* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture Slides for Carbohydrates
Cell membrane wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cell growth wikipedia , lookup
Protein moonlighting wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Signal transduction wikipedia , lookup
Cytokinesis wikipedia , lookup
Endomembrane system wikipedia , lookup
Proteolysis wikipedia , lookup
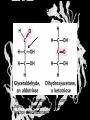
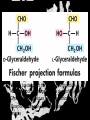
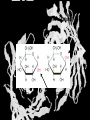
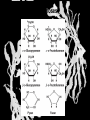
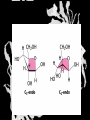
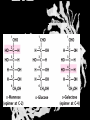
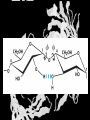
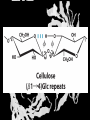
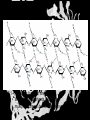
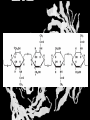
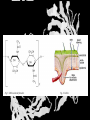
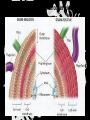
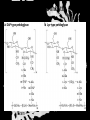
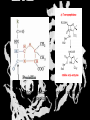
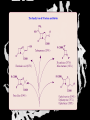
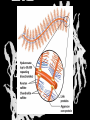
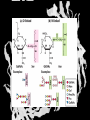
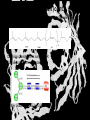
Carbohydrates CH339K What Makes a Carbo a Carbo? Overall formula CH2On Where n ≥ 3 Etymology: From their general formula Cn(H2O)n; they were once thought to be hydrates of carbon. Simplest Carbos: N=3 2 Tautomers Tautomers are isomers of organic compounds that readily interconvert by a chemical reaction called tautomerization. (Wikipedia) Vertical bonds go into the plane of the projection Horizontal bonds emerge from the plane of projection Glyceraldehyde exists as a pair of enantiomers Enantiomers are non-superimposable mirror images R-S Nomenclature (for the Organic guys) With n > 3, you can form diastereomers Diastereomers are stereoisomers that are not mirror images Hemiacetals and Hemiketals An aldehyde or a ketone can react wth an alcohol Ring formation Ring formation in a ketose Anomers Differ at the hemiacetal or hemiketal carbon a-D-Glucose b-D-Glucose Furanoses and Pyranoses Multiple possible rings in a C6-Ketose Multiple Possible Rings in a C5-Aldose Ring pucker Epimers Differ at only one carbon, but not the anomeric carbon Sugar Derivatives -2H+,e- +H2O aldonolactone Aldonic acid Cu2+ +NH3, -H2O Acetic anhydride Alduronic acid Amino sugar Substituted Amino sugar Sugar Derivatives Glycosides Glycoside: Any of a group of organic compounds that yield a sugar and one or more nonsugar substances on hydrolysis. Natural Glycosides Amygdalin is a poison found in the seeds of bitter almonds. It releases cyanide upon hydrolysis. Amygdalin is also known as Laetrile or “Vitamin B17,” a quack (excuse me, “alternative”) cancer treatment. Natural Glycosides Digitoxin is used as a heart stimulant in medicine; related componds are used as poisons in murder mysteries Polymerization Activation – couple to UDP Disaccharides Storage Polysacharides amylopectin Amylose Structure Iodine Test for Starch – Molecular Basis I2 + I- I3 Amylopectin: a-1,4 + a-1,6 Glycogen : a-1,4 + a-1,6 Glycogen in liver cells – stained with carmin Storage Polysaccharides Polysaccharide Linkage Branching Size (residues) Amylose a-1,4 unbranched Up to 5,000 Amylopectin a-1,4 and a-1,6 25-30 residues Up to 1,000,000 Glycogen a-1,4 and a-1,6 8-12 residues Up to 50,000 Structural Polysaccharides Cellulose Cellulose complexes β(1-4) linkages form flat sheets • analogous to β-sheets in proteins • multiple hydrogen bonds between strands. Chitin Insect cuticles Self-Nonself Recognition – Blood Groups Bacterial Cell Walls Gram + Staphylococcus aureus Gram - Escherischia coli Bacterial Cell Walls Gram - Gram + Bacterial Cell Walls – another view Peptidoglycan Components E. coli S. aureus What the heck is diaminopimelate? Lysine with an extra carboxyl attached to Ce. Transpeptidase cross-links the individual chains Gram - Gram + Cross Linking E. Coli S. aureus Spore formation Figure 1. Stages of sporulation. (A) Stage 0/I, (B) stage II, (C) stage III, (D) stage IV, (E) released spore. The hatched line around the spore in panels D and E is the coat. From: Cell. Mol. Life Sci. 59 (2002) Bacillus anthracis Production Do not try this at home!!!!! 1. It will not work as shown 2. You will die 3. Everyone you know will go to Gitmo Delivery The M33 500-lb biological cluster bomb, which held 108 of the M114 bombs. Photograph: Chemical and Biological Defense Command Historical Research and Response Team, Aberdeen Proving Ground, Md. Pilot anthrax plant at Ft. Detrick, MD (shut down in 1969) Al Hakam “Single-Cell Protein” Plant (demolished under UN supervision, 1996) Whoopsie! 