* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download L14_Adv06PDHwebCT
Lipid signaling wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Microbial metabolism wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Electron transport chain wikipedia , lookup
Butyric acid wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Catalytic triad wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
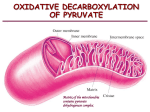
control of metabolic reactions •making +ve DGo‘ reactions happen •points of control: DGo‘ and equilibrium •multi-active enzymes: enzyme complexes and multiple active sites reactions with +ve DGo‘ can occur by: • coupling with a reaction with –ve DGo‘ • –ve physiological DG due to cellular low ratio [products]/[reactants] 1. reactions with +ve DGo‘ occur by coupling with a reaction with -ve DGo‘ • Thus, ATP ADP +Pi (DG<0) is coupled with non-spontaneous reactions (DG>0) Glucose glucose-6-P + H20 DG = 13.8 kJ.mol-1 hexokinase DG = -30.5 kJ.mol-1 ATP +H20 ADP +Pi DG = -16.3 kJ.mol-1 Glucose + ATP overall glucose-6-P + ADP 2. Recall: For a reaction A + B C + D [C] [D] DG = DGº' + RT ln [A] [B] DGo' of a reaction may be positive, and DG negative, depending on cellular concentrations of reactants and products. Many reactions for which DGo' is positive are spontaneous in vivo because other reactions cause [products] or [substrate]. any [products] or [substrate] that moves the reaction away from equilibrium ratio causes reaction to proceed spontaneously forward to restore equilibrium free energy change is related to the equilibrium constant (K'eq) = the ratio of DG = DGº' + RT ln [C] [D] [A] [B] = DGº' + RT ln [C] [D] [A] [B] [C] [D] DGº' = - RTln [A] [B] [C] [D] defining K'eq = [A] [B] DGº' = - RT ln K'eq [products]/[reactants] at equilibrium At equilibrium, no net change so DG = 0. I won’t be asking you to solve any of these equations! many reactions are near equilibrium • then DG ~0 (no net change in free energy) • easily reversed by changing ratio of [products]/[substrate] as don’t need to overcome high DG For A+B ↔C+D product A+B C+D substrate A+B C+D • enzymes that catalyse such reactions act to restore equilibrium • rate regulated by [products]/[reactants] Implication: a reaction near equilibrium may have +ve DGo' but be spontaneous in the cell -ve DG because other reactions cause [products] or [substrate]. Other reactions are FAR from equilibrium • enzyme rate is too slow to allow products to build to equilibrium concentration • [substrate] builds up in excess of Keq DG <<<0 (highly negative) • not affected by D[substrate] (saturated) • essentially irreversible • rate controlled by changing activity of enzyme (eg allosteric interactions) • reactions with DG <<0 are often sites of regulation reactions with DG <<0 are often sites of regulation 1. Often occur early as a “committed step” in metabolic pathways (eg AcetylCoA carboxylase) 2. most metabolic pathways are irreversible • ≥ 1 step with -ve DG required to drive: eg PDH, pyruvate carboxylase) • one way street: return by a different street 3. catabolic and anabolic pathways are separate independent control (eg glycolysis and gluconeogenesis (eg pyruvate carboxylase) use different enzymes) Pyruvate dehydrogenase a pretty, pink multi-enzyme complex ‘gatekeeper’ to entry to citric acid cycle http://www.brookscole.com/chemistry_d/templates/student_resources/shared_resources/animations/pdc/pdc.html Pyruvate dehydrogenase 2 NADH pyruvate dehydrogenase 2 NADH 6 NADH regulates entry into the citric acid cycle of metabolites leaving glycolysis Summary 1. structure of PDH complex 3 enzymes (E1, E2, E3) 2. reactions of PDH complex 5 reactions, 5 cofactors 3. mechanism of PDH complex lipoamide swinging arm 4. regulation of PDH complex de/phosphorylation of E1 product inhibition of E2 and E3 Excellent animation of PDH reactions if you can access it: (not examinable, but might help understanding!) http://www.brookscole.com/chemistry_d/templates/student_resources/shared_resources/animations/pdc/pdc.html PDH = multi-enzyme complex 3 different ENZYMES (non-covalently associated) 5 COFACTORS E1: pyruvate dehydrogenase +TPP E2: dihydrolipoyl transacetylase + lipoamide E3: dihydrolipoyl dehydrogenase + FAD catalyse 5 sequential reactions + NADH +CoenzymeA overall….. NAD+ high energy bond CoA (2C) pyruvate AcCoA CoA (3C) NADH CO2 irreversible multi-enzyme complex (E. coli) • a) dihydrolipoyl transacetylase (E2) • arranged as corners of a core cube surrounded by an outer cube: • b) pyruvate dehydrogenase (E1) edges • c) dihydrolipoyl dehydrogenase (E3) faces Note that there are many copies of each enzyme in each complex PDH structure is more complex in other organisms dodecahedron core= • 12 pentagon faces • 20 vertices (E2 trimers) • in mammals = + kinase + phosphatase E2 core of B. stearothermophilus each enzyme uses a cofactor TPP in each E1 FAD in each E3 2 lipoate binding domains in each E2 pyruvate dehydrogenase (E1) 5 sequential reactions In summary: 1) pyruvate is decarboxylated hydroxyethyl, requires TPP to stabilise the intermediate. 2) hydroxyethyl oxidised to acetyl, collected by lipoamide of E2, which gets reduced. 3) lipoamide of E2, passes acetyl to coenzyme A acetyl CoA. 4) lipoamide of E2, gets re-oxidised, gives its electrons to FAD in E3 which 5) passes electrons to NAD NADH dihydrolipoyl transacetylase (E2) dihydrolipoyl dehydrogenase (E3) 5 4 1 2 3 1. decarboxylation by E1 loss of CO2 conversion of pyruvate to a 2 carbon moiety hydroxyethyl(2C) Pyruvate (3C) E1 has a bound coenzyme (TPP) that attacks pyruvate and stabilises the intermediate i. TPP forms a carbanion H+ readily dissociates (due to adjacent N+) N+ stabilises the carbanion H+ ii. nucleophilic attack by TPP carbanion on electron-deficient C2 of pyruvate hydroxyethyl-TTP CO2 releases CO2 iii. TTP stabilises the carbanion intermediate after CO2 is lost. can’t just remove CO2 highly unstable intermediate I won’t ask you to recreate bond rearrangements! CO2 2. formation of acetyl by E1 + TTP regenerated hydroxyethyl acetylOXIDATION REDUCTION - R - lys gain of hydrogen lipoamide - R - lysine E2 dihydrolipoamide hydroxyethyl is transferred the lipoamide group of E2, Lipoamide (= lipoic acid linked covalently to Lysine) contains a cyclic disulfide reactive group that can be reversibly reduced dihydro-lipoamide E2 uses lipoamide as a cofactor cyclic disulphide reversibly reduced and oxidised lipoic acid acts as a long flexible arm that can transfer substrates between active sites there are actually 2 lipoate-binding domains in each E2. lipoamide = lipoic acid covalently bound to lysine in E2 3. trans-esterification acetyl group transferred by E2 to CoA = high energy thioester bond Thioesters: high energy bond • Form between carboxylic acid (COOH) and a thiol (SH) eg thiol in CoenzymeA • eg Acetyl-CoA is common to CHO, fat and protein metabolism • eg.In citric acid cycle, cleavage of thioester in succinyl-CoA provides energy for synthesis of GTP Lipoamide cofactor in E2 So far…. • disulfide swings to outer shell to collect hydroxyethyl from TPP in E1 • swings to E2 to transfer acetyl to CoA now we have acetyl-CoA Remember: there are multiple copies of Kreb’s, FA synthesis each enzyme in complex next must