* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Substitution Rxns-a-Sn2-12-quesx
Kinetic isotope effect wikipedia , lookup
Elias James Corey wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Marcus theory wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ene reaction wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
Discodermolide wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Petasis reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Hydroformylation wikipedia , lookup
Stille reaction wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
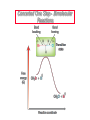
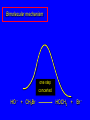
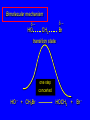
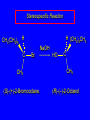
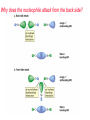
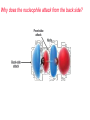
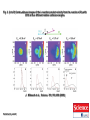
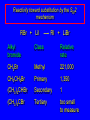
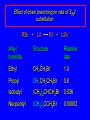
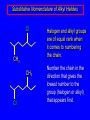
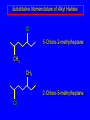
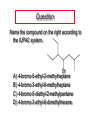
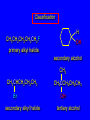
Nucleophilic Substitution Reactions: SN2 Mechanism The SN2 Mechanism of Nucleophilic Substitution Concerted One Step - Bimolecular Reactions Kinetics Many nucleophilic substitutions follow a second-order rate law. CH3Br + HO – CH3OH + Br – rate = k [CH3Br] [HO – ] What is the reaction order of each starting material? What can you infer on a molecular level? What is the overall order of reaction? Bimolecular mechanism one step concerted HO – + CH3Br HOCH3 + Br – Bimolecular mechanism one step concerted HO – + CH3Br HOCH3 + Br – Bimolecular mechanism dHO dBr CH3 transition state one step concerted HO – + CH3Br HOCH3 + Br – Question Assuming the reaction below takes place by a concerted process, which mechanistic scheme is correct? NaCN CN Cl + NaCl A. NaCN Cl CN + NaCl CN + NaCl CN + NaCl NC B. CN NaCN Cl CN Cl C. CN NaCN Cl Stereochemistry of SN2 Reactions Generalization Nucleophilic substitutions that exhibit second-order kinetic behavior are stereospecific and proceed with inversion of configuration. Inversion of Configuration nucleophile attacks carbon from side opposite bond to the leaving group Inversion of Configuration nucleophile attacks carbon from side opposite bond to the leaving group three-dimensional arrangement of bonds in product is opposite to that of reactant Inversion of configuration (Walden inversion) in an SN2 reaction is due to back side attack Stereospecific Reaction A stereospecific reaction is one in which stereoisomeric starting materials give stereoisomeric products. The reaction of 2-bromooctane with NaOH (in ethanol-water) is stereospecific. (+)-2-Bromooctane (–)-2-Octanol (–)-2-Bromooctane (+)-2-Octanol Stereospecific Reaction H (CH2)5CH3 CH3(CH2)5 H NaOH C Br CH3 (S)-(+)-2-Bromooctane HO C CH3 (R)-(–)-2-Octanol 1) Draw the Fischer projection formula for (+)-S-2-bromooctane. 2) Write the Fischer projection of the (–)-2-octanol formed from it by nucleophilic substitution with inversion of configuration. CH3 H CH3 Br CH2(CH2)4CH3 HO H CH2(CH2)4CH3 A.) R- ? or B.) S- ? A conceptual view of SN2 reactions Why does the nucleophile attack from the back side? Why does the nucleophile attack from the back side? “Roundabout” SN2 Reaction Mechanism SN2 Reaction Mechanisms: Gas Phase (2008) http://pubs.acs.org/cen/news/86/i02/8602notw1.html Traditional Physicist Roland Wester and his team in Matthias Weidemüller's group at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected mechanism. In this new pathway, called the roundabout mechanism, chloride bumps into the methyl group and spins the entire methyl iodide molecule 360° before chloride substitution. Data at lower collision energies support the traditional SN2 mechanism. However, at higher collision energies, about 10% of the iodide ions fell outside of the expected distribution. Roundabout Roundabout SN2 Mechanism Traditional SN2 Mechanism Fig. 1. Calculated MP2(fc)/ECP/aug-cc-pVDZ Born-Oppenheimer potential energy along the reaction coordinate g = RC-I - RC-Cl for the SN2 reaction Cl- + CH3I and obtained stationary points J. Mikosch et al., Science 319, 183 -186 (2008) Published by AAAS Fig. 2. (A to D) Center-of-mass images of the I- reaction product velocity from the reaction of Cl- with CH3I at four different