* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 4 . The imino tautomer of adenine can pair with cytosine
Agarose gel electrophoresis wikipedia , lookup
Expanded genetic code wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Eukaryotic transcription wikipedia , lookup
RNA silencing wikipedia , lookup
Epitranscriptome wikipedia , lookup
Community fingerprinting wikipedia , lookup
Gene expression wikipedia , lookup
Holliday junction wikipedia , lookup
Promoter (genetics) wikipedia , lookup
Non-coding RNA wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Molecular cloning wikipedia , lookup
Genetic code wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Cre-Lox recombination wikipedia , lookup
Non-coding DNA wikipedia , lookup
DNA supercoil wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
Biochemistry wikipedia , lookup
Molecular evolution wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Base-mispairing due to tautomerism to the rare lactim and imino
forms
The bases of DNA can exist in rare tautomeric forms. The fraction of each
type of base in the form of these imino and enol tautomers is about10-4. The
imino tautomer of adenine can pair with cytosine, eventually leading to a
transition from A-T to G-C; ditto for 5BrU replacing T for T-A to C-G.
Spontaneous mutation rate is about 1 in 10 billion / replication / base in E. coli
Deamination of bases: Chemical mutagenesis and possibly carcinogenesis
Nitrous acid (HNO2) hydrolyzes amino groups on bases via diazotization. Adenine is
deaminated to hypoxanthine, cytosine to uracil, and guanine to xanthine.
Hypoxanthine pairs with cytosine, inducing a mutation of A-T to G-C. It is likely that
mutations in DNA repair genes will lead to the accumulation of mutations throughout
the genome. In time, genes important in controlling cell proliferation become altered,
resulting in the onset of cancer.
Therefore, beware of nitrite as food preservative, but don’t go to extremes as
nitrate is converted to nitrite in our body by gut and oral bacteria.
Nature’s strategy to protect from natural deamination:
Use thymine base in DNA
Why is a methylated base employed in DNA and not in RNA?
Heterocyclic
amine
imine….
or
Because cytosine
canhydrolyzes
be convertedvia
to uracil
diazotization. Cytosine in DNA spontaneously deaminates
at a perceptible rate to form uracil.
For DNA as codon, this reaction has grave
consequence in base pairing. With thymine instead
of uracil in DNA, U can be recognized and repaired
Rationaloe for the ribose-phosphate backbone in nucleic acids
1. Why ribosyl but not glucosyl phosphate as part of duplex chain
Steric effect of hexopyranosyl units ("too many atoms") affect base
pairing
Hexopyranosyl-(4'6') oligonucleotides vs. RNA duplexes
Homo-DNA, an artificial oligonucleotide containing 6-mem. pyranose
ring with an additional CH2 group, shows much stronger base pairing
due to the higher rigidity of pyranose vs. that of furanose rings, resulting
in a preorganization of the single strand's backbone conformation
toward the double strand's pairing conformation. But the system also
has pronounced adenine and guanine self-pairing.
For the family of hexopyranosyl-(4'6') oligonucleotides, whose structures
differ from that of homo-DNA by 2 additional OH groups and relate to
the natural hexoses in the same way that RNA relates to ribose, none
exhibit discernible Watson-Crick base pairing between adenine and uracil
Instead, some purine-purine self-pairing occurs, although much weaker
than in homo-DNA. Guanine-cytosine pairing is far weaker than in RNA,
hence incompetent for informational base pairing.
Note: additional OH at 2' equatorial results in a steric clash between
this OH and the neighboring nucleobase.
Why are phosphate groups used to form
the nucleic acid chain
●
A coding tape with polyanions for ionic interactions
Phosphoric acid is specially suited for its role in
nucleic acids because it can link two nucleotides and
still ionize. The resulting diester is stable to
hydrolysis because there is still a negative charge to
stabilize it against hydrolysis. The ionic interactions
in water serve to retain the molecules within a lipid
membrane.
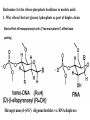
Why not polyamide backbone as illustrated in PNA
(Peptide Nucleic Acid)
TOO MUCH inter-chain binding (H-bonds and other
forces as found in protein secondary structures; also
no electrostatic repulsion as present in phosphate
anion) negates specific template base-pairing.