. Identical amino acid residues are boxed. The eight amino acid “pentraxin family signature” is marked in bold. The p-strand regions defined by x-ray crystallography of human SAP (Emsley et al., 1994) are indicated by letters&0 and a single overline above the corresponding residues. Residues involved in calcium binding are indicated by a dot: Asp-58, Asn-59, Glu-136, Gln-137, Asp-138, and Gln-148. Conserved cysteine residues 36 and 95 are highlighted by an asterisk. B, Full-length Narp is compared to guinea pig Apexin and human NPII. Regions that are identical in all three sequences are boxed, and regions identical between two are shaded. Figure 2mcontinues. pig homolog of Narp (Fig. 2B). The nucleotide homology between Narp and Apexin continues beyond the end of the ORF into the 3’-untranslated sequence (82% identity in ORF, 25% identity in the 5’-untranslated sequence, and 58% identity in the 3’untranslated sequence), further suggesting that Nalp is the rat homolog of Apexin. Apexin is expressed at high levels in the mature sperm acrosome and is hypothesized to play a role in protein aggregation during acrosome biogenesis. Regulation relative studies to transcript tween species termed clone testis size. Narp have but not does Accordingly, been not reported yield the a conclusive difference in this system. comparison transcript of size be- could be attributable to differences in The fourth novel pentraxin, NPII (Fig. 2B; also termed NPTX2), is a human genomic that was identified by a low-stringency screen with NP (Hsu or and Apexin in tissue-specific expression. identical Perin, to and It is notable that the reported Apexin cDNA is only 1572 nucleotides compared to the Narp cDNA, which is 2561. The Apexin sequence appears to be full-length because Northern analysis of Apexin identifies a prominent band of 1.6 kb in testis (brain RNA was not examined) (Reid and Blobel, 1994), which is consistent with the size of their reported cDNA. Our Northern blot (see below) indicates that the Nurp mRNA transcript is enriched in brain functional 1995). NPII-predicted amino acid sequence is 94% Narp. This degree of identity, together with its similar mRNA size and distribution of expression, suggests that NPII may be the human homolog of Narp. Consistent with its possible regulation as an IEG, we note that the putative promoter region of NPII possesses consensus binding sites for the transcription factors CREB and Zif268. and Nat-p mRNA is enriched in brain and is rapidly induced by seizure The expression of Narp mRNA was examined by Northern blot analysis of total RNA from rat cerebral cortex, hippocampus, and different peripheral tissues (Fig. 3). We also assayed Narp mRNA in hippocampus and cortex after MECS. Narp is enriched in cortex and hippocampus relative to peripheral tissues. Lower levels of Narp mRNA were also detected in the testis (and ovary; data not shown). Narp mRNA is induced in the hippocampus within 1 hr after MECS and remains elevated for as long as 8 hr. Densitometry of the autoradiogram indicates that Narp mRNA is induced at least fivefold by MECS. Increased levels of mRNA in the hippocampus after MECS are associated primarily with granule cell neurons (Fig. 4). Nurp mRNA is induced in animals Tsut et al. l Narp Expression and Regulation in Bram J. Neuroscl., April 15, 1996, 76(8):2463-2478 2469 Narp Apexin NPTXZ Nary, Apexin NPTXZ 100 99 98 Narp Apexin NPTX2 Narp LGEL ERQLLRKVAE Apexin NPTX2 Narp Apexin NPTX2 LNALLQRV LNALLQRV TELERGNSAF TELERGNSAF KSPDAFKVSL KSPDAFKVSL LEDEKSLLHN TNYLYGK TNYLYGK ETSA IKKTLPEL IKKTLPEL 200 193 198 250 243 248 Narp Apexin NPTX2 300 293 298 Narp Apexin NPTX2 350 343 348 Narp Apexin NPTX2 LILGQEQDTV GGRFDATQAF VGELSQFNIW Narp Apexin NPTX2 DRVL 400 393 398 432 425 430 Figure 2 Continued pretreated with cycloheximide,indicating that the induction does not require new protein synthesis(data not shown).mRNA induction in the absenceof new protein synthesisis a defining characteristicof IEGs (Lau and Nathans,1987). Narp is rapidly regulated by physiological synaptic activity The regulation of Nulp mRNA was also analyzed usingthe paradigmof in vivo LTP. As shownin Figure 4 (fop), Nalp mRNA is strongly induced4 hr after an HF synapticstimulusthat produces LTP (n = 2). The time courseof Nurp mRNA increasesafter LTP induction was also assayed.Increasesin Narp mRNA were detected asearly as30 min (n = 6), appearednear-maximalafter 1 hr (n = 2), 2 hr (n = 2) and 4 hr (n = 2), and returned to basal level after 24 hr (n = 2) (data not shown). This time course is similar to that after MECS (Fig. 3). Induction of Narp mRNA after LTP is dependent on the activation of NMDA receptors becauseprevious treatment of rats with the noncompetitive NMDA receptor antagonistMK-801 (1 mg/kg, i.p.) blocked increasesin both Nuvp mRNA and LTP (n = 3; data not shown). Nurp mRNA is readily detected by in situ hybridization in normal cortex and is enriched in cortical layers 2/3 and 5/6. To determinewhether this basalexpressionis regulated by synaptic activity, we monitored Nurp mRNA levels in visual cortex after monocularinjection of TTX. This manipulationreducesafferent activity to the contralateral visual cortex and resultsin a rapid decreasein the expressionof several of the IEGs known to be expressedin cortex, indicating that their basalexpressionis continuously maintainedby natural afferent synapticactivity (Worley et al., 1991). Rapid responsegenesthe expressionof which is dynamicallyregulated in this paradigminclude~$268 (Worley et al., 1990,1991),prostaglandinsynthase(Yamagata et al., 1993), Egr-3 (Yamagataet al., 1994a),Arc (Lyford et al., 1995),and PA activin (Andreassonand Worley, 1995). Becausethere are no intracranial manipulationsandbecausethe perturbation resultsin 2470 J. Neurosci., April 15, 1996, 76(8):2463-2478 Tsui et al. 3. Narp mRNA induction in hippocampus and cortex by MECS and expression in peripheral tissues. Northern analysis of Narp mRNA after an MECS-induced seizure in the hippocampus and cortex and expression in peripheral tissues. In the hippocampus, NaT mRNA is induced as early as 1 hr after MECS, and expression remains elevated for 8 hr. Narp mRNA is also induced by MECS in the cortex. Narp mRNA is enriched in control hippocampus and cortex (highlighted with a dot) relative to peripheral tissues. Figzwe a reduction of natural activity rather than a stimulation of activity, as results with MECS and in viva LTP, dynamicregulation in this monoculardeprivation paradigmprovides someof the strongest evidence of a role for the gene in normal synaptic physiology. As demonstratedby Figure 5, Nalp mRNA is reduced in the deafferented visual cortex, most noticeably in cortical layers 213 (top), within 4 hr after TTX injection. The reduction of Nalp mRNA is mostevident in comparisonsof the contiguousassociationcortex and primary visual cortex where