* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Plant Cell Growth and Elongation
Survey
Document related concepts
Biochemical switches in the cell cycle wikipedia , lookup
Tissue engineering wikipedia , lookup
Cytoplasmic streaming wikipedia , lookup
Cell membrane wikipedia , lookup
Signal transduction wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Programmed cell death wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Transcript
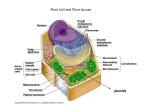
Plant Cell Growth and Elongation Secondary article Article Contents . Introduction Maureen C McCann, John Innes Centre, Norwich, UK Keith Roberts, John Innes Centre, Norwich, UK Nicholas C Carpita, Purdue University, West Lafayette, Indiana, USA . Cell Expansion Can Be Uniform (Isodiametric) or Directional . New Wall Synthesis is Needed for Cell Expansion . The Water Potential Provides the Driving Force for Cell Expansion The plant cell wall is a strong material that resists expansion when water is taken up by the protoplast; for cells to expand irreversibly, the existing wall architecture must be loosened to permit the insertion of newly synthesized wall polymers. Biochemical approaches have identified changes in wall composition that occur during growth and some of the enzymes involved in wall loosening, whilst the study of mutants is beginning to identify genes involved in plant cell growth and elongation. Introduction Plants enlarge by the coordinated expansion of individual cells. In some cases, such as the large storage cells in potatoes or metaxylem elements, the final cell size can be 100 000 or 1 000 000 times greater than the original size of the cell, when born in the meristem. Most of the volumetric increase in the protoplast is accounted for by expansion of the vacuole (up to 95% cell volume). The existing cell wall architecture must change during cell expansion to incorporate new material, increasing the surface area of the cell by as much as 10 000 times and inducing water uptake by the protoplast. As the rate of cell wall expansion is ratelimiting for growth, plant form derives from two processes: first, establishing the planes of division in cells, thus determining the positions in which new walls are created, and second, targeting new cell wall material to particular areas of the cell surface. The primary cell wall is born in the cell plate during cell division and is capable of cell expansion and elongation. At differentiation, many cells elaborate, on the membrane side of the primary wall, a separate, secondary cell wall containing complex structures uniquely suited to the cell’s function. Many mature cells, then, have both a primary and a distinct secondary cell wall. In plants, the expansion must be coordinated among all neighbouring cells. Organ size, and thus plant stature and form, is controlled genetically, but entrained by environmental factors. Leaf size may be equal between wild-type and mutant maize plants, but the number of cells may differ. However, while the final size of leaf may be genetically predetermined, whether a plant attains its potential will depend upon extrinsic factors. The supply of water and nutrients through vascularized tissue may limit organ size. Plants must be exquisitely responsive to environmental signals, such as light, water stress and nutrient status, which all modulate the pattern of cell . Proteins Participate in Controlled Cell Wall Loosening . How Does Growth Stop? . Mutations That Alter Cell Growth and Elongation Are Beginning to Tell Us More About the Genes Involved growth and ultimate cell size. The developmental pathways to various cell types involve several growth factors and their dependent signal transduction mechanisms. Thus the cells that comprise plant organs must be able to integrate complex networks of intracellular and extracellular signals to start growth, expand, and then to stop growth in a coordinated fashion. Plant cell growth is a cellular process that integrates the ‘loosening’ of the cell wall, the outward push of the protoplast driven by osmotic forces, and the deposition and intercalation of new wall to maintain thickness as the existing wall extends. The orientation of cellulose microfibrils spooled on the inner surface of the wall establishes the eventual direction of cell expansion and the cytoskeleton is an essential determinant of this orientation. The direction of cell growth may be influenced by cortical microtubules whereas the rate of growth is regulated by wall loosening and wall deposition. The cessation of growth is a mysterious process but equally as important. Cell size is coupled to chromosome copy number: chromosome doubling, endoreduplication or polyteny may be required to support a specific volume of cytoplasm. Arabidopsis leaf trichome increases from 16n to 128n during its growth to its final cell size (Traas et al., 1998). At the end of growth, various crosslinking mechanisms in the wall are activated that lock the cell into its specific size and shape. Several model systems have been employed to examine the physiological mechanisms of cell expansion, including the grass coleoptile and the dicot epicotyl and hypocotyl. Additionally, several Arabidopsis thaliana developmental mutants have contributed new molecular and genetic tools to probe cell expansion, from genes that determine cell identity to those that constitute the biochemical and physiological mechanisms of expansion and differentiation. