* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download A Type of Basket Cell in Superficial Layers of the Cat Visual Cortex
Survey
Document related concepts
Nervous system network models wikipedia , lookup
Optogenetics wikipedia , lookup
Environmental enrichment wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Electrophysiology wikipedia , lookup
Development of the nervous system wikipedia , lookup
Subventricular zone wikipedia , lookup
Apical dendrite wikipedia , lookup
Synaptic gating wikipedia , lookup
Anatomy of the cerebellum wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Neuroanatomy wikipedia , lookup
Channelrhodopsin wikipedia , lookup
Synaptogenesis wikipedia , lookup
Transcript
Brain Research, 244 (1982) 9-16
Elsevier Biomedical Press
9
A Type of Basket Cell in Superficial Layers of the Cat Visual Cortex.
A Golgi-Electron Microscope Study
JAVIER DeFELIPE and ALFONSO FAIRI~N*
Unidad de Neuroanatomia, Instituto Cajal, CSIC, Veldzquez 144, Madrid 6 (Spain)
(Accepted December 24th, 1981)
Key words: cerebral cortex - - interneurons - - basket cells - - Golgi-EM technique
The axonal arborizations of the basket cells in the cerebral neocortex have long been considered as the source of the
presynaptic terminals contacting the cell bodies of pyramidal cells. Given that the concept of the cortical basket cell is based upon
indirect evidence only, it was deemed worthwhile to re-investigate this problem using the Golgi-EM technique. This approach
permits one to trace the presynaptic terminals back to their parent cell body, so that it allows for a positive identification of basket
cells, i.e. cells which produce axosomatic synapses by preference. A type of interneuron in layer l I - l l l of the cat visual cortex is
described. Its axon terminals form multiple synaptic contacts, of the symmetrical type, on cell bodies and proximal dendrites of
pyramidal and non-pyramidal cells. On the basis of this efferent synaptic pattern, this interneuron is considered to be a basket cell.
The possible correspondence of this interneuronal type with other putative basket cells described in previous Golgi studies is
discussed. In addition, a simple re-sectioning method for semithin sections is described, which has been used to identify individual
Golgi-impregnated axonal boutons in electron microscopy.
INTRODUCTION
Cell bodies o f p y r a m i d a l cells in the cerebral
n e o c o r t e x receive synapses which are o f the symmetrical type2,6A2,17,18,19. All evidence indicates that
these a x o s o m a t i c synapses are GABAergicg,22, 24
a n d thus, inhibitoryg,ls, 23. M o r e o v e r , symmetrical
G A B A e r g i c 23 synapses have been r e p o r t e d on cell
bodies o f n o n - p y r a m i d a l cells12,17,12, 2°.
The pericellular plexuses f o r m e d by the axons o f
b a s k e t cells have been considered as the source o f
these a x o s o m a t i c synapses on p y r a m i d a l cells~, 10,
13,14,22,27. These plexusesS, 15 are s u p p o s e d l y form e d b y the convergence o f an i n d e t e r m i n a t e n u m b e r
o f b a s k e t cell axonsl,8,12,15, b u t it is implied that
each i n d i v i d u a l i n t e r n e u r o n m a k e s a substantial
c o n t r i b u t i o n to the pericellular plexus. To date,
however, there is no direct evidence to substantiate
such assumptions. G o l g i - E M studies4,7,12A7,18,25,26
have shown that s y m m e t r i c a l synapses derive from
the a x o n terminals o f aspinous n o n - p y r a m i d a l cells.
One class o f these i n t e r n e u r o n s are m u l t i p o l a r cells
* To whom correspondence should be addressed.
0006-8993/82/0000-0000/$02.75 © Elsevier Biomedical Press
whose axons f o r m descending arcades is. Their a x o n
terminals c o n t r i b u t e to the a x o s o m a t i c synapses,
but, in addition, an a m p l e variety o f p o s t s y n a p t i c
elements are contacted. M o r e o v e r , no multiple axosomatic contacts on a given p y r a m i d are f o r m e d t h a t
derive f r o m a single interneuron.
This r e p o r t presents G o l g i - E M evidence for a
type o f b a s k e t cell, located in layers I I - I I I o f the cat
area 17. The axons o f this i n t e r n e u r o n a l type f o r m
multiple synaptic contacts on cell bodies and proxim a l dendrites o f p y r a m i d a l a n d n o n - p y r a m i d a l
cells.