there is a prominent reduction of hybridization. In control cortex, the level of hybridization is uniform acrossthis sameboundaryregion. Becausevisualprojections in the rodent are -90% crossed(Zilles et al., 1984) the effect of deprivation is almost exclusively contralateral. The effect of monocular‘ITX injection on basalexpressionof Narp mRNA is lessdramatic than is evident with certain other IEGs suchaszif268. This differencein the degreeof reduction of Narp andzif268 mRNAs doesnot appearto be attributable to a slower rate of degradationof Narp becausesimilar differences in the degreeof mRNA reduction were alsoobserved18 hr after ITX injection (n = 5; data not shown).Narp mRNA in cortex is less abundant than zif268, and technical differences in detection may contribute to their differential responsiveness. Alternatively, basal expressionof Narp in cortex may be regulated by signalsin addition to those generated by afferent activity. mRNA expression is developmentally regulated and enriched in limbic structures and sensory ganglia The developmentalexpressionof Narp mRNA was assayedby Northern blot analysisof total RNA isolatedfrom whole brainsof embryonic day 14 to adult (Fig. 6A). Narp mRNA was first detected at embryonic day 14 and increasedmonotonically to peak levels at postnatal day 21. Levels remain high in adult animals.In situ hybridization wasusedto examinethe anatomical distribution of Narp mRNA in embryonic day 19 rats. Nalp mRNA is detected in the habenula,dorsalmedial hypothalamus, hippocampus,and trigeminal ganglia (Fig. 6B). Lower levelsare Narp Narp Expression and Regulation In Brain cortex Hippocampus monocular l 4. Narp mRNA in hippocampal granule cells by LTP stimulation. Rats were chronically implanted for in viva recording and were stimulated as described in Materials and Methods. Natp mRNA was analyzed by in situ hybridization. The top brain is from a rat that received a unilateral high-frequency LTP stimulus to the right perforant path and an identical number of low-frequency stimuli to the left hippocampus 4 hr before killing. Narp mRNA is markedly increased in the granule cell layer that received the LTP stimulus. The bottom brain is a composite of half brains from a naive control rat (left) and a rat that received a MECS seizure 4 hr before killing (right). MECS results in Narp mRNA induction in the superficial and deep layers of the neo- and pyriform cortices as well as the dentate gyrus. Densitometry of the autoradiographic image indicates a 2.5-fold increase in the grain density over the granule cell layer of the dentate gyrus in the 4 hr LTP hippocampus compared with control. Figure presentin cortex and other forebrain structures.A more detailed analysis of the developmental expressionwill be published separately. In vitro synthesis and post-translational modification of Narp Zn vitro TNT wasusedto confirm the predicted ORF. The longest cDNA clone produceda protein with an M, of -46 kDa, which is identical to the predicted size(Fig. 7A). TNT usinga cDNA clone that begins-30 nucleotides3’ to the predicted initiator methionine produced an -43 kDa protein @‘-truncated Narp), which correspondsto the predicted product size synthesizedfrom the first internal methionine(Fig. 7A). Restriction of the samecDNA before TNT with BglI, which cuts 67 nucleotides 5’ to the C terminus of the predicted ORF (nucleotide 1242), results in a product of reducedsizeconsistentwith the position of the restriction sitein the ORF (data not shown).Theseexperimentssupport the predicted ORF. The ORF of Nalp encodesa putative secretory signalpeptide. To assess the presenceof a functional signalsequence,full-length Narp wasprepared by TNT in the presenceof dog microsomes. The microsomepreparation hasthe necessaryactivity to translocate the nascentprotein into the microsomeand cleavesthe signal sequence.Narp prepared in the presenceof dog microsomes yields severalproductsthat migrate on SDS-PAGE with an M, of -44 to -58 kDa. The -44 kDa product is slightly smallerthan the -46 kDa product prepared without microsomes (Fig. 7B), consistent with microsome-dependent signal peptide cleavage. Demonstration that Nalp is secretedby COS-1 cells (below) is also consistentwith the hypothesisthat Narp possesses a functional signalsequence. Tsui et al. l Narp Expression and Regulation in Brain J. Neurosci., A Pmnatal Tissues April 15, 1996, Postnatal 76(8):2463-2478 2471 Tissues 18s Figure 5. Regulation of Nap mRNA by natural synaptic activity in visual cortex. Postnatal day 21 rat received a monocular injection of TTX 4 hr before killing. In situ analysis of Nurp mRNA demonstrates reduced expression in the deafferented visual cortex (between arrowheads, top). A prominent difference in expression is evident between contiguous primary and association visual cortices (boundary is indicated by the lateral arrow), whereas in control cortex Nurp mRNA expression is uniform across this boundary. Densitometry of the autoradiographic image indicates a 1% fold higher grain density over the temporal cortex relative to the contiguous deprived visual cortex. Identical results were observed in four additional 21-d-old rats and three adult rats killed 4 hr after TTX injection. Narp has three potential N-glycosylation sites. Other pentraxins are known to be glycosylated and this modification may be important in their saccharide-binding properties (Emsley et al., 1994). In addition to the product that migrates slightly faster than Narp prepared without microsomes described above (-44 kDa), a second product is observed that migrates with an M, of -58 kDa. To determine whether mature Narp is glycosylated,we examined the effect of treating the TNT/microsomeproduct with endoglycosidaseH. As shownin Figure X, endoglycosidase H converts the -58 kDa band to an -44 kDa product, consistentwith the interpretation that the -58 kDa band is glycosylatedNap. Narp protein is secreted or COS-1 cells were transiently transfected with pRK.5 Nap vector alone. Forty hours after transfection, cellswere metabolically labeledwith [““Slmethionine 3 hr before samplingmedia. Conditionedmediafrom cellswere analyzed by SDS-PAGE and autoradiography.A prominent band of -58 kDa is presentin the mediaof cellstransfectedwith pRK5 + Nalp that is not present in cellstransfectedwith vector alone(Fig. &i). The -58 kDa band is enriched in the media and was not detected in the pelleted COS-1cells(data not shown).To confirm that the -58 kDa band representsNarp, we performed immunoprecipitationsfrom the conditioned media using a polyclonal rabbit antiseragenerated againsta full-length Nalp bacterial fusion protein and demonstratedthat the -58 kDa band is selectivelyprecipitated (Fig. 8s). Thesedata strongly suggestthat Narp is secretedinto the media 6. Developmental regulation of Nqv mRNA expression. A, Northern analysis of total RNA (10 pg/lane) prepared