* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ocular Dominance in Human V1 Demonstrated by Functional
Artificial intelligence for video surveillance wikipedia , lookup
Neurolinguistics wikipedia , lookup
Affective neuroscience wikipedia , lookup
Neuromarketing wikipedia , lookup
Neurophilosophy wikipedia , lookup
Time perception wikipedia , lookup
Cortical cooling wikipedia , lookup
Neuroeconomics wikipedia , lookup
Haemodynamic response wikipedia , lookup
Metastability in the brain wikipedia , lookup
Emotional lateralization wikipedia , lookup
Human brain wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Aging brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neurostimulation wikipedia , lookup
Magnetoencephalography wikipedia , lookup
C1 and P1 (neuroscience) wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Ocular Dominance in Human V1 Demonstrated by Functional
Magnetic Resonance Imaging
RAVI S. MENON, 1 SEIJI OGAWA, 2 JOHN P. STRUPP,3 AND KÂMIL UǦURBIL 3
1
Advanced Imaging Laboratories, The John P. Robarts Research Institute, London, Ontario N6A 5K8, Canada; 2 Lucent
Technologies Bell Laboratories, Murray Hill, New Jersey 07974; and 3Centre for Magnetic Resonance Research,
University of Minnesota, Minneapolis, Minnesota 55455
Menon, Ravi S., Seiji Ogawa, John P. Strupp, and Kâmil Uǧurbil. Ocular dominance in human V1 demonstrated by functional
magnetic resonance imaging. J. Neurophysiol. 77: 2780–2787,
1997. Very high resolution functional magnetic resonance imaging
(fMRI) at a 4 Tesla (T) magnetic field was used to map ocular
dominance regions in the human visual cortical layers using the
blood oxygen level dependent (BOLD) contrast mechanism. The
fMRI response from primary visual cortex (V1) exhibited a distribution of ocular dominance reminiscent of the single-cell recordings of Hubel and Wiesel. Pixels could be grouped into seven
categories varying from left-only response to binocular-only response to right-only responses. Nonspecific responses were found
in the MRI-visible draining veins as well as in the parenchyma.
Although large vessel BOLD signals are easily detectable, regardless of field strength, they demonstrate a fMRI response to photic
input that could not be used to distinguish ocular dominance. The
difference in BOLD response between a region activated by one
eye and that activated by the other is only 2.9% on average. This
necessitates the use of a difference paradigm to visualize the regions of ocular dominance accurately. The data show that BOLDbased fMRI is sensitive to neuronal activity in cortical columns
when using differential techniques, opening up the possibility of
mapping specialized populations of neurons in humans that are not
accessible to electrophysiological or other methods of invasive
mapping.
INTRODUCTION
In primates, axons from the left and right eyes terminate
in monocular laminae of the lateral geniculate body. From
this nucleus, geniculostriate projections to primary visual
cortex (V1) continue to reflect either left or right eye input
and terminate in layer IVC of V1 where they are arranged
in a system of roughly parallel alternating stripes known as
ocular dominance columns (ODCs). In cortical layers above
and below IVC, cortical neurons vary in the strength of their
response to inputs from the two eyes. This response can vary
from exclusive domination by one eye to equal influence of
both as one makes a tangential penetration with an electrode
through the striate cortex (Hubel and Wiesel 1962). Only
in layer IVC do the cells receive innervation from exclusively one eye, and hence the classical periodic columnar
pattern is only observed in layer IVC. In nonhuman primates,
the organization of ODCs has been studied by histological
stains and autoradiography (Hubel and Wiesel 1977; LeVay
et al. 1985; Kennedy et al. 1976), by microelectrode recordings (Hubel and Wiesel 1976), real-time optical imaging using voltage-sensitive dyes (Salzberg et al. 1973),
2780
and optical imaging of intrinsic signals (Grinvald et al. 1986,
1991; Tso et al. 1990). In humans, the ODCs have been
demonstrated as interdigitated stripes of Ç1 mm in width
by postmortem histochemical staining for cytochrome oxidase in striate cortex by Horton et al. (1984, 1990), but a
noninvasive technique for examining human striate cortex
organization on the scale of cortical functional subunits has
not been available.