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 1 Plant Cell Growth and Elongation Cell Expansion Can Be Uniform (Isodiametric) or Directional Plant cell growth, an irreversible increase in cell volume, can occur by expansion (increase in cell size in two or three dimensions) or by elongation (expansion constrained to one dimension). Variety in cell shape may result if either of these two processes occur only at specific regions of the cell surface. A few plant cells, such as root hairs and pollen tubes, grow by tip growth, in which the mechanisms of wall loosening and deposition of new wall material occur only at the tip of an elongating cell. However, these two mechanisms operate along the entire wall in the vast majority of plant cells. A variation of elongation growth, called intrusive growth, has the appearance of tip growth. This type of growth describes the tapering elongation at the tips of some types of vascular cells, such as inter- and intraxylary fibre cells, as they extend through the middle lamellae of their neighbours above and below their zone of elongation. Cell expansion involves widespread changes to cell wall architectures, in both mass and composition. The osmotic pressure exerted by the protoplast is necessary to drive cell expansion, but because growth can begin and end with almost imperceptible changes in turgor pressure, wall loosening is considered as the primary determinant of cell expansion. The cell wall architecture must be extensible, that is, biochemical loosening of the cell wall matrix permits insertion of newly synthesized polymers. Cells may extend their original length several orders of magnitude with little change in wall thickness. Thus, loosening and the continued cutting and pasting of new material into the texture of the wall must be tightly integrated events. Cellulose forms the main scaffolding framework of the cell wall and accounts for 20% to 30% of the dry mass of elongating cell walls. Cellulose forms microfibrils, paracrystalline assemblies of several dozen (1!4)b-d-glucan chains hydrogen bonded to one another along their length (Figure 1). During cell growth, the orientation of inextensible cellulose microfibrils controls the direction of elongation. In cells that grow by isodiametric expansion, the microfibril orientation within successive lamellae is relatively random, but in cells that grow by elongation, microfibrils in each lamella align in a net transverse orientation to the axis of elongation. Plant cells possess arrays of cortical microtubules underlying and connected to the plasma membrane. As the orientation of the cortical microtubule array often precedes new cellulose microfibril deposition, it is thought by many to act as a template for the orientation of newly synthesized microfibrils (Baskin, 2000). Cellulose synthesis is catalysed at multimeric enzyme complexes located in the plasma membrane. Microtubules may define tracks in which the cellulose synthase complexes are constrained to move through the plasma membrane, or more direct connections, proteins 2 Figure 1 The fast-freeze, deep-etch, rotary-shadowed replica technique is used to image cell wall architecture without the use of chemical fixatives and dehydrants. Pectins have been extracted from this onion parenchyma cell wall to expose the cellulose–xyloglucan network. One cellulose microfibril is drawn out. Cellulose microfibrils are paracrystalline arrays of several dozen (1!4)b-D-glucan chains that tightly hydrogen bond to each other, both side-to-side and top-to-bottom. The arrangement of the glucan chains in a cross-section of a single microfibril, and the arrangement of atoms in the unit structure of the microfibril core, are shown. The glucan chains in the core of the microfibril have a precise spacing as determined by X-ray diffraction. (From R. J. Preston (1974) Physical Biology of the Plant Cell Wall, Chapman and Hall, London.) From studies involving solid-state nuclear magnetic resonance (NMR) spectroscopy, glucan chains at the surface of the microfibril are thought to adopt a slightly