regenerate lipoamide and produce NADH • So… lipoamide swings to E3 to be reoxidised and transfer electrons to NADH via FAD 4. regeneration of lipoamide (E2) by FAD (E3) OXIDATION in E2 REDUCTION in E3 5. redox • FAD funnels electrons to NAD+ NADH • regeneration of FAD in E3 OXIDATION in E3 REDUCTION in E2 NAD+ NADH + H+ ENZYME E1: pyruvate dehydrogenase E2: dihydrolipoyl transacetylase E3: dihydrolipoyl dehydrogenase COFACTOR +TPP + lipoamide + FAD PDH controlled by covalent modification and product inhibition • mammalian complex also contains kinase and phosphatase P ATP Ser inactive E1 PDH phosphatase PDH kinase active E1 pyruvate NAD+ AcCoA NADH CO2 inhibit PDH • high energy state P ATP Ser inactive E1 PDH phosphatase PDH kinase activates active E1 pyruvate NAD+ AcCoA NADH CO2 inhibition by products in addition to activating PDH kinase, NADH and acetyl-CoA: • compete with substrates for binding sites • drive E2 and E3 in reverse (these reactions are close to equilibrium) • E2 not available to collect hydyrxyol from TPP • TPP cannot accept pyruvate activate PDH • low cell energy, or high available fuel glucose Insulin P ADP Ser inactive E1 activates PDH phosphatase PDH kinase active E1 pyruvate AcCoA NAD+ NADH CoA CO2 activate PDH pyruvate overrides NADH, AcCoA still make AcCoA for fat when pyruvate P Ser inactive E1 PDH phosphatase PDH kinase activates active E1 pyruvate AcCoA NAD+ NADH CoA CO2 glucose in high energy: (high ATP, high AcCoA, high NADH) gluconeogenesis, fatty acid synthesis in low energy (low ATP, low AcCoA, ) glycolysis PEP PK pyruvate Pyr carbox CO2 PDH CO2 AcCoA AC Carbox malonylCoA FA Synthase fatty acids CO2 OAA citric acid cycle We now look at 3 other enzymes that use ‘swinging arm’ cofactors Pyruvate carboxylase AcetylCoA carboxylase Fatty acid synthase pyruvate carboxylase ATP pyruvate (3C) + HCO3- ADP oxaloacetate (4C) glucose (6C) • first reaction in gluconeogenesis • with PEPCK to bypass pyruvate kinase (DG<<0 in glycolysis) • requires ATP to overcome –ve DGo‘ of glycolysis another good animation, if you can access it: (not examinable, but might help understanding!) http://www.bmb.uga.edu/8010/moremen/weblinks/nucleotide/PyrCarb/PyrCarb.html pyruvate carboxylase •tetramer •each monomer has 2 active sites •uses biotin as swinging arm in active site 1 HCO3ATP biotin ADP carboxyphosphate carboxybiotin biotin’s swinging arm Biotin carboxylation is catalyzed at one active site : first, ATP reacts with HCO3(bicarbonate) to yield carboxyphosphate. The carboxyl from this high energy phosphate intermediate is transferred to the nucleophilic N of the biotin ring At active site 1: 1. bicarb + ATP high energy carboxyphosphate intermediate 2. -ve DG transfer of CO2 to biotin = carboxylation I won’t ask you to recreate bond rearrangements! HCO3ATP biotin ADP carboxyphosphate oxaloacetate (4C) 2. biotin arm swings to the 2nd active site, active CO2 is transferred from carboxybiotin to pyruvate OAA pyruvate (3C) carboxybiotin at active site 2: 1. CO2 leaves biotin, 2. biotin accepts a proton from pyruvate 3. pyruvate attacks CO2 pyruvate loses a proton, becomes an enolate nucleophile (donates e-) I won’t ask you to recreate bond rearrangements! OAA HCO3ATP biotin ADP carboxyphosphate biotin’s swinging arm carboxybiotin oxaloacetate (4C) pyruvate (3C) Overall: at active site 1: biotin + ATP + HCO3- carboxybiotin + ADP + Pi at active site 2: carboxybiotin + pyruvate OAA + biotin AcetylCoA carboxylase ATP AcetylCoA (2C) ADP + HCO3- malonylCoA (4C) fatty acids • first reaction committed step in fatty acid synthesis •Also uses biotin as swinging arm between two active