* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
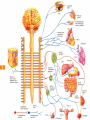
Download 44 Nociceptive sensation. Somatic sensory analyzer
Neural coding wikipedia , lookup
Central pattern generator wikipedia , lookup
Endocannabinoid system wikipedia , lookup
Synaptogenesis wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Development of the nervous system wikipedia , lookup
Synaptic gating wikipedia , lookup
Optogenetics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroanatomy wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Neuroregeneration wikipedia , lookup
Perception of infrasound wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Circumventricular organs wikipedia , lookup
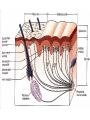
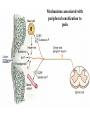
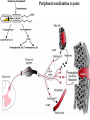
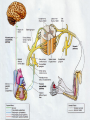
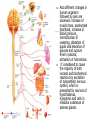
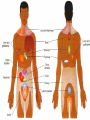
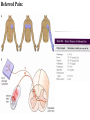
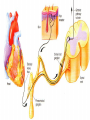
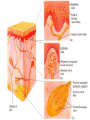
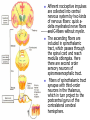
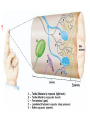
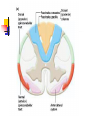
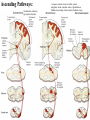
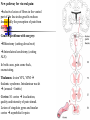
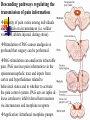
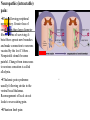
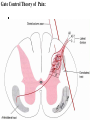
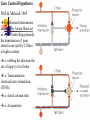
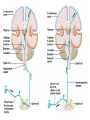
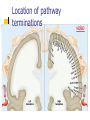
Nociceptive sensation. Nociceptors (Free nerve ending) Mechanical nociceptors: activated by strong stimuli such as pinch, and sharp objects that penetrate, squeeze, pinch the skin. sharp or pricking pain, via A-delta fibers. Thermal nociceptors: activated by noxious heat (temp. above 45°C), noxious cold (temp. below 5°C), and strong mechanical stimuli. via A-delta fibers. Polymodal nociceptors: activated by noxious mechanical stimuli, noxious heat, noxious cold, irritant chemicals. slow dull burning pain or aching pain, via nonmyelinated C fibers. Persists long after the stimulus is removed. Research for a transduction protein: capsaicin (from chili peppers) bind to capsaicin receptor on nociceptor endingstransducer for noxious thermal and chemical stimuliburning sensation associated with spicy food. Knockout mouse lacking capsaicin receptor drinks solution of capsaicin, has reduced thermal hyperalgesia Mechanisms associated with peripheral sensitization to pain Agents that Activate or Sensitize Nociceptors: Cell injury arachidonic acid prostaglandins vasc. permeability (cyclo-oxygenase) sensitizes nociceptor Cell injury arachidonic acid leukotrienes vasc. permeability (lipoxygenase) sensitizes nociceptor Cell injury tissue acidity kallikrein bradykinin vasc. permeability activates nociceptors synthesis & release of prostaglandins Substance P (released by free nerve endings) sensitize nociceptors vasc. perm., plasma extravasation (neurogenic inflammation) releases histamine (from mast cells) Calcitonin gene related peptide (free nerve endings) dilation of peripheral capillaries Serotonin (released from platelets & damaged endothelial cells) activates nociceptors Cell injury potassium activates nociceptors Peripheral sensitization to pain: CGRP CGRP Pain input to the spinal cord: -Projecting neurons in lamina I receive A-delta and C fibers info. -Neurons in lamina II receive input from C fibers and relay it to other laminae. -Projecting neurons in lamina V (wide-dynamic range neurons) receive A-delta, C and A-beta (low threshold mechanoceptors) fibers information. How is pain info sent to the brain: hypotheses pain is signaled by lamina I and V neurons acting together. If lamina I cells are not active, the info about type and location of a stimulus provided by lamina V neurons is interpreted as innocuous. If lamina I cells are active then it is pain. Thus: lamina V cells details about the stimulus, and lamina I cells whether it is painful or not -A-delta and C fibers release glutamate and peptides on dorsal horn neurons. -Substance P (SP) is co-released with glutamate and enhances and prolongs the actions of glutamate. -Glutamate action is confined to nearby neurons but SP can diffuse and affect other populations of neurons because there is no specific reuptake. Neurotransmitters Chemical substances that allow nerve impulses to move from one neuron to another Found in synapses Norepinephrine Substance P Acetylcholine Enkephalins Endorphins Serotonin Can be either excitatory or inhibitory Fast pain (acute) occurs rapidly after stimuli (.1 second) sharp pain like needle puncture or cut not felt in deeper tissues larger A nerve fibers Slow pain (chronic) begins more slowly & increases in intensity in both superficial and deeper tissues smaller C nerve fibers Also different changes in human organism