* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 8b Questions
Cytoplasmic streaming wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Cell growth wikipedia , lookup
Node of Ranvier wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Chemical synapse wikipedia , lookup
SNARE (protein) wikipedia , lookup
Cell encapsulation wikipedia , lookup
Lipid bilayer wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Signal transduction wikipedia , lookup
Action potential wikipedia , lookup
Cytokinesis wikipedia , lookup
List of types of proteins wikipedia , lookup
Endomembrane system wikipedia , lookup
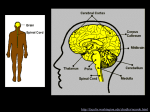
1 Chapter 6: Nervous Systems Revised 20 September 2011 for 12th Edition Section B1 and B2 What term is given to opposite charges that are separated from each other and have the ability to do work when they are allowed to come together? What is the definition of electrical current? If there is no potential difference between two points, will there be any current? How is resistance related to current? According to Ohm’s law, if the voltage is held constant and the resistance is suddenly decreased, will current be increased or decreased? In cells, is the lipid portion of the cell membrane a good conductor of electricity (i.e. does current flow easily through the lipid bilayer?) Is the resistance of the lipid bilayer considered “high” or “low?” What component of a cell membrane accounts for the variable resistivity across cell membranes? What is the normal range for resting membrane potential in neurons? Which side (inside or outside) of a cell membrane has the most negatively charged particles? What is the most abundant (major) extracellular cation? What is the most abundant (major) extracellular anion? What is the major intracellular cation? Which intracellular molecules are negatively charged? Based solely on its concentration gradient across a resting cell membrane, in which direction would Na+ diffuse (in or out) if the membrane becomes more permeable to Na+? Based solely on its concentration gradient across a resting cell membrane, in which direction would K+ diffuse (in or out) if the membrane becomes more permeable to K+? What establishes and maintains the concentration gradients for Na+ and K+ ions across the cell membrane? How is the concentration of Na+ kept elevated outside of the cell? 2 How is the concentration of K+ kept elevated inside the cell? What two factors determine the magnitude of the resting membrane potential? Given the situation in Figure 6-10 (p. 144) where the membrane is permeable only to K+, what force is responsible for the movement of K+ from Compartment 2 to Compartment 1? Eventually, there will be no net movement of K+ from C2 to C1. What force prevents the additional net flux of K+ from C2 to C1? What term is used when the inward flux of an ion is exactly equal but opposite to the outward flux of that ion (no net flux)? Which compartment, C1 or C2, corresponds to the intracellular fluid in this example? Would the equilibrium potential for K+ (also known as the Nernst potential for K+ and abbreviated as E K+) be positive or negative? One cannot detect a change in the concentration of K+ on the two sides of the membrane once the equilibrium potential has been reached. What does this imply about the number of ions that must move in order to establish a membrane potential? In the above example, how would E K+ be affected if the concentration of K+ in C2 was made greater? What would the membrane potential be if K+ were the only permeable ion and the concentration of K+ was exactly the same on both sides of a membrane? Given the situation in Figure 6-11 (p. 145) where the membrane is permeable only to Na+, would the equilibrium potential for Na+ (E Na+) be a positive or negative value? Once Na+ has reached its equilibrium potential, what prevents the movement of more Na+ ions from the outside of the cell to the inside? What is the Nernst equation used for? What information is required to solve the Nernst equation? The Nernst equation can be simplified to Ex = 61mV log [X]outside/[X]inside at body temperature of humans. Use this simplified version of the Nernst equation with the information from Table 6-2 to calculate the equilibrium potential for K+ and then for Na+. Using the concentration gradients from Table 6-2, what would the equilibrium potential be for Na+? for K+? What types of molecules are mostly responsible for the net negativity of the inside of a cell membrane? Why are they called “fixed” anions? How does the Goldman equation differ from the Nernst equation? When should the Goldman equation be used? What information in addition to concentration gradients for permeable ion species is required to solve the Goldman equation? 3 Why is the resting membrane potential of a cell not equal to the equilibrium potential for K+? Why is the resting membrane potential of a cell closer to the equilibrium potential for K+ that for Na+? Theoretically, if a cell membrane were permeable only to Na+, what would the membrane potential be? What is the role of the Na+/K+ATPase pump? Why is the Na+/K+ATPase pump called electrogenic? In a living cell, is the resting membrane potential more accurately described as a steady state or an equilibrium condition? (Recall that energy is required for a steady state.) Why is it unnecessary to be concerned with Cl- when thinking about membrane potentials in most types of cells?