* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Notes 5.1 Osmosis in Action
Signal transduction wikipedia , lookup
Cell membrane wikipedia , lookup
Tissue engineering wikipedia , lookup
Endomembrane system wikipedia , lookup
Extracellular matrix wikipedia , lookup
Programmed cell death wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell growth wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Cytokinesis wikipedia , lookup
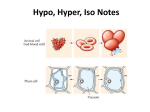
Notes 5.1 Osmosis in Action Pages 99-100 Table Talk •Watch this video. •As a group discuss the answers to these questions: • • • What organism is this? What kind of environment do you think it’s in? What is happening? Standards •CLE 3210.1.5 - Compare different models to explain the movement of materials into and out of the cell. SPI 3210.1.8 - Compare active and passive transport. RLE 2010.2.1 - Recognize the importance of homeostasis as a survival mechanism. • • Objectives 1.Compare different models to explain the movement of materials into and out of the cell and the role of the solution the cells exist within. • • • Diffusion Facilitated diffusion osmosis 2.Recognize the importance of homeostasis as a survival mechanism. Quick Review •Solutions • • have: Solvent: The liquid that dissolves the solute. Solute(s): The substance that gets dissolved. •Concentration: the amount of a substance in a given volume •Osmosis: When water moves from high to low concentration. So What? •Cells live in solutions…. So we’ve named the three types of solutions: • • • Isotonic Hypertonic Hypotonic Isotonic •Isotonic: Concentrations of solutes are the same inside and outside of the cell. •What happens: Water is at an equilibrium so it moves freely into and out of the cell at the same rate. Hypertonic •Hypertonic Concentrations of solutes are higher outside the cell than inside. •What happens? Water moves outside the cell in order to create a dynamic equilibrium. Cells shrivel up and may die (in plants it’s called plasmolysis.) Hypotonic Hypotonic: Concentrations of solutes are lower outside the cell than inside. What happens: Water moves into the cell in order to create a dynamic equilibrium. Cells expand and may explode (called cytolysis.) Summary Good graphic on page 99. Review •Watch this video again. •As a group discuss the answers to these questions: • • • What organism is this? What kind of environment do you think it’s in? What is happening? •Stopped here for the day Quick Review Equal •Isotonic – solutes are __________ within the solution in comparison to the cell Higher •Hypertonic – solutes are __________ within the solution in comparison to the cell Lower •Hypotonic – solutes are ___________ within the solution in comparison to the cell Objectives 1.Compare different models to explain the movement of materials into and out of the cell and the role of the solution the cells exist within. • • • Diffusion Facilitated diffusion osmosis 2.Recognize the importance of homeostasis as a survival mechanism. Cell Responses to Osmosis Land Organisms: Cells inside our bodies are in slightly hypotonic environments. Saltwater Organisms: No problems under normal conditions. Freshwater organisms: Live in a hypotonic environment. Hypotonic Environment Defenses Single-cell organisms: Contractile Vacuole: a sac that stores water and when it is full the sac contracts and the water is expelled. (If it fails the paramecium will die.) Multi-celled organisms: Solute Pumps: Proteins in the cell membrane can pump solutes out of the cell, making the concentrations of water more equal. Plant Cells Hypotonic Environments: Turgor Pressure As the plant cell swells, the pressure of the water is not enough to break the cell wall. This gives soft plants rigidity. Hypertonic Environments: Plasmolysis As plant cells lose water, the cell membrane pulls away from the cell wall and turgor pressure is lost and the plant wilts. No Defenses Some cells have no defense against osmosis. Example: Red Blood Cells - hypotonic Red Blood Cells - hypertonic Review •Watch this video again. •As a group discuss the answers to these questions: • • • What organism is this? What kind of environment do you think it’s in? What is happening? Review the Objectives 1.Compare different models to explain the movement of materials into and out of the cell and the role of the solution the cells exist within. • • • Diffusion Facilitated diffusion osmosis 2.Recognize the importance of homeostasis as a survival mechanism.