* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
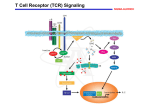
Download The Glial Cell–Derived Neurotrophic Factor Signaling Pathway
Survey
Document related concepts
Cytokinesis wikipedia , lookup
NMDA receptor wikipedia , lookup
Cell growth wikipedia , lookup
Nerve growth factor wikipedia , lookup
Phosphorylation wikipedia , lookup
Purinergic signalling wikipedia , lookup
Cellular differentiation wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Programmed cell death wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Tyrosine kinase wikipedia , lookup
VLDL receptor wikipedia , lookup
Biochemical cascade wikipedia , lookup
Transcript
Part F. The Glial Cell–Derived Neurotrophic Factor Signaling Pathway 35 Signaling Pathways of Glial Cell–Derived Neurotrophic Factor LOUIS F. REICHARDT G lial cell–derived neurotrophic factor (GDNF), a distant relative of transforming growth factor- (TGF-), was purified as a factor that promotes the survival and differentiation of embryonic mesencephalic dopaminergic neurons (Lin et al., 1993). A closely related protein, neurturin, was purified as a factor that promotes the survival of sympathetic neurons (Kotzbauer et al., 1996). Subsequently, two additional members of the GDNF family of ligands—artemin and persephin—were identified in EST databases (Baloh et al., 1998; Milbrandt et al., 1998; Masure et al., 1999). Each of these proteins has been shown to function as a survival and differentiation factor for subpopulations of cultured neurons (reviewed in Airaksinen et al., 1999; Baloh et al., 2000a; Bennett, 2001; Butte, 2001; Manie et al., 2001; Takahashi, 2001). The search for GDNF receptors was initially frustrating and led to the characterization of a glycosylphosphatidylinositol (GPI)-anchored binding protein now named GFR1 that seemed unlikely to function as a single subunit receptor for this neurotrophic factor (Treanor et al., 1996). But when mice lacking GDNF were examined (Moore et al., 1996; Pichel et al., 1996), they proved to have an almost identical phenotype to mice that lacked the orphan receptor tyrosine kinase c-ret (Schuchardt et al., 1994). In each instance, severe deficits were seen in development of the metanephric kidney and the enteric nervous system, suggesting that they act through the same signaling path- 410 way. Indeed, it was rapidly demonstrated that GFR1 is the major ligand-binding subunit in a signaling complex that includes c-ret (e.g. Durbec et al., 1996a; Jing et al., 1996). More recent work has demonstrated the existence of four members of the GFR family (GFR1–4) that, after ligand engagement, activate the c-ret tyrosine kinase (Airaksinen et al., 1999; Klein et al., 1997; Enokido et al., 1998; Lindahl et al., 2001) with GDNF, neurturin, artemin, and persephin functioning as reasonably specific ligands for GFR1, GFR2, GFR3, and GFR4, respectively. In prior work, inactivating mutations of cret had been shown to be responsible for many cases of Hirschsprung’s disease, which is caused by a failure of enteric neuron precursors to populate normally the intestine (Edery et al., 1994). In addition, mutations or gene fusions that activate the tyrosine kinase activity of cret had been shown to be oncogenic, resulting in multiple endocrine neoplasia and medullary thyroid carcinoma (Goodfellow, 1994). Consequently, the signaling pathways activated by GDNF have been of keen interest to both developmental biologists and oncologists. LIGAND–RECEPTOR INTERACTIONS The members of the GDNF family are secreted disulfide-bonded homodimers that are distant relatives of TGF- (Lin et al., 1993; Kotzbauer et al., 1996; Baloh et al., 1998; Milbrandt et al., 1998; Ma- Signaling Pathways of Glial Cell–Derived Neurotrophic Factor Fig1 sure et al., 1999; Rosenblad et al., 2000). The three-dimensional structure of GDNF has been determined at high resolution (Eigenbrot and Gerber, 1997). This structural information has made it possible to identify the surface residues in GDNF that are responsible for mediating interactions with GFR1. The structural information has guided experiments in which domains are swapped between GDNF family members. Individual amino acids within domains involved in receptor interactions have been mutated to confirm their functions (Baloh et al., 2000b; Eketjall et al., 1999). The GFR family (GFR1–4) consists of four glycosylphosphatidylinositol (GPI)-anchored membrane proteins that bind preferentially to GDNF, neurturin, artemin, and persephin, respectively (Airaksinen et al., 1999; Scott and Ibanez, 2001). Although each member of this family has a single preferred ligand, several have been shown to bind to additional members of the GFR family with lower affinity. While association of a GFR receptor with c-ret is promoted by ligand engagement (Treanor et al., 1996), in the absence of ligand some of the GFR receptors also interact with c-ret with lower affinities (Sanicola et al., 1997; Eketjall et al., 1999). Association between a GFR subunit and c-ret has been shown to broaden the ligand-binding ability of the complex (Sanicola et al., 1997). For example, some mutants of GDNF are defective in binding to GFR1 and are able to bind and activate signaling in cells that express both GFR1 and cret (Eketjall et al., 1999). When first isolated, clones of GFR1 were shown to encode a 461 amino acid long protein that includes a cleavable signal sequence. The presence of a C-terminal hydrophobic sequence without any C-terminal hydrophilic region, preceded by a group of three small amino acids, defined a cleavage and recognition site for possible addition of a glycosylphosphatidylinositol (GPI)-linked membrane anchor (Jing et al., 1996; Treanor et al., 1996). GFR1 was shown to be associated with the membrane from which it was released by phosphatidylinositolspecific phospholipase C. The presence of GFR1 was essential for autophosphorylation of c-ret by GDNF. Interestingly, both soluble and membrane-attached GFR1 were effective in mediating GDNF-promoted c-ret autophosphorylation, suggesting that GFR receptors can act in trans. Each of the other members of the GFR family has subsequently been shown to have similar properties (Buj-Bello et al., 1997; Thompson et al., 1998). In addition, transcription of the mouse GFR4 gene generates interesting developmentally regulated and tissue-specific splice variants, several of which are predicted to result in expression of secreted soluble proteins and one of which is predicted to generate a trans-membrane isoform (Lindahl et al., 2000). Splice variants predicted to result in expression of both GPI-attached and secreted receptors have also been reported in rat and human tissues (Masure et al., 2000; Lindahl et al., 2001). The functional importance of these variants has not yet been investigated. In initial characterizations, the extracellular domains of GFR subunits were observed to be cysteine-rich. In subsequent analyses, three domains in the extracellular portions of the GFR receptors have been defined (Lindahl et al., 2000; Scott and Ibanez, 2001). The central domain, which is the most conserved, has been shown to determine ligand-binding specificity for GDNF and for neurturin. Because there are different crucial ligand-binding determinants for these two trophic factors, it was possible to construct a GFR1–GFR2 chimeric receptor that binds both ligands with similar efficiency (Scott and Ibanez, 2001). Although GFR1–3 in mammals and GFR1–4 in chick share these three domains, the mouse, rat, and human GFR4 receptors lack the most N-terminal cysteine-rich domain (Lindahl et al., 2000, 2001; Masure et al., 2000; Zhou et al., 2001). None of the splice variants of GFR4 alter the central domain. RET was originally identified as an oncogene activated by fusion of a tyrosine kinase domain to other proteins (Takahashi et al., 1985). When first sequenced, the protooncogene c-ret appeared to be a receptor tyrosine kinase that contained a cleavable signal sequence, an approximately 600 amino acid extracellular domain, a transmembrane domain, and a cytoplasmic domain with a tyrosine kinase plus several tyrosines that could potentially serve after phosphorylation as recruitment sites for signaling proteins (Takahashi et al., 1988, 1989) (Fig. 35–1). C-ret was unusual because it lacked the immunoglobu- 411 lin, leucine-rich repeats and the fibronectin-type III repeats that are present in the extracellular domains of other members of the receptor tyrosine kinase family (Schneider, 1992; Iwamoto et al., 1993). In addition, differential splicing 3 of exon 19 generates short (9 a.a.), medium (43 a.a.), and long (51 a.a.) isoforms of the cytoplasmic domain, all of which contain a functional tyrosine kinase but differ in their docking sequences for adapter proteins (Tahira et al., 1990; Lorenzo et al., 1995, 1997). Of these, only the short and long isoforms are expressed at significant levels in vivo. Initial analyses indicated that the approximately 600 amino acid long extracellular domain lacked homology to known proteins. Subsequent analysis, though, indicated the presence of conserved repeats homologous in position and sequence to the Ca2-binding domains of cadherins (Schneider, 1992; Hill et al., 2001). Recent molecular modeling of the extracellular domain of c-ret has confirmed the presence of four cadherin-like domains, followed by a cysteine-rich, membraneproximal domain that is similar to the membrane-proximal domain of the cadherin-related protein Flamingo (Anders et al., 2001). Similar to cadherins, c-ret binds Ca2, and the presence of Ca2 is essential for crosslinking of 125I-GDNF to c-ret, but not to GFR1, on cells that express both receptor subunits (Anders et al., 2001). This suggested that Ca2 stabilizes the structure of the c-ret extracellular domain. The presence of Ca2 is also essential for surface expression of c-ret, suggesting that Ca2 is essential for stabilization of c-ret inside the endoplasmic reticulum (van Weering et al., 1998). Since GFR and GDNF homologs have not been identified in the Drosophila genome, c-ret may have evolved from a cell adhesion molecule. Although GDNF family ligands provide the most direct pathway for activation through GFR subunits of c-ret, an alternative pathway has recently been described in neurons that coexpress trkA, a receptor tyrosine kinase that is activated by nerve growth factor (NGF) (Tsui-Pierchala et al., 2002). Mature sympathetic neurons have lost their dependence on NGF for survival, but they continue to require it for process outgrowth and differentiation. NGF-mediated activation of TrkA results in delayed phosphorylation of c-ret in mature sympathetic neurons both in vitro and in vivo (Tsui-Pierchala et al., 2002). Only the long isoform Ret 51, not the short isoform Ret 9, is phosphorylated in response to NGF. Optimal responses to NGF in vitro by these neurons depends on c-ret, but not on the GFR subunit function. Thus, in some neurons NGF transactivates the long isoform of c-ret, which, in turn, facilitates the normal maturation of these cells. The mechanism by which this occurs is not known, but the slow kinetics of c-ret phosphorylation suggest that trkA does not directly phosphorylate c-ret. SIGNALING ACTIVATED BY c-ret Because of its