The hemodynamic-response mechanism that allows visualization of orientation columns and ODCs in awake monkeys by optical imaging of intrinsic signals demonstrates
that corticovascular responses to visual stimuli can be localized to the columnar level in several mammalian species
(Grinvald et al. 1986, 1991; Malonek and Grinvald 1996;
Tso et al. 1990). The blood oxygen level dependent (BOLD)
technique (Ogawa et al. 1990a,b; Turner et al. 1991) on
which the vast majority of cortical mapping using functional
magnetic resonance imaging (fMRI) is based (Bandettini et
al. 1992; Kwong et al. 1992; Ogawa et al. 1992), is also
sensitive to the hemodynamic changes in the local vasculature, which suggests that, in principle, cortical column organization could be mapped noninvasively with the use of
fMRI as well. Although the optical data demonstrate that
the capillary bed hemodynamic response is sufficiently confined to layer IVC of the cortical column (the ODC), localization of the mapping signal in fMRI with respect to the
active cortical area and vascular tree is still quite controversial (Cohen and Bookheimer 1994; Duyn et al. 1994; Frahm
et al. 1994; Kim et al. 1994; Kwong 1995; Lai et al. 1993;
Menon et al. 1993). At issue here is the belief that BOLD
signals coming from large vessels may dominate those coming from the microvasculature, particularly on conventional
MRI scanners. This is problematic, because it raises concern
that macrovascular changes distal to the actual site of neuronal activity can occur. This would place a fundamental
limit on correlation of fMRI activation maps and neuronal
activity. Because cells above and below the ODC defined in
layer IVC can also respond in varying degrees as mentioned
above, the vascular response may not be confined to layer
IVC, but might traverse the entire depth of the cortical ribbon. Thus it may be expected, particularly with the resolution
used in fMRI, that each pixel would contain variable degrees
of left or right eye dominance, and that confinement of the
fMRI mapping signal to layer IVC may not be feasible.
When one compares previous optical mapping experiments on awake nonhuman primates (Grinvald et al. 1991;
0022-3077/97 $5.00 Copyright q 1997 The American Physiological Society
/ 9k11$$my37 J096-6
08-08-97 12:57:57
neupa
LP-Neurophys
MAPPING OCULAR DOMINANCE WITH FMRI
Malonek and Grinvald 1996) with considerably lower resolution fMRI experiments performed at a magnetic field
strength of 4 T (Menon et al. 1995), the temporal response
and sign of the optical signal from the capillary beds in V1
of awake monkeys parallels that of the fMRI time course in
certain regions of striate cortex in humans. The remarkable
similarity of the results obtained from these two techniques,
along with other theoretical and experimental evidence for
increasing capillary bed BOLD contributions at higher magnetic field strengths (Bandettini et al. 1994; Gati et al. 1997;
Menon et al. 1993, 1994; Ogawa et al. 1993; Song et al.
1994) suggest that a component of the fMRI mapping signal
arises from microvasculature, at least at the highest magnetic
fields available for human research (4 T).
For fMRI studies using clinically available hardware, Ç3mm in-plane resolution and Ç5-mm slices are typical because of the limited signal-to-noise ratio (SNR) available
in high temporal resolution imaging sequences (Callaghan
1993). Using a high-resolution fMRI pulse-sequence with
imaging hardware and parameters optimized at three different field strengths, we have found that the SNR at 4 T is at
least three times higher than at the much more commonly
available 1.5 T field strength (Gati et al. 1997). This increase
is sufficiently large to attempt imaging of ocular dominance
in human V1, where ODCs are Ç0.8–1 mm on a side for a
column and 5–10 mm long in humans (Horton and HedleyWhite 1984; Horton et al. 1990). Using a simple visual
paradigm in combination with an optimized radio-frequency
(RF) coil, head restraint, and the enhanced SNR provided
by 4 T, we have been able to demonstrate adjacent image
pixels in human V1 that respond predominantly to left or
right eye photic input, as well as many that respond only to
binocular input.
METHODS
Subjects
Five normal subjects [4 right eye and right hand dominant (3
male, 1 female), 1 left eye and left hand dominant (1 male), ages
25–32] gave informed consent before participating in this study.
All had previous fMRI experience. Approval for this protocol was
given by the University of Western Ontario Review Board for
Health Sciences Research involving human subjects, and radio
frequency power deposition guidelines established for clinical
scanners by the Food and Drug Administration were adhered to.
Activation tasks
A single round red (635 nm, 300 mcd, Radio Shack ‘‘Jumbo’’)
light-emitting diode (LED), placed above the subject’s head and
driven by a stimulator (GRASS Instruments, Quincy, MA) at 8
Hz was used for photic stimulation. The LED subtended Ç107 of
visual field. The subjects were directed to fixate on the position of
the LED, which was gated on or off by the scanner, but to generate
monocular input, subjects were instructed via a special headset
(Resonance Technology, Van Nuys, CA) when to close either the
left or the right eyelid gently as the scanning progressed. Binocular
stimulation (‘‘B’’ state), sandwiched between periods of darkness
(‘‘D’’ state), was used to identify primary visual cortex. Monocular stimulation using the open left eye (‘‘L’’ state) or open right
eye (‘‘R’’ state) was used to delineate ocular dominance regions.