from forebrains of embryonic (E) days 14, 15, 17, 19, and 21, and postnatal (P) days 1, 4, 8, 12,16,5 week-, 8 week-, and 3-month-old rats. Nurp mRNA is detected as early as El4 and increases steadily to peak levels between P16 and P21. The slight difference in motility of the E21 RNA sample may be attributable to excess salt in this preparation. B, In situ analysis of expression of Nurp mRNA in El9 rat forebrain (magnification, .5X). Nurp mRNA is detected in the hippocampus (h), habenula (b), ventromedial hypothalamus (v), and the trigeminal ganglia (t). C, Cresyl violet counterstaining of the in situ section of El9 rat. Figure by the transfected COS-1 cells. Although it remains formally possiblethat Narp is passivelyreleasedinto the mediaby COS-1 cells that are injured or dying, this is unlikely becausethe metabolic label, which is incorporated into newly synthesizedprotein, is added40 hr after transfectionat a time when cytotoxic effectsof this manipulation should be resolved. Additionally, media is sam- pled 3 hr after addition of the metaboliclabel, thereby limiting the interval during which cellsmight undergospontaneouscell death and releaseof intracellular contents. Biochemical studies of Narp: demonstration of calcium-dependent binding to a complex saccharide matrix Pentraxins bind specifically to derivatized saccharides,and this lectin property is thought to be essentialfor their biological function (Emsley et al., 1994). The close homology of Narp to other pentraxins, particularly at the Ca’+- and carbohydratebinding domains, suggeststhat Narp may also function as an endogenouslectin. Substratestypically usedin pentraxin-binding assaysare modified agar. Agar is a polysaccharidethat contains agarobiose,sulfate, and pyruvate (Hind et al., 1984), and agaroseis prepared from agar by removing sulfated sugars.The 2472 J. Neuroscl., April 15, 1996, 76(8):2463-2478 Tsui et al. l Narp Expression and Regulation in Brain C kDa EndoH - + ld)a -68 -18 l 18 Figure Z In vitro synthesis of Nurp protein: post-translational modification of Nurp protein by dog, microsomes. A, Circular and linearized Nary cDNA plasmids subcloned in pBluescript SK+ vector were used as templates to synthesis l\iarp pro&in b; tn vifro TNT. Translated ““S-labeled prod&s were analyzed by SDS-PAGE. An -46 kDa protein product is detected using the circular or linearized full-length Num1 olasmids farrow‘l. An -43 kDa ~~~ nrotein r~ - .-... I \ is synthesized from a plasmid, .5’-trundated N&u, that lacks the puta&e initiator methionine but includes the first internal methionine. The observed product size is consistent with translation initiation from the first internal methionine. Two bands, -34 and -27 kDa, are nonspecific products from the pBluescript vector alone (data not shown). No product is detected in the absence of added DNA (d-H,0 control). B, Cleavage of the putative signal sequence of Nurp protein by dog microsomes. Full-length Nurp was synthesized by in vitro TNT reactions in the presence or absence of dog microsomes (DM). Translation is more efficient in the presence of microsomes, and nonspecific bands at -34 and -27 kDa are not evident. A slight reduction in the size of the Nulp protein is detected compared to the Nurp protein synthesized in the absence of dog microsomes, consistent with cleavage of the signal sequence (arrowheads). C, N-glycosylation of Nurp protein. Nurp prepared by in vitro synthesis in the presence of dog microsomes yields major products of -58 and -44 kDa (arrows). Incubation of Nurp with 2 mU of endoglycosidase H shifts the -58 kDa protein to -44 kDa, supporting the inference that the -58 kDa band is a glycosylated form of Narp. polysaccharide backbone of agarose (marketed under trade name Sepharose) can be derivatized by covalent attachment to a variety of molecules. CRP and SAP are known to bind to phosphatidylethanolamine-Sepharose (PE-Sepharose) and phosphatidylcholine-Sepharose (PC-sepharose), and these substrates are commonly used to purify CRP and SAP from body fluids (Schwalbe et al., 1992). In all cases, binding to these substrates is calcium-dependent. To examine the hypothesis that Nurp is a calcium-dependent lectin, a full-length C-terminal myctagged Nalp expression construct was prepared in pRK5 and expressed in COS-1 cells for binding studies. The COS-1 cellderived myc-tagged Narp is biologically active in the neurite outgrowth assay described below and was used in preference to native Narp because of the relative insensitivity of the Narp antisera for immunoblots. As demonstrated in Figure 9, myctagged Narp binds specifically to agar in the presence of Cazt and quantitatively elutes in the presence of the divalent cationic chelator EDTA. The estimated size of the EDTA-elutable band is -60 kDa and corresponds closely to the size of the glycosylated Nalp prepared by TNT with dog microsomes. No EDTA-elutable band was detected in parallel experiments that used conditioned media from COS-1 cells transfected with the expression vector lacking the Narp insert. Myc-tagged Nalp did not bind in a calcium-dependent manner to agarose, Sepharose, or PC-Sepharose (data not substrate specificity of Narp is distinct The difference in binding to agar and pyruvate or sulfated side chains present in binding to Narp. low-melting agarose, PEshown), indicating that the from either CRP or SAP. agarose suggests that the in agar may be important Narp promotes neurite outgrowth of cortical explant neurons Pentraxins possess structural and biochemical similarities to the class of calcium-dependent plant-derived lectins that include concanavalin A (Emsley et al., 1994). Concanavalin A produces marked effects on a variety of cell types including neurons (Lin and Levitan, 1987, 1991), and we have examined the possibility that Nalp may mimic certain of these effects. Concanavalin A is highly active in promoting neurite outgrowth (Lin and Levitan, 1987). Accordingly, we examined the possibility that Nalp might also promote neurite outgrowth. Cortical explants were prepared from postnatal day 1 rat pups as described previously. One day after plating cortical explants, COS-1 cells, which had been transfected previously with either a Narp expression construct or the vector alone, were added to the culture. In cocultures expressing Narp, cells at the periphery of the TSUI et al. l Narp Expresslon and Regulation J. Neurosci., m Bram B A xP 2uQ kDa kDa -200 -97 -68 4 -43 -29 -29 -18 -14 Supernatant -14 lmmunoprecipitation Figure 8. Transient expression and secretion of Nurp by COS-1 cells. COS-1 cells were transientlv transfected with 10 ~g of plasmid DNA (pRK5 vector alone or pRR5 + Nqu). Forty hours after transfection, COS-1 cells were labeled with 250 uCi/ml 1”‘Slmethionine for 3 hr in Met- DMEM supplemented with 1’% dialyied’fetal bovine serum. A, Supernatants from pRK5 vector alone and