different alignment from 1808. (Courtesy of M. Jarvis) linking the complexes to microtubules, have been proposed. Treatment with the cellulose synthesis inhibitor ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Plant Cell Growth and Elongation isoxaben results in disorganized microtubules in elongating cells. The multinet growth hypothesis was developed to explain how cellulose microfibrils in the cotton fibre are deposited in a shallow helical orientation net transverse to the elongation axis but are displaced longitudinally as elongation progresses. New microfibrils deposited in lamellae on the inner surface of the wall in a generally transverse orientation functionally replace older microfibrils. Reorientation of the helical microfibrils occurs primarily in the inner layers of the wall close to the plasma membrane, and the older microfibrils to the exterior of the wall are passively reoriented in a longitudinal direction as the cell elongates. The driving force for wall extension is generally viewed to be the turgor generated by the protoplast, but it is the tension created on the microfibrils 908 to the outward push of the protoplast that leads to separation of the microfibrils. A turgor pressure of a few atm (about 100 kPa) can generate several thousand atm of tensile forces in the wall because the internal pressure exerted by an increase in volume of a relatively large protoplast is resisted by a very thin cell wall (Figure 2). The multinet growth hypothesis describes wall growth in many cells, but microfibrils do not necessarily reorient axially, and significant extension is possible with little reorientation if many lamellae contribute to the expansion. Cellulose microfibrils woven in a shallow helix around the cell prevent the growing cell from becoming spherical. By analogy, the springlike toy Slinky1 or Flexi1 stretches easily along its axis, but resists attempts to increase its diameter. Similarly, helical cellulose microfibrils would offer little resistance to cell stretching in the longitudinal axis – once crosslinks between adjacent turns are weakened – but withstand an enormous tension across this axis. When stretched, the spring extends substantially with only a small change in the helical angle, but with a wide separation of the coils. Extension of a cell wall might be viewed as a series of tightly interacting concentric Slinkys in both right-handed and left-handed orientations that reorient at crossed angles during separation. Given the estimated thickness of the primary wall and the dimensions of the matrix components, only about four to ten lamellae make up the wall. Microfibrils from inner lamellae must move into outer lamellae as the wall is remodelled, to fill in the gaps (Figure 3). New Wall Synthesis is Needed for Cell Expansion Biochemically, there are three classes of polymers that constitute nearly independent determinants of strength in elongating cells: (1) the microfibrils arranged in the transverse axis, (2) the crosslinking glycans in the longitudinal axis, and (3) networks involving structural Figure 2 The original multinet growth hypothesis explains that as walls stretch during growth, the microfibrils reorient passively from a transverse direction on the inner wall to a longitudinal direction at the outer wall. The hydrostatic pressure developed by the protoplasm is resisted by a relatively thin cell wall, and the tensile force pulling the microfibrils apart is several orders of magnitude higher than cell turgor pressure. For example, a spherical cell with a radius (t) of 50 mm and 10 atm of turgor (P), enveloped by a cell wall only 0.1 mm thick (t), develops 2500 atm of tension in the wall (s1). This enormous tension changes as the cell geometry changes. When this cell begins to elongate and become cylindrical, the tension increases to 5000 atm tangentially (s2) simply because of the change in cell dimension. Whereas the Slinky is difficult to pull outward because of the orientation of the coils, it is easily pulled longitudinally. Hence, cell shape is controlled in plants similarly. Altering the interaction between the tethering crosslinking glycans and cellulose is the principal determinant of cell expansion. proteins or phenylpropanoid compounds, or elements of the pectin network. As well as new cellulose microfibrils, other wall polymers must be synthesized and deposited during growth. A direct relationship exists between the amount of new wall material made and deposited by a cell, and the extent of corresponding cell growth. The Golgi ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 3 Plant Cell Growth and Elongation Figure 3 Wall loosening and incorporation of new wall polymers is integrated so that wall thickness is maintained during cell