Each of the states, B, D, L, and R were 15 s in duration.
/ 9k11$$my37 J096-6
2781
MRI studies
All MRI experiments were performed on a 4 T whole-body
human imager [varian (Palo Alto, CA)/Siemens (Erlangen, Germany)] with a 7.6-cm diam double-balanced, distributed capacitance transmit/receive RF coil built into the bottom of a rigid
Plexiglas head-holder. Subject head motion was restrained with an
integrated foam padded vice that pressed against the sides of the
head, around the headphones. In some cases, the surface coil was
removed and a head coil inserted for anatomic imaging, without
disturbing the subject.
Images were usually acquired with parameters (256 complex
points/readout window, 256 phase-encoding steps) that yielded a
resolution of 547 by 547 mm when using a 14 by 14-cm field of
view. To locate the calcarine sulcus at the beginning of each scanning session, we acquired multislice sagittal images with graywhite matter contrast (T 1-weighted anatomic images) at 0.5-cm
spatial increments centered about the midline using an imaging
pulse sequence we have described in detail previously (Kim et al.
1994; Menon et al. 1993; Ogawa et al. 1992). Briefly, 256 centrically ordered phase-encoding steps segmented in 4 blocks of 64
interleaved steps with a magnetization preparation inversion time
(TI) of 1.2 s were used for this high-resolution FLASH sequence
(Haase et al. 1986). For these anatomic images, the echo time
(TE) was 5 ms, the repetition time (TR) was 10 ms, the slice
thickness was 4 mm, and the segment interleave time was 4 s.
From these multislice sagittal images the calcarine fissure was
easily identified (Menon et al. 1993). Five equally spaced and
abutting oblique slices of 4-mm thickness were chosen parallel to
the calcarine fissure at the posterior occipital pole. This location
and orientation was chosen on the basis of previous MRI myeloarchitectonic analysis of layer IVC in striate cortex (Clark et al.
1992). The chosen orientation allows the columns to run perpendicular to the slice plane. Because the columns are expected to be
5–10 mm in length in humans and 0.8–1.2 mm on a side (Horton
et al. 1990), it is expected that they will be perpendicular to the
4-mm-thick slice in at least a few local regions, but not necessarily
in the whole slice, given that the calcarine sulcus is rarely straight
in human subjects. Anatomic imaging in the same manner as described above was also performed on these five slices.
All fMRI was carried out by the use of serial repetitions of a
centric ordered phase-encoded FLASH pulse sequence (TE Å 30
ms, TR Å 50 ms, slice thickness Å 4 mm, Flip Angle Ç227 ), with
an interimage spacing of 2.2 s for a total of 15 s per image. It is
worthwhile to note that the fMRI images and the anatomic images
are exactly coincident, because they are made with the same imaging sequence and resolution in the same session. We functionally
localized primary visual cortex in each subject using a pilot fMRI
protocol using binocular photic stimulation while scanning the five
slice planes selected above. To identify the maximally activated
part of primary visual cortex, three serial sets of the five slices
were made with the LED off, and three sets were made while the
LED was on for binocular stimulation. In this mapping protocol,
the dark and light states each lasted 225 s. Regions of the cortex
that responded to binocular stimulation in this multislice paradigm
were determined by a t-test as described in the analysis section
below. From these multislice maps of binocular stimulation, we
determined the imaging slice location that demonstrated maximum
striate cortex activation. Although not as elegant as other fMRI
V1 delineation techniques (Sereno et al. 1995; Tootell et al. 1995),
this simple paradigm allows rapid identification of activated primary visual cortex, which is essential to set up a single slice study.
The scanning session was then continued (within 5 min) using
the slice location that showed maximum activation. To map cortical
columns, we obtained 24 serial (in time) fMRI images of this slice
in V1 during which time different states involving both binocular
and alternating monocular stimuli were presented. In four of five
08-08-97 12:57:57
neupa
LP-Neurophys
2782
R. S. MENON, S. OGAWA, J. P. STRUPP, AND K. UǦURBIL
subjects, the column mapping protocol was repeated twice. The 24
state combination shown in Fig. 1A was used to obtain the data
presented in this paper.