MATERIALS AND METHODS
Seven cats, 3 m o n t h s old, were used in this study.
U n d e r N e m b u t a l anaesthesia all cats were perfused
with a solution o f l ~ g l u t a r a l d e h y d e - l ~o p a r a f o r m a l d e h y d e in a p h o s p h a t e buffer 16. Pieces o f cerebral cortex were rinsed in buffer and then G o l g i
i m p r e g n a t e d 6. Slices, 150/zm thick, were o b t a i n e d
with a sliding m i c r o t o m e a n d stored in a n h y d r o u s
10
I,
2
3
t
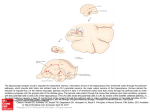
lO.um']
Fig. 1. C a m e r a lucida d r a w i n g o f an i n t e r n e u r o n in layer I I - I I I of the cat area 17. The arrow points to the origin of the axon. See
text for details.
Fig. 2. Enlarged view of the boxed area in Fig. I. Note the axonal swellings I. 2 a n d 3 a n d a connecting axom~l braid {arrowhead L
A n ascending dendrite is labelled as d.
11
glycerol. Those slices containing well impregnated
interneurons were illuminated while in glycerol at 24
°C for 90 rain, using a 150 W photoflood lamp ,~.
Then they were gold-toned, de-impregnated, and
embedded in Epon-Araldite 6. Besides illumination
of the slices prior to gold toning, some technical
modifications were used 5,7 which significantly increase the yield of axonal arborizations that can be
followed in their entirety after de-impregnation.
After plastic embedding, drawings were made using
a camera lucida, and then the slices were serially
sectioned at 3 # m in an ultramicrotome. Semithin
sections were picked up individually from the trough
and mounted on slides made from the same EponAraldite used for embedding. For that purpose, the
sections were placed on the slides with a drop of
distilled water and allowed to dry at 60 °C for at
least 1 h; no special adhesive was used. Later on,
they were stained with l ~ toluidine blue in 1 ~o
borax. The sections were inspected in the light
microscope to ascertain which parts of the axonal
arborization would appear in each section. Using
this method, absolute certainty as to which Golgistained boutons belong to the axonal plexus under
study was obtained (Figs. 1-6). With the semithin
sections facing out, the plastic slide pieces were
attached to plastic blanks using cyanoacrylic glue
and trimmed with glass knives. Serial ultrathin
sections were then obtained. When compared to
previous techniques for re-sectioning semithin sections 2,s,11, the present one offers the advantage of
its obvious simplicity.
RES U LTS
The interneurons that were considered for the
present study were multipolar neurons with local
axonal arborizations, located in layer l l - l l l of the
cat visual cortex. These cells were systematically
inspected in semithin sections to ascertain whether
their axonal boutons were preferentially located at a
perisomatic site. When this was the case, the semithin sections were re-sectioned in the ultrathin range
and examined under the electron microscope in
order to prove the existence of axosomatic synapses
effected by these axons, and to define the form of the
synaptic contacts.
One example has been chosen to illustrate the
features of these cells that give origin to perisomatic
axonal boutons. Fig. 1 depicts a layer l I - I I l cell that
has a multipolar appearance with a rather wide
dendritic field. The axon (arrow) emerges from the
base of a major dendritic trunk. It is primarily
descending and originates as a dense plexus in the
lower region of the dendritic field. Some collaterals
turn upwards and spread in a fan-like fashion to
distribute in the upper part of the dendritic domain.
Boutons, mainly 'en passant', are seen along the
axonal branches.
It is noteworthy that the axonal arborization
shows no signs of target selectivity (see ref. 7), i.e. no
basket-like formations are visible when the Golgi
sections are examined at the light microscope level.
Under the electron microscope, however, labelled
neurons belonging to the axonal plexus of this
interneuron are consistently seen against the cell
bodies of pyramidal cells (Figs. 4, 7, 9 and 10). Such
distribution is also evident in semithin sections (Fig.
3). One distinguishing characteristic of this interneuronal type is that its axonal arborizations form
multiple axosomatic contacts on a given postsynaptic cell. The number of pericellular boutons each
single pyramidal cell body receives from the cell
shown in Fig. 1 can be rather large; for example, 7 in
the case shown in Figs. 9 and 10.
