* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Kristen Ray - USD Biology
Discovery and development of antiandrogens wikipedia , lookup
Pharmacognosy wikipedia , lookup
CCR5 receptor antagonist wikipedia , lookup
Drug interaction wikipedia , lookup
Toxicodynamics wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Serotonin syndrome wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
NMDA receptor wikipedia , lookup
5-HT3 antagonist wikipedia , lookup
5-HT2C receptor agonist wikipedia , lookup
Neuropharmacology wikipedia , lookup
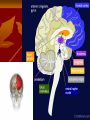
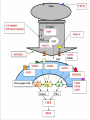
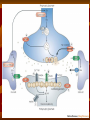
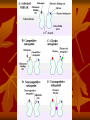
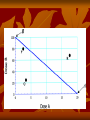
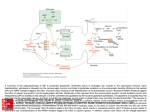
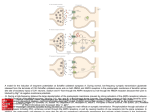
OCD Neurochemical dysfunction (abnormalities in serotonin (5-HT), dopamine (DA), and glutamatergic transmitter systems) Drug Therapy in OCD Strategies targeting these systems Role of serotonin (5-HT) neurotransmitter system and glutamatergic system SSRIs Block action of 5HT transporter (5-HTT) protein Responsible for uptake of intrasynaptic 5-HT released following an action potential Prevent reuptake of 5-HT into the pre-synaptic neuron More serotonin left in synapse to bind with postsynaptic receptors Serotonin receptors are activated by 5-HT Receptors modulate the release of many neurotransmitters, including glutamate, GABA, dopamine, epinephrine/norepinephrine, and acetylcholine, as well as many hormones. Influence biological and neurological processes such as aggression, anxiety, appetite, cognition, learning, and memory Targeting 5-HT receptor subtypes Drugs that block the 5-HT2 family of receptors Augment action of SSRIs or have therapeutic efficacy by themselves Blockade of 5-HT2A receptors and activation of non-5HT2A receptors may have similar effects Synergistic treatment with 5-HT2A antagonist/SSRI combination Risperidone Potent 5-HT2A antagonist blocks α2 adrenoreceptors Presynaptic heteroreceptors on 5-HT neurons that regulate release of 5-HT Further enhance SSRI therapy through desensitization of 5-HT1D terminal autoreceptor 5-HT2C receptor agonism Psilocybin; mixed 5-HT2C/2A/1A receptor agonist 5-H1D/B receptor Regulate release of 5-HT from presynaptic terminal by reducing 5-HT neurotransmission Activation of 5-HT1D/B receptor by an agonist compound worsen OCD symptoms Chronic deficits in 5-HT functioning Agonist m-CPP Silent polymorphism of G861C gene 5-HT1D/B receptors supersensitive, resulting in chronic reductions in synaptic levels of 5-HT 5-HT1D/B antagonist compounds expected to hasten onset of therapeutic action of SSRIs in OCD rapidly producing state of 5-HT1D/B receptor insensitivity Glutamatergic System Glutamate most abundant excitatory neurotransmitter in the vertebrate nervous system. Nerve impulses trigger release of glutamate from pre-synaptic cell Opposing post-synaptic cell, glutamate receptors (NMDA receptors) Role in synaptic plasticity learning and memory in the brain Glutamate transporters rapidly remove glutamate from extracellular space In brain injury or disease, work in reverse and excess glutamate accumulates outside cells Causes calcium ions to enter cells via NMDA receptor channels Excitotoxicity- overstimulation of receptors leads to neuronal damage and eventual cell death “The Combined Effects of Memantine and Fluoxetine on an Animal Model of Obsessive Compulsive Disorder” Effective class of drugs used to treat OCD Fluoxetine First in a generation of SSRIs for treatment of major depressive disorder and anxiety disorders (OCD) Good overall safety and tolerability Better than most other antidepressants Memantine First used in treatment of Alzheimer’s dementia low-affinity voltage-dependent antagonist at glutamatergic NMDA receptors Uncompetitive antagonist channel blocker Aim of Study Use mouse model of OCD Examine whether a synergistic, as opposed to additive relationship between NMDA antagonists and SSRIs in treatment of OCD Combining relatively low doses of both drugs Low enough where do not decrease compulsive behavior when administered alone Isobologram Graph of equally effective dose pairs (isoboles) for a single effect level Particular effect level is selected 50% of the maximum Doses of drug A and drug B (each alone) that give this effect are plotted as axial points in a Cartesian plot Straight line connecting A and B is the locus of points (dose pairs) that will produce this effect in a simply additive combination Line of additivity allows a comparison with the actual dose pair that produces this effect level experimentally Isobologram Third point plotted on graph Combination points below the line of additivity Synergism Combination points along line of additivity dose combinations of two drugs necessary to produce same effect size Simply Additive Combination points above line of additivity Subadditivity Method Male Swiss Webster mice 18-63 g depending on age Kept at 68 to 72 °F in 12h/12h light-dark cycle Ad libitum access to water and food pellets Scratching Assay Established as effective model compulsive behavior in dogs with allergic dermatitis Intradermal injection in mice Serotonin Compound 48-80 Promotes histamine release and mast cell degranulation Effect of fluoxetine on serotonin-induced scratching compared to controls Effect of memantine on serotonin-induced scratching compared to controls 5,10,15,30 mg/kg Effect of combination fluoxetine and memantine on serotonin-induced scratching 5,10,15,30 mg/kg 5mg/kg fluoxetine 5mg/kg memantine Effect of fluoxetine (10 mg/kg) and memantine (5 mg/kg) by compound 48-80 both alone and in combination Experimental mice intraperitoneal injection of varying doses of fluoxetine and/or memantine in saline (0.9% NaCl) containing ascorbic acid (1mg/ml) Control mice Intraperitoneal injection of varying doses of saline and ascorbic acid 0.1ml/10g of body weight 5 minutes later Control and experimental mice subcutaneously injected behind neck 0.1 ml of 0.4 mg/ml serotonin or 0.1 ml of compound 48-80 1 mg/ml in saline and ascorbic acid to induce scratching Each mouse then placed individually in separate cage paired with control mouse in separate cage given saline pretreatment Cumulative number of scratches counted continuously using manual counter Cumulative scratches recorded every 5 minutes for 30 minutes Other behaviors noted motor activity, sedation, licking, and rearing Statistical Analysis Mean scratching scores for pretreated mice compared to their saline controls Cumulative scratches in mice injected with drug were expressed as % of scratches compared to saline controls Results Discussion Combined doses of fluoxetine and memantine required to produce 90% reduction in scratching much lower than doses of either drug alone necessary to produce same effect Synergistic relationship Present study mechanisms most likely serotonergic and glutamatergic Basis for synergism between fluoxetine and memantine SSRIs increase amount of serotonin in synapses and NMDA antagonist block glutamate binding at NMDA receptors Both decreased serotonergic activity and increased glutamatergic activity have been linked to the expression of impulsive behaviors