important roles in normal development and in oncogenesis, signaling pathways regulated by c-ret and oncogenic ret variants have been intensely studied and are frequently reviewed (van Weering and Bos, 1998; Mason, 2000; Saarma, 2000; Manie et al., 2001; Takahashi, 2001). Ligand engagement of a GFR receptor in cells that also express c-ret results in dimerization and activation of the c-ret tyrosine kinase activity. Immediate substrates of this kinase include six tyrosines in the c-ret cytoplasmic domain (Liu et al., 1996) (Fig. 35–1). Of these, four tyrosines (Y-905, Y-1015, Y-1062, Y-1096) have been shown to provide docking sites for cytoplasmic signaling proteins (Hayashi et al., 2000; Coulpier et al., 2002). Phosphorylated Y905 has been shown to provide a docking site for Grb-7 and Grb-10 (Pandey et al., 1995, 1996). It is not certain what roles the Grb-7/10/14 family of adaptor proteins play in c-ret-mediated signaling, but these proteins have been shown to regulate cell migration and survival (Han et al., 2001). Recent work, for example, indicates that Grb-10 functions as a coactivator of Akt (Jahn et al., 2002). Y-1096 is present in the long, but not in the short or middle, isoforms of c-ret and has been shown to provide a docking site for the adaptor Grb-2 (Liu et al., 1996). Recruitment of Grb-2 provides a potential mechanism for activation of the PI-3 kinase–Akt and Ras–Map kinase pathways. Phosphorylated Y-1015 mediates docking of PLC-1, which is activated by phosphorylation and then acts to hydrolyze phosphatidy- 412 DEFINED PATHWAYS Figure 35–1. Interactions of GDNF with GFR1 and c-ret. Depicted on the left is GDNF binding to GFR1 that is preferentially localized in cholesterol and sphingolipid-rich lipid rafts. GDNF binding to GFR1 results in activation of src-family tyrosine kinases that are also present in lipid rafts. Downstream signaling events (not shown) include activation of MAP kinase, CREB, and phospholipase-C. Shown also in the left panel is c-ret attached to two proteins—ACAP and ENIGMA—that associate with this receptor irrespective of its activation state. ACAP provides a docking site for protein kinase A that has been shown to phosphorylate S-696, thereby regulating signaling through Y687. Depicted on the right is the recruitment by the GDNF–GFR1 complex of c-ret to the lipid raft. Recruitment of c-ret results in activation of its tyrosine kinase and phosphorylation of several tyrosines in its cytoplasmic domain. Phosphorylation of Y-905 results in recruitment of two adapters Grb-7 and Grb-10 that activate poorly characterized downstream signaling pathways. Phosphorylation of Y-1015 permits recruitment and activation of phospholipase-C1. Activation of PLC results in increased cytoplasmic Ca2 and activation of Ca2- and diacylglycerol-regulated protein kinases. Phosphorylation of Y-1062 results in recruitment of several different adaptors, including IRS1, FRS2, Shc, Dok-4, and Dok-5. These adaptors have been shown to activate several intracellular signaling pathways, including cascades controlled by Ras, Erk-1/2, Erk-5, PI-3 kinase, and rac (see text for details). Phosphorylation of Y-1096 permits recruitment of Grb-2 and results in activation of ras through SOS. Localization of c-ret in lipid rafts has been shown to promote the recruitment of FRS2. Localization of c-ret to rafts is essential for normal activation of several of the signaling pathways summarized above and described in more detail in the text. linositides to generate inositol tris-phosphate and diacylglycerol. Inositol tris-phosphate induces release of Ca2 stores, increasing levels of cytoplasmic Ca2. This results in activation of the various enzymes that are regulated by cytoplasmic Ca2, including Ca2-calmodulinregulated protein kinases and phosphatases and Ca2-regulated isoforms of protein kinase C. Formation of diacylglycerol (DAG) stimulates the activities of DAG-regulated isoforms of protein kinase C. One of these DAG-regulated isoforms, PKC, is required for activation of the mitogen activated protein kinase (ERK) cascade and ERKdependent differentiation in many neuronal cells (Corbit et al., 1999, 2000). It appears to act between Raf and MEK in the ERK signaling cascade. Activation of PLC-1 through Y-1015 is essential to observe the full oncogenic and proliferative activities of ret (Borrello et al., 1996). By far the most important mediator of signaling by c-ret appears to be Y-1062 (Fig. 35–1). Interestingly, while Y-1062 is present in the long, medium, and short isoforms of c-ret, splicing introduces different sequences immediately 3 of the codon for Y-1062, so the amino acid sequence immediately C-terminal of Y-1062 differs between the RET 9, RET 43, and RET 51 isoforms, thus providing the potential for differential recognition of signaling proteins at this site (Tahira et al., 1990; Myers et al., 1995; Lorenzo et al., 1997). The presence of Y-1062 is required for the oncogenic and proliferative activities of cret isoforms (Melillo et al., 2001b). The signaling pathways activated by phosphorylation of this site are extensive and include the Ras, PI3 kinase, Jun kinase, p38MAP kinase, Erk-1/2 and Erk-5 kinases, and Rac (Hayashi et al., 2001; Fukuda et al., 2002). Depending on cell type, phosphorylation of this site promotes cell survival, proliferation, differentiation, motility, or oncogenesis. When phosphorylated, this site has been shown to recruit several different adaptor proteins, including IRS1, FRS2, Shc, Dok-4 and Dok-5 (Arighi et al., 1997; Hayashi et al., 2000; Grimm et al., 2001; Melillo et al., 2001a, 2001b). Shc recruitment provides pathways through Grb-2 and the Ras exchange factor SOS (son of sevenless) for activation of Ras. Activation of Ras by SOS has many downstream consequences, including stimulation of PI-3 kinase, activation of the c-raf/ERK pathway, and stimulation of the p38MAP kinase pathway (Xing et al., 1998). Alternatively, Shc recruitment of Grb-2 has also been shown to promote association of the adapter Gab-1, which, in turn, binds and facilitates activation through phosphorylation of PI-3 kinase and the downstream signaling pathways. Recruited IRS1 has been shown to associate directly with, thereby facilitating, activation of PI-3 kinase Signaling Pathways of Glial Cell–Derived Neurotrophic Factor (Melillo et al., 2001a). FRS2 recruitment and phosphorylation provides multiple docking sites for the Grb-2–SOS complex, thereby promoting activation of RAS, the ERKs, and PI-3 kinase (Melillo et al., 2001b). FRS2 also provides a site for recruitment of Crk that, in turn, recruits the exchange factor C3G, thereby activating the small G protein Rap1 (York et al., 2000). This provides a mechanism for activation of B-raf and the ERKs that does not depend on Ras. Because Rap1 is localized to endosomes, efficient activation of this pathway appears to require endocytosis of ligand–receptor complexes (York et al., 2000; Howe et al., 2001). In PC-12 cells, FRS2-mediated signaling has been shown to be essential for the prolonged activation of the ERK cascade that is required to promote differentiation of these cells (Grewal et al., 1999; York et al., 2000). FRS2 also provides a docking site for the tyrosine phosphatase Shp2 (Hadari et al., 1998). Shp2 has been shown to enhance activation of the Ras-Raf-MEK-ERK pathway by a mechanism that is not clear. Gab-1 also has been shown to nucleate formation of a complex that includes Shp2 (Shi et al., 2000). P62dok is an adaptor protein that has been shown to associate with several receptor tyrosine kinases. P62dok also associates with RasGAP and has been shown to inhibit ERK kinase activation. There are at least five dok homologs that associate with c-ret in a two-hybrid assay (Grimm et al., 2001). Two of these, dok-4 and dok-5, are novel. Association of these adaptors with c-ret depends on phosphorylation of Y-1062. In contrast to p62dok, these two adaptors do not interact with RasGAP, and overexpression enhances rather than inhibits ERK activation. Ligand engagement of c-ret in PC-12 cells results in neurite outgrowth that requires Y-1062. Fusion of sequences from either dok-4 or dok-5, but not dok-2, to a mutated c-ret lacking Y-1062 also permits GDNF-dependent neurite outgrowth by PC-12 cells (Grimm et al., 2001). The same fusions also are able to mediate GDNF-dependent survival of these cells in a serum-free medium. Thus these two adaptors are likely to be important in mediating neuronal responses to GDNF and its family members. The differentiation-promoting responses observed in PC-12 cells and neurons differ fundamentally from the proliferative responses observed in other cells. Clearly, responses to c-ret activation are celltype specific, depending on the repertories of the intracellular signaling proteins that are present in different cell types. Responses of different cell types to neurotrophins also depend on cell type, with some cells showing proliferative and others differentiative responses (Huang and Reichardt, 2001). Among other targets of probable importance for promoting differentiation, activation of c-ret has been shown to up-regulate the cyclin-dependent kinase inhibitor p27kip1 (Baldassarre et al., 2002). In addition to classical adaptors, the Y-1062 site is also essential for recruiting a PDZ protein named ENIGMA (Durick et al., 1996, 1998). ENIGMA is an interesting protein that consists of an N-terminal PDZ domain plus three Lim domains, one of which associates specifically with c-ret in an interaction that depends on Y-1062. This interaction does not depend on phosphorylation of this tyrosine, however. While the function of ENIGMA in c-ret signaling is uncertain, it is essential for the mitogenic activity of a cAMP-dependent kinase RI–c-ret fusion protein named Ret/Ptc2 that causes papillary thyroid carcinoma (Durick et al., 1998). ENIGMA is believed to promote mitogenesis and oncogenesis by localizing Ret/Ptc2 to the membrane through its PDZ domain. Since Y-1062 is essential for recruitment of adaptors, such as Shc, that are essential for the mitogenic and oncogenic activities of Ret/Ptc2, dimerization is essential not only to activate the tyrosine kinase activity but also to permit simultaneous localization of Ret/Ptc2 to the membrane through ENIGMA and recruitment of these adaptors. Because Y-1062 recruits so many different adaptors, which, in turn, nucleate formation of different signaling complexes that affect cell function, it will be a major challenge for future scientists to understand the factors that bias c-ret-mediated signaling responses into specific pathways, such as proliferation versus differentiation. The protein kinase A–anchoring protein ACAP-79 also associated with c-ret in a ligand-independent manner (Fukuda et al., 2002). ACAP-79 provides a high-affinity docking site for the type II regula- 413 tory subunit of cAMP-dependent protein kinase. In response to cAMP, S-696 in the cytoplasmic domain of c-ret is phosphorylated, and phosphorylation at this site promotes a guanine nucleotide exchange factor activity for Rac-1 and promotes lamellipodia formation in the presence of GDNF. Mutation of S-696 or Y-1062 decreases Rac-1 guanine nucleotide exchange factor activity, but mutation of Y-687 rescues the phenotype of the S-696 