* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download AP Biology Chapter 9.2016
Amino acid synthesis wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biosynthesis wikipedia , lookup
Epitranscriptome wikipedia , lookup
Metalloprotein wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Butyric acid wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Mitochondrion wikipedia , lookup
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Phosphorylation wikipedia , lookup
Light-dependent reactions wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
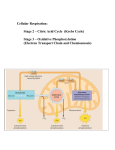
Cellular Respiration AP Biology Chapter 9 – start… • Quick Write… • On the card you picked up at the beginning of class write all you know about “cellular respiration” … its parts, what are the main components, reactants and products, its purpose, where it occurs and which organisms carry out this process! Warm Up • Which atom has a stronger affinity for electrons (ie holds its electrons closer and tighter to its nucleus) – Carbon? – Oxygen? OR • How do you know? Fig. 9-1 How do these leaves power the work of life for this chimpanzee? Coupled Reactions • What do you remember about the Cotransport of H+ and Sucrose through the plasma membrane? • Photosynthesis • Cellular respiration – Are they coupled in an ecosystem? Fig. 9-2 Light energy p163 ECOSYSTEM Photosynthesis in chloroplasts CO2 + H2O Organic +O molecules 2 Cellular respiration in mitochondria ATP ATP powers most cellular work Heat energy Cellular Respiration • The breakdown of glucose is exergonic • ATP is the result of – Fermentation (Glycolsis)Anaerobic Respiration) – Cellular Respiration (Aerobic Respiration) • ATP is used Cellular work… drives all the work! Page 149 examples… (ch8) Respiration Overview • Photosynthesis is the process of incorporating energy from light into energy-rich molecules like glucose. • Cellular Respiration is the opposite process extracting that stored energy from glucose to for ATP. • The chemical equation: energy ENERGY C6H12O6 + 6O2 6CO2 + 6 H2O Background Chemistry • OXIDATION-REDUCTION reaction = chemical reactions which involve a partial or complete TRANSFER of electrons from one reactant to another. (REDOX reactions) • OXIDATION = partial or complete LOSS of electrons • REDUCTION= a partial or complete GAIN of electrons Fig. 9-UN1 p164 becomes oxidized (loses electron) becomes reduced (gains electron) Fig. 9-UN2 becomes oxidized becomes reduced Fig. 9-3 p165 Reactants Products becomes oxidized becomes reduced Methane (reducing agent) Oxygen (oxidizing agent) Carbon dioxide Water **Cell Respiration LAB 5 • Practice Writing Lab Reports NEEDED! • Tomorrow you will need to have your lab notebook prepared with complete lab format and data table ready to record • Follow the ARHS Lab Format Guidelines ALWAYS (blue form you should have received in September/ more available) • EXAMPLE on how to start: next slide **EXAMPLE Title: Cell Respiration Purpose: (Read the full procedure; using the Overview, Objectives, Introduction and noting our discussion on previous slide--- write out in your own words the Purpose of the Lab procedure; Sometimes their will be need to make a prediction/hypothesis) Please predict which temperature will have a higher rate of cellular respiration. Cold or room temp?? Materials: “as on procedure sheet” Procedure: “as on procedure sheet” is OK but it is recommended to draw the procedural set up OR flow chart the procedure **Pre Lab Questions Answer before DATA • What is the purpose of the KOH in the procedure? • Why do we use Water Displacement when setting up the tubes? ***** • DATA: (Draw in OR tape in the data table given to you in the procedure) (There is a special table made especially for that purpose) Each lab group will be assigned a particular temperature Blue (stations 1, 2, 6) --- Cold Temp [You will need ice] Silver (stations 3, 4, 5) --- Room Temp. Lab Debrief – • • • • • 11/29/16 Record your group’s data average. Determine the class average data. What does the class data tell us? Did the data support your hypothesis? Note what needs to be included for lab writeup! – LAB Due Tuesday, 11/30/16 – What question could you ask to learn more about Cellular Respiration? Design a lab??? Fig. 9-UN3 P164 11/29/16 Cellular Respiration Loses electrons becomes oxidized becomes reduced Gains electrons Rate of Cellular Respiration • What are ways that we could measure this rate? 1. Product produced? Carbon dioxide OR Water 2. Reactant used? Glucose OR Oxygen 3. Energy produced? how would we measure energy? Video clip • Cellular Respiration • 2 PHASES – Glycolysis (anaerobic) Fermentation – Aerobic • Acetyl CoA production • Citric Acid Cycle (Krebs Cycle) • Electron Transport Chain (ETC) • Phosphorylation – ATP Cell Resp - Video Clip • What are the key players in this process? • Who does this process? • What is the purpose of this process? • What did we measure in our cell respiration lab? Carbon cycle - video clip • What does the Law of Conservation of Mass state? • How are Carbon atoms conserved? • How does cellular respiration aide in this cycle? • Could you have carbon atoms that once were part of Einstein? • 11/30/16 White Board Share 11/30/16 • Using your lab report (Analysis #14) • Record on white board your scientific question for a new lab studying Cellular Respiration… • Each student will present a very brief explanation of what you are testing for in your designed experiment – If possible share your hypothesis as well Electron Shuttle [NAD+] p166 • Nicotinamide Adenine Dinucleotide (NAD+): A dinucleotide that functions as a coenzyme in the redox reactions of metabolism • Coenzyme: small non protein organic molecule that is required for certain enzymes to function • Dinucleotide: molecules with two nucleotides Fig. 9-4 2 e– + 2 H+ 2 e– + H+ NADH H+ Dehydrogenase p166 Reduction of NAD+ NAD+ + + H+ 2[H] Oxidation of NADH Nicotinamide (reduced form) Nicotinamide (oxidized form) Fig. 9-5 p166 H2 + 1/2 O2 2H (from food via NADH) Controlled release of + – 2H + 2e energy for synthesis of ATP 1/ 2 O2 Explosive release of heat and light energy 1/ (a) Uncontrolled reaction (b) Cellular respiration 2 O2 NAD+ in Cellular Respiration • During oxidation (loss of electrons) of glucose, NAD+ functions as an oxidizing agent by trapping energyrich electrons from glucose or food. These reactions are catalyzed by enzymes called DEHYDROGENASES, which: Remove a pair of hydrogen atoms (2 eand 2p) from a substrate Deliver the 2e- and 1p to NAD+ NADH Release the remaining hydrogen proton into the surrounding solutions H+ Cellular Respiration • These high energy