followed by pain are observed: increase of muscle tone, accelerated heartbeat, increase of blood pressure, intensification of sweating, dilatation of pupils and elevation of glucose and cuprum level in plasma, activation of hemostasis. It considered to cause the majority of both visceral and biochemical reactions by excitation of sympathetic nervous system, which is presented by neurons of hypothalamus, hypophisis and cells in medullar substance of adrenal glands. Referred Pain: Afferent nociceptive impulses are collected into central nervous system by two kinds of nervous fibers: quick adelta myelinated nerve fibers and C-fibers without myelin. The ascending fibers are included in spinothalamic tract, which passes through the spinal cord and reach medulla oblongata. Here there are second order sensory neurons of spinomesencephalic tract. Fibers of spinothalamic tract synapse with third-order neurons in the thalamus, which in turn project to the postcentral gyrus of the contralateral cerebral hemisphere. Sensory pathways: 3 neurons 1st: enters spinal cord from periphery 2nd: crosses over (decussates), ascends in spinal cord to thalamus 3rd: projects to somatosensory cortex Ascending Pathways: ->localization, intensity, type of pain stimulus ->arousal, emotion; involves limbic system, amygdala, insula, cingulate cortex, hypothalamus. Mediate descending control of pain (feedback loop) New pathway for visceral pain: selective lesion of fibers in the ventral part of the fasciculus gracilis reduces dramatically the perception of pain from the viscera. General problems with surgery: Rhizotomy (cutting dorsal root) Anterolateral cordotomy (cutting ALS) In both cases, pain come back, excruciating. Thalamus: lesion VPL, VPM thalamic syndrome. Intralaminar nuclei (arousal + limbic) Cortex: S1 cortex localization, quality and intensity of pain stimuli. Lesion of cingulate gyrus and insular cortex asymbolia for pain Descending pathways regulating the transmission of pain information: intensity of pain varies among individuals and depends on circumstances (i.e. soldier wounded, athlete injured, during stress). Stimulation of PAG causes analgesia so profound that surgery can be performed. PAG stimulation can ameliorate intractable pain. PAG receives pain information via the spinomesencephalic tract and inputs from cortex and hypothalamus related to behavioral states and to whether to activate the pain control system. PAG acts on raphe & locus ceruleus to inhibit dorsal horn neurons via interneurons and morphine receptors. Application: Intrathecal morphine pumps Neuropathic (intractable) pain: Pain following peripheral nerve injury. Greater loss of small fibers than large diameter fibers. Axons of surviving Abeta fibers sprout new branches and make connection to neurons vacated by the lost C fibers . Nonpainful stimuli become painful. Change from innocuous to noxious sensation is called allodynia. Thalamic pain syndrome: usually following stroke in the ventral basal thalamus. Rearrangement of local circuit leads to excruciating pain. Phantom limb pain: A-beta Pain Signaling neurons C fibers N Gate Control Theory of Pain: Gate Control Hypothesis: Wall & Melzack 1965 Hypothesized interneurons activated by A-beta fibers act as a gate, controlling primarily the transmission of pain stimuli conveyed by C fibers to higher centers. i.e. rubbing the skin near the site of injury to feel better. i.e. Transcutaneous electrical nerve stimulation (TENS). i.e. dorsal column stim. i.e. Acupuncture Location of pathway terminations video Analgesics: 1) May act at the site of injury and decrease the pain associated with an inflammatory reaction (e.g. non-steroidal anti-inflammatory drugs (NSAID) such as: aspirin, ibuprofen, diclofenac). Believed to act through inhibition of cyclo-oxygenase (COX). COX-2 is induced at sites of inflammation. Inhibition of COX-1 causes the unwanted effects of NSAID, i.e. gastrointestinal bleeding and nephrotoxicity. Selective COX-2 inhibitor are now used. 2) May alter nerve conduction (e.g. local anesthetics): block action potentials by blocking Na channels. Used for surface anesthesia, infiltration, spinal or epidural anesthesia. Used in combination to steroid to reduce local swelling (injection near nerve root). Local anesthetic preferentially blocks C fiber conduction, cold decreases firing of C fibers, ischemia blocks first the large myelinated fibers. 3) May modify transmission in the dorsal horn (e.g. opioids: endorphin, enkephalin, dynorphin…). Opioids act on G-protein coupled receptors: Mu, Delta and Kappa. Opioid agonists reduce neuronal excitability (by increasing potassium conductance) and inhibit neurotransmitter release (by decreasing presynaptic calcium influx) 4) May affect the central component and the emotional aspects of pain (e.g. opioids, antidepressant). Problems of tolerance and dependence