mutation. These data suggest that guanine nucleotide exchange factor activity is promoted by signals depend on phosphorylation of Y-1062, but is inhibited by a signal that emanates from phosphorylated Y-687. cAMP-dependent phosphorylation of S696 prevents signaling from phosphorylated Y-687. These data illuminate a novel mechanism through which cAMP regulates a tyrosine kinase–mediated signaling pathway. SIGNALING THROUGH GFR SUBUNITS GFR subunits function in large part as binding partners for GDNF family ligands. Initially, GFR subunits were believed to only function as binding subunits of the c-ret receptor complex. However, recent work has demonstrated that these subunits have broader and more direct roles in mediating signaling by GDNF (Saarma, 2001). Among other functions, the presence of a GFR subunit appears to inhibit basal activation of the c-ret kinase in the absence of physiological ligands (Trupp et al., 1998). Since inhibition has only been observed in COS cells in which c-ret was overexpressed alone or in combination with a GFR subunit, the physiological significance of this observation is uncertain. Similar to other GPI-anchored membrane proteins, GFR subunits are preferentially localized to cholesterol and sphingolipid-rich lipid rafts (Poteryaev et al., 1999; Trupp et al., 1999), where src-family kinases are also concentrated (Lee et al., 2001). GDNF application to cells expressing GFR1, but not c-ret, has been shown to activate src kinase (Trupp et al., 1999). In turn, GFR1-mediated src kinase promotes phosphorylation of MAP kinase, CREB, and phospholipaseC1 (Trupp et al., 1998; Poteryaev et al., 1999). Activation of the pathways through GFR1 alone is much less prolonged than activation through c-ret (Trupp et al., 1998), but since many cells express GFR subunits that do not coexpress c-ret, this signaling pathway probably has physiological consequences. A recent report suggests that ligand engagement of GFR2 does not activate the signaling pathways that are activated by ligand engagement of GFR1 in the absence of c-ret (Pezeshki et al., 2001), so it is not certain that c-ret-independent signaling is promoted by all four of the GFR subunits. One of the most important functions of ligand-bound GFR subunits now appears to be recruitment of c-ret to lipid rafts, where it acquires new signaling properties. Disruption of the specialized lipid raft domains by cholesterol depletion has been shown to severely attenuate c-ret-dependent GDNF signaling (Tansey et al., 2000). GDNF binding to the GFR1 subunit results in relocalization of c-ret from the bulk membrane to the detergent-resistant lipid raft domain (Tansey et al., 2000). In this specialized domain, c-ret interacts with signaling proteins that it does not associate with outside of the rafts. For example, c-ret outside of rafts is found in association with Shc, but not FRS2, while the c-ret in rafts is preferentially associated with FRS2, but not Shc (Paratcha et al., 2001). In addition, raft-associated c-ret interacts with and activates c-src, but not other src-related tyrosine kinases. Despite the theoretical existence of other pathways to PI-3 kinase through Y-1062, c-src is essential in neurons for activation of the PI-3 kinase–Akt pathway and GDNF-dependent survival and neurite outgrowth (Tansey et al., 2000; Encinas et al., 2001). Intriguingly, both soluble and membrane-tethered GFR subunits are able to recruit c-ret to lipid rafts and potentiate downstream signaling by GDNF (Paratcha et al., 2001). The mechanism by which ligand-bound GFR in trans recruits c-ret to rafts is not certain, but it must differ from the mechanism through which ligand-bound, membrane-tethered GFR subunits recruit c-ret in cis, because trans recruitment requires an active c-ret kinase, while cis-recruitment does not. Since GFR subunits have been shown to be released from cells that do not express c-ret (Trupp et al., 1997; Yu et al., 1998; Worley et al., 2000; Paratcha et 414 DEFINED PATHWAYS al., 2001), the ability of a ligand-bound GFR subunit to promote activation and localization of c-ret to lipid rafts is very likely to be biologically important. FUNCTIONS OF GDNF FAMILY-MEDIATED SIGNALING PATHWAYS GDNF is strongly expressed in the developing gut (Moore et al., 1996). In GDNF, GFR1, and c-ret mutants, enteric neurons fail to populate the intestine, and only a few of these neurons are present in the stomach and esophagus (Schuchardt et al., 1994; Durbec et al., 1996b; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Cacalano et al., 1998; Enomoto et al., 1998). The phenotypes of these mice are similar to, but more severe than, those of humans with Hirschsprung’s disease in which deficits in enteric neuron populations in the lower intestine result in intestinal obstruction (Edery et al., 1997; Gabriel et al., 2002). Indeed, Hirschsprung’s disease is often associated with heterozygous mutations that result in deficits in signaling through c-ret or components of the c-ret signaling pathway (Edery et al., 1994; Romeo et al., 1994; Amiel et al., 1996; Attie et al., 1996; Kusafuka and Puri, 1998; Parisi and Kapur, 2000; Gabriel et al., 2002). Mice heterozygous for GDNF have been shown to have frequent obstructions of the lower intestine that result in very high levels of postnatal lethality (Shen et al., 2002), and some humans with Hirschsprung’s disease also have mutations in GDNF (Eketjall and Ibanez, 2002). The GDNF to c-ret signaling pathway appears to act at many steps in differentiation of the enteric nervous system (Gershon, 1998; Pachnis et al., 1998). A pool of neural crest cells from the hindbrain gives rise to most of the enteric nervous system. In the absence of GDNF signaling, there are deficits in the initial appearance of these cells in the gut anlage, reflecting defects in migration, as well as increases in apoptosis of this pool (Durbec et al., 1996b; Taraviras et al., 1999). In culture and in intestinal explants, GDNF and neurturin have been shown to promote survival, proliferation, and differentiation of enteric neuron precursors (Hearn et al., 1998; Heuckeroth et al., 1998; Taraviras et al., 1999). Localized extrinsic sources of GDNF also attract these cells in what is most likely a chemotropic response (Young et al., 2001). Activation of the PI-3/Akt pathway has been shown to be essential for the proliferative response of quail enteric precursors (Focke et al., 2001). In transplantation experiments it has been possible to show that c-ret-expressing neural precursors can invade the gut and populate it efficiently from a c-ret mutant embryo, demonstrating that the GDNF to c-ret pathway is required in the neural crest–derived progenitor cells and not in the nonneural cells of the developing gut (Natarajan et al., 1999). Intriguingly, with development, enteric precursors shift from a proliferative to a trophic and differentiative response to GDNF (Worley et al., 2000). At later stages of development, c-ret expression is restricted to a population of enteric precursors that is committed to differentiate into neurons (Lo and Anderson, 1995). Interestingly, the short and long isoforms of c-ret differ in their abilities to support development of the enteric nervous system (de Graaff et al., 2001). By targeted exon deletion, mice have been generated that only express the short or long isoform. Animals that express RET-51, but not RET-9, lack enteric neurons in the colon, while animals that only express RET-9 appear completely normal. During development, enteric neural precursors expressing only RET-51 never populate the distal gut. This is not simply because they migrate more slowly than normal precursors. The same deficit is observed in organ cultures of the embryonic gut in which the overall growth and elongation of the gut is quite limited. Consequently, the observations suggest that there are differences in the signaling required to permit invasion of the proximal and distal segments of the gut. Absence of signaling by GDNF family members also results in deficits in additional neuronal populations, including sensory, motor, and autonomic populations (Moore et al., 1996; Sanchez et al., 1996; Heuckeroth et al., 1999; Nishino et al., 1999; Rossi et al., 1999, 2000; Enomoto et al., 2000, 2001; Laurikainen et al., 2000; Hashino et al., 2001). Analyses of targeted mutants have demonstrated survival requirements for c-ret, two of the GDNF family members, and three of the GFR subunits. GDNF family members can be transported retrogradely from targets (Leitner et al., 1999) and can function as targetderived trophic factors, similar to nerve growth factor (Oppenheim et al., 1995; Hashino et al., 2001). In addition, c-ret signaling has been shown to be required for migration of neural precursors of sympathetic neurons (Enomoto et al., 2001). As a result, the superior cervical ganglion is misplaced in both the GFR3 and c-ret mutants (Nishino et al., 1999; Enomoto et al., 2001). Deficits in neuronal number and delays in neuronal differentiation are seen throughout the sympathetic chain in the c-ret mutant (Enomoto et al., 2001). In addition, sympathetic axons are misrouted, almost certainly because the axons fail to respond to artemin. Artemin is expressed in the vasculature that provides an initial guidance cue for these axons and has been shown to be chemotropic for these axons in vitro. In both wild-type and mutant embryos, almost all cell death in sympathetic ganglia occurs after down-regulation of c-ret expression. In the mutant, there is a similar elevation in cell death in both c-ret-expressing and non-expressing neurons. As a result, the elevated cell death observed in the mutant has been attributed to failure of neurons to contact sources of other trophic factors. It does not appear to be caused by direct dependence on artemin or other members of the GDNF family. Both GDNF and neurturin are required for normal development of several parasympathetic ganglia, with GDNF required early and neurturin later in development (Enomoto et al., 2000; Rossi et al., 2000; Hashino et al., 2001). The sequential requirement for GDNF and neurturin correlates with changes in their expression in target tissues and with changes in expression of the GFR1 and GFR2 receptors in the neurons that innervate these tissues (Rossi et al., 2000; Hashino et al., 2001). In addition to these striking effects on development of sympathetic and parasympathetic neurons, GDNF mutants have been shown to have deficits in both neural crest–derived and placode-derived sensory ganglia (Moore et al., 1996; Sanchez et al., 1996). It is not certain whether these deficits reflect a requirement for trophic factor support by either neural precursors or mature neurons, or both. GDNF also regulates the survival of motor and autonomic neurons in the spinal cord. In the GDNF mutant, deficits were seen in some, but not all, cranial motor nuclei and in motor neurons in the spinal cord (Moore et al., 1996). Because there are distinct profiles of GFR subunit expression in various motor neuron populations, it has seemed attractive to imagine that different muscles express different GDNF family members that provide trophic support for specific populations of motor neurons (Mikaels et al., 2000). Absence of GDNF or GFR1 has