expansion. As the walls are only a few strata thick, loosening of the wall with no insertion of new wall material would very quickly thin the wall during growth and cause rupture. In contrast, deposition without loosening would increase wall thickness, because the walls would not expand. stacks are the sites of synthesis for all noncellulosic polysaccharides, which are packaged in secretory vesicles and exported to the cell surface where they are assembled onto and around the growing cellulose microfibrils (Figure 4) (Samuels et al., 1995). At least two types of primary cell walls are made by angiosperms ‘Type I’ walls, which are found in dicots and the noncommelinoid monocots, contain about equal amounts of cellulose and crosslinking xyloglucans (XyGs), polymers with a glucan backbone decorated with xylose-containing side-chains (Carpita and Gibeaut, 1993). XyGs occur in two distinct locations in the wall: binding tightly to exposed faces of glucan chains in the cellulose microfibrils, and spanning the distance between adjacent microfibrils or simply linking to other XyGs to space and lock the microfibrils into place. The cellulose–XyG framework is embedded in a pectin matrix that controls, among other physiological properties, wall porosity. The pectic polysaccharides that comprise the gel matrix are some of most complex polysaccharides known and fall into two major classes. 4 Figure 4 Biosynthesis of the wall requires a coordination of the synthesis of cellulose microfibrils at the plasma membrane surface, with the synthesis and glycosylation of proteins and wall-modifying enzymes at the rough endoplasmic reticulum and the synthesis of all noncellulosic polysaccharides at the Golgi apparatus. Material destined for the cell wall is packaged into secretory vesicles, transported to the cell surface and integrated with the newly synthesized microfibrils. It is estimated that assembly of the new wall stratum begins when no more than 10 glucose residues of a cellulose chain are made. The two major pectins are homogalacturonans (HGs), polymers with a backbone of galacturonic acid residues, and rhamnogalacturonan I (RG-I), polymers with a backbone of alternating rhamnose and galacturonic acid residues. HGs are thought to be secreted as highly methyl esterified polymers, and the enzyme pectin methylesterase (PME) located in the cell wall removes some of the methyl groups to initiate binding of the carboxylate ions to Ca2 1 . The helical chains of HGs can condense by crosslinking with Ca2 1 to form gels. The extent of methyl esterification may remain high in the walls of some cells, and a type of gel may form with highly esterified parallel chains of HGs. Some HGs and RGs are crosslinked by ester linkages to pectins or other polymers held more tightly in the wall matrix and can only be released from the wall by deesterifying agents. Neutral polymers (arabinans or galactans) are pinned at one end to the pectic backbone, but extend into, and are highly mobile in, the wall pores. Some type I walls contain several types of structural proteins. Maize and other commelinoid monocots possess a different kind of primary wall, a ‘type II’ wall (Carpita and Gibeaut, 1993). They contain cellulose microfibrils of the same structure as those of the type I wall, but glucuronoarabinoxylans (GAXs) are the principal polymers that interlock the microfibrils. Unbranched GAXs can hydrogen bond to cellulose or to each other. The attachment of arabinose and glucuronic acid side groups to ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Plant Cell Growth and Elongation the xylan backbone of GAXs prevents the formation of hydrogen bonds, diminishing the extent of crosslinking between two unbranched GAX chains or GAX to cellulose. In general, grasses are pectin-poor, but the pectins they do contain are similar in structure to those of dicots. When grass cells begin to elongate, they accumulate mixed-linked b-glucans in addition to GAX. Grasses, which have very little structural protein compared with dicots and non-commelinoid monocots, can have extensive interconnecting networks of phenylpropanoids that generally form after cells stop expanding. The Water Potential Provides the Driving Force for Cell Expansion When plant growth regulators, such as auxin and gibberellin, change the dimension of growth, they do so through changes in the orientation of cortical microtubules and cellulose microfibrils. When they change the rate of growth, their mechanisms include dissociation or breakage of the tethering molecules, XyGs or GAXs, between microfibrils. A mathematical formula that describes the growth rate of plant cells allows us to define the cell wall properties that must be modified to permit growth (Taiz, 1984). James Lockhart was one