electrons transferred from substrate (H) to NAD+ are then passed down the ELECTRON TRANSPORT CHAIN to oxygen, powering the ATP SYNTHESIS (oxidative phosporylation) Cell Respiration Overview • Respiration in the presence of oxygen is called: aerobic respiration • Aerobic respiration is divided into three components: – Glycolysis – Krebs Cycle – Chemiosmosis -- Electron Transport Chain with Oxidative phosphorylation (oxidative respiration) Figure 9.6-3 p. 167 Electrons carried via NADH and FADH2 Electrons carried via NADH Glycolysis Glucose Pyruvate CYTOSOL Pyruvate oxidation Acetyl CoA Citric acid cycle Oxidative phosphorylation: electron transport and chemiosmosis MITOCHONDRION ATP ATP ATP Substrate-level phosphorylation Substrate-level phosphorylation Oxidative phosphorylation Fig. 9-7 p168 Enzyme Enzyme ADP P Substrate + Product ATP Glycolysis • Glycolysis is the decomposition (lysis) of glucose (glyco) to pyruvate. • Catabolic pathway during which Glucose is split into two 3-carbon sugars, which are then oxidized and rearranged by a step-wise process that produces pyruvate & ATP • Follow the next few slides on pages 169 Specifics – “must know facts” • Occurs in Cytoplasm • Catalyzed by enzymes • Occurs with or without oxygen • No CO2 is released Fig. 9-9-1 p169 Glucose ATP 1 Hexokinase ADP Glucose Glucose-6-phosphate ATP 1 Hexokinase ADP Glucose-6-phosphate Fig. 9-9-2 Glucose ATP 1 Hexokinase ADP Glucose-6-phosphate 2 Phosphoglucoisomerase Fructose-6-phosphate Glucose-6-phosphate 2 Phosphoglucoisomerase Fructose-6-phosphate Fig. 9-9-3 Glucose ATP 1 Hexokinase ADP Fructose-6-phosphate Glucose-6-phosphate 2 Phosphoglucoisomerase ATP 3 Phosphofructokinase Fructose-6-phosphate ATP 3 Phosphofructokinase ADP ADP Fructose1, 6-bisphosphate Fructose1, 6-bisphosphate Fig. 9-9-4 Glucose ATP 1 Hexokinase ADP Glucose-6-phosphate 2 Phosphoglucoisomerase Fructose1, 6-bisphosphate 4 Fructose-6-phosphate ATP Aldolase 3 Phosphofructokinase ADP 5 Isomerase Fructose1, 6-bisphosphate 4 Aldolase 5 Isomerase Dihydroxyacetone phosphate Dihydroxyacetone phosphate Glyceraldehyde3-phosphate Glyceraldehyde3-phosphate Fig. 9-9-5 2 NAD+ 2 NADH + 2 H+ 6 Triose phosphate dehydrogenase 2 Pi 2 1, 3-Bisphosphoglycerate Glyceraldehyde3-phosphate 2 NAD+ 2 NADH Energy Payoff Phase 6 Triose phosphate dehydrogenase 2 Pi + 2 H+ 2 1, 3-Bisphosphoglycerate Fig. 9-9-6 2 NAD+ 2 NADH + 2 H+ 6 Triose phosphate dehydrogenase 2 Pi 2 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP 2 1, 3-Bisphosphoglycerate 2 ADP 2 3-Phosphoglycerate 2 ATP 2 7 Phosphoglycerokinase 3-Phosphoglycerate Fig. 9-9-7 2 NAD+ 2 NADH + 2 H+ 6 Triose phosphate dehydrogenase 2 Pi 2 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP 2 3-Phosphoglycerate 8 2 3-Phosphoglycerate Phosphoglyceromutase 2 8 Phosphoglyceromutase 2-Phosphoglycerate 2 2-Phosphoglycerate Fig. 9-9-8 2 NAD+ 2 NADH + 2 H+ 6 Triose phosphate dehydrogenase 2 Pi 12/1/16 2 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP 2 3-Phosphoglycerate 2 2-Phosphoglycerate 8 Phosphoglyceromutase 9 2 2 H2O 2-Phosphoglycerate Enolase 9 Enolase 2 H2O 2 Phosphoenolpyruvate 2 Phosphoenolpyruvate Fig. 9-9-9 2 NAD+ 6 Triose phosphate dehydrogenase 2 Pi 2 NADH + 2 H+ 2 1, 3-Bisphosphoglycerate 2 ADP 7 Phosphoglycerokinase 2 ATP 2 Phosphoenolpyruvate 2 ADP 2 3-Phosphoglycerate 8 Phosphoglyceromutase 