been shown to result in selective loss of defined subpopulations of motor neurons that express GFR1 and c-ret (Garces et al., 2000; Oppenheim et al., 2000). Interestingly, no deficits were observed in GFR2 mutants (Garces et al., 2000). In zebrafish, GFR1 is expressed in a specific primary motor neuron, CaP, while GDNF is expressed specifically in the muscle that it innervates (Shepherd et al., 2001). Ectopic expression of GDNF results in perturbations in growth by the CaP axon. Depletion of GDNF with morpholino antisense oligos did not, though, result in death or abnormal axon growth by the CaP neuron. GDNF also has been shown to prevent the death of preganglionic neurons in the intermediolateral column of the spinal cord after the removal of their target, the adrenal gland (Schober et al., 1999). Since GDNF is expressed in adrenal chromaffin cells, it is almost certainly functioning as a target-derived trophic factor. GDNF-dependent survival of chick motor neurons depends on signaling through the PI-3 kinase–Akt pathway, similar to survival of other neurons promoted by neurotrophins, such as nerve growth factor (NGF) or brain derived growth factor (BDNF) (Soler et al., 1999; Huang and Reichardt, 2001). There are differences in the essential survival pathways activated by GDNF and neurotrophins, however. In contrast to BDNF, GDNF has been shown to elevate levels of X-linked inhibitor of apoptosis (XIAP) and neuronal inhibitor of apoptosis (NAIP) proteins in motor neurons after sciatic nerve axotomy (Perrelet et al., 2002). Inhibition of NAIP or XIAP activity prevents GDNF-, but not BDNF-mediated neuroprotective effects. The GDNF family of ligands has numerous additional effects that Signaling Pathways of Glial Cell–Derived Neurotrophic Factor are of functional importance in the mature nervous system. They have been shown to protect neuronal subpopulations from excitotoxicity (Pérez-Navarro et al., 1999; Bonde et al., 2000; Gratacos et al., 2001; Marco et al., 2002). As described, they are also neuroprotective after axotomy (Perrelet et al., 2002). In cell culture they have been shown to promote synapse formation (Wang et al., 2002). In vivo, overexpression of GDNF in muscle increases the number of fibers innervated by single motor neurons and promotes constant remodeling of neuromuscular junctions (Keller-Peck et al., 2001). Similar to neurotrophins, GDNF and its relatives have been shown to regulate expression of a variety of ion channels and receptors (Bradbury et al., 1998; Fjell et al., 1999; Brene et al., 2000; Cummins et al., 2000; Boettger et al., 2002). In some instances, GDNF appears to regulate the same channels as neurotrophins, but there are also numerous examples of differential regulation. Of potential clinical importance, GDNF has been shown to be analgesic in a model of neuropathic pain and is believed to act by regulating expression of Na channels in nociceptive sensory neurons (Boucher et al., 2000). In addition to chronic effects, which are mediated largely through gene expression, GDNF has been shown to acutely regulate the activity of channels in subpopulations of neurons. For example, GDNF has been shown to potentiate the excitability of cultured midbrain dopaminergic neurons through reversible inhibition of transient A-type K channels (Yang et al., 2001). GDNF activates the MAP kinase cascade in these neurons, and MAP kinase has been shown to directly phosphorylate these channels. Inhibition of the MAP kinase cascade prevents the effect of GDNF, so it is likely that phosphorylation by MAP kinase directly regulates the activity of these channels. Similar to BDNF, GDNF has been shown to enhance Ca2 entry and potentiate release of acetylcholine in Xenopus spinal cord–skeletal muscle co-cultures (Wang et al., 2001). Long-term exposure to GDNF increases expression of a Ca2-binding protein, frequenin. Interestingly, inhibition of frequenin expression with anti-sense oligos or of its function with a specific antibody prevents the effects of GDNF on Ca2 entry. Overexpression of frequenin results in a similar phenotype to that observed following GDNF application, and the response to each occludes the response to the other. Both GDNF and frequenin have been shown to increase the opening probability of N-type, but not L-type, Ca2 channels. Thus GDNF acts through frequenin to regulate Ca2 channel function by a mechanism that is not yet understood. Signaling mediated by GDNF family members also is important for development outside of the nervous system. In particular, GDNF signaling through GFR1 and c-ret is essential for normal development of the metanephric kidney where GDNF is expressed at high levels in the metanephric mesenchyme while GFR1 and c-ret are expressed in the ureteric bud (Pachnis et al., 1993; Hellmich et al., 1996; Kreidberg, 1996; Moore et al., 1996). In the absence of any of these three proteins, the ureteric bud fails to invade and branch within the metanephric mesenchyme, so mature kidneys are absent from the vast majority of animals (Schuchardt et al., 1994, 1996; Moore et al., 1996; Pichel et al., 1996; Sanchez et al., 1996; Cacalano et al., 1998; Enomoto et al., 1998; Tomac et al., 2000). Even mice with only one copy of the GDNF gene have frequent unilateral kidney agenesis, and the average size of their kidneys is reduced by 25% with fewer nephrons and a reduced ureteric duct volume (Moore et al., 1996; Cullen-McEwen et al., 2001). Intriguingly, despite the similarities in inductive interactions in formation of the pro, meso, and metanephric kidneys, in mice GDNF/GFR1/c-ret signaling is essential only for development of the metanephros. Consistent with this, no deficit in pronephric kidney development is observed following GDNF depletion in zebrafish using anti-sense morpholino-oligonucleotides (Shepherd et al., 2001). In contrast, GDNF signaling through GFR1 has been shown to be essential for normal migration of pronephric duct cell precursors in axolotl (Drawbridge et al., 2000). The observations in axolotl suggest that GDNF may contribute to pronephric development in other species, but that other factors may compensate when it is absent or present at abnormally low concentrations. In rodents, GDNF signaling appears to regulate initial formation of the ureteric bud because application of GDNF is capable of inducing 415 bud formation from other regions of the wolffian duct (Sainio et al., 1997). GDNF signaling through GFR1 and c-ret, however, is important throughout kidney development, not just during initial invasion of the ureteric bud. In organ culture, interference with GDNF signaling inhibits branching of the ureteric bud, while application of exogenous GDNF strongly promotes ureteric bud growth and branching (Vega et al., 1996; Pepicelli et al., 1997; Ehrenfels et al., 1999). Interestingly, the short and long isoforms of c-ret also differ in their abilities to support kidney development (de Graaff et al., 2001). In the presence of only RET-51 the ureteric bud initiates invasion of the metanephric mesenchyme, but later steps in kidney development do not occur normally. In contrast, expression of RET-9 alone supports all steps in kidney development. In the c-ret mutant background, forced expression of ligand-dependent or constitutively active RET-9, but not RET-51, rescues kidney development. Thus there must be differences in the essential c-ret-mediated signaling pathways at various stages of kidney development. GDNF acts in part by stimulating and directing cell motility. GDNF has been shown to be chemotactic, attracting ureteric buds to localized sources of GDNF (Sainio et al., 1997). GDNF also stimulates the motility of kidney-derived epithelial cells (Tang et al., 1998). Some groups have observed that GDNF increases cell proliferation in the tips of the branches of the ureteric bud (Pepicelli et al., 1997). Activation of the PI-3 kinase pathway is required for the responses of both kidney-derived epithelial cells and ureteric buds to GDNF (Tang et al., 2002). Many of the growth factors and transcription factors that regulate kidney development act, at least partly, through regulation of GDNF or c-ret expression. Once the primary ureteric bud invades the metanephric mesenchyme, expression of c-ret is focused in the tips of the branches of the ureteric bud, while GDNF expression is induced in the mesenchyme surrounding these tips. Ectopic expression of cret throughout the ureteric bud–derived epithelia, for example, alters the normal pattern of kidney morphogenesis, possibly because inappropriately localized c-ret competes with bud-tip-localized c-ret for GDNF (Srinivas et al., 1999). Expression of GDNF or c-ret (or both) is strongly reduced in several mutants that prevent normal kidney development, such as Emx2 (Miyamoto et al., 1997), Eya1 (Xu et al., 1999), Pax2 (Brophy et al., 2001), double mutants of Hoxa11 and Hoxd11 (Patterson et al., 2001), and a double mutant of two retinoic acid receptors Rara and Rarb2 (Batourina et al., 2001). In the latter instance, c-ret expression in the ureteric bud has been shown to depend indirectly on vitamin A–mediated signaling through receptors localized in the metanephric stromal cells (Lelievre-Pegorier et al., 1998; Moreau et al., 1998; Mendelsohn et al., 1999; Batourina et al., 2001). Forced Hoxb7 promoter-driven expression of c-ret in the kidney anlage rescues kidney development in the absence of normal vitaminA–mediated signaling. Therefore, the reduction in c-ret expression clearly explains the deficits observed in vitamin A–deficient animals and in animals that lack the two retinoic acid receptors. In contrast, the domain of expression of GDNF in the intermediate mesenchyme is expanded anteriorly in double Foxc1 and Foxc2 mutants, resulting in frequent presence of duplex kidneys and double ureters (Kume et al., 2000). Expression of several genes believed to be important in kidney development, including Wnt-11, Wnt-4, and Ld (limb deformity), are down-regulated when GDNF to c-ret signaling in the kidney is inhibited through use of a Ret–Ig fusion protein (Pepicelli et al., 1997; Ehrenfels et al., 1999). By combined use of expression analysis and genetics, signaling pathways upstream and downstream of GDNF signaling are gradually being assembled. Signaling mediated by GDNF family members is also essential for normal differentiation of other tissues. For example, GDNF and its receptors are expressed in the anlage of teeth, and GDNF has recently been shown to be essential for their normal development (de Vicente et al., 2002). In its absence, ameloblasts and odontoblasts fail to develop fully, resulting in an absence of the enamel matrix and predentin layers. Expression of GDNF, neurturin, and their receptors in skin changes in correlation with different phases of hair follicle growth and regression (Botchkareva et al., 2000). Hair follicle regression is delayed by exogenous GDNF or neurturin and is accelerated in 416 DEFINED PATHWAYS GFR1/ and GFR2/ mice (Botchkareva et al., 2000), indicating that these factors control the phase shift from growth to regression. As a final example of special interest, GDNF is expressed in Sertoli’s cells in the testes, where it is induced by follicle-stimulating hormone (Meng et al., 2000; Tadokoro et