relative collision energies J. Mikosch et al., Science 319, 183 -186 (2008) Published by AAAS Fig. 3. View of a typical trajectory for the indirect roundabout reaction mechanism at 1.9 eV that proceeds via CH3 rotation J. Mikosch et al., Science 319, 183 -186 (2008) Published by AAAS Steric Effects in SN2 Reactions Crowding at the Reaction Site The rate of nucleophilic substitution by the SN2 mechanism is governed by steric effects. Crowding at the carbon that bears the leaving group slows the rate of bimolecular nucleophilic substitution. Reactivity toward substitution by the SN2 mechanism RBr + LiI RI + LiBr Alkyl bromide Class Relative rate CH3Br Methyl 221,000 CH3CH2Br Primary 1,350 (CH3)2CHBr Secondary 1 (CH3)3CBr Tertiary too small to measure A bulky substituent in the alkyl halide reduces the reactivity of the alkyl halide: steric hindrance Decreasing SN2 Reactivity CH3Br CH3CH2Br (CH3)2CHBr (CH3)3CBr Decreasing SN2 Reactivity CH3Br CH3CH2Br (CH3)2CHBr (CH3)3CBr Reaction coordinate diagrams for (a) the SN2 reaction of methyl bromide and (b) an SN2 reaction of a sterically hindered alkyl bromide Crowding Adjacent to the Reaction Site The rate of nucleophilic substitution by the SN2 mechanism is governed by steric effects. Crowding at the carbon adjacent to the one that bears the leaving group also slows the rate of bimolecular nucleophilic substitution, but the effect is smaller. Effect of chain branching on rate of SN2 substitution RBr + LiI RI + LiBr Alkyl bromide Structure Relative rate Ethyl CH3CH2Br 1.0 Propyl CH3CH2CH2Br 0.8 Isobutyl (CH3)2CHCH2Br 0.036 Neopentyl (CH3)3CCH2Br 0.00002 Question Which reaction will have the fastest rate of reaction? A) B) C) Putting things together IUPAC Nomenclature of Alkyl Halides IUPAC Nomenclature There are several kinds of IUPAC nomenclature. The two that are most widely used are: functional class nomenclature substituent nomenclature Both types can be applied alkyl halides and to alcohols. Functional Class Nomenclature of Alkyl Halides Name the alkyl group and the halogen as separate words (alkyl + halide). CH3F CH3CH2CHCH2CH2CH3 Br CH3CH2CH2CH2CH2Cl H I Functional Class Nomenclature of Alkyl Halides Name the alkyl group and the halogen as separate words (alkyl + halide). CH3F CH3CH2CH2CH2CH2Cl Methyl fluoride Pentyl chloride CH3CH2CHCH2CH2CH3 Br 1-Ethylbutyl bromide H I Cyclohexyl iodide Substituent Nomenclature of Alkyl Halides Name as halo-substituted alkanes. Number the longest chain containing the halogen in the direction that gives the lowest number to the substituted carbon. CH3CH2CH2CH2CH2F CH3CHCH2CH2CH3 Br CH3CH2CHCH2CH3 I Substitutive Nomenclature of Alkyl Halides Name as halo-substituted alkanes. Number the longest chain containing the halogen in the direction that gives the lowest number to the substituted carbon. CH3CH2CH2CH2CH2F 1-Fluoropentane CH3CH2CHCH2CH3 I 3-Iodopentane CH3CHCH2CH2CH3 Br 2-Bromopentane Substitutive Nomenclature of Alkyl Halides Cl CH3 CH3 Cl Halogen and alkyl groups are of equal rank when it comes to numbering the chain. Number the chain in the direction that gives the lowest number to the group (halogen or alkyl) that appears first. Substitutive Nomenclature of Alkyl Halides Cl 5-Chloro-2-methylheptane CH3 CH3 2-Chloro-5-methylheptane Cl Question Name the compound on the right according to the IUPAC system. A) B) C) D) 4-bromo-5-ethyl-2-methylheptane 4-bromo-3-ethyl-6-methylheptane 4-bromo-5-diethyl-2-methylpentane 4-bromo-3-ethyl-6-dimethylhexane Question Br Cl What is the correct IUPAC name for the ABOVE structure? A. (3S,4S)-3-bromo-4-chlorohexane B. (3S,4S)-3,4-dibromochloroheptane C. (3R,4R)-3-chloro-4-bromohexane D. (3R,4R)-3-bromo-4-chlorohexane E. (3S,4S)-4-bromo-3-chlorohexane Classes of Alkyl Halides Classification Alkyl halides & alcohols are classified as primary secondary tertiary according to their "degree of substitution." Degree of substitution is determined by counting the number of carbon atoms directly attached to the carbon that bears the halogen or hydroxyl group. Different Kinds of Alkyl Halides Classification H CH3CH2CH2CH2CH2F OH primary alkyl halide secondary alcohol CH3 CH3CHCH2CH2CH3 Br secondary alkyl halide CH3CCH2CH2CH3 OH tertiary alcohol Question What type of alcohol is 2-methyl-3-pentanol? A) primary (1°) B) secondary (2°) C) tertiary (3°) D) quaternary (4°)