* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Transport/Diffusion
Cytoplasmic streaming wikipedia , lookup
Tissue engineering wikipedia , lookup
Signal transduction wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Cell culture wikipedia , lookup
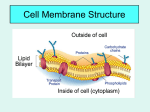
Cell membrane wikipedia , lookup
Cytokinesis wikipedia , lookup
Endomembrane system wikipedia , lookup
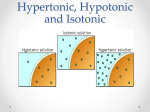
Transport/Diffusion Law of diffusion: Kinetic energy: (Thermal motion) Equilibrium: Passive Transport: Osmosis: (dialysis) Solution: Solute: ( ) Solvent: Universal Solvent: Honors Biology Trembly Brownian Motion: Cells must live in a proper water balance. Meaning, if they do not have enough water or if they have too much water they will die. They must have the correct water concentration to live. To study how cells live, we must consider the following 2 processes: 1) Solution concentration: 2) Membrane permeability: The concentration of a solution involves the total concentration of all the dissolved solutes within a solution: We do not consider the cell as part of the solution. Ex: Solution Concentration: If a cell lives in a beaker of water containing sodium 15% and potassium 10% molecules, then the solution has 25% solute or salts and the rest of the solution contains water (75% water). Membrane permeability: This cell’s membrane has pores for sodium but not for potassium. The cell allows sodium molecules to move in or out of the cell. However potassium is only found outside the cell (a non-penetrating solute) Influences on permeability: Tonicity: Tonic: In biology tonic means solutes in the solution. The tonic concentration is always compared to the concentration inside the cell. Hypotonic Solution: Hypertonic Solution: Isotonic Solution: The cell itself has its own concentration of water and solutes, this is the cells cytoplasm. Most cells cytoplasm has a concentration of about ______ solutes and ______ water. The left side of the tube is initially hypotonic The right side of the tube is initially hypertonic Since this membrane is impermeable to solutes, the water will cross the membrane to the hypertonic side. Why does the water go to the hypertonic side? All cells need to be in some degree of an aqueous (water) solution to maintain life. Biology is concerned with living systems. Cells in the body are in a solution, the extracellular solution (aka. Interstitial fluid) which is a watery fluid found just under the skin and all the spaces outside of the body’s organs. Below is a diagram of what happens to a blood cell in different types of solutions: Known as cytolysis Equilibrium Known as plasmolysis There are three tonic conditions Outside- Outside- Outside- Salts- high conc Salts- low conc Salts- equal conc H2O- low conc H2O- high conc H2O- equal conc Inside- Inside- Inside- Salts- low Salts- high Salts- equal H2O- high H2O- low H2O- equal Hypertonic Hypotonic Isotonic Environment high in salt Environment low in salt Cell loses water Cell gains water Leads to ___________ ` Plant cells in salt water Leads to __________ Environment and cell of equal salinity No net gain or loss of water No harm to cell RBCs in freshwater Saline solution Osmoreguolation: Dealing With Tonicity- Ecological Strategies InSW- InSW- OutSW- OutSW- Saltwater Organisms Freshwater Organisms Problems1. Problems1. 2. 2. Solutions Solutions What about? Anadromous fishes- ex: Catadromous fishes- ex: Cytolysis: Plasmolysis: Our blood cells need to be in an isotonic environment. Doctors often get a report on a person’s electrolytes. Electrolytes are the solutes or salts in our blood. If the electrolyte count is high (hypertonic condition) if the electrolyte count is low (hypotonic) if the electrolytes are equal to the cell’s solute concentrations (isotonic). *Plant cells have a cell wall which protects them from bursting *Animal cell will burst when placed in a hypotonic solution. When plant cells are placed in a hypertonic solution, water molecules will move out of the cell. This dehydrates the cell and the cell becomes flaccid Turgor pressure: Protists deal with osmosis using this organelle ________________________ Water movement Cytoplasm Outside cell Environmental Tonicity: Tonicity refers to the saltiness of the fluid environment a cell exists in. In nature a similar measurement is salinity, such as in oceans (saltwater) and lakes (freshwater). Saltwater has more salinity or a higher salt concentration. Passive Transport 1. Simple Diffusion 2. Facilitated diffusion Ex: insulin and glucose 3. Carrier Transport Across Membrane: 4. G-Protein Linked Receptors: Name comes from ____________________________ Gated Ion Channels: Some pores open and close 1. Voltage Gated: 2. Chemical Gated: Active Transport: Sodium / Potassium Pump: Diagram- Integrated Transport SystemsHow do you get large polar molecules through the membrane when they are too large to fit through the protein passages? ENDOCYTOSIS: How large solutes or large amounts of solutes get into the cell. STEPS 1. 2. 3. 4. 2 Types of Endocytosis Phagocytosis: Pinocytosis: EXOCYTOSIS: STEPS 1. 2. 3. 4.