FMRI analysis
All image analysis was done using Stimulate v5.0 (Strupp 1996)
provided by the University of Minnesota, running on a Sun UltraSparc 140. To initially identify primary visual cortex in the pilot
multislice scanning protocol, a Student’s t-test was done, on a pixel
by pixel basis, to compare the dark and binocularly driven states
for each slice. Only pixels with a statistically significant difference
between B and D states (P õ 0.05) were included. The functional
maps were overlaid on the corresponding anatomic slices. The
ocular dominance mapping experiment was then performed on the
single slice determined most suitable from this analysis.
Once the data from the selected single-slice column mapping
study were acquired, a map of the binocular phase of the paradigm
was made by cross-correlation of the model time course (Bandettini
et al. 1992) shown in Fig. 1B with slightly Gaussian blurred image
data. An extremely high correlation value of 0.75 was used. Because a centric phase-encoding scheme was used, the image intensity is dominated by the first few lines of the acquisition, which
occur well within the hemodynamic lag time of 5–8 s. Therefore
the reference waveform was shifted by one image to account for
the hemodynamic lag in response to visual stimulation as we have
demonstrated previously (Menon et al. 1995). The net result is a
clean map (‘‘mask’’) of the cortical ribbon and vasculature as
shown in Fig. 2B in which the pixels that we detect are activated
by either or both eyes. The blurring ensures that all layers of the
striate cortex are included in the mask. This mask was then used
as a template on the original (unfiltered) image data to generate
time courses from each 0.55 mm by 0.55 mm by 4 mm voxel
that was deemed activated by this procedure. No other image or
background thresholding, pixel clustering, or region-of-interest
(ROI) limitation was used.
The time courses of the pixels identified above were then binned
into seven categories ranging from those that responded solely to
left eye visual input, to those that responded to both eyes, to those
that responded to right eye input as has been done previously in
neurophysiology (Hubel and Wiesel 1962). Binning was accomplished by doing a Student’s t-test for significant differences between right and left eye stimulation periods at the P Å 0.05 level.
Those pixels that had significant differences were then binned ac-
cording to the percentage difference between the two monocular
conditions. Differences in right minus left response or left minus
right response that were õ1.5% were not significant at this P value
and were considered binocularly responding, those between 1.5
and 4.5% were deemed partially dominated by the appropriate eye,
and those exceeding 4.5% were considered monocular. Maps of
ocular dominance were made based on these histograms for each
subject.
RESULTS
An oblique T 1-weighted anatomic image parallel to the
calcarine fissure, acquired as described above, is shown in
Fig. 2A. Overlayed on this image, in Fig. 2B, is the functional map determined for binocular stimulation. This highresolution functional map of binocular activation was derived from the first four periods of the visual paradigm shown
in Fig. 1A using the cross-correlation function in Fig. 1B.
The activation was observed to be almost exclusively confined to the occipital pole and to coregister extremely well
with the cortical gray matter in the primary visual areas.
Areas that appeared yellow in the color scale ( ú10% change
on binocular stimulation) were in regions of cortex with
visible venous vasculature.
From such a map, we generated a time series through the
whole fMRI data set for each activated pixel, including those
overlying visible veins. Typically, this might include 2,500
pixels. These were analyzed sulcus by sulcus, and signals
from visible veins (contributing Ç 1/3 of the activated pixels)
were excluded. For example, from the ROI shown in green
in Fig. 2B, we extracted the time course shown in Fig. 3,
representing temporal data from Ç60 activated cortical pixels. This time course showed activity during binocular activation as expected because of the correlation performed, but
it also showed well-correlated activity with the paradigm
(Fig. 1A) when either eye was stimulated in isolation. Typically, six such regions (3 sulci on each side of the midline)
were used in each subject, averaging Ç500 pixels per subject
in total. For each subject, the pixel time courses were sorted
according to the histogram binning procedure described
above. The distribution of activation in each subject and the
FIG . 1. Visual paradigm and binocular
reference vector. A: the visual paradigm
consisted of 24 serial states of darkness
(D), binocular stimulation (B), and monocular stimulation of either the left (L) or
right (R) eye as shown at top. Each state
lasted 15 s during which time 1 image was
acquired. B: to identify pixels that responded to photic stimulation in either or
both eyes, a cross-correlation of images 1
to 9 was performed with the reference vector shown, shifted by 1 image to account
for the hemodynamic lag time.
/ 9k11$$my37 J096-6
08-08-97 12:57:57
neupa
LP-Neurophys
MAPPING OCULAR DOMINANCE WITH FMRI
2783
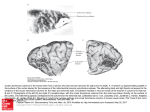
FIG . 2. Functional magnetic resonance imaging (fMRI) maps of striate cortex to binocular and monocular visual input.