pRJS5 + Nq were collected and fractionated on SDS-polyacrylamide gels. The armwhead indicates an -58 kDa band expressed only by COS-1 cells transfected with pRK5 + Narp, suggesting that Nurp is secreted into the media. B, lmmunoprecipitations’were performed using polyclonal antisera generated against the recombinant bacterial protein as described in Materials and Methods. The -58 kDa band is selectively immunoprecipitated by immune serum. explant exhibited exuberant outgrowth of processes within 24 hr of the addition of the COS-1 cells. Processoutgrowth was observedsurroundingthe perimeter of each of 26 separateexplants from three dishesin cocultures that expressedNurp, whereas explantscultured with control COS-1 cells (17 separateexplants from 3 dishes)or without COS-1 cells(16 explantsfrom 3 dishes) April 15, 1996, 76(8):2463-2478 2473 exhibited no processes.By 48 hr in coculturewith Narp, processes were longer and more prominent than at 24 hr, and isolatedcells with elongatedprocesses were seensurroundingthe explant that appearedto have migrated out of the body of the explant. In control cocultures,only infrequent short processeswere present by 48 hr without evidence of cellular migration. Similar results were obtained in four additional experiments.As a control for the specificity of Nalp in this assay,we examinedthe effect of coculturing cortical explantswith COS-1 cellsthat expressand secrete amyloid precursor protein-like protein (APLP-2) (Slunt et al., 1994).Theseexplantsappearedidentical to control explantscultured either without COS-1 cellsor with COS-1 cellstransfected with vector alone (data not shown). To determine the cellular compositionof the Narp-dependent processoutgrowth, we performed immunohistochemistryusing antiserafor the neuron-specificproteins MAP2 (Pennypackeret al., 1991), which is selectively expressedin neuronal dendrites, and tau, which is expressedin neuronal axons (Binder et al., 1985),aswell asfor the glial-specificprotein GFAP (Rinamanet al., 1993). In control explants, MAP2-positive cell bodies and processes werepresentwithin the explant, and few processes were seen extending beyond the edge of the explant (Fig. l&4). By contrast, explants cocultured with Nary secreting COS-1 cells exhibited MAP2-positive cells with exuberant processesboth at the border of the explant and extendingmanycell body diameters from the edge of the explant (Fig. 1OB). The morphology and immunohistochemicalproperties of thesecellsindicate that they are neurons.Tau immunostainingwasdifficult to detect in control explants and was not increasedin explantscocultured with Narp secretingCOS-1 cells (data not shown).GFAP-stained glial cells appearedto be largely restricted to the body of the explant at the 24 and 48 hr time points (data not shown). We concludefrom theseexperimentsthat Nalp-secretingCOS-1 cellspromote neurite outgrowth andmigration of neuronsfrom the cortical explant. This effect is not accompaniedby prominent growth or migration of glial cells, suggestingthat the effect is primarily on neurons. We usedthe neurite ,outgrowth promoting effect of Nalp as a bioassayof its function to test the activity of C-terminal myctagged Narp. Cortical microexplants grown in coculture with COS-1 cellsexpressingmyc-Nalp demonstratedthe samegrowth of neurites and migration of neuronsas explantscoculturedwith COS-1 cells secretingnatural Nurp (data not shown). We conclude that the myc epitope tag doesnot interfere with the biological activity of Narp in this assay. Using the COS-1 cell-generatedmyc-Nurp, we performed two additional setsof experimentsto confirm that the active principle in the coculture paradigmis Narp. In the first set of experiments, agar column chromatography was used to prepare partially puri- fied myc-taggedNurp. Addition of this material to the microexplant culture reproducedthe neurite outgrowth effect of coculture with Narp-secretingCOS-1 cells (Fig. lL4). In control experiments,identical agarcolumn fractionspreparedfrom COS-1cells transfectedwith the pRK5 vector alone did not induce neurite outgrowth. The secondsetof experimentsuseda myc monoclonalantisera to immunodepletemyc-taggedNurp from the agar columneluate. Addition of myc mAb and subsequent precipitation with G-protein-Sepharoseremoved neurite outgrowth promoting activity (Fig. 11B). In control experiments,addition of mouseascites fluid followed by G-protein precipitation did not block this activity. In the final set of experiments,we sought to establishthe 2474 J. Neurosci., April 15, 1996, 16(8):2463-2478 Tsui et al. l Narp Expression and Regulation in Brain kDa kDa 43.6 - - 70.8 - 43.6 - 28 Figure 9. Ca’+-dependent binding of Narp protein to an agar column. Narp was synthesized by COS-1 cells transiently transfected with an expression vector for myc-tagged Narp. A shows the Western blot analysis using mouse anti-myc Abs (1:500) of the COS-1 cell supernatant @e-flow Through), flow-through fraction, wash fractions (0.15 M NaCI, 0.05 M Tris, pH 7.4), EDTA elution fractions (0.15 M NaCl, 0.05 M Tris, 0.02 M EDTA, pH 7.4), and SDS-loading buffer fraction. Myc-tagged Narp protein (arrowhead; -60 kDa) binds to the agar column in a Ca*+ -dependent manner and is quantitatively eluted in the second and third EDTA elution fractions. Serum contains proteins that cross-react during development of the anti-myc immunoblot and are seen in the Pre-flow Through and Flow Through fractions. B, Identical agar column fractions from conditioned media of COS-1 cells transiently transfected with the pRK5 vector alone. Note similar cross-reacting bands in the Pre-flow Through and Flow Through lanes but the absence of EDTA-elutable myc-immunoreactive protein. concentration of Narp necessary to promote neurite outgrowth. Again, we used myc-tagged Narp and established a direct competitive ELISA. Aliquots of agar column fractions were assayed in parallel for biological activity, and levels of myc-tagged Narp were quantitated. These studies indicate that full activity is produced by -40 @ml myc-tagged Narp (Fig. 11B). Based on our estimated molecular weight of the Narp monomer (-58 kDa), this translates to an effective concentration of -0.8 nM. Higher concentrations were without additional effect. In conjunction, these experiments establish that Narp promotes neurite outgrowth and establishes the concentration range of its activity to be comparable to that of growth factor-induced effects in other systems. Chromosomal localization of Nay The mouse chromosomal location of Narp was determined by interspecific backcross analysis using progeny derived from matings of [(C57BL/6J X Mus spretc~)F, X C57BL/6J] mice. The mapping results indicate that Narp is located in the distal region of mouse chromosome 5 linked to Epo, Pd&a, and Flt3. Although 159 mice were typed for every marker, up to 191 mice were typed for some pairs of