of the pioneers in efforts to apply such a biophysical approach to characterize the rate of plant cell enlargement. The driving force for water uptake in a symmetrical elongating cell can be quantified by the equation: dl/dt 5 Lp (DCw) where dl/dt is the change in length per unit time, Lp is hydraulic conductivity, i.e. the rate at which water can flow across the membrane, and DCw is the water potential difference between the cell and the external medium. The difference in water potential is the driving force for water movement and comprises two components, Cp 1 Cp, which are the osmotic potential and pressure potential (turgor), respectively. The equation is revised to include any type of growth by: dV/dt 5 A Lp (DCw) where growth is reflected as a change in volume per unit time, and dependent on the surface area (A) of the plasma membrane available for water uptake. Thus, in this equation, the rate of growth is proportional to membrane surface area, the conductivity of the membrane, and the water potential difference driving water uptake. However, the equation fails to account fully for some biophysical properties of the wall. In nongrowing cells (dV/dt 5 0), DC is zero, because the rigid cell wall prevents an increase in cell volume and the turgor pressure rises to a value equal to that of the cell’s osmotic potential. As we have seen earlier (Figure 2), small changes in turgor pressure can result in the generation of enormous tensions on the microfibrils. However, in growing cells the DC does not quite reach zero because the wall tethers have been loosened and the volume increases irreversibly. This event is called stress relaxation; it is a wall-localized event that serves as the fundamental difference between growing and nongrowing cells. Actually, when turgor is reduced in growing cells by an increase in the external osmotic potential, growth ceases before turgor reaches zero. This value is called the yield threshold. Lockhart noted that the increment of growth rate change above the yield threshold was dependent not only on turgor but also on a factor ‘m’, called wall extensibility, which is the slope of a general equation: rate 5 m(Cp 2 Y) where Y is the yield threshold. Much of the work that remains to be done is to assign biochemical determinants of yield threshold and extensibility. As we shall see next, some likely candidates have been identified. Proteins Participate in Controlled Cell Wall Loosening The extraction of several kinds of polysaccharide hydrolases from the cell walls of tissues rich in growing cells raised the possibility that the regulation of these enzymes was a mechanism by which auxin could cause wall expansion. A major breakthrough came with the discovery that the growth-promoting activity of auxin could be substituted by mere H 1 , and, further, auxin caused an acidification of the culture medium in which elongating tissue sections were bathed. From these observations sprang the acid-growth hypothesis, the essence of which is that auxin activates a proton pump in the plasma membrane that acidifies the apoplast, and there, growthspecific hydrolases are activated that cleave load-bearing bonds of polysaccharides that tether the cellulose microfibrils. Cleavage of these bonds results in loosening of the wall, and the water potential difference causes uptake of water. Relaxation of the wall, i.e. separation of the microfibrils, passively leads to an increase in cell size. The basic tenets have stood the test of time, but two problems persist. First, no enzymes have been found that hydrolyse wall crosslinking glycans exclusively at pH lower than 5.0, and second, no reasonable explanation exists for how growth is kept in check once the hydrolases are activated. Furthermore, no hydrolases extracted from the wall and added back to the isolated tissue sections, regardless of external pH, cause extension in vitro. There are two new candidates for wall-loosening enzymes. An enzyme activity was discovered that, under conditions of excess XyG as substrate, could carry out a ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 5 Plant Cell Growth and Elongation transglycosylation rather than hydrolysis. Hence, one chain of XyG can be cleaved and reattached to the nonreducing terminus of another XyG chain. The enzyme is called xyloglucan endotransglycosylase (XET) (Nishitani and Tominaga, 1992). In such a mechanism, microfibrils could undergo a transient slippage but overall tensile strength of the wall would not diminish (Figure 5). XETs may also function in the realignment of XyG chains in different lamellae during growth, and in the assembly of the wall as newly synthesized XyGs are incorporated. In some cases, the correlation between growth and XET activity is not clear, and