A: T 1-weighted MRI image of subject 1. B: map of pixels that satisfy the cross-correlation criteria shown in Fig. 1 B at a
correlation value of 0.75. Activation is coded by color [red (1%) r yellow ( ú20%)] overlaid on a high-resolution T 1
weighted MRI image of the same subject. No significant negative changes were observed. C: map demonstrating pixels
responding predominantly to left eye monocular stimulation in reds [red (1%) r yellow ( ú10%)], whereas those responding
primarily to right eye monocular stimulation are shown in blues [blue (1%) r violet ( ú10%)]. Pixels were chosen on the
basis of the ocular dominance histograms shown in Fig. 4. D: expansion of part of C. In A–C the field of view is 14 by 14
cm.
average across all subjects is shown in Fig. 4. The distribution appears quite symmetrical from regions on either side
of the midline, precluding hemifield effects.
Maps consisting of pixels corresponding to the two left
most bins and the two right most bins of these histograms
/ 9k11$$my37 J096-6
were made, such as that shown in Fig. 2C and its expansion
in Fig. 2D. These are maps of ocular dominance, but the
responses are not necessarily confined to layer IVC as discussed earlier. Figure 5 shows the averaged temporal responses of the four left dominant and four right dominant
08-08-97 12:57:57
neupa
LP-Neurophys
2784
R. S. MENON, S. OGAWA, J. P. STRUPP, AND K. UǦURBIL
FIG . 3. Time course of image intensity
corresponding to pixels activated during the
binocular states. The ROI from which they
are extracted is shown in Fig. 2B. Contributions from visible vessels have been excluded. The fractional signal change in this
region was 2.48%.
FIG . 4. Histogram of ocular dominance
for all 5 subjects and the average across
subjects. Positive differences indicate right
eye dominance, and negative changes indicate left eye dominance. The bins are 0–
1.5% (binocular or no significant difference), 1.6–2.5%, 2.6–3.5%, and 3.5–
4.5% (monocular). At a P value of 0.05,
differences under 1.5% could not be detected in single pixels. The few pixels
whose changes were ú4.5% were placed
in the 4.5% categories.
/ 9k11$$my37 J096-6
08-08-97 12:57:57
neupa
LP-Neurophys
MAPPING OCULAR DOMINANCE WITH FMRI
2785
FIG . 5. Time course of image intensity
corresponding to pixels activated during the
monocular states. The average time course
of the 4 red pixels (left eye) and the 4 blue
pixels (right eye) marked in Fig. 2D are
shown. These pixels are chosen on the basis
of the ocular dominance histogram for subject 1 in Fig. 4 and have changes ú2.5%.
pixels indicated in Fig. 2D. To determine the mean size of
the regions demonstrating ocular dominance, we reprocessed
the image data with increasingly wider Gaussian filters until
pixels that were significantly monocularly activated were
blurred together and 50% of the left-right differentiation was
lost. This procedure is shown as a function of filter width
for two subjects in Fig. 6.
DISCUSSION
To assess the significance of the changes measured, we
first examine their magnitude relative to the noise. The mean
fractional image intensity change of the pixels we characterize as responding during binocular stimulation is 2.7 { 0.5%
(mean { SE; n Å 5). The mean fractional change in the
same regions of cortex during monocular stimulation was
similar to the binocular case at 2.9 { 0.5% (n Å 5). We
have excluded visible veins from this measurement. In the
local draining veins, changes ú20% can be observed. We
observe the maximum gray matter changes between binocular and dark stimulus conditions to be Ç5% with our slice
thickness of 4 mm, and, taking this as the maximum expected
parenchymal change, we require the fMRI mapping procedure to be sensitive to changes of õ5% in a single pixel to
detect ocular dominance regions. Given the stability of the
head and our instrument, the signal-to-noise ratio in the images and our desired confidence levels, differences of õ1.5%
between states, are not significant in a single pixel, using
FLASH, at the P Å 0.05 level. Therefore we sort our L-R
differences into bins of 0–1.5% (binocular or no significant
/ 9k11$$my37 J096-6
difference), 1.6–2.5%, 2.6–3.5%, and 3.5–4.5% (monocular). The difference could be positive or negative depending
on which eye is dominant. A positive difference indicated
right eye dominance.