markers. Each locus was analyzed in pairwise combinations for recombination frequencies using the additional data. The ratios of the total number of mice exhibiting recombinant chromosomes to the total number of mice analyzed for each pair of loci, and the most likely gene order is: centromere - Epo 71191 - Pdgfa - 31177 - Flt3 - O/l60 - Narp. The recombination frequencies [expressed as genetic distances in centiMorgans (CM) + SE] are: Epo, 3.7 k 1.4; Pdgfa, 1.7 + 1.0 [Flt3, Nalp]. No recombinations were detected between Flt3 and Narp in 160 animals typed in common, suggesting that the two loci are within 1.9 CM of each other (95% confidence limit). We have compared our interspecific map of chromosome 5 with a composite mouse linkage map that reports the map location of many uncloned mouse mutations [compiled by M. Davisson, T. Roderick, A. Hillyard, and D. Doolittle and provided by GBASE, a computerized database maintained at The Jackson Laboratory (Bar Harbor, ME)]. Nalp mapped in a region of the composite map that lacks mouse mutations with a phenotype that might be expected for an alteration in this locus. The distal region of mouse chromosome 5 shares a region of homology with human chromosomes 7 and 13. DISCUSSION We have identified and characterized a novel IEG, termed Narp, that is a member of the pentraxin family. Nurp mRNA is rapidly and transiently induced in neurons by electrically evoked seizures. Narp is also dynamically regulated by physiological synaptic activity in the adult hippocampus and visual cortex. In the hippocampus, synaptic induction of Nav is associated with the production of LTP and is dependent on NMDA receptor activation. In this paradigm, Nulp mRNA induction, like the induction of other IEGs, is not simply linked to synaptic activity but, rather, to specific forms of activity that activate the NMDA receptor (Worley et al., 1993). Identical numbers of synaptic stimuli adminis- Tsui et al. l Narp Expression and Regulation m Brain J. Neurosci., April 15, 1996, 76(8):2463-2478 2475 initial reports that is suggestiveof function. Biochemicalcharacterization of Nalp protein demonstratesthat it binds to agar, a complex polysaccharidematrix, in a calcium-dependentmanner. Calcium-dependentbinding to sugarsis a shared property of biochemicallycharacterizedmembersof the pentraxin family and is consistentwith the high degreeof conservationbetweenNalp and other pentraxinsin the essentialdomainsinvolved in calcium and saccharidebinding. The binding specificity of Narp appearsto be distinct from that of CRP or SAP in that it bindsagarbut not phosphatidylcholine or phosphatidylethanolamine derivatized agarose.The physiologicalsaccharidespecificityof Narp in brain remainsto be determined.Like other pentraxin family members, Narp possesses a functional signalsequenceand is secretedinto the media when expressedby COS-1 cells. Presumably,Natp is also secretedby neurons,consistentwith a possiblefunction in intercellular signaling. An interesting aspectof the pentraxin family is that it shares secondaryand tertiary structurewith the plant lectin concanavalin A (Emsleyet al., 1994).Although the precisecellular functionsof membersof the pentraxin family remain controversial,their multivalency and calcium-dependentlectin properties appear to be important. CRP is thought to play a role in non-Ab-mediated immune responsesby binding and aggregatingbacteria or other pathogens(Siegel et al., 1974, 1975). SAP is believed to coat moleculespresent in the basementmembraneand protect them from proteolytic degradation (Emsley et al., 1994). SAP is also reported to be a component in all known forms of amyloid, including the amyloid of Alzheimer’s disease,and may therefore play a role in certain diseaseprocesses(Coria et al., 1988;Pepys et al., 1994). Evidence that the lectin property of pentraxins is central to their function supportsthe hypothesisthat Narp may alsofunction in vivo asan endogenous,calcium-dependentlectin. Severallines of evidencesuggesta role for specificinteractions betweenlectins and glycosylatedproteins or lipids in neurobiolFigure IO. Nurpinduces neuriteoutgrowthfromcorticalexplantcultures. ogy. Complexoligosaccharideantigensand carbohydrate-binding Corticalexplantspreparedfrompostnatalday1 rat pupsw&e cocultured proteins have been localized to specific developing neurons for 48hr withCOS-1cellsthat hadoreviouslv withaNuro * beentransfected 1 (Dodd et al., 1984;Dodd and Jessell,1985;Blum and Barnstable, expression construct(B) or thevector alone(control;A) asdescribed in MaterialsandMethods.Cultureswerestainedwith the neuron-specific 1987). Endogenous“soluble lectins” have been purified from anti-MAP2mAb. In explantscoculturedwith controlCOS-1cells(A), brain and peripheraltissues(Kobiler et al., 1978;Barondes,1984) MAP2-positivecellsare largelyrestrictedto the bodyof the explant.By and are capableof aggregatingcells, and they can alter growth contrast,exuberantMAPZpositiveneuriteoutgrowthisevidentfrom the properties of neuronsin culture (Joubert et al., 1987;Kuchler et corticalexplantscocultured with COS-1cellsexpressing Narp (B). MAPZ and coworkers have characterized a soluble positivecellbodiesarevisualized beyondthe edgeof theexplant,suggest- al., 1988). Jesse11 ingmigrationfrom the explant.Magnification,400X. lectin, termed RL-14.5, that is expressedby neuronsin the developing spinal cord, brainstem,and dorsal root ganglia(Regan et al., 1986; Hynes et al., 1990). These studiessupport a role for tered at low frequency, which do not activate the NMDA receplectins in the developmentof specificcellular interactionsin the tor, do not induce Narp. Also like other IEGs, Narp mRNA is expressedat relatively high levels by neuronsin the adolescent nervous system. Solublelectins,purified from either brain or peripheraltissues, and adult neocortexwhere natural synaptic activity is dependent, are highly homologous,and their saccharidebinding is indepento a substantialdegree,on NMDA receptor activation (Miller et dent of calcium(Barondes,1988;Clerch et al., 1988;Hynes et al., al., 1989;Fox and Daw, 1993).Basalexpressionof Narp in cortex 1990). Narp is structurally distinct from the family of soluble is dependenton natural synapticactivity as demonstratedby the lectins,and its lectin property iscalcium-dependent.Studiesusing rapid reduction in expressionin the primary visual cortex after plant lectins, which as noted are structurally and biochemically interruption of afferent visual input. Accordingly, Nav is exrelated to pentraxins, support a role for this subclassof calciumpressedby neuronsand is dynamically regulated in responseto dependentlectinsin neurobiology.Binding sitesfor concanavalin specifictypes of synaptic activity that are effective in producing A and pea lectin are enriched in the developingand adult brain long-term changes in cellular and synaptic