Pyramidal cell bodies are not the only targets for
this axonal arborization. The perikaryon of a cell,
which, according to established criteriaas,2 0, is classified as an aspinous non-pyramidal cell, is shown in
Figs. 13 and 14. This cell, located in the bottom of
Fig. 3. A selected area ofa semithin section, is photographed here at the same scale as Fig. 2. Fragments of dendrite d, boutons 2
and 3, and the axonal braid (arrowhead) can be recognized. Axonal boutons 2 and 3 are against the perikaryon of a pyramidal cell
(P) of layer 11.
Fig. 4. After re-sectioning the semithin section shown in Fig. 3., the same structures can be recognized in an ultrathin section.
Fig. 5. In an adjacent section, boutons 2 and 3 are seen surrounding the perikaryon, close to the axon hillock, is - axon initial segment.
Fig. 6. In another section of the series, bouton 2 is seen at a higher magnification. The symmetrical synapse (arrow) formed by this
bouton on the soma has been sectioned obliquely.
12
Fig. 7. L o w power electron m i c r o g r a p h of a n ultrathin section obtained f r o m a semithin section adjacent to the one s h o w n in Fig, 3,
B o u t o n s 1 a n d 2 (see Fig. 2) are in contact with the perikaryon of the s a m e p y r a m i d a l n e u r o n (P) as in Fig. 4,
Fig. 8. Symmetrical synapse (arrow) formed by bouton t on the pyramidal cell perikaryon.
Figs. 9-12. A x o n a l b o u t o n s fi'om the interneuron of Fig. I, s u r r o u n d i n g the perikaryon of a layer 11 pyramidal cell, T h e low powe~
m i c r o g r a p h s of Figs. 9 a n d 10 belong to ultrathin sections taken f r o m two adjacent semithin sections. A ~ot~fl of 7 boutoTls (I 7) ~re
visible. I n Figs. 1 1 a n d 12 the symmetrical synapses (arrows) formed by bouton~ I and 4 are show~.
13
.
Figs. 13-18. Synapses on the perikaryon of a smooth stellate cell.
Fig. 13. Low power micrograph of the postsynaptic neuron. The nucleus is eccentrically located, and the cytoplasm is rich in
organelles.
(continued on page 14)
14
Fig. 19. Low power micrograph of a fusiform cell (A). The perikaryal cytoplasm is dark and contrasts with the appearance of that
of a contiguous neuron (B), which is the same shown in Fig. 13. A labelled bouton contacts the perikaryon of the fusiform
neuron (arrow).
Fig. 20. In a serial section, a narrow band of the perikaryat cytoplasm of the fusiform neuron is seen (A). Note the high number of
clusters of ribosomes. A band of cytoplasm of cell B is seen. Two additional labelled boutons synapse on the fusiform cell perikaryom
layer II1, receives 5 stained b o u t o n s on its cell body
which e m a n a t e from the same source. In addition, 3
labelled b o u t o n s contact the p e r i k a r y o n of a cell
with a different m o r p h o l o g y (Figs. 19 and 20). It is a
fusiform cell with a dark cytoplasm due to its high
c o n t e n t of free ribosomes grouped into clusters. The
p e r i k a r y o n receives only a few unlabelled synapses.
The nucleus is elongated a n d shows, in other sec-
tions t¥om the series, a n i n d e n t e d envelope. These
features make this cell c o m p a r a b l e to the bitufted
cell described by Peters et al. "~'t in the rat visual
cortex. Moreover, proximal dendrites of pyramidal
cells have also been identified as postsynaptic.
W i t h o u t exceptions, the b o u t o n s form symmetrical synapses on the cell bodies and the proximal
dendrites they contact (Figs. 6, 8, 11, 12, 15b, 17, 18
Fig. 14. A large number of synapses (arrowheads) contact the perikaryon. Four boutons (14), labelled by their content of gold
particles, are seen in this ultrathin section.
Fig. 15. In two consecutive sections from the series, an additional bouton (5) contacts the same perikaryon, The symmetrical
synapse (s2) formed by this bouton is seen in b; in a, an unlabelled asymmetrical synapse (sl) is visible On the perikaryon.
Fig. 16. The perikaryon receives both asymmetrical (s~) and symmetrical (s2) synapses. Bouton 4 (see Fig. 14) is against the
membrane; synaptic contact is not visible in this ultrathin section.