some XETs appear to function hydrolytically. Other proteins catalyse wall extension in vitro without any detectable hydrolytic or transglycolytic events (McQueen-Mason et al., 1992). Called expansins, these proteins allow extension of paper strips under tension, indicating that they probably catalyse breakage of hydrogen bonds. Such an activity could disrupt the tethering of cellulose by XyGs in type I walls and by GAXs and b-glucans in type II walls. XET and expansin may not be the only wall-loosening agents, and work continues to determine the role, if any, of the hydrolases. Recognition of the differences in wall composition between the grasses and all other flowering plant species has also partitioned the studies appropriately to the xyloglucanase, which cleaves XyG selectively, and the exo- and endo-b-d-glucanases, which hydrolyse the grass b-d-glucans to glucose. Addition of purified exo- and endoglucanases to heat-killed coleoptiles cannot induce extension. However, when antibodies directed against these enzymes are added to enzyme-active walls, they inhibit growth. Biochemical changes in the cell wall accompany cell enlargement If XET and expansin activities are indeed growth-inducing factors in the cell wall, then how are they regulated? The cellulose/crosslinking glycan network lies embedded in a network of pectins which may control access of these enzymes to their substrates. The self-hydrolysis of isolated walls by nascent enzymes, termed autolysis, yields substantial amounts of Ara and Gal from type I walls, suggesting that changes in the neutral sugar side-branches of RG-I or arabinogalactan proteins (AGPs) occur during growth. From biochemical analyses, the most marked change is the increase in methyl esterification of the wall as newly synthesized pectins are deposited. This seems to be succeeded by a de-esterification event when growth stops. The wall Ca2 1 content of the meristem and elongation zones is actually quite low, and intermediate types of esterfree acid gel can also form between partially de-esterified HGA chains in the absence of Ca2 1 . Thus, Ca2 1 –HG junction zones are structures more likely to be found in 6 Figure 5 Stress-relaxation is considered to be the underlying basis of cell expansion. When an elongating cell is stretched by turgor, the longitudinal stress is borne more or less equally by the glycans tethering the cellulose microfibrils. If some of the tethers are dislodged from the microfibrils or hydrolysed, then they temporarily ‘relax’ and the yield threshold is breached because the other tethers are strained. Water uptake results in expansion of the microfibrils, which attach to the relaxed glycans, and they again are placed under tensile stress. Microfibril separation driven by osmotic pressure of the cell is facilitated by loosening of the crosslinking glycans that tether them. This may be accomplished by coordinate action of expansins, which break the steric interactions between the crosslinking glycans and cellulose, and xyloglucan endotransglycosylase (XET), which hydrolyses a glycan and reattaches one part of the chain to the nonreducing terminus of another. This action by XET may also function in forming new tethers as microfibrils from inner lamellae merge with microfibrils of the outermost lamellae as they are pulled apart during wall extension. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net Plant Cell Growth and Elongation cells after cell elongation has stopped. Runs of HG at the ends or within RG could link these two types of polymers, but the Rha units of RG-I and their side-chains interrupt the Ca2 1 junctions. In addition to the Ca2 1 -binding junction zones, pectins in some species may be crosslinked to other pectins and noncellulosic polysaccharides by ester linkages with dihydroxycinnamic acids such as diferulic acid. Location of the de-esterification of HG, the size and frequency of junction zones, and the size and conformation of the side-chains attached to the RG could all influence the porosity of the pectin gel to the extent that movement and activity of enzymes for wall metabolism could be controlled (Goldberg et al., 1996). More obvious biochemical changes occur with the crosslinking glycans of the type II walls of commelinoid monocots. A highly substituted GAX (HS-GAX), with six out of every seven Xyl units bearing an appendant group, is associated with the maximum growth rate of coleoptiles. In dividing and elongating cells, highly branched GAXs are abundant, whereas after elongation and differentiation, more and more unbranched GAX accumulates. Cleavage of the Ara and other side groups from contiguously branched Xyl units could yield runs of unbranched xylan capable of binding to other unbranched xylans or to cellulose