One could argue that in any regions where the cortical
ribbon is not roughly perpendicular to the slice, a single
pixel could contain contributions from both sets of ODCs
and the layers that lie above and below them, and that pixel
will appear to respond to both eyes to a greater or lesser
extent. Because of this partial volume effect, the distribution
of ocular preference shown in Fig. 5 may be distorted, but
probably randomly so, because our voxel size is smaller than
the column cross-section. Nevertheless, measured over five
subjects and several thousand pixels, our individual and average distributions bear a strong resemblance to those from
single-unit recordings first derived by Hubel and Weisel. It
is interesting to note a slight preponderance of right eye
dominant pixels, consistent with the eye dominance of our
group of subjects. The pixels labeled as being binocular (less
than {1.5% change) should be in layers other than IVC in
the striate cortex. However, the pixels showing a preference
for one eye could either be in layer IVC (particularly those
that seem to respond exclusively to one eye) or in other
layers of the cortex. It is likely that the BOLD hemodynamic
response from one column ‘‘spills over’’ into adjacent columns to a certain extent (i.e., the local change in deoxyhemoglobin concentration is not perfectly confined to an electrically active column). This is nicely demonstrated by the
recent intrinsic optical imaging work of Malonek and Grinvald. Like their technique, our analysis method looks at rela-
08-08-97 12:57:57
neupa
LP-Neurophys
2786
R. S. MENON, S. OGAWA, J. P. STRUPP, AND K. UǦURBIL
FIG . 6. Effects of filtering on ocular
dominance response. For pixels such as
those shown in Fig. 2D, whose time
courses are seen in Fig. 5, the difference
between left and right responses was observed as a function of blurring. Half the
contrast between eyes is lost at a Gaussian
filter full-width at half-maximum (FWHM)
of 1.6 pixels (875 mm).
tive differences between two stimulated states (L and R).
To demonstrate the importance of such a subtraction paradigm, we note that in Fig. 2B, where the binocular and dark
states were effectively subtracted, large changes are seen in
the draining veins. However, the effective subtraction of the
L from R state (or vice versa) in Figs. 2C efficiently suppresses the draining veins because the large vessel changes
are common to both L and R states. With our resolution, we
cannot preclude that there are still some macrovascular effects that appear in our maps of ocular dominance. In the
same manner, global nonspecific parenchymal changes also
subtract out when comparing two activated states, but not
when comparing an activated state with a passive state.
Several observations can be made to establish that the
maps in Fig. 2 and the time courses shown in Fig. 5 are not
caused by random correlations of the fMRI signal with the
reference vectors. First, at the statistical thresholds used, the
BOLD changes appear confined to the cortical gray matter
and a few vessels but are not seen outside the brain nor in
the white matter. Our philosophy in determining the correlation or t-test value to use is based on setting a level that
eliminates all random activation in the background noise.
Thus the changes we observed are well above chance. Second, the activated regions are of a similar size to that expected from postmortem studies (Horton and Hedley-White
1984; Horton et al. 1990). Our blurring procedure shown
in Fig. 6 demonstrates that 50% of left-right differentiation
is wiped out by a Gaussian filter of full-width at half-maxi-
/ 9k11$$my37 J096-6
mum of 875 mm (1.6 pixels). Thus, on average, the ocular
dominance regions are 875 mm on a side. Third, the ocular
dominance areas appear confined to the primary visual cortex
as expected. Fourth, we can demonstrate that the temporal
behavior of adjacent pixels of assigned ocular dominance
are consistent with that of the stimulus paradigm (Fig. 5).
These four observations lend strong support to our identification of the activated regions as regions of ocular dominance and not random noise.
Our data demonstrate that mapping of ocular dominance
in humans is possible in a noninvasive manner. The fMRI
time courses show that the hemodynamic response can be
used as a direct indicator of neuronal activity in cortical
columns, opening up the possibility of mapping specialized
populations of neurons in humans that are not accessible to
electrophysiological or other methods of invasive mapping.
Advances in motion correction, image processing, and MRI
hardware should allow more detailed cortical mapping on
this scale.
We thank J. Gati and P. Andersen for technical assistance.
This work was supported by Grant MT13350 and a salary support award
from the Medical Research Council of Canada to R. S. Menon and National
Institutes of Health Grants 1 RO1 EY-11551-01 to R. S. Menon and RR08079 to K. Uǧurbil.
Address for reprint requests: R. S. Menon, Advanced Imaging Laboratories, The John P. Robarts Research Institute, PO Box 5015, 100 Perth
Drive, London, Ontario N6A 5K8, Canada.
Received 6 February 1996; accepted in final form 24 January 1997.
08-08-97 12:57:57
neupa
LP-Neurophys
MAPPING OCULAR DOMINANCE WITH FMRI
REFERENCES
BANDETTINI, P. A., WONG, E. C., HINKS, R. S., TIKOFSKY, R. S., AND HYDE,
J. S. Time course EPI of human brain function during task activation.
Magn. Reson. Med. 25: 390–397, 1992.