1979 – Sverdvlosk 66 people die Aerosol plume from “Military Compound 19” Somebody left the vent to the outside open. b-Lactams This carbon forms covalent bond with active-site serine on transpeptidase enzyme Glycosaminoglycans Aka Mucopolysaccharides - Unbranched polysaccharides - Repeating disaccharide - Hexose linked to aminohexose - Typically with many negative charges -Acidic -Slimy Structure Name/Composition Hyaluronates: composed of D-glucuronate + GlcNAc linkage is b(1, 3) Localization synovial fluid, vitreous humor, ECM of loose connective tissue Dermatan sulfates: composed of L-iduronate (many are sulfated) + GalNAc-4-sulfate linkages is b(1, 3) skin, blood vessels, heart valves Chondroitin 4- and 6-sulfates : composed of D-glucuronate + GalNAc-4- or 6-sulfate linkage is b(1, 3) (the figure contains GalNAc 4-sulfate) cartilage, bone, heart valves most abundant GAG Heparin and Heparan sulfates: composed of D-glucuronate-2-sulfate (or iduronate-2-sulfate) + N-sulfo-Dglucosamine-6-sulfate linkage is a(1, 4) (heparans have less sulfate than heparins) Heparin component of intracellular granules of mast cells lining the arteries of the lungs, liver and skin Heparan Sulfate basement membranes, components of cell surfaces cornea, bone, cartilage aggregated with chondroitin sulfates Heparin more sulfated than heparan sulfates Heparan Sulfate contains higher acetylated glucosamine than heparin Keratan sulfates: composed of galactose + GlcNAc-6sulfate linkage is b(1, 4) Comments large polymers, shock absorbing cornea, bone, cartilage aggregated with chondroitin sulfates Proteoglycans • Cell surface or extracellular matrix • Glycosaminoglycan(s) bound to core protein • Noncovalent attachments to help bind extracellular matrix Disease from Failure to Metabolize Glycosaminoglycans (One example of many) Disease Hurler syndrome Deficient enzyme Accumulated products α-L-iduronidase Heparan sulfate Dermatan sulfate Symptoms Incidence Mental retardation 1 in 100.000 Micrognathia Coarse facies Macroglossia Retinal degeneration Corneal clouding Cardiomyopathy Hepatosplenomegal y Proteoglycan - Photo Glycoproteins Glycoprotein Functions Function Glycoproteins Structural molecule Collagens Lubricant and protective agent Mucins Transport molecule Transferrin, ceruloplasmin Immunologic molecule Immunoglobins, histocompatibility antigens Hormone Chorionoic gonadotropin, thyroid-stimulating hormone (TSH) Enzyme Various, eg, alkaline phosphatase Cell attachment-recognition site Various proteins involved in cell-cell (eg, sperm-oocyte), virus-cell, bacterium-cell, and hormone cell interactions Antifreeze Certain plasma proteins of coldwater fish Interact with specific carbohydrates Lectins, selectins (cell adhesion lectins), antibodies Receptor Various proteins involved in hormone and drug action Affect folding of certain proteins Calnexin, calreticulin Regulation of development Notch and its analogs, key proteins in development Hemostasis (and thrombosis) Specific glycoproteins on the surface membranes of platelets N-Linked Glycosylation • • • • All eukaryotes make N-linked glycoproteins Usually Cotranslational Consensus sequence Asn – X – Ser/Thr Usually when there’s an accessible loop in the folding protein structure N-Linked Glycosylation (cont.) • Core oligosaccharide synthesized on cytoplasmic side of ER • Attached to dolichol in ER membrane • Core oligo transferred to lumen of ER • Core oligo transferred to protein • Further processing occurs in ER / Golgi N-Linked Glycosylation O-Linked Glycosylation • • • • • • • True posttranslational modification No oligo precursor Frequently starts with GalNAc Secretory cells Zona pellucida around mammalian eggs Hematopoeisis Inflammatory response Common (not universal!!) core Frozen Fish (Not!) Chaenocephalus aceratus