* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hypertonic solution
Cytokinesis wikipedia , lookup
Cell growth wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Tissue engineering wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
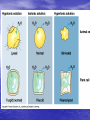
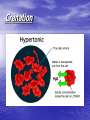
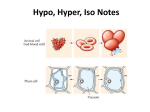
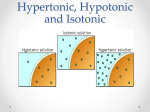
• Isotonic solutions: • if the osmotic concentrations of both solutions are equal than no net movement of water occurs • Examples include most interstitial fluid and most intravenous solutions • Hypertonic solution: the solution has more dissolved particles, salts, sugar, etc., than is in the cells, so cells will shrivel up or shrink as water moves out of the cell • If red blood cells are exposed to a hypertonic solution- a solution that contains more solutes, or dissolved substances, than there are inside cellsthe cells will shrink, or crenate as water moves outside the cell (this is because water is in higher concentration inside the cell than outside, so it follows its concentration gradient and leaves the cell) http://highered.mcgrawhill.com/sites/0072495855/student_view0/chapter21/animation__hemolysis_and_crenation.html • Crenation is the contraction of a cell after exposure to a hypertonic solution, due to the loss of water through osmosis Crenation Would patients ever be given hypertonic solutions? • Yes, if they have edema (swelling of • the feet and hands due to fluid retention) Such solutions draw water out of the tissue spaces into the bloodstream so that the kidneys can eliminate excess fluid Hypotonic solution: • Solution has less dissolved particles, salts, sugar, etc., than is in the cell; cells swell and can burst as water enters the cells Hypotonic solution: • Distilled water is an • example of this type of solution (there are no solutes). Water will enter cells until they finally burst, or lyse. Given intravenously to rehydrate tissues of extremely dehydrated patients