microfibrils. Type II walls are also notably poor in pectin. Chemically, pectins of the type II wall comprise both HG and RG, but HS-GAX is closely associated with these pectins. The HGs of maize pectins also contain nonmethyl esters, whose formation and disappearance coincide with the most rapid rate of cell elongation. We do not yet know the chemical nature of the nonmethyl esters. Some arabinans, particularly the 5-linked arabinans, are found in the walls of dividing cells but are not made during cell expansion. When grass cells begin to elongate, they accumulate bglucans in addition to GAX (Carpita, 1996). b-Glucans are unique to the Poaceae. b-Glucan is one of the few known developmental stage-specific polysaccharides. Absent from the meristems and dividing cells, b-glucans accumulate to almost 30% of the noncellulosic cell wall material during the peak of cell elongation, and then are largely hydrolysed by the cells during differentiation. The appearance of b-glucans during cell expansion and the acceleration of their hydrolysis by growth regulators all implicate direct involvement of the polymer in growth. How Does Growth Stop? Once elongation is complete, the primary wall locks the cell into shape. One component of the locking mechanism for type I walls may be hydroxyproline-rich glycoproteins (HRGPs). HRGP monomers accumulate early in the cell cycle and become insoluble during cell elongation and differentiation. But how HRGPs are crosslinked in the wall, either in homo-networks or in hetero-networks with other proteins, is not known. Type II walls contain a threonine-rich protein with sequences reminiscent of the typical HRGP structure. This protein is prevalent in vascular tissue and in special, reinforced wall structures, such as the pericarp. However, much of the crosslinking function in type II walls probably rests with the esterified and etherified phenolic acids, and formation of these crosslinkages accelerates at the end of the growth phase. Mutations That Alter Cell Growth and Elongation Are Beginning to Tell Us More About the Genes Involved Cell elongation and differentiation are developmental programmes that are under strong control by light intensity and quality, and, in turn, by several growth regulators. It is not surprising that mutations that alter expression of phytochrome, cryptochrome, and blue light photoreceptors greatly alter growth responses, from the shortening of elongation responses to phototrophic bending. Likewise, mutations in the synthesis of growth regulators such as auxin, gibberellin, cytokinin, abscisic acid, ethylene and their putative receptors, lead to aberrant growth, usually manifest in dwarfism or miniaturization. Other than confirming the role of such growth regulators, such mutations have not been exceptionally informative in gaining an understanding of the biochemical or physical mechanisms of growth. On the other hand, some dwarf mutations have ultimately led to the discovery of an entirely new class of growth regulators called brassinosteroids, whose action is epistatic to the action of the oldest known growth regulator, auxin. The de-etiolated (det) mutants are an interesting group of mutants that undergo the first stages of phytochromeactivated morphogenesis in darkness. The defect of one of these mutants was traced to a defect in a vacuolar protonpumping gene whose absence altered cytosolic pH. The pH status, in fact, an acidification of the cytosolic compartment, has been postulated to be the primary signal for auxin-induced elongation, and it appears that some nominal support for this hypothesis has come from the discovery of a positive-control mutant. Other kinds of dwarf or miniaturization mutants do not appear to fall within the domain of growth regulators per se, but the defects are closer to the mechanisms of cell expansion. For example, lilliputian mutants appear to be derived from defects in microtubule (MT) assembly, and cells that cannot escape cell division stage may have impaired MT organizing centres required for proper ordering of the MTs at the plasma membrane. Other cytoskeletal mutants appear to affect polarity rather than cell growth proper. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net 7 Plant Cell Growth and Elongation Mutations that affect the cell wall directly have been harder to track down. Polysaccharides are not primary gene products, and it has proven difficult to analyse genetically the role that each component (and its modifications) plays in the overall mechanical and functional properties of the cell wall, or of the tissues that contain them. Over 1000 gene products are probably involved in cell wall biosynthesis, assembly and turnover, and genetically defined variation offers the most comprehensive approach to addressing function. Arabidopsis mutants with altered carbohydrate components in the primary wall have now been defined. Over three dozen mutants have been classified, mapping to 11 different loci in which one or several specific sugars are over- or under-represented compared with the sugar composition in wild-type plants (Reiter et al., 1997). Of these, the mur1 defect has been traced to a GDP-mannose 4,6 dehydratase, mur2 to a fucosyl transferase, and mur4 to a C-4 epimerase. However, many cell wall mutants have also been selected on the basis of a growth or developmental phenotype. A temperature-sensitive mutant in primary wall cellulose synthase, rsw1, was selected by a root radial swelling phenotype at restrictive temperatures, while a secondary wall cellulose synthase mutant, irx3, was selected by a collapsed xylem phenotype. A dwarf hypocotyl mutant, korrigan, results from a lesion in a membrane-bound endoglucanase, while another dwarf, acaulis, is an XET mutant. The tch4 mutant is also an XET mutant but remains elongated rather than becoming dwarfed, as wildtype plants do when they are mechanically stressed. When the genes of many cell wall modifying enzymes and structural proteins become available, the function of their products will be tested in suppression and over-expression studies, to identify the key players in determining the rate of plant cell growth. References Baskin TI (2000) The cytoskeleton. In: Buchanan BB, Gruissem W and Jones RL (eds) Biochemistry and Molecular Biology of Plants, chap 5. Rockville, MD: American Society of Plant Physiology. Carpita NC (1996) Structure and biogenesis of the cell walls of grasses. Annual Review of Plant Physiology and Plant Molecular Biology 47: 445–476. Carpita NC and Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. The Plant Journal 3: 1– 30. 8 Goldberg R, Morvan C, Jauneau A and Jarvis MC (1996) Methylesterification, de-esterification and gelation of pectins in the primary cell wall. In: Visser J and Voragen AGJ (eds) Pectins and Pectinases, pp. 151–171. Amsterdam: Elsevier Science. McQueen-Mason S, Durachko DM and Cosgrove DJ (1992) 2 endogenous proteins that induce cell-wall extension in plants. Plant Cell 4: 1425–1433. Nishitani K and Tominaga R (1992) Endoxyloglucan transferase, a novel class of glycosyltransferase that catalyzes transfer of a segment of xyloglucan molecule to another xyloglucan molecule. Journal of Biological Chemistry 267: 21058–21064. Reiter WD, Chapple C and Somerville CR (1997) Mutants of Arabidopsis thaliana with altered cell wall polysaccharide composition. The Plant Journal 12: 335–345. Samuels AL, Giddings TH and Staehelin LA (1995) Cytokinesis in tobacco BY-2 and root-tip cells – a new model of cell plate formation in higher plants. Journal of Cell Biology 130: 1345–1357. Taiz L (1984) Plant cell expansion: Regulation of cell wall mechanical properties. Annual Review of Plant Physiology 35: 585–657. Traas J, Hulskamp M, Gendreau E and Hofte H (1998) Endoreduplication and development: rule without dividing? Current Opinion in Plant Biology 1: 498–503. Further Reading Carpita NC and McCann MC (2000) The cell wall. In: Buchanan BB, Gruissem W and Jones RL (eds) Biochemistry and Molecular Biology of Plants, chap 2. Rockville, MD: American Society of Plant Physiology. Cassab GI (1998) Plant cell wall proteins. Annual Review of Plant Physiology and Plant Molecular Biology 49: 281–309. Cosgrove DJ (1997) Assembly and enlargement of the primary cell wall in plants. Annual Review of Cell Development and Biology 13: 171–201. Delmer DP (1999) Cellulose biosynthesis: Exciting times for a difficult field of study. Annual Review of Plant Physiology and Plant Molecular Biology 50: 245–276. Gunning B and Steer M (1996) Plant Cell Biology. Structure and Function. Sudbury, MA: Jones and Bartlett Publishers. McCann MC and Roberts K (1991) Architecture of the primary cell wall. In: Lloyd CW (ed.) The Cytoskeletal Basis of Plant Growth and Form, pp. 109–129. London: Academic Press. McCann MC, Wells B and Roberts K (1990) Direct visualization of cross-links in the primary plant cell wall. Journal of Cell Science 96: 323–334. Nishitani K (1995) Endo-xyloglucan transferase, a new class of transferase involved in cell-wall construction. Journal of Plant Research 108: 137–148. Reiter W-D (1998) Arabidopsis thaliana as a model system to study synthesis, structure, and function of the plant cell wall. Plant Physiology and Biochemistry 36: 167–176. Staehelin LA and Moore I (1995) The plant Golgi apparatus: structure, functional organization and trafficking mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology 46: 261–288. ENCYCLOPEDIA OF LIFE SCIENCES / & 2001 Macmillan Publishers Ltd, Nature Publishing Group / www.els.net