BANDETTINI, P. A., WONG, E. A., JESMANOWICZ, A., PROST, R., COX, R. W.,
HINKS, R. S., AND HYDE, J. S. MRI of human brain activation at 0.5 T,
1.5 T and 3.0 T: comparison of DR2 * and functional contrast to noise
ratio. Proc. Soc. Mag. Reson. 2: 434, 1994.
CALLAGHAN, P. T. Principles of Nuclear Magnetic Resonance Microscopy.
New York: Oxford Univ. Press, 1993, p. 93–226.
CLARK, V. P., COURCHESNE, E., AND GRAFE, M. In vivo myeloarchitonic
analysis of human striate and extrastriate cortex using magnetic resonance
imaging. Cereb. Cortex 2: 417–424, 1992.
COHEN, M. S. AND BOOKHEIMER, S. Y. Localization of brain function using
magnetic resonance imaging. Trends Neurosci. 17: 268–277, 1994.
DUYN, J. H., MOONEN, C.T.W., VAN YPEREN, G. H., DE BOER, R. W., AND
LUYTEN, P. R. In-flow versus deoxyhemoglobin effects in BOLD functional MRI using gradient echos at 1.5 T. NMR Biomed. 7: 83–88, 1994.
FRAHM, J., MERBOLDT, K.-D., HANICKE, W., KLEINSCHMIDT, A., AND
BOECKER, H. Brain or vein-oxygenation or flow? On signal physiology
in functional MRI of human brain activation. NMR Biomed. 7: 45–53,
1994.
GATI, J. S., MENON, R. S., UGURBIL, K., AND RUTT, B. K. Experimental
determination of the BOLD field dependence in tissue and vessels. Magn.
Reson. Med. In press.
GRINVALD, A., FROSTIG, R. D., SIEGEL, R. M., AND BARTFELD, E. Highresolution optical imaging of functional brain architecture in the awake
monkey. Proc. Natl. Acad. Sci. USA 88: 11559–11563, 1991.
GRINVALD, A., LIEKE, E., FROSTIG, R. D., GILBERT, C. D., AND WIESEL,
T. N. Functional architecture of cortex revealed by optical imaging of
intrinsic signals. Nature Lond. 324: 361–364, 1986.
HAASE, A., FRAHM, J., HANICKE, W., AND MERBOLDT, K.-D. FLASH imaging. Rapid NMR imaging using low flip angle pulses. J. Magn. Reson.
67: 257–266, 1986.
HORTON, J. C., DAGI, L. R., AND MCCRANE, E. P. Arrangement of ocular
dominance columns in human visual cortex. Arch. Ophthalmol. 108:
1025–1031, 1990.
HORTON, J. C. AND HEDLEY-WHITE, E. T. Mapping of cytochrome oxidase
patches and ocular dominance columns in human visual cortex. Philos.
Trans. R. Soc. Lond. B Biol. Sci. B304: 255–272, 1984.
HUBEL, D. H. AND WIESEL, T. N. Receptive fields, binocular interaction and
functional architecture in the cat’s visual cortex. J. Physiol. Lond. 160:
106–154, 1962.
HUBEL, D. H. AND WIESEL, T. N. Receptive field and functional architecture
of monkey striate cortex. J. Physiol. Lond. 195: 215–243, 1968.
HUBEL, D. H. AND WIESEL, T. N. Functional architechture of macaque monkey visual cortex. Proc. R. Soc. Lond. B Biol. Sci. B198: 1–59, 1977.
KENNEDY, C., DES ROSIERS, M. H., SAKURADA, O., SHINOHARA, M., REIVICH, M., JEHLE, J., AND SOKOLOFF, L. Metabolic maps of the primary
visual system of the monkey by means of autoradiographic 14C-deoxyglucose technique. Proc. Natl. Acad. Sci. USA 73: 4230–4234, 1976.
KIM, S.-G., HENDRICH, K., HU, X., MERKLE, H., AND UGURBIL, K. Potential
pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR Biomed. 7: 69–74, 1994.
KWONG, K. K. Functional magnetic resonance imaging with echo planar
imaging. Magn. Reson. Quart. 11: 1–20, 1995.
KWONG, K. K., BELLIVEAU, J. W., CHESLER, D. A., GOLDBERG, I. E., WEISSKOFF, R. M., PONCELET, B. P., KENNEDY, D. N., HOPPEL, B. E., COHEN,
M. S., TURNER, R., CHENG, H.-M., BRADY, T., AND ROSEN, B. R. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA 89: 5675–5679,
1992.
/ 9k11$$my37 J096-6
2787
LAI, S., HOPKINS, A. L., HAACKE, E. M., LI, D., WASSERMAN, B. A., BUCKLEY, P., FRIEDMAN, L., MELTZER, H., HEDERA, P., AND FRIEDLAND, R.
