* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download oxidation
Polyclonal B cell response wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemical cascade wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Electron transport chain wikipedia , lookup
Signal transduction wikipedia , lookup
Microbial metabolism wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Proteolysis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Blood sugar level wikipedia , lookup
Phosphorylation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
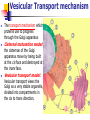
Cells and Their Housekeeping Functions – Metabolic Process Shu-Ping Lin, Ph.D. Institute of Biomedical Engineering E-mail: [email protected] Website: http://web.nchu.edu.tw/pweb/users/splin/ Date: 11.08.2010 Metabolism Cell metabolism: sum of all chemical reactions in living cell used for production of useful energy and subsequent synthesis of cell constituents Intake of food by cells from bloodstream: messenger substances (hormones) released from endocrine glands into blood stream to affect metabolism of cells that have receptors for that hormone Hormone: Anabolism: cells steadily remodel and replace their structures Catabolism: structures worn out and no longer needed are broken down into small molecules and either reused or excreted Alter permeability of cell membrane to extracellular substances, Ex: glucose Alter activity of key intracellular enzymes (pacemaker enzymes) controlling major chemical pathways, Ex: insulin increases glucose uptake by muscle cells and increases storage of glycogen. Type 1 diabetes (insulin deficiency) – depress glucose uptake and increase glycogen breakdown, causing abnormally high levels of glucose in blood ↑osmotic pressure Remove tissue water, cellular dehydration, and electrolyte loss Cells break down structural lipids and proteins when glucose starves. Protein deficiency and weight loss in type 1 diabetes Animals in low blood glucose levels Secreting epinephrine (adrenaline) from adrenal gland and glucagon from pancreas Lead to an increase in conversion of glycogen to glucose in liver. Opposite directions: glucagon and insulin establish levels in circulation Excess nutrients not immediately used are stored as glycogen (in liver and skeletal muscle; sufficient for a few hours) and fat reserve-triglyceride (sufficient fat stored for several weeks of starvation) Generation of Useful Energy from Food First stage of metabolism: large molecules split into smaller units in digestive tract, no useful energy is produced; Ex: proteins 20 amino acids, carbohydrates glucose, fats glycerol and fatty acids Second stage: occurs in cytoplasm small organic units convert into simple units, Ex: sugars, fatty acids, glycerol, and amino acids are converted into acetyl unit of acetyl CoA; process does not require oxygen, yields small amount of ATP Third stage: useful food energy, citric acid cycle and oxidative phosphorylation carried out under aerobic conditions in mitochondria Oxygen for contracting muscle cells is insufficient, pyruvate convert to lactate releasing useful energy But accumulation of lactate in muscle tissues is responsible for muscle cramps. Yeast (anaerobic organisms), pyruvate transform into ethanol Oxidation-Reduction Reactions Oxidation-reduction or redox reaction: food degradation, chemical reactions in which one or more electrons are transferred from one reactant to another, each reaction requires an electron donor and an electron acceptor Oxidation Give up e- by removing H, loss of electrons Adds O Called an electron donor or a reducing agent (reduces the accepting molecule, makes it more -) Releases energy - exergonic Reduction Gain e-(more -), addition of electrons Removes O Called an electron acceptor or oxidizing agent (oxidizes donor molecule) Stores energy - endergonic Oxidation Xe- + Y X + YeReduction Xe- is being oxidized (losing e-), acts as a reducing agent because it reduces Y Y is being reduced (gaining e-), acts as an oxidizing agent because it oxidizes Xe- Electron-transfer potential of NADH convert into phosphate-transfer potential of ATP NAD+(oxidized form) +RH2 NADH (reduced form) +H++R Redox potential: Oxidized form X reduced form X- G 0' nFE 0' Chemical reactions: degradation of food are exergonic redox (△G<0) NAD+ Oxidized form NADH Reduced form Degradation of Glucose Glucose metabolism: C6H12O6 + 6O2 6CO2 + 6H2O + energy Blocks indicate 4 separate pathways in cellular energy process, each pathway is composed of multiple consecutive reactions catalyzed by enzyme. Anaerobic Respiration Glycolysis in cytoplasm, others in mitochondria (called (Respiration without O2) cellular respiration) Glycolysis: consists of 10 reactions to convert glucose into 2 molecules of 3-carbon compounds (i.e. pyruvic acid and pyruvate) First 5 reactions consume energy: 2 ATP molecules are used to phosphorylate and activate glucose to 3-carbon sugar phosphate Aerobic Respiration nd 2 set of reactions: hydrogen atoms are removed (Respiration using O2) (oxidation) by NAD+ forming NADH (reduction): 2NAD+ + 4H (oxidation) 2 NADH (that’s a total of 4 e) -- Four ATP were produced from energy released by substrate-level phosphorylation Exergonic process with ∆G = -140kcal/mol Glycolytic pathway do not involve oxygen Glucose+ 2Pi+ 2ADP+ 2NAD+ 2 pyruvate+ 2ATP+ 2NADH+ 2H++ 2H2O Glycolysis is highly regulated. blood to meet the need for ATP. Cells acquire enough glucose from Vesicular Transport mechanism The transport mechanism which proteins use to progress through the Golgi apparatus Cisternal maturation model: the cisternae of the Golgi apparatus move by being built at the cis face and destroyed at the trans face. Vesicular transport model: Vesicular transport views the Golgi as a very stable organelle, divided into compartments in the cis to trans direction. Vesicular Transport Type Description Exocytotic vesicles Example Vesicle contains proteins destined for extracellular release. After packaging the vesicles bud off and immediately move (continuous) towards the plasma membrane, where they fuse and release the contents into the extracellular space in a process known as constitutive secretion. Antibody release by activated plasma B cells Secretory vesicles Vesicle contains proteins destined for extracellular release. After packaging the vesicles bud off and are stored in the cell until a signal is given for their release. When the appropriate signal is received they move towards the membrane and fuse to release their contents. This process is known as regulated secretion. Neurotrans mitter release from neurons Lysosomal vesicles Vesicle contains proteins destined for the lysosome, an organelle of degradation containing many acid hydrolases, or to lysosome-like storage organelles. These proteins include both digestive enzymes and membrane proteins. The vesicle first fuses with the late endosome, and the contents are then transferred to the lysosome via unknown mechanisms Digestive proteases destined for the lysosome (regulated)