Fig. 17. In a consecutive section, bouton 4 forms a symmetrical synapse (arrow) on the perikaryot~
Fig. 18. Axosomatic synapse (arrow) of the symmetrical type, formed by bouton I (see Fig. 14)
15
and 20). These synapses show a very thin postsynaptic density and are similar in m o r p h o l o g y to those
formed by the axon o f other types of smooth stellate
cells4,7,12,17,18,25,26.
DISCUSSION
This paper presents, for the first time, evidence for
a type of interneuron whose axon terminals form
multiple synaptic contacts o f the symmetrical type
on cell bodies of both pyramidal and non-pyramidal
cells in superficial layers of the cat visual cortex. On
the basis of this efferent synaptic pattern, this interneuron is considered to be a basket cell. Although it
is clear that convergence must play a role in the
building up of a pericellular basket1,8,13, is, the
existence of multiple synaptic contacts of a c o m m o n
origin indicates that each basket cell axon makes a
substantial contribution to the pericellular plexus.
Thus, a high degree of selectivity with regard to the
postsynaptic partners is apparent which could be
compared to that shown by chandelier cells7, 25. If,
as postulated, basket and chandelier cells exert an
inhibitory action7,9,18,zz,24, 25, the selective distribution of their axon terminals would make these two
neuronal types very efficient functionally. A n interesting finding, moreover, is that the axons of the
present type of basket cell form multiple synaptic
contacts on cell bodies of aspinous non-pyramidal
cells, which are considered to be inhibitorylS,20, 23.
Such a synaptic arrangement is consistent with
physiological data revealing inhibition of first-order
REFERENCES
1 Cajal, S. R., Histologie du SystOme Nerveux de l'Homme
et des Vertdbrds, Vol. lI, A. Maloine, Paris, 1911, 993 pp.
2 Christensen, B. N., Morphological correlates of synaptic
transmission in lamprey spinal cord, J. NeurophysioL, 39
(1976) 197 212.
3 Colonnier, M., Synaptic patterns on different cell types in
the different laminae of the cat visual cortex. An electron
microscope study, Brain Research, 9 (1968) 268-287.
4 DeFelipe, J. and Fair6n, A., Interneurones with axonal
arcades in the cat visual cortex. A Golgi-EM study,
Neurosci. Lett., Suppl. 7 (1981) S 399.
5 Fair6n, A., DeFelipe, J. and Martinez-Ruiz, R., The
Golgi-EM procedure: a tool to study neocortical interneurons. In E. Acosta Vidrio and S. Fedoroff (Eds.),
Glial and Neuronal Cell Biology, Progress in Clinical and
Biological Research, Vol 59A, Alan R. Liss, New York,
1981, pp. 219-301.
inhibitory neurons after retinal stimulation zs, as has
been recently discussed 26 in relation to a different
type of local interneuron in the cat visual cortex.
Several types of presumed basket cells have been
described1,1°,14, zT. The interneurons of the present
report may correspond to the short-range basket
cells o f Szenfftgothai 27 and to the sparsely spinous,
spherical, multipolar neurons with a local axonal
plexus described by Peters and Regidor z2, although
they do not classify these neurons as basket cells.
The Jones' type 6 interneuron 10 has some features in
c o m m o n with the interneurons we are describing
and, in fact, is considered by this author as a type o f
basket cell. However, type 6 cells have longer horizontal axonal branches and the local plexus seems to
be less profuse. Nevertheless, since electron microscope data have not been reported on these interneurons, comparisons are difficult. To this end, further
studies are necessary to confirm the postulated
specificity of the axonal arborizations of the diverse
types o f basket cells described in Golgi studies.
ACKNOWLEDG EM ENTS
We wish to thank Drs. A. Peters and J. Regidor
for allowing the use o f their unpublished results,
Miss R. Martinez-Ruiz for her technical assistance,
and Mr. S. J. Jones for linguistic advice. Helpful
discussions on neuronal typology with Dr. J. Regidor
are gratefully acknowledged. This work was supported by Grant 3344/79 from CA1CYT,
6 Fair6n, A., Peters, A. and Saldanha, J., A new procedure
for examining Golgi impregnated neurons by light and electron microscopy, J. Neuroeytol., 6 (1977) 311-337.
7 Fair6n, A. and Valverde, F., A specialized type of neuron
in the visual cortex of cat: a Golgi and electron microscope study of chandelier cells, J. Comp. Neurol., 194
(1980) 761-779.
8 Hollfinder, H. and Vanegas, H., Identification of pericellular baskets in the cat striate cortex: light and electron
microscopic observations uptake of horseradish peroxidase, J. Neurocytol., 10 (1981) 577-587.