al., 2002). GDNF promotes proliferation of undifferentiated spermatogonia and decreases their sensitivity to differentiation signals. As a result, the testes of mice with one copy of a GDNF null allele are depleted in stem cell reserves, while the testes of males in which GDNF is overexpressed accumulate undifferentiated spermatogonia (Meng et al., 2000). In older mice, this results in development of malignant tumors expressing germ cell markers (Meng et al., 2001). GDNF SIGNALING PATHWAYS AND HUMAN DISEASE The human genetics of the GDNF signaling pathway has provided interesting insights of general interest into the anatomy of receptor tyrosine kinase signaling. This has been possible because abnormalities in this signaling pathway are associated with Hirschsprung’s disease, which is caused by deficiencies in signaling through this pathway, and several forms of cancer, which are associated with excessive activation of this pathway (reviewed in Hansford and Mulligan, 2000; Jhiang, 2000; Manie et al., 2001; Takahashi, 2001; Gabriel et al., 2002; McCabe, 2002; Passarge, 2002). Hirschsprung’s disease is a human disorder caused by failure of enteric neurons to populate the caudal intestine. Long-segment and short-segment Hirschsprung’s disease are distinguished by the length of intestine not populated by enteric neurons (Gabriel et al., 2002). Four classes of mutations in c-ret have been identified that are associated with Hirschsprung’s disease. Each reduces or compromises signaling through this receptor tyrosine kinase. One class of mutations alters important amino acid residues in the extracellular domain of c-ret that reduce c-ret maturation and transport to the surface membrane (Iwashita et al., 1996; Ito et al., 1997; Takahashi et al., 1999; Manie et al., 2001). Molecular modeling of the cadherin domains indicates that each of the amino acids mutated in this class of Hirschsprung’s disease is important for stabilizing the structure of a domain, so these mutations almost certainly reduce maturation and surface expression through effects on protein folding (Anders et al., 2001). Thus there is comparatively little c-ret available to be activated by GDNF family members. Not surprisingly, the severity of Hirschsprung’s disease correlates inversely with the amount of mutant ret transport to the surface with the transport of mutants, resulting in long-segment disease more severely impaired than the transport of mutants and giving rise to shortsegment disease (Iwashita et al., 1996). A second class of mutants is located in the kinase domain of c-ret; they reduce or abolish c-ret’s tyrosine kinase activity (reviewed in Takahashi, 2001). These mutations occur in conserved amino acids shared with other tyrosine kinases. Finally, two classes of mutations affect signal transduction initiated by c-ret. Several have been shown to inhibit the activation of PLC1. Others affect amino acid residues close to the crucial Y-1062 site and have been shown to inhibit Shc, FRS2, and IRS1 binding to this site and to impair activation of the PI-3 kinase–Akt and Ras–Erk pathways (Geneste et al., 1999; Ishiguro et al., 1999; Melillo et al., 2001a, 2001b). It seems likely that they also affect binding by some of the other adaptor proteins known to recognize this site, such as Dok-4 and Dok-5. Thus, analysis of a genetically inherited human disease has resulted in dissection of the essential domains of a receptor tyrosine kinase. Finally, recent work indicates that overexpression of c-ret induces apoptosis in some cells, which is suppressed by the presence of GDNF (Bordeaux et al., 2000). Overexpression of several of the mutants associated with Hirschsprung’s disease also promotes apoptosis, but the apoptosis induced by these mutants cannot be suppressed by GDNF. C-ret-promoted apoptosis appears to involve cleavage of the cytoplasmic domain by a caspase-dependent mechanism. The molecular details of this signaling pathway are not yet understood. Mutations in other components of the GDNF signaling pathway may also contribute to appearance of a Hirschsprung’s disease phenotype (Gabriel et al., 2002). Several mutations in GDNF and neur- turin have been identified in patients with Hirschsprung’s disease, and some of the GDNF mutants have been shown to result in expression of a GDNF with reduced binding affinity for GFR1 (Doray et al., 1998; Eketjall and Ibanez, 2002). While none of these alone seems likely to cause Hirschsprung’s disease, they may contribute to pathogenesis in collaboration with other genetic alterations. It seems possible that contributions to the Hirschsprung’s disease phenotype could also be made by alleles of genes encoding proteins in the c-ret signaling pathway. Ret was first identified as an oncogene present in a human lymphoma that was the product of recombination between two unlinked DNA segments, generating a fusion protein including a tyrosine kinase (Takahashi et al., 1985; Takahashi and Cooper, 1987). Since this initial discovery, mutations or fusions of c-ret have been associated with several different types of cancer, including papillary thyroid carcinomas (PTC), multiple endocrine neoplasia type 2 (MEN2), pheochromocytoma, and parathyroid hyperplasia (Jhiang, 2000). Analysis of oncogenic forms of c-ret has identified several classes of mechanisms that render the activity of its tyrosine kinase partially or completely independent of the ligand. In the first, the cytoplasmic domain of c-ret is fused to the product of another gene, resulting in formation of a constitutively dimerized fusion protein (Takahashi et al., 1985). Eight different genes have been identified that are rearranged to form fusions with ret in PTCs (Jhiang, 2000). Approximately 5% to 30% of spontaneous papillary thyroid carcinomas are associated with gene fusions of this type. As discussed previously, proliferative and oncogenic responses by one of these fusion proteins have been shown to depend on interaction of the soluble fusion protein with the LIM and PDZ-domain-containing protein ENIGMA that relocalizes ret from the cytoplasm to the membrane (Durick et al., 1996, 1998). It seems likely that other fusion proteins of this type also require ENIGMA to promote oncogenesis efficiently. A second type of mutation generates an unpaired cysteine in the extracellular domain of c-ret that results in the constitutive dimerization and activation of membrane-associated ret (Santoro et al., 1995). These are dominant oncogenic mutations that result in MEN2 class A disease. In at least some cases, full activation of the mutant receptor requires the presence of a GDNF ligand, indicating that dimerization alone does not create an optimal conformation for activation of the ret tyrosine kinase activity (Mograbi et al., 2001). In the third class of mutation, an alteration in the c-ret kinase domain (M918T) increases the basal kinase activity of c-ret and alters its substrate preferences without promoting constitutive dimerization and results in MEN2 class B disease (Santoro et al., 1995; Liu et al., 1996). Ret (M918T) can be further activated by GDNF family ligands, so the pattern of expression of these ligands may contribute to the neoplastic phenotype. Not unexpectedly, many of the tyrosines phosphorylated in c-ret in response to GDNF application are also phosphorylated in these oncogenic ret proteins, including notably Y-1062 (Liu et al., 1996). Phosphorylation of Y-1096 is not observed in the MEN2B mutant (M918T), but there is increased phosphorylation of Y-1062, and a new phosphorylation site is created (Liu et al., 1996; Salvatore et al., 2001). Thus, differences in tyrosine kinase activity and substrate specificity are both thought to contribute to differences in the cancers that are associated with different ret mutations. Many studies have shown that adapters such as Shc, Grb-2, FRS2, IRS1, and PLC-1 are recruited by oncogenic mutants of Ret protein, thereby activating the Ras-Map kinase, PI-3 kinase–Akt, PLC, and other signaling pathways within affected cells (Hansford and Mulligan, 2000; Jhiang, 2000; Takahashi, 2001). CONCLUSION GDNF and its relatives have proven to have unusually interesting signaling pathways that are important in normal development of the nervous system and other organs. Because of its involvement in both development and oncogenesis, studies have provided a model of how human genetics can contribute to an understanding of receptor function, cell biology, and disease. Characterization of mouse mutants lacking these proteins or their receptors have illuminated the diversity Signaling Pathways of Glial Cell–Derived Neurotrophic Factor of mechanisms through which trophic factors regulate cell behavior and development, both inside and outside of the nervous system. These proteins have also been shown to regulate the electrical properties of neurons through control of transmitter receptors and ion channels. Because they protect neurons against a variety of toxic insults, they are strong candidates to be developed as therapeutic agents to treat Parkinson’s and other diseases. Uncontrolled activity of the common receptor c-ret contributes to development of several different cancers. The signaling cascades characterized as part of the effort to understand the oncogenic activity of ret have proven to be of general interest and, in the future, may provide targets for therapeutic treatments of many cancers in addition to those involving c-ret and the GDNF signaling pathway. ACKNOWLEDGMENTS I wish to thank Drs. Ursula Funfschilling, Beatriz Rico, and Sam Pleasure for critical review of the manuscript. I also thank Ms. Sonia Brown for design of Figure 35–1. Work in my laboratory has been supported by grants from the National Institutes of Health and Howard Hughes Medical Institute. I am an investigator in the Howard Hughes Medical Institute. REFERENCES AU1 Airaksinen MS, Titievsky A, Saarma M (1999). GDNF family neurotrophic factor signaling: four masters, one servant? Mol Cell Neurosci 13: 313–325. Amiel J, Attie T, Jan D, Pelet A, Edery P, Bidaud C, Lacombe D, Tam P, Simeoni J, Flori E, et al. (1996). Heterozygous endothelin receptor B (EDNRB) mutations in isolated Hirschsprung disease. Hum Mol Genet 5: 355–357. Anders J, Kjar S, Ibanez CF (2001). Molecular modeling of the extracellular domain of the RET receptor tyrosine kinase reveals multiple cadherin-like domains and a calciumbinding site. J Biol Chem 276: 35808–35817. Arighi E, Alberti L, Torriti F, Ghizzoni S, Rizzetti MG, Pelicci G, Pasini B, Bongarzone I, Piutti C, Pierotti MA, Borrello MG (1997). Identification of Shc docking site on Ret tyrosine kinase. Oncogene 14: 773–782. Attie T, Amiel J, Jan D, Edery P, Pelet A, Salomon R, Munnich A, Lyonnet S, NihoulFekete C (1996). [Genetics of Hirschsprung’s disease]. Ann Chir 50: 538–541. Baldassarre G, Bruni P, Boccia A, Salvatore G, Melillo RM, Motti ML, Napolitano M, Belletti B, Fusco A, Santoro M, Viglietto G (2002). Glial cell line-derived neurotrophic factor induces proliferative inhibition of NT2/D1 cells through RET-mediated up-regulation of the cyclin-dependent kinase inhibitor p27(kip1). Oncogene 21: 1739–1749. Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM Jr, Milbrandt J (1998). Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFR3RET receptor complex. Neuron 21: 1291–1302. Baloh RH, Enomoto H, Johnson EM Jr, Milbrandt J (2000a). The GDNF family ligands and receptors: implications for neural development. Curr Opin Neurobiol 10: 103–110. Baloh RH, Tansey MG, Johnson EM Jr, Milbrandt J (2000b). Functional mapping of receptor specificity domains of glial cell line–derived neurotrophic factor (GDNF) family ligands and production of GFR1 RET-specific agonists. J Biol Chem 275: 3412–3420. Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C (2001). Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27: 74–78. Bennett DL (2001). Neurotrophic factors: important regulators of nociceptive function. Neuroscientist 7: 13–17. Boettger MK, Till S, Chen MX, Anand U, Otto WR, Plumpton C, Trezise DJ, Tate SN, Bountra C, Coward K, et al. (2002). Calcium-activated potassium channel SK1- and IK1like immunoreactivity in injured human sensory neurones and its regulation by neurotrophic factors. Brain 125: 252–263. Bonde C, Kristensen BW, Blaabjerg M, Johansen TE, Zimmer J, Meyer M (2000). GDNF and neublastin protect against NMDA-induced excitotoxicity in hippocampal slice cultures. Neuroreport 11: 4069–4073. Bordeaux MC, Forcet C, Granger L, Corset V, Bidaud C, Billaud M, Bredesen DE, Edery P, Mehlen P (2000). The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J 19: 4056–4063. Borrello MG, Alberti L, Arighi E, Bongarzone I, Battistini C, Bardelli A, Pasini B, Piutti C, Rizzetti MG, Mondellini P, et al. (1996). The full oncogenic activity of Ret/ptc2 depends on tyrosine 539, a docking site for phospholipase C. Mol Cell Biol 16: 2151–2163. Botchkareva NV, Botchkarev VA, Welker P, Airaksinen M, Roth W, Suvanto P, MullerRover S, Hadshiew IM, Peters C, Paus R (2000). New roles for glial cell line-derived neurotrophic factor and neurturin: involvement in hair cycle control. Am J Pathol 156: 1041–1053. Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB (2000). Potent analgesic effects of GDNF in neuropathic pain states. Science 290: 124–127. Bradbury EJ, Burnstock G, McMahon SB (1998). The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci 12: 256–268. Brene S, Messer C, Okado H, Hartley M, Heinemann SF, Nestler EJ (2000). Regulation of GluR2 promoter activity by neurotrophic factors via a neuron-restrictive silencer element. Eur J Neurosci 12: 1525–1533. Brophy PD, Ostrom L, Lang KM, Dressler GR (2001). Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: 4747–4756. Buj-Bello A, Adu J, Pinon LG, Horton A, Thompson J, Rosenthal A, Chinchetru M, Buch- 417 man VL, Davies AM (1997). Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature 387: 721–724. Butte MJ (2001). Neurotrophic factor structures reveal clues to evolution, binding, specificity, and receptor activation. Cell Mol Life Sci 58: 1003–1013. Cacalano G, Fariñas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, et al. (1998). GFR1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron 21: 53–62. Corbit KC, Foster DA, Rosner MR (1999). Protein kinase C mediates neurogenic but not mitogenic activation of mitogen-activated protein kinase in neuronal cells. Mol Cell Biol 19: 4209–4218. Corbit KC, Soh JW, Yoshida K, Eves EM, Weinstein IB, Rosner MR (2000). Different protein kinase C isoforms determine growth factor specificity in neuronal cells. Mol Cell Biol 20: 5392–5403. Coulpier M, Anders J, Ibanez CF (2002). Coordinated activation of autophosphorylation sites in the RET receptor tyrosine kinase: importance of tyrosine 1062 for GDNF mediated neuronal differentiation and survival. J Biol Chem 277: 1991–1999. Cullen-McEwen LA, Drago J, Bertram JF (2001). Nephron endowment in glial cell linederived neurotrophic factor (GDNF) heterozygous mice. Kidney Int 60: 31–36. Cummins TR, Black JA, Dib-Hajj SD, Waxman SG (2000). Glial-derived neurotrophic factor upregulates expression of functional SNS and NaN sodium channels and their currents in axotomized dorsal root ganglion neurons. J Neurosci 20: 8754–8761. de Graaff E, Srinivas S, Kilkenny C, D’Agati V, Mankoo BS, Costantini F, Pachnis V (2001). Differential activities of the RET tyrosine kinase receptor isoforms during mammalian embryogenesis. Genes Dev 15: 2433–2444. de Vicente JC, Cabo R, Ciriaco E, Laura R, Naves FJ, Silos-Santiago I, Vega JA (2002). Impaired dental cytodifferentiation in glial cell-line derived growth factor (GDNF) deficient mice. Ann Anat 184: 85–92. Doray B, Salomon R, Amiel J, Pelet A, Touraine R, Billaud M, Attie T, Bachy B, Munnich A, Lyonnet S (1998). Mutation of the RET ligand, neurturin, supports multigenic inheritance in Hirschsprung disease. Hum Mol Genet 7: 1449–1452. Drawbridge J, Meighan CM, Mitchell EA (2000). GDNF and GFR-1 are components of the axolotl pronephric duct guidance system. Dev Biol 228: 116–124. Durbec P, Marcos-Gutierrez CV, Kilkenny C, Grigoriou M, Wartiowaara K, Suvanto P, Smith D, Ponder B, Costantini F, Saarma M, et al. (1996a). GDNF signalling through the Ret receptor tyrosine kinase. Nature 381: 789–793. Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V (1996b). Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development 122: 349–358. Durick K, Wu RY, Gill GN, Taylor SS (1996). Mitogenic signaling by Ret/ptc2 requires association with enigma via a LIM domain. J Biol Chem 271: 12691–12694. Durick K, Gill GN, Taylor SS (1998). Shc and Enigma are both required for mitogenic signaling by Ret/ptc2. Mol Cell Biol 18: 2298–2308. Edery P, Lyonnet S, Mulligan LM, Pelet A, Dow E, Abel L, Holder S, Nihoul-Fekete C, Ponder BA, Munnich A (1994). Mutations of the RET proto-oncogene in Hirschsprung’s disease. Nature 367: 378–380. Edery P, Eng C, Munnich A, Lyonnet S (1997). RET in human development and oncogenesis. Bioessays 19: 389–395. Ehrenfels CW, Carmillo PJ, Orozco O, Cate RL, Sanicola M (1999). Perturbation of RET signaling in the embryonic kidney. Dev Genet 24: 263–272. Eigenbrot C, Gerber N (1997). X-ray structure of glial cell–derived neurotrophic factor at 1.9 A resolution and implications for receptor binding. Nat Struct Biol 4: 435–438. Eketjall S, Ibanez CF (2002). Functional characterization of mutations in the GDNF gene of patients with Hirschsprung disease. Hum Mol Genet 11: 325–329. Eketjall S, Fainzilber M, Murray-Rust J, Ibanez CF (1999). Distinct structural elements in GDNF mediate binding to GFR1 and activation of the GFR1-c-Ret receptor complex. EMBO J 18: 5901–5910. Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM Jr. (2001). c-Src is required for glial cell line–derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci 21: 1464–1472. Enokido Y, de Sauvage F, Hongo JA, Ninkina N, Rosenthal A, Buchman VL, Davies A. M (1998). GFR -4 and the tyrosine kinase Ret form a functional receptor complex for persephin. Curr Biol 8: 1019–1022. Enomoto H, Araki T, Jackman A, Heuckeroth RO, Snider WD, Johnson EM Jr., Milbrandt J (1998). GFR 1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron 21: 317–324. Enomoto H, Heuckeroth RO, Golden JP, Johnson EM, Milbrandt J (2000). Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development 127: 4877–4889. Enomoto H, Crawford PA, Gorodinsky A, Heuckeroth RO, Johnson EM Jr., Milbrandt J (2001). RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development 128: 3963–3974. Fjell J, Cummins TR, Dib-Hajj SD, Fried K, Black JA, Waxman SG (1999). Differential role of GDNF and NGF in the maintenance of two TTX-resistant sodium channels in adult DRG neurons. Brain Res Mol Brain Res 67: 267–282. Focke PJ, Schiltz CA, Jones SE, Watters JJ, Epstein ML (2001). Enteric neuroblasts require the phosphatidylinositol 3-kinase pathway for GDNF-stimulated proliferation. J Neurobiol 47: 306–317. Fukuda T, Kiuchi K, Takahashi M (2002). Novel mechanism of regulation of Rac activity and lamellipodia formation by RET tyrosine kinase. J Biol Chem 277: 19114–19121. Gabriel SB, Salomon R, Pelet A, Angrist M, Amiel J, Fornage M, Attie-Bitach T, Olson JM, Hofstra R, Buys C, et al. (2002). Segregation at three loci explains familial and population risk in Hirschsprung disease. Nat Genet 31: 89–93. Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, deLapeyriere O (2000). GFR 1 is required for development of distinct subpopulations of motoneuron. J Neurosci 20: 4992–5000. Geneste O, Bidaud C, De Vita G, Hofstra RM, Tartare-Deckert S, Buys CH, Lenoir GM, 418 DEFINED PATHWAYS Santoro M, Billaud M (1999). Two distinct mutations of the RET receptor causing Hirschsprung’s disease impair the binding of signalling effectors to a multifunctional docking site. Hum Mol Genet 8: 1989–1999. Gershon MD (1998). Genes, lineages, and tissue interactions in the development of the enteric nervous system. Am J Physiol 275: G869–873. Goodfellow PJ (1994). Inherited cancers associated with the RET proto-oncogene. Curr Opin Genet Dev 4: 446–452. Gratacos E, Perez-Navarro E, Tolosa E, Arenas E, Alberch J (2001). Neuroprotection of striatal neurons against kainate excitotoxicity by neurotrophins and GDNF family members. J Neurochem 78: 1287–1296. Grewal SS, York RD, Stork PJ (1999). Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol 9: 544–553. Grimm J, Sachs M, Britsch S, Di Cesare S, Schwarz-Romond T, Alitalo K, Birchmeier W (2001). Novel p62dok family members, dok-4 and dok-5, are substrates of the c-Ret receptor tyrosine kinase and mediate neuronal differentiation. J Cell Biol 154: 345–354. Hadari YR, Kouhara H, Lax I, Schlessinger J (1998). Binding of Shp2 tyrosine phosphatase to FRS2 is essential for fibroblast growth factor–induced PC12 cell differentiation. Mol Cell Biol 18: 3966–3973. Han DC, Shen TL, Guan JL (2001). The Grb7 family proteins: structure, interactions with other signaling molecules and potential cellular functions. Oncogene 20: 6315–6321. Hansford JR, Mulligan LM (2000). Multiple endocrine neoplasia type 2 and RET: from neoplasia to neurogenesis. J Med Genet 37: 817–827. Hashino E, Shero M, Junghans D, Rohrer H, Milbrandt J, Johnson EM Jr. (2001). GDNF and neurturin are target-derived factors essential for cranial parasympathetic neuron development. Development 128: 3773–3782. Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, et al. (2000). Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene 19: 4469–4475. Hayashi Y, Iwashita T, Murakamai H, Kato Y, Kawai K, Kurokawa K, Tohnai I, Ueda M, Takahashi M (2001). Activation of BMK1 via tyrosine 1062 in RET by GDNF and MEN2A mutation. Biochem Biophys Res Commun 281: 682–689. Hearn CJ, Murphy M, Newgreen D (1998). GDNF and ET-3 differentially modulate the numbers of avian enteric neural crest cells and enteric neurons in vitro. Dev Biol 197: 93–105. Hellmich HL, Kos L, Cho ES, Mahon KA, Zimmer A (1996). Embryonic expression of glial cell-line derived neurotrophic factor (GDNF) suggests multiple developmental roles in neural differentiation and epithelial–mesenchymal interactions. Mech Dev 54: 95–105. Heuckeroth RO, Lampe PA, Johnson EM, Milbrandt J (1998). Neurturin and GDNF promote proliferation and survival of enteric neuron and glial progenitors in vitro. Dev Biol 200: 116–129. Heuckeroth RO, Enomoto H, Grider JR, Golden JP, Hanke JA, Jackman A, Molliver DC, Bardgett ME, Snider WD, Johnson EM Jr, Milbrandt J (1999). Gene targeting reveals a critical role for neurturin in the development and maintenance of enteric, sensory, and parasympathetic neurons. Neuron 22: 253–263. Hill E, Broadbent ID, Chothia C, Pettitt J (2001). Cadherin superfamily proteins in Caenorhabditis elegans and Drosophila melanogaster. J Mol Biol 305: 1011–1024. Howe CL, Valletta JS, Rusnak AS, Mobley WC (2001). NGF signaling from clathrincoated vesicles: evidence that signaling endosomes serve as a platform for the Ras-MAPK pathway. Neuron 32: 801–814. Huang EJ, Reichardt LF (2001). Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci 24: 677–736. Ishiguro Y, Iwashita T, Murakami H, Asai N, Iida K, Goto H, Hayakawa T, Takahashi M (1999). The role of amino acids surrounding tyrosine 1062 in ret in specific binding of the shc phosphotyrosine-binding domain. Endocrinology 140: 3992–3998. Ito S, Iwashita T, Asai N, Murakami H, Iwata Y, Sobue G, Takahashi M (1997). Biological properties of Ret with cysteine mutations correlate with multiple endocrine neoplasia type 2A, familial medullary thyroid carcinoma, and Hirschsprung’s disease phenotype. Cancer Res 57: 2870–2872. Iwamoto T, Taniguchi M, Asai N, Ohkusu K, Nakashima I, Takahashi M (1993). cDNA cloning of mouse ret proto-oncogene and its sequence similarity to the cadherin superfamily. Oncogene 8: 1087–1091. Iwashita T, Murakami H, Asai N, Takahashi M (1996). Mechanism of ret dysfunction by Hirschsprung mutations affecting its extracellular domain. Hum Mol Genet 5: 1577–1580. Jahn T, Seipel P, Urschel S, Peschel C, Duyster J (2002). Role for the adaptor protein Grb10 in the activation of Akt. Mol Cell Biol 22: 979–991. Jhiang SM (2000). The RET proto-oncogene in human cancers. Oncogene 19: 5590–5597. Jing S, Wen D, Yu Y, Holst PL, Luo Y, Fang M, Tamir R, Antonio L, Hu Z, Cupples R, et al. (1996). GDNF-induced activation of the ret protein tyrosine kinase is mediated by GDNFR-, a novel receptor for GDNF. Cell 85: 1113–1124. Keller-Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD (2001). Glial cell line–derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci 21: 6136–6146. Klein RD, Sherman D, Ho WH, Stone D, Bennett GL, Moffat B, Vandlen R, Simmons L, Gu Q, Hongo JA, et al. (1997). A GPI-linked protein that interacts with Ret to form a candidate neurturin receptor. Nature 387: 717–721. Kotzbauer PT, Lampe PA, Heuckeroth RO, Golden JP, Creedon DJ, Johnson EM, Jr, Milbrandt J (1996). Neurturin, a relative of glial-cell-line-derived neurotrophic factor. Nature 384: 467–470. Kreidberg JA (1996). Gene targeting in kidney development. Med Pediatr Oncol 27: 445–452. Kume T, Deng K, Hogan BL (2000). Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127: 1387–1395. Kusafuka T, Puri P (1998). Genetic aspects of Hirschsprung’s disease. Semin Pediatr Surg 7: 148–155. Laurikainen A, Hiltunen JO, Thomas-Crusells J, Vanhatalo S, Arumae U, Airaksinen MS, Klinge E, Saarma M (2000). Neurturin is a neurotrophic factor for penile parasympathetic neurons in adult rat. J Neurobiol 43: 198–205. Lee H, Woodman SE, Engelman JA, Volonte D, Galbiati F, Kaufman HL, Lublin DM, Lisanti MP (2001). Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-Src tyrosine kinase: targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-Src and caveolin-1 (TYR-14). J Biol Chem 276: 35150–35158. Leitner ML, Molliver DC, Osborne PA, Vejsada R, Golden JP, Lampe PA, Kato AC, Milbrandt J, Johnson EM Jr (1999). Analysis of the retrograde transport of glial cell line–derived neurotrophic factor (GDNF), neurturin, and persephin suggests that in vivo signaling for the GDNF family is GFR coreceptor-specific. J Neurosci 19: 9322–9331. Lelievre-Pegorier M, Vilar J, Ferrier ML, Moreau E, Freund N, Gilbert T, Merlet-Benichou C (1998). Mild vitamin A deficiency leads to inborn nephron deficit in the rat. Kidney Int 54: 1455–1462. Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F (1993). GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260: 1130–1132. Lindahl M, Timmusk T, Rossi J, Saarma M, Airaksinen MS (2000). Expression and alternative splicing of mouse Gfra4 suggest roles in endocrine cell development. Mol Cell Neurosci 15: 522–533. Lindahl M, Poteryaev D, Yu L, Arumae U, Timmusk T, Bongarzone I, Aiello A, Pierotti MA, Airaksinen MS, Saarma M (2001). Human glial cell line–derived neurotrophic factor receptor 4 is the receptor for persephin and is predominantly expressed in normal and malignant thyroid medullary cells. J Biol Chem 276: 9344–9351. Liu X, Vega QC, Decker RA, Pandey A, Worby CA, Dixon JE (1996). Oncogenic RET receptors display different autophosphorylation sites and substrate binding specificities. J Biol Chem 271: 5309–5312. Lo L, Anderson DJ (1995). Postmigratory neural crest cells expressing c-RET display restricted developmental and proliferative capacities. Neuron 15: 527–539. Lorenzo MJ, Eng C, Mulligan LM, Stonehouse TJ, Healey CS, Ponder BA, Smith DP (1995). Multiple mRNA isoforms of the human RET proto-oncogene generated by alternate splicing. Oncogene 10: 1377–1383. Lorenzo MJ, Gish GD, Houghton C, Stonehouse TJ, Pawson T, Ponder BA, Smith DP (1997). RET alternate splicing influences the interaction of activated RET with the SH2 and PTB domains of Shc, and the SH2 domain of Grb2. Oncogene 14: 763–771. Manie S, Santoro M, Fusco A, Billaud M (2001). The RET receptor: function in development and dysfunction in congenital malformation. Trends Genet 17: 580–589. Marco S, Perez-Navarro E, Tolosa E, Arenas E, Alberch J (2002). Striatopallidal neurons are selectively protected by neurturin in an excitotoxic model of Huntington’s disease. J Neurobiol 50: 323–332. Mason I (2000). The RET receptor tyrosine kinase: activation, signalling and significance in neural development and disease. Pharm Acta Helv 74: 261–264. Masure S, Geerts H, Cik M, Hoefnagel E, Van Den Kieboom G, Tuytelaars A, Harris S, Lesage AS, Leysen JE, Van Der Helm L, et al. (1999). Enovin, a member of the glial cell-line-derived neurotrophic factor (GDNF) family with growth promoting activity on neuronal cells: existence and tissue-specific expression of different splice variants. Eur J Biochem 266: 892–902. Masure S, Cik M, Hoefnagel E, Nosrat CA, Van der Linden I, Scott R, Van Gompel P, Lesage AS, Verhasselt P, Ibanez CF, Gordon RD (2000). Mammalian GFR-4, a divergent member of the GFR family of coreceptors for glial cell line-derived neurotrophic factor family ligands, is a receptor for the neurotrophic factor persephin. J Biol Chem 275: 39427–39434. McCabe ER (2002). Hirschsprung’s disease: dissecting complexity in a pathogenetic network. Lancet 359: 1169–1170. Melillo RM, Carlomagno F, De Vita G, Formisano P, Vecchio G, Fusco A, Billaud M, Santoro M (2001a). The insulin receptor substrate (IRS)-1 recruits phosphatidylinositol 3-kinase to Ret: evidence for a competition between Shc and IRS1 for the binding to Ret. Oncogene 20: 209–218. Melillo RM, Santoro M, Ong SH, Billaud M, Fusco A, Hadari YR, Schlessinger J, Lax I (2001b). Docking protein FRS2 links the protein tyrosine kinase RET and its oncogenic forms with the mitogen-activated protein kinase signaling cascade. Mol Cell Biol 21: 4177–4187. Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J (1999). Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126: 1139– 1148. Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, RaatikainenAhokas A, Sainio K, Rauvala H, Lakso M, et al. (2000). Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science 287: 1489–1493. Meng X, de Rooij DG, Westerdahl K, Saarma M, Sariola H (2001). Promotion of seminomatous tumors by targeted overexpression of glial cell line-derived neurotrophic factor in mouse testis. Cancer Res 61: 3267–3271. Mikaels A, Livet J, Westphal H, De Lapeyriere O, Ernfors P (2000). A dynamic regulation of GDNF-family receptors correlates with a specific trophic dependency of cranial motor neuron subpopulations during development. Eur J Neurosci 12: 446–456. Milbrandt J, de Sauvage FJ, Fahrner TJ, Baloh RH, Leitner ML, Tansey MG, Lampe PA, Heuckeroth RO, Kotzbauer PT, Simburger KS, et al. (1998). Persephin, a novel neurotrophic factor related to GDNF and neurturin. Neuron 20: 245–253. Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S (1997). Defects of urogenital development in mice lacking Emx2. Development 124: 1653–1664. Mograbi B, Bocciardi R, Bourget I, Juhel T, Farahi-Far D, Romeo G, Ceccherini I, Rossi B (2001). The sensitivity of activated Cys Ret mutants to glial cell line–derived neurotrophic factor is mandatory to rescue neuroectodermic cells from apoptosis. Mol Cell Biol 21: 6719–6730. Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A (1996). Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79. Moreau E, Vilar J, Lelievre-Pegorier M, Merlet-Benichou C, Gilbert T (1998). Regula- Signaling Pathways of Glial Cell–Derived Neurotrophic Factor tion of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am J Physiol 275: F938–945. Myers SM, Eng C, Ponder BA, Mulligan LM (1995). Characterization of RET proto-oncogene 3 splicing variants and polyadenylation sites: a novel C-terminus for RET. Oncogene 11: 2039–2045. Natarajan D, Grigoriou M, Marcos-Gutierrez CV, Atkins C, Pachnis V (1999). Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development 126: 157–168. Nishino J, Mochida K, Ohfuji Y, Shimazaki T, Meno C, Ohishi S, Matsuda Y, Fujii H, Saijoh Y, Hamada H (1999). GFR 3, a component of the artemin receptor, is required for migration and survival of the superior cervical ganglion. Neuron 23: 725–736. Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S (1995). Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature 373: 344–346. Oppenheim RW, Houenou LJ, Parsadanian AS, Prevette D, Snider WD, Shen L (2000). Glial cell line–derived neurotrophic factor and developing mammalian motoneurons: regulation of programmed cell death among motoneuron subtypes. J Neurosci 20: 5001–5011. Pachnis V, Mankoo B, Costantini F (1993). Expression of the c-ret proto-oncogene during mouse embryogenesis. Development 119: 1005–1017. Pachnis V, Durbec P, Taraviras S, Grigoriou M, Natarajan D (1998). Role of the RET signal transduction pathway in development of the mammalian enteric nervous system. Am J Physiol 275: G183–186. Pandey A, Duan H, Di Fiore PP, Dixit VM (1995). The Ret receptor protein tyrosine kinase associates with the SH2-containing adapter protein Grb10. J Biol Chem 270: 21461– 21463. Pandey A, Liu X, Dixon JE, Di Fiore PP, Dixit VM (1996). Direct association between the Ret receptor tyrosine kinase and the Src homology 2-containing adapter protein Grb7. J Biol Chem 271: 10607–10610. Paratcha G, Ledda F, Baars L, Coulpier M, Besset V, Anders J, Scott R, Ibanez CF (2001). Released GFR1 potentiates downstream signaling, neuronal survival, and differentiation via a novel mechanism of recruitment of c-Ret to lipid rafts. Neuron 29: 171–184. Parisi MA, Kapur RP (2000). Genetics of Hirschsprung disease. Curr Opin Pediatr 12: 610–617. Passarge E (2002). Dissecting Hirschsprung disease. Nat Genet 31: 11–12. Patterson LT, Pembaur M, Potter SS (2001). Hoxa11 and Hoxd11 regulate branching morphogenesis of the ureteric bud in the developing kidney. Development 128: 2153–2161. Pepicelli CV, Kispert A, Rowitch DH, McMahon AP (1997). GDNF induces branching and increased cell proliferation in the ureter of the mouse. Dev Biol 192: 193–198. Pérez-Navarro E, Arenas E, Marco S, Alberch J (1999). Intrastriatal grafting of a GDNFproducing cell line protects striatonigral neurons from quinolinic acid excitotoxicity in vivo. Eur J Neurosci 11: 241–249. Perrelet D, Ferri A, Liston P, Muzzin P, Korneluk RG, Kato AC (2002). IAPs are essential for GDNF-mediated neuroprotective effects in injured motor neurons in vivo. Nat Cell Biol 4: 175–179. Pezeshki G, Franke B, Engele J (2001). Evidence for a ligand-specific signaling through GFR-1, but not GFR-2, in the absence of Ret. J Neurosci Res 66: 390–395. Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, et al. (1996). Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382: 73–76. Poteryaev D, Titievsky A, Sun YF, Thomas-Crusells J, Lindahl M, Billaud M, Arumae U, Saarma M (1999). GDNF triggers a novel ret-independent Src kinase family-coupled signaling via a GPI-linked GDNF receptor 1. FEBS Lett 463: 63–66. Romeo G, Ronchetto P, Luo Y, Barone V, Seri M, Ceccherini I, Pasini B, Bocciardi R, Lerone M, Kaariainen H, et al. (1994). Point mutations affecting the tyrosine kinase domain of the RET proto-oncogene in Hirschsprung’s disease. Nature 367: 377–378. Rosenblad C, Gronborg M, Hansen C, Blom N, Meyer M, Johansen J, Dago L, Kirik D, Patel UA, Lundberg C, et al. (2000). In vivo protection of nigral dopamine neurons by lentiviral gene transfer of the novel GDNF-family member neublastin/artemin. Mol Cell Neurosci 15: 199–214. Rossi J, Luukko K, Poteryaev D, Laurikainen A, Sun YF, Laakso T, Eerikainen S, Tuominen R, Lakso M, Rauvala H, et al. (1999). Retarded growth and deficits in the enteric and parasympathetic nervous system in mice lacking GFR alpha2, a functional neurturin receptor. Neuron 22: 243–252. Rossi J, Tomac A, Saarma M, Airaksinen MS (2000). Distinct roles for GFR1 and GFR2 signalling in different cranial parasympathetic ganglia in vivo. Eur J Neurosci 12: 3944–3952. Saarma M (2000). GDNF: a stranger in the TGF- superfamily? Eur J Biochem 267: 6968–6971. Saarma M (2001). GDNF recruits the signaling crew into lipid rafts. Trends Neurosci 24: 427–429. Sainio K, Suvanto P, Davies J, Wartiovaara J, Wartiovaara K, Saarma M, Arumae U, Meng X, Lindahl M, Pachnis V, Sariola H (1997). Glial-cell-line-derived neurotrophic factor is required for bud initiation from ureteric epithelium. Development 124: 4077–4087. Salvatore D, Melillo RM, Monaco C, Visconti R, Fenzi G, Vecchio G, Fusco A, Santoro M (2001). Increased in vivo phosphorylation of ret tyrosine 1062 is a potential pathogenetic mechanism of multiple endocrine neoplasia type 2B. Cancer Res 61: 1426–1431. Sanchez MP, Silos-Santiago I, Frisen J, He B, Lira SA, Barbacid M (1996). Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382: 70–73. Sanicola M, Hession C, Worley D, Carmillo P, Ehrenfels C, Walus L, Robinson S, Jaworski G, Wei H, Tizard R, et al. (1997). Glial cell line–derived neurotrophic factor-dependent RET activation can be mediated by two different cell-surface accessory proteins. Proc Natl Acad Sci USA 94: 6238–6243. Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA, Grieco M, Fusco A, Vecchio G, Matoskova B, Kraus MH, et al. (1995). Activation of RET as a dominant transforming gene by germline mutations of MEN2A and MEN2B. Science 267: 381–383. 419 Schneider R (1992). The human protooncogene ret: a communicative cadherin? Trends Biochem Sci 17: 468–469. Schober A, Hertel R, Arumae U, Farkas L, Jaszai J, Krieglstein K, Saarma M, Unsicker K (1999). Glial cell line–derived neurotrophic factor rescues target-deprived sympathetic spinal cord neurons but requires transforming growth factor- as cofactor in vivo. J Neurosci 19: 2008–2015. Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V (1994). Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383. Schuchardt A, D’Agati V, Pachnis V, Costantini F (1996). Renal agenesis and hypodysplasia in ret-k-mutant mice result from defects in ureteric bud development. Development 122: 1919–1929. Scott RP, Ibanez CF (2001). Determinants of ligand binding specificity in the glial cell line–derived neurotrophic factor family receptor S. J Biol Chem 276: 1450–1458. Shen L, Pichel JG, Mayeli T, Sariola H, Lu B, Westphal H (2002). Gdnf haploinsufficiency causes Hirschsprung-like intestinal obstruction and early-onset lethality in mice. Am J Hum Genet 70: 435–447. Shepherd IT, Beattie CE, Raible DW (2001). Functional analysis of zebrafish GDNF. Dev Biol 231: 420–435. Shi ZQ, Yu DH, Park M, Marshall M, Feng GS (2000). Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol 20: 1526–1536. Soler RM, Dolcet X, Encinas M, Egea J, Bayascas JR, Comella JX (1999). Receptors of the glial cell line–derived neurotrophic factor family of neurotrophic factors signal cell survival through the phosphatidylinositol 3-kinase pathway in spinal cord motoneurons. J Neurosci 19: 9160–9169. Srinivas S, Wu Z, Chen CM, D’Agati V, Costantini F (1999). Dominant effects of RET receptor misexpression and ligand-independent RET signaling on ureteric bud development. Development 126: 1375–1386. Tadokoro Y, Yomogida K, Ohta H, Tohda A, Nishimune Y (2002). Homeostatic regulation of germinal stem cell proliferation by the GDNF/FSH pathway. Mech Dev 113: 29–39. Tahira T, Ishizaka Y, Itoh F, Sugimura T, Nagao M (1990). Characterization of ret protooncogene mRNAs encoding two isoforms of the protein product in a human neuroblastoma cell line. Oncogene 5: 97–102. Takahashi M (2001). The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev 12: 361–373. Takahashi M, Cooper GM (1987). ret transforming gene encodes a fusion protein homologous to tyrosine kinases. Mol Cell Biol 7: 1378–1385. Takahashi M, Ritz J, Cooper GM (1985). Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell 42: 581–588. Takahashi M, Buma Y, Iwamoto T, Inaguma Y, Ikeda H, Hiai H (1988). Cloning and expression of the ret proto-oncogene encoding a tyrosine kinase with two potential transmembrane domains. Oncogene 3: 571–578. Takahashi M, Buma Y, Hiai H (1989). Isolation of ret proto-oncogene cDNA with an amino-terminal signal sequence. Oncogene 4: 805–806. Takahashi M, Iwashita T, Santoro M, Lyonnet S, Lenoir GM, Billaud M (1999). Co-segregation of MEN2 and Hirschsprung’s disease: the same mutation of RET with both gain and loss-of-function? Hum Mutat 13: 331–336. Tang MJ, Worley D, Sanicola M, Dressler GR (1998). The RET-glial cell-derived neurotrophic factor (GDNF) pathway stimulates migration and chemoattraction of epithelial cells. J Cell Biol 142: 1337–1345. Tang MJ, Cai Y, Tsai SJ, Wang YK, Dressler GR (2002). Ureteric bud outgrowth in response to RET activation is mediated by phosphatidylinositol 3-kinase. Dev Biol 243: 128–136. Tansey MG, Baloh RH, Milbrandt J, Johnson EM Jr. (2000). GFR-mediated localization of RET to lipid rafts is required for effective downstream signaling, differentiation, and neuronal survival. Neuron 25: 611–623. Taraviras S, Marcos-Gutierrez CV, Durbec P, Jani H, Grigoriou M, Sukumaran M, Wang LC, Hynes M, Raisman G, Pachnis V (1999). Signalling by the RET receptor tyrosine kinase and its role in the development of the mammalian enteric nervous system. Development 126: 2785–2797. Thompson J, Doxakis E, Pinon LG, Strachan P, Buj-Bello A, Wyatt S, Buchman VL, Davies AM (1998). GFR-4, a new GDNF family receptor. Mol Cell Neurosci 11: 117–126. Tomac AC, Grinberg A, Huang SP, Nosrat C, Wang Y, Borlongan C, Lin SZ, Chiang YH, Olson L, Westphal H, Hoffer BJ (2000). Glial cell line–derived neurotrophic factor receptor 1 availability regulates glial cell line–derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neuroscience 95: 1011–1023. Treanor JJ, Goodman L, de Sauvage F, Stone DM, Poulsen KT, Beck CD, Gray C, Armanini MP, Pollock RA, Hefti F, et al. (1996). Characterization of a multicomponent receptor for GDNF. Nature 382: 80–83. Trupp M, Belluardo N, Funakoshi H, Ibanez CF (1997). Complementary and overlapping expression of glial cell line–derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor- indicates multiple mechanisms of trophic actions in the adult rat CNS. J Neurosci 17: 3554–3567. Trupp M, Raynoschek C, Belluardo N, Ibanez CF (1998). Multiple GPI-anchored receptors control GDNF-dependent and independent activation of the c-Ret receptor tyrosine kinase. Mol Cell Neurosci 11: 47–63. Trupp M, Scott R, Whittemore SR, Ibanez CF (1999). Ret-dependent and -independent mechanisms of glial cell line-derived neurotrophic factor signaling in neuronal cells. J Biol Chem 274: 20885–20894. Tsui-Pierchala BA, Milbrandt J, Johnson EM Jr. (2002). NGF utilizes c-Ret via a novel GFL-independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron 33: 261–273. van Weering DH, Bos JL (1998). Signal transduction by the receptor tyrosine kinase Ret. Recent Results Cancer Res 154: 271–281. van Weering DH, Moen TC, Braakman I, Baas PD, Bos JL (1998). Expression of the re- 420 AU2 DEFINED PATHWAYS ceptor tyrosine kinase Ret on the plasma membrane is dependent on calcium. J Biol Chem 273: 12077–12081. Vega QC, Worby CA, Lechner MS, Dixon JE, Dressler GR (1996). Glial cell line–derived neurotrophic factor activates the receptor tyrosine kinase RET and promotes kidney morphogenesis. Proc Natl Acad Sci USA 93: 10657–10661. Wang CY, Yang F, He X, Chow A, Du J, Russell JT, Lu B (2001). Ca2 binding protein frequenin mediates GDNF-induced potentiation of Ca2 channels and transmitter release. Neuron 32: 99–112. Wang CY, Yang F, He XP, Je HS, Zhou JZ, Eckermann K, Kawamura D, Feng L, Shen L, Lu B (2002). Regulation of neuromuscular synapse development by glial cell line–derived neurotrophic factor and neurturin. J Biol Chem 277: 10614–10625. Worley DS, Pisano JM, Choi ED, Walus L, Hession CA, Cate RL, Sanicola M, Birren SJ (2000). Developmental regulation of GDNF response and receptor expression in the enteric nervous system. Development 127: 4383–4393. Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME (1998). Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol 18: 1946–1955. AU1: Ref. Arighi—if not cited, pls delete. AU2: Xing ref—if not cited, pls. delete. Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R (1999). Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23: 113–117. Yang F, Feng L, Zheng F, Johnson SW, Du J, Shen L, Wu CP, Lu B (2001). GDNF acutely modulates excitability and A-type K channels in midbrain dopaminergic neurons. Nat Neurosci 4: 1071–1078. York RD, Molliver DC, Grewal SS, Stenberg PE, McCleskey EW, Stork PJ (2000). Role of phosphoinositide 3-kinase and endocytosis in nerve growth factor–induced extracellular signal-regulated kinase activation via Ras and Rap1. Mol Cell Biol 20: 8069–8083. Young HM, Hearn CJ, Farlie PG, Canty AJ, Thomas PQ, Newgreen DF (2001). GDNF is a chemoattractant for enteric neural cells. Dev Biol 229: 503–516. Yu T, Scully S, Yu Y, Fox GM, Jing S, Zhou R (1998). Expression of GDNF family receptor components during development: implications in the mechanisms of interaction. J Neurosci 18: 4684–4696. Zhou B, Bae SK, Malone AC, Levinson BB, Kuo YM, Cilio MR, Bertini E, Hayflick SJ, Gitschier JM (2001). hGFR-4: a new member of the GDNF receptor family and a candidate for NBIA. Pediatr Neurol 25: 156–161.











![[pdf]](http://s1.studyres.com/store/data/008806779_1-709ec10357a7e0d52ffd9b5d02228d42-150x150.png)