properties. Nalp mRNA is alsoexpressedearly in the developingnervous system and are associatedwith specific neuronal and glial populations and is enriched in neurons of the limbic system and sensory (Simpsonet al., 1977;DeGrauw and Liwnicz, 1986;Waters et al., 1990;Adam et al., 1993).Plant lectins modify the rate of desenganglia,suggestinga role in early stagesof neuronal development sitization of glutamatereceptor channelsin both invertebrate and that is not presentlyknown to be driven by synapticactivity. vertebrate systems(Kehoe, 1978; Shinozaki and Ishida, 1979; Narp appearsto representthe rat homologof humanNPII and Huettner, 1990; Thio et al., 1993). In the rodent hippocampus, guineapig Apexin. However, there islittle information from these 2476 J. Neurosci., April 15, 1996, 76(8):2463-2478 Tsui et al. l Narp Expression and Regulation in Brain 60 VECTOR Fluid Ascites ALONE 0 50 pl 100 pl 200 pl (20) (40) (60) 400 pl (80) Anti-myc Abs Narp rig/ml Il. Neurite outgrowth assaywith partially purified myc-tagged Nurp. A, Partially purified myc Nurp induces neurite outgrowth. Cortical explants from postnatal day 1 rat pups were cultured on poly-L-ornithine-coated plates. Myc-tagged Nalp was partially purified from COS-1 cell supernatant, and aliquots were added to culture media. The amount of myc-tagged Nurp was determined by ELISA. After 24 hr, the number of explants with neurite outgrowth was determined and expressed as the percent of the total number of cortical explants. Data are from three separate experiments, each performed in duplicate. Ncurite outgrowth is observed with -40 ngiml Nurp. Control experiments used identical volumes of agar column fractions prepared from conditioned media of COS-1 cells transfected with vector alone. Explants treated with the control fractions demonstrate a 20-30% spontaneous outgrowth. B, Immunodepletion with myc Ab blocks neurite outgrowth activity. Partially purified myc-tagged Nurp (-60 ng in 200 ~1) was immunodepleted using mouse anti-myc Ab conjugated to G-protein-agarose beads. Control experiments substituted mouse ascites for anti-myc Ab. Immunodepleted and control fractions were added to media, and neurite outgrowth was assayed after 24 hr. Data are from two separate experiments, each perfoimed in quadruplicate. Figure several plant lectins, including concanavalin A, selectively block desensitization of the AMPA receptor without altering NMDA, GABA,, or nicotinic acetylcholine receptor responses (Thio et al., 1992). Evidence that glutamate receptor function can be selectively modified by plant lectins provides a precedent for understanding how Narp might function on a molecular level. Narp is unique among the known set of endogenous lectins present in neural tissues in that it is regulated as an IEG and that its expression is tightly linked to physiological neuronal activity. Studies examining the cellular function of Narp demonstrate that it promotes neuronal dendritic outgrowth and migration of neurons from cortical explant cultures. These observations suggest that Nalp may function as a novel, neuron growth-promoting molecule that is tightly coupled to physiological activity. In this regard, Nay is similar to more conventional growth factors such as brain-derived growth factor or nerve growth factor, which are also rapidly regulated by neuronal activity (Bachoo et al., 1992; Patterson et al., 1992). Although the mechanism of the effect of Narp has not been determined, there are several clues as to how it might act. The effect of Narp is most obvious when microexplants are grown on a poly-t.-ornithine substrate, which is more supportive of neurite outgrowth than untreated plastic or glass but less supportive than poly-L-lysine, collagen, or NCAM. In our assays, Narp did not appear to promote neurite outgrowth when explants were cultured on collagen. Accordingly, it is possible that Narp is simply a better substrate for growth than polyornithine. Interactions between the extracellular matrix and membrane receptors of the integrin family can alter cell growth properties via modulation of intracellular kinase-dependent mechanisms similar to conventional receptor tyrosine kinases (Bachoo and Polosa, 1991). The major argument against the notion that Narp acts via a substrate effect is its potency. The potency of Narp is remarkable in that 40 r&ml (0.8 nM) is fully effective in promoting neurite outgrowth, whereas substrates such as NCAM and laminin are used in the concentration range of 1 mgiml (Lemmon et al., 1992). Moreover, we have been unsuccessful in reproducing the growthpromoting effects of Nalp by pretreating either plastic- or polyornithine-coated substrates with the same preparations of Nalp that are effective when added to explants in media (our unpublished observations). Finally, we note that concanavalin A and pea lectin have similar, marked substrate-dependent effects on neuronal process outgrowth and are effective at concentrations in the range of 10 nM to 1 PM (Lin and Levitan, 1987; Carrow and Levitan, 1989; Lin and Levitan, 1991; Outenreath and Jones, 1992). Based on similar arguments as presented for Narp, it is believed that the growth-promoting effects of plant lectins are attributable to the binding of specific receptors on the surface of neurons and are not attributable to a substrate effect. Pentraxins and plant lectins are multivalent lectins, and their binding efficacy, as well as activity in physiological assays, is dependent on their multivalency (Lin and Levitan, 1991). Accordingly, Narp might bind to glycoprotein targets on the cell surface to effect clustering of receptors or changes in cell-cell or cell-substrate interactions. In either event, whether it is mediated by a substrate effect or by a more classical receptor-mediated mechanism, the growthpromoting effects of Narp, in combination with its dynamic expression in the brain, suggest an important role in developmental and activity-dependent plasticity. REFERENCES Adam E, Dziegielewska KM, Saunders NR, Schumacher U (1993) Neuraminic acid specific lectins as markers of early cortical plate neurons. Int J Dev Neurosci 11:451-460. Agranoff B (1981) Learning and memory: biochemical approaches. In: Basic neurochemistry, 3rd Ed, p 801. Boston: Little & Brown. Tsui et al. l Narp Expression and Regulation in Brain Andreasson K, Worley P (1995) Induction of P-A activin expression by synaptic activity and during neocortical development. Neuroscience 69:781-796. Bachoo M, Polosa C (1991) Long-term potentiation of nicotinic transmission by a heterosynaptic mechanism in the stellate ganglion of the cat. J Physiol (Lond) 65:639-647. Bachoo M, Heppner T, Fiekers J, Polosa C (1992) A role for protein kinase C in long-term potentiation of nicotinic transmission in the superior cervical ganglion of the rat. Brain Res 585:299-302. Bailey CH, Montarolo PG, Chen M, Kandel