9 Houser, C. R., Vaughn, J. E., Jones, E. G. and Hendry,
S. H. C., GABA neurons of monkey motor and sensory
cortex: an immunocytochemical study, Neurosei. Abstr.,
6 (1980) 159.
10 Jones, E. G., Varieties and distribution of non-pyramidal
ceils in the somatic sensory cortex of the squirrel monkey,
J. Comp. Neurol., 160 (1975) 205-268.
II Kaplan, M. S. and Hinds, J. W., Neurogenesis in the
16
12
13
14
15
16
17
18
19
20
adult rat: electron microscopic analysis of light radioautographs, Science, 197 (1977) 1092-1094.
LeVay, S., Synaptic patterns in the visual cortex of the cat
and monkey. Electron microscopy of Golgi preparations,
J. Comp. NeuroL, 150 (1973) 53-86.
Marin-Padilla, M., Origin of the pericellular baskets of
the pyramidal cells of the h u m a n motor cortex: a Golgi
study, Brain Research, 14 (1969) 633-646.
Marin-Padilla, M., Prenatal and early postnatal ontogenesis of the human motor cortex: a Golgi study. II. The
basket-pyramidal system, Brain Research, 23 (1970) 185 191.
Marin-Padilla, M., Three-dimensional reconstruction of
the pericellular nests (baskets) of the motor (area 4) and
visual (area 17) areas of the h u m a n cerebral cortex. A
Golgi study, Z. Anat. EntwickL-Gesch., 144 (1974) 123-135.
Palay, S. L. and Chan-Palay, V., Cerebellar Cortex. Cytology and Organization, Springer-Verlag, Berlin, Heidelberg,
New York, 1974, p. 327.
Parnavelas, J. G., Sullivan, K., Lieberman, A. R. and
Webster, K. E. Neurons and their synaptic organization in
the visual cortex of the rat. Electron microscopy of Golgi
preparations, Cell Tiss. Res., 183 (1977) 499-517.
Peters, A. and Fairdn, A., Smooth and sparsely-spirted
stellate cells in the visual cortex of the rat : a study using
a combined Golgi-electron microscope technique, J.
eomp. Neurol., 181 (1978) 129-172.
Peters, A. and Kaiserman-Abramof, 1. R., The small
pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines, Amer. J. Anat., 127 (1970)
321-356.
Peters, A. and Proskauer, C. C., Smooth or sparsely spi-
21
22
23
24
25
26
27
28
ned cells with myelinated axons in rat visual cortex, Ne~,roscience, 5 (1980) 2079-2092.
Peters, A., Proskauer, C. C., Feldman, M. L. and Kimcrer, L., The projection of the lateral geniculate nucleus to
area 17 of the rat cerebral cortex, V. Degenerating axon
terminals synapsing with Go!gi impregnated neurons, J.
Neurocytol., 8 (1979) 331- 357.
Peters, A. and Regidor, J., A reassessment of the forms of
non-pyramidal neurons in area ~7 of cat visual cortex. J.
comp. NeuroL, 203 (1981) 685-716.
Ribak, C. E., Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase, J. Neuroc:vtol., 7 (1978)461-478~
Ribak, C. E., Harris, A. B., Vaughn, J. E. and Roberts, E.,
Inhibitory, GABAergic nerve terminals decrease at sites of
focal epilepsy, Science, 205 (1979) 21 t-214.
Somogyi, P., A specific 'axo-axonal' interneuron in the
visual cortex of the rat, Brain Research, 136 (1977) 345
350.
Somogyi, P. and Cowey, A., Combined Golgi and electron
microscopic study on the synapses formed by double
bouquet cells in the visual cortex of the cat and monkey,
J. comp. NeuroL, 195 (198t) 547 566.
Szent~igothai, J., Synaptology of the visual cortex. In R.
Jung (Ed.), Handbook of Sensory Physiology, Vol. VIII3,
Central Processing of Visual Infortnation, Part B, Visual
Centers in the Brain, Springer-Verlag, Berlin, Heidelberg,
New York, 1973, p.p. 269-324.
Toyama, K., Maekawa, K. and Takeda, F., Convergence
of retinal inputs onto visual cortical cells: 1. A study of
the ceils monosynaptically excited from the lateral geniculate body, Brain Research, 137 (1977) 207~220.