Identification of vascular structures as a major source of signal contrast
in high resolution 2D and 3D functional activation imaging of the motor
cortex at 1.5 T: preliminary results. Magn. Reson. Med. 30: 387–392,
1993.
LEVAY, S., CONNOLLY, M., HOUDE, J., AND VAN ESSEN, D. C. The complete
pattern of ocular dominance stripes in the striate cortex and visual field
of the macaque monkey. J. Neurosci. 5: 486–501, 1985.
MALONEK, D. AND GRINVALD, A. Interactions between electrical activity
and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science Wash. DC 272: 551–554,
1996.
MENON, R. S., HU, X., ADRIANY, G., ANDERSEN, P., OGAWA, S., AND UGURBIL, K. Conparison of SE-EPI, ASE-EPI and conventional EPI applied
to functional neuroimaging: the effect of flow crushing gradients on the
bold signal. Proc. Soc. Mag. Reson. 2: 622, 1994.
MENON, R. S., OGAWA, S., STRUPP, J. P., ANDERSEN, P., AND UGURBIL, K.
BOLD based functional MRI at 4 Tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using
intrinsic signals. Magn. Reson. Med. 33: 453–459, 1995.
MENON, R. S., OGAWA, S., TANK, D. W., AND UGURBIL, K. 4 Tesla gradientrecalled echo characteristics of photic stimulation-induced signal changes
in the human primary visual cortex. Magn. Reson. Med. 30: 380–386,
1993.
OGAWA, S., LEE, T. M., NAYAK, A. S., AND GLYNN, P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn. Reson. Med. 14: 68–78, 1990.
OGAWA, S., MENON, R. S., TANK, D. W., KIM, S.-K., MERKLE, H., ELLERMANN, J. M., AND UGURBIL, K. Functional brain mapping by blood oxygenation level-dependent contrast magnetic resonance imaging. Biophys.
J. 64: 803–812, 1993.
OGAWA, S., TANK, D. W., MENON, R., ELLERMANN, J. M., KIM, S.-G., MERKLE, H., OGAWA, S., LEE, T. M., KAY, A. R., AND TANK, D. W. Brain
magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 87: 9868–9872, 1990.
OGAWA, S., TANK, D. W., MENON, R., ELLERMANN, J. M., KIM, S.-G., MERKLE, H., AND UGURBIL, K. Intrinsic signal changes accompanying sensory
stimulation: functional brain mapping with magnetic resonance imaging.
Proc. Natl. Acad. Sci. USA 89: 5951–5955, 1992.
SALZBERG, B. M., DAVILA, H. V., AND COHEN, L. B. Optical recording of
impulses in individual neurons of an invertebrate central nervous system.
Nature Lond. 246: 508–509, 1973.
SERENO, M. I., DALE, A. M., REPPAS, J. B., KOWNG, K. K., BELLIVEAU,
J. B., BRADY, T. J., ROSEN, B. R., AND TOOTELL, R.B.H. Borders of multiple visual areas in humans revealed by functional magnetic resonance
imaging. Science Wash. DC 268: 889–893, 1995.
SONG, A. W., WONG, E. C., BANDETTINI, P. A., AND HYDE, J. S. The effect
of diffusion weighting on task-induced functional MRI. Proc. Soc. Mag.
Reson. 2: 643, 1994.
STRUPP, J. P. Stimulate, A Gui based fmri Analysis Software Package.
NeuroImage 3: S607, 1996.
TOOTELL, R.B.H., REPPAS, J. B., KWONG, K. K., MALACH, R., BORN, R. T.,
BRADY, T. J., ROSEN, B. R., AND BELLIVEAU, J. W. Functional analysis
of human MT and related visual cortical areas using magnetic resonance
imaging. J. Neurosci. 15: 3215–3230, 1995.
TSO, D. Y., FROSTIG, R. D., LIEKE, E. E., AND GRINVALD, A. Functional
organization of primate visual cortex revealed by high resolution optical
imaging. Science Wash. DC 249: 417–420, 1990.
TURNER, R., LE BIHAN, D., MOONEN, C. T. W., DESPRES, D., AND FRANK,
J. Echo-planar time course mri of cat brain oxygenation changes. Magn.
Reson. Med. 22: 159–166, 1991.
WOOTEN, G. F. AND COLLINS, R. C. Metabolic effects of unilateral lesion
of the substantia niagra. J. Neurosci. 1: 285–291, 1981.
08-08-97 12:57:57
neupa
LP-Neurophys