ER, Schacher S (1992) Inhibitors of protein and RNA synthesis block the structural changes that accompany long-term facilitation in Aplysiu. Neuron 9:749-758. Barondes SH (1984) Soluble lectins: a new class of extracellular proteins. Science 223:1259-1264. Barondes SH (1988) Bifunctional properties of lectins: lectins redefined. Trends Biol Sci 13:480-482. Binder LI, Frankfurter A, Rebhun LI (1985) The distribution of tau in the mammalian central nervous system. J Cell Biol 101:1371-1378. Bliss TVP, Collingridge GL (1993) A synaptic model of memory: longterm potentiation in the hippocampus. Nature 361:31-39. Blum AS, Barnstable CJ (1987) 0acetylation of a cell-surface carbohydrate creates discrete molecular patterns during neural development. Proc Nat1 Acad Sci USA 84:8716-8720. Breviara F, d’Aniello EM, Golay J, Peri G, Bottazzi B, Bairoch A, Saccone S, Marzella R, Predazzi V, Rocchi M, Valle GD, Dejana E, Mantovani A, Introna M (1992) Interleukin-l-inducible genes in endothelial cells. J Biol Chem 267:22190-22197. Chen C, Okayamam H (1987) High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7:2745-2752. Clerch LB, Whitney P, Hass M, Drew K, Miller T, Werner R, Massaro D (1988) Sequence of a full-length cDNA for rat lung P-galactosidasebinding protein: primary and secondary structure of the lectin. Biochemistry 27:692-699. Cohen C, Parry D (1994) a-helical coiled coils: more facts and better predictions. Science 263:488-489. Cole A, Abu-Shakra S, Saffen D, Baraban J, Worley P (1990) Rapid rise in transcription factor messenger RNAs in rat brain after electroshock induced seizures. J Neurochem 55:1920-1927. Copeland NG, Jenkins NA (1991) Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet 7:113-118. Coria F, Castano E, Prelli F, Larrondo-Lillo M, van Duinen S, Shelanski ML, Frangione B (1988) Isolation and characterization of amyloid P component from Alzheimer’s disease and other types of cerebral amyloidosis. Lab Invest 58:454-458. Davis HP, Squire LR (1984) Protein synthesis and memoty: a review. Physiol Bull 96:518-559. DeGrauw TJ, Liwnicz BH (1986) Lectins are markers of neuronal migration and differentiation in rat brain. Dev Neurosci 8:236-242. Dodd J, Jesse11 TM (1985) Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to superficial dorsal horn of rat spinal cord. J Neurosci 5:3278-3294. Dodd J, Solter D, Jesse11 TM (1984) Monoclonal antibodies against carbohydrate differentiation antigens identify subsets of primary sensory neurons. Nature 311:469-472. Dowton SB, McGrew SD (1990) Rat serum amyloid P component. Biothem J 270:553-556. Emsley J, White HE, O’Hara BP, Oliva G, Srinivasan N, Tickle IJ, Blundell TL, Pepys MB, Wood SP (1994) Structure of pentameric human serum amyloid P component. Nature 367:338-345. Flexner JB, Flexner LB, Stellar E (1963) Memory in mice as affected by intracerebral puromycin. Science 141:57-59. Fox K, Daw NW (1993) Do NMDA receptors have a critical function in visual cortical plasticity? Trends Neurosci 16:116-122. Frey U, Huang YY, Kandel ER (1993) Effects of CAMP stimulate a late stage of LTP in hippocampal CA1 neurons. Science 260:1661-1664. Goelet P, Castellucci V, Schacher S, Kandel E (1986) The long and the short of long-term memory: a molecular framework. Nature 3221419-422. Green EL (1981) Linkage, recombination and mapping. New York: Oxford UP. Hind CRK, Collins PM, Renn D, Cook RB, Caspi D, Baltz ML, Pepys MB (1984) Binding specificity of serum amyloid P component for the pyruvate acetal of galactose. J Exp Med 159:1058-1069. J. Neurosci., April 15, 1996, 16(8):2463-2478 2477 Hsu YC, Perin MS (1995) Human neuronal pentraxin II (NPTX2): conservation, genomic structure, and chromosomal localization. Genomics 28:220-227. Huettner JE (1990) Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron 5~255-266. Hynes MA, Gitt M, Barondes SH, Jesse11 TM, Buck LB (1990) Selective expression of an endogenous lactose-binding lectin gene in subsets of central and peripheral neurons. J Neurosci 10:1004-1013. Jenkins NA, Copeland NG, Taylor BA, Lee BK, (1982) Organization, distribution, and stability of endogenous ecotropic murine leukemia virus DNA sequences in chromosomes of Mus musculuus. J Virol 43126-36. Jordan CA (1990) In situ hybridization in cells and tissue sections: a study of myelin gene expression during CNS myelination and remyelination. In: In situ hybridization histochemistry, pp 39-70. Boca Raton, FL: CRC. Joubert R, Caron M, Bladier D (1987) Brain lectin-mediated agglutinability of dissociated cells from embryonic and postnatal mouse brain. Dev Brain Res 36:146-150. Kaufmann WE, Worley PF, Pegg J, Bremer M, Isakson Cox P (1996) Cox-2, a synaptically-induced enzyme, is expressed by excitatory neurons at postsynaptic sites in rat cerebral cortex. Proc Nat1 Acad Sci USA, in press. Kehoe J (1978) Transformation by concanavalin A of the response of molluscan neurons to t-glutamate. Nature 274:866-869. Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M (1994) Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 78:425-435. Kobiler D, Beyer EC, Barondes SH (1978) Developmentally regulated lectins from chick muscle, brain, and liver have similar chemical and immunological properties. Dev Biol 64:265-272. Kozak M (1987) At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J Mol Biol 196:947-950. Kuchler S, Fressinaud C, Sarlieve LL, Vincendon G, Zanetta JP (1988) Cerebellar soluble lectin is responsible for cell adhesion and participates in myelin compaction in cultured rat oligodendrocytes. Dev Neurosci 10:199-212. Lau LF, Nathans D (1987) Expression of a set of growth-related immediate early genes in BALB/c3T3 cells: coordinate regulation with c-fos or c-myc. Proc Nat1 Acad Sci USA 84:1182-1186. Lee G, Lee TH, Vicek J (1993) TSG-14, a tumor necrosis factor- and IL-l-inducible protein, is a novel member of the pentraxin family of acute phase proteins. J Immunol 150:1804-1812. Lemmon V, Burden SM, Payne HR, Elmslie GJ, Hlavin ML (1992) Neurite growth on different substrates: permissive versus instructive influences and the role of adhesive strength. J Neurosci 12:818-826. Lin SS, Levitan IB (1987) Concanavalin A alters synaptic specificity between cultured Aplysia neurons. Science 237:648-650. Lin SS, Levitan IB (1991) Concanavalin A: a tool to investigate neuronal plasticity. Trends Neurosci 14:273-277. Linzer DIH, Nathans D (1983) Growth-related changes in specific mRNAs of cultured mouse cells. Proc Nat1 Acad Sci USA 80:4271-4275. Lyford G, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF (1995) Arc, a growth factor and activity-regulated gene encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 14:433-445. Miller KD, Chapman B, Stryker MP (1989) Visual responses in adult cat visual cortex depend on N-methyl-D-aspartate receptors. Proc Natl Acad Sci USA 86:5183-5187. Montarolo PG, Goelet P, Castellucci V, Morgan J, Kandel E, Schacher S (1986) A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science 234:1249-1254. Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y (1993) Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature 363:713-722. Nguyen PV, Abel T, Kandel ER (1994) Requirement of a critical period of transcription for induction of a late phase of LTP. Science 265:1104-1106. Noland TD, Friday BB, Maulit MT, Gerton GL (1994) The sperm acrosomal matrix contains a novel member of the pentraxin family of calcium-dependent binding proteins. J Biol Chem 269:32607-32614. Patterson SL, Grover LM, Schwartzkroin PA, Bothwell M (1992) Neurotrophin expression in rat hippocampal slices: a stimulus paradigm 2478 J. Neurosci., April 15, 1996, 76(8):2463-2478 inducing LTP in CA1 evokes increases in BDNF and NT-3 messengerRNAs. Neuron 9:1081-1088. Pennypacker K, Fischer I, Levitt P (1991) Early in vitro genesis and differentiation of axons and dendrites by hippocampal neurons analyzed quantitatively with neurofilament-H and microtubule-associated protein 2 antibodies. Exp Neurol 111:25. Pepys MB, Rademacher TW, Amatayakul-Chantler S, Williams P, Nobel GE, Hutchinson WL, Hawkins PN, Nelson SR, Gallimore JR, Herbert J, Hutton T, Dwek RA (1994) Human serum amyloid P component is an invariant constituent of amyloid deposits and has a uniquely homogeneous glycostructure. Proc Nat1 Acad Sci USA 91:5602-5606. Pongor S, Hatsagi Z, Degtyarenko K, Fabian P, Skerl V, Hegyi H, Murvai J, Bevilacqua V (1994) The SBASE protein domain library, release 3.0: a collection of annotated protein sequence segments. Nucleic Acids Res 22:3610-3615. Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D (1993) Tissueplasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature 361:453-457. Rassouli M, Sambasivam H, Azadis P, Dell A, Morris HR, Nagpurkar A, Mookerjea S, Murray RK (1992) Derivation of the amino acid sequence of rat C-reactive protein from cDNA cloning with additional studies on the nature of its dimeric component. J Biol Chem 267~2947-2954. Regan LJ, Dodd J, Barondes SH, Jesse11 TM (1986) Selective expression of endogenous lactose-binding lectins and lactoseries glycoconjugates in subsets of rat sensory neurons. Proc Nat1 Acad Sci USA 83:2248-2252. Reid M, Blobel CP (1994) Apexin, an acrosomal pentaxin. J Biol Chem 269:32615-32620. Rinaman L, Card JP, Enquist LW (1993) Spatiotemporal responses of astrocytes, ramified microglia, and brain macrophages to central neuronal infection with pseudorabies virus. J Neurosci 13:685-720. Rost B, Sander C (1993) Prediction of protein structure at better than 70% accuracy. J Mol Biol 232:584-599. Rost B, Sander C (1994) Combining evolutionary information and neural networks to predict protein secondary structure. Proteins 19:55-72. Saffen DW, Cole AJ, Worley PF, Christy BA, Ryder K, Baraban JM (1988) Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Nat1 Acad Sci USA 85:7795-7799. Schlimgen AK, Helms JA, Vogel H, Perin MS (1995) Neuronal pentraxin, a secreted protein with homology to acute phase proteins of the immune system. Neuron 14:519-526. Schwalbe RA, Dahlback B, Coe JE, Nelsestuen GL (1992) Pentraxin family of proteins interact specifically with phosphorylcholine and/or phosphotylethanolamine. Biochemistry 31:4907-4915. Shatz C (1990) Impulse activity and the patterning of connections during CNS development. Neuron 5:745-756. Shaw G, Kamen R (1986) A conserved AU sequence from the 3’ untranslated region of GM-CSF mRNA mediates selective mRNA degredation. Cell 46:659-667. Sheng M, Greenberg M (1990) The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 4:477-485. Shinozaki H, Ishida M (1979) Pharmacological distinction between the excitatory junctional potential and the glutamate potential revealed by concanavalin A at the crayfish neuromuscular junction. Brain Res 161:493-501. Tsui et al. l Narp Expression and Regulation in Brain Siegel J, Rent R, Gewurz H (1974) Interactions of C-reactive protein with the complement. J Exp Med 140:631-647. Siegel J, Osmand AP, Wilson MR (1975) Interaction of C-reactive protein with the complement system. J Exp Med 142:709-721. Silva AJ, Giese PK (1994) Plastic genes are in! Curr Opin Neurobiol 4:413-420. Simpson DL, Thorne DR, Loh HH (1977) Developmentally regulated lectin in neonatal rat brain. Nature 266:367-369. Singh G, Kaur S, Stock JL, Jenkins NA, Gilbert DJ, Copeland NG, Potter SS (1991) Identification of 10 murine homeobox genes. Proc Nat1 Acad Sci USA 88:10706-10710. Sisodia SS, Koo EH, Beyreuther K, Unterbeck A, Price D (1990) Evidence that beta-amyloid protein in Alzheimer’s disease in not derived by normal processing. Science 248:492-495. Slunt HH, TG, Von Koch C, Lo A, Tanzi RE, Sisodia SS (1994) Expression of a ubiquitous, cross-reactive homologue of the mouse fi-amyloid precursor protein (APP). J Biol Chem 269:2637-2644. Thio LL, Clifford DB, Zorumski CF (1992) Blockade of ionotropic quisqualate receptor desensitization in rat hippocampal neurons by wheatgerm agglutinin and other lectins. J Neurol Sci 52:35-44. Thio LL, Clifford DB, Zorumski CF (1993) Blockade of ionotropic quisqualate receptor desensitization in rat hippocampal neurons by wheatgerm agglutinin and other lectins. Neuroscience 52:35-44. von Heijne G (1986) A new method for predicting signal sequence cleavage sites. Nucleic Acids Res 14:4683-4690. Waters RS, McCandlish CA, Cooper NG (1990) Early development of Sl cortical barrel subfield representation of forclimb in normal and deafferented neonatal rat as delineated by peroxidasc conjugated lcctin, peanut agglutinin (PNA). Exp Brain Res 81:234-240. Worley PF, Cole AJ, Murphy TM, Christy BA, Nakabeppu Y, Baraban JM (1990) Synaptic regulation of immediate early genes in brain. Cold Spring Harb Symp Quant Biol 55:213-223. Worley PF, Cole AJ, Murphy TM, Christy BA, Nakabeppu Y, Baraban JM (1991) Constitutive expression of ~$68 in neocortex is regulated by synaptic activity. Proc Nat1 Acad Sci USA 88:5106-5110. Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA (1993) Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci 13:4776-4786. Yamagata K Andreasson KI, Kaufmann WE, Barnes CA, Worley PF (1993) Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron 11:371-386. Yamagata K, Kaufmann WE, Lanahan A, Papapavlou M, Barnes CA, Worley PF (1994a) Egr3/Pilot, a zinc-finger transcription factor, is rapidly regulated by activity in brain neurons and co-localizes with Egrl/ Zif268. Learn Memory 1:140-152. Yamagata K, Sanders LK, Kaufmann WE, Barnes CA, Nathans D, Worley PF (1994b) Rheb, a growth factor and synaptic activity regulated gene, encodes a novel Ras-related protein. J Biol Chem 269:16333-16339. Zilles K, Wree A, Schleicher A, Divac I (1984) The monocular and binocular subfields of the rat’s primary visual cortex: a quantitative morphological approach. J Comp Neurol 226:391-402.