2 ATP 2 10 Pyruvate kinase 2-Phosphoglycerate 9 2 H2O Enolase 2 Phosphoenolpyruvate 2 ADP 10 Pyruvate kinase 2 ATP 2 2 Pyruvate Pyruvate Fig. 9-8 Energy investment phase p168 Glucose 2 ADP + 2 P 2 ATP used 4 ATP formed Energy payoff phase 4 ADP + 4 P 2 NAD+ + 4 e– + 4 H+ 2 NADH + 2 H+ 2 Pyruvate + 2 H2O Net Glucose 4 ATP formed – 2 ATP used 2 NAD+ + 4 e– + 4 H+ 2 Pyruvate + 2 H2O 2 ATP 2 NADH + 2 H+ Krebs Cycle – IF Oxygen is Present… • The Krebs cycle details what happens to the pyruvate end product of glycolysis. • Remember, 2 pyruvate enter the Krebs cycle, so it is actually run twice for each molecule of glucose. • The steps are detailed next… • Follow on page 170-171 Krebs Cycle • Specifics: 1. Hans Krebs, German-British scientist, 1930s 2. Also known as Citric Acid Cycle 3. Process occurs in Mitochondria Krebs Cycle / Citric Acid Cycle Part 1 (p 170) Before going into cycle: Pyruvate is converted to Acetyl CoA. In a step leading up to the actual Krebs cycle, pyruvate combines with coenzyme A (CoA). In that reaction, 1 NADH and 1 CO2 are also produced. Fig. 9-10 p170 CYTOSOL MITOCHONDRION NAD+ NADH + H+ 2 1 Pyruvate Transport protein 3 CO2 Coenzyme A Acetyl CoA Krebs – part 2 • Krebs Cycle: 3 NADH, 1 FADH2 (another electron carrier), 1 ATP, and 1 CO2 • The Krebs cycle begins when acetyl CoA combines with OAA (oxaloacetic acid) to form citric acid [citrate]. Along the way, 3 NADH and 1 FADH2 are made and CO2 is released. – Because the first product from acetyl CoA is citric acid, Krebs is sometimes referred to as the citric acid cycle. – Let’s follow along pages 170-171 Fig. 9-11 Pyruvate p170 CO2 NAD+ CoA NADH + H+ Acetyl CoA CoA CoA Citric acid cycle FADH2 2 CO2 3 NAD+ 3 NADH FAD + 3 H+ ADP + P i ATP Fig. 9-12-1 p171 Acetyl CoA CoA—SH 1 Oxaloacetate Citrate Citric acid cycle Fig. 9-12-2 Acetyl CoA CoA—SH H2O 1 Oxaloacetate 2 Citrate Isocitrate Citric acid cycle Fig. 9-12-3 Acetyl CoA CoA—SH 1 H2O Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle 3 NADH + H+ CO2 -Ketoglutarate Fig. 9-12-4 Acetyl CoA CoA—SH 1 H2O Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle NADH + H+ 3 CO2 CoA—SH -Ketoglutarate 4 NAD+ Succinyl CoA NADH + H+ CO2 Fig. 9-12-5 Acetyl CoA CoA—SH 1 H2O Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle NADH + H+ 3 CO2 CoA—SH -Ketoglutarate 4 CoA—SH 5 NAD+ Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Fig. 9-12-6 Acetyl CoA CoA—SH H2O 1 Oxaloacetate 2 Citrate Isocitrate NAD+ Citric acid cycle NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Fig. 9-12-7 Acetyl CoA CoA—SH H2O 1 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH -Ketoglutarate 4 6 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Fig. 9-12-8 Acetyl CoA CoA—SH NADH +H+ H2O 1 NAD+ 8 Oxaloacetate 2 Malate Citrate Isocitrate NAD+ Citric acid cycle 7 H2O NADH + H+ 3 CO2 Fumarate CoA—SH 6 -Ketoglutarate 4 CoA—SH 5 FADH2 NAD+ FAD Succinate GTP GDP ADP ATP Pi Succinyl CoA NADH + H+ CO2 Summary of Pathway 1. Pyruvate enters inner compartment of mitochondrion • Gives off CO2 • Converts NAD+ to NADH 2. Acetyl CoA is now a 2-carbon molecule which enters the cycle by attaching to 4carbon oxaloacetate to form 6-carbon citrate or citric acid 3. Each pyruvate or Acetyl CoA enters the cycle, thus it occurs twice for every Glucose molecule 4. As the cycle progresses: • • • • • 2