sites •reactions very similar to pyruvate carboxylase HCO3ATP biotin ADP carboxyphosphate WOW look! mechanism of carboxylation (addition of COO-) is the same as for pyruvate carboxylase!!! ATP-dependent carboxylation of the biotin, carried out at active site 1 , is followed by transfer of the carboxyl group to acetyl-CoA at a second active site 2 . only difference is COO- is added to acetylCoA rather than to pyruvate biotin’s swinging arm malony-lCoA (3C) Acetyl-CoA (2C) carboxybiotin regulation of AcCoA-Carboxylase The mammalian enzyme is regulated, by phosphorylation by cAMP dependent kinase inhibition when energy (cAMP) allosteric control by local metabolites. Conformational changes with regulation: active = multimeric filamentous complexes. inactive = dissociation to = monomeric form P fatty acid synthase • dimer • 6 active sites are individual domains of a large protein – ? developed from gene fusion – has more catalytic activities than any enzyme! • has two prosthetic groups thioester bonds – thiol of cysteine (in condensing domain) – thiol of P-pantetheine (in acyl carrier domain) • acts as a long flexible arm transferring substrates between active sites has two prosthetic groups H H3N+ C COO- CH2 SH thiol of cysteine in condensing domain cysteine SH thiol of P-pantetheine phosphopantetheine of acyl carrier protein CH2 CH2 -mercaptoethylamine NH Phosphopantetheine is covalently linked to a serine of the acyl carrier protein domain C O CH2 CH2 pantothenate NH The long flexible arm of phosphopantetheine allows its thiol to move between active sites C O HO C H H3C C CH3 O H2C forms thioesters like CoA does O P NH O O- phosphate CH2 CH C serine residue O phosphopantetheine is part of CoA SH phosphopantetheine of acyl carrier protein CH2 CH2 SH Coenzyme A CH2 CH2 -mercaptoethylamine -mercaptoethylamine NH NH C C O CH2 CH2 CH2 CH2 NH pantothenate HO O C H C H2C CH3 O O P NH O O- phosphate CH2 CH C serine residue O NH2 O HO C H H3C C CH3 O H2C H3C pantothenate C NH C O O ADP-3'phosphate P N N O O O- P N N CH2 O O- O H H O H OH H phosphopantetheine - O P O O- fatty acid synthase 2NADPH H20 2 3 2)Thioester bond between malonyl and pantetheine 3)The condensation reaction * involves decarboxylation of the malonyl carbanion attacks carbonyl carbon of the acetyl. Uses swinging arm of pantotheine You will have done these reactions in Dr Denyer’s lectures dimer of the multi-domain enzyme are probably aligned in antiparallel In the transfer step: the growing fatty acid chain is preferentially passed from the pantetheine thiol of one subunit cysteine thiol of the other ? intra-subunit substrate transfers also occur by swinging arm of pantetheine Pant-SH HS-Cys Cys-SH HS-Pant Fatty Acid Synthase dimer essential dietary cofactors: cannot be made by mammals • thiamine = vitamin B1 (in TPP) – deficiency = beri-beri – eg alcohol reduced uptakeof thiamine brain symptoms (brain glucose metabolism) • • • • • riboflavin = vitamin B2 (for FAD) niacin = vitamin B3 (NAD) lipoic acid biotin pantothenic acid (vitamin B5) advantages of multi-active site enzymes and multi enzyme complexes • diffusion distance between substrate and active sites (usually the limiting factor in determining the reaction rate) reaction rate • chance of side reactions – substrates stay within complex • coordinated control of sequential reactions Voet, Voet and Pratt (2nd Ed) • • • • • • • .DG and equilibrium pg 401 PDH pg 519 -524, regulation pg 533 TPP mechanism pg 450 thioester bonds pg 413 Pyruvate carboxylase pg 502, pg AcetylCoA carboxylase pg 651 Fatty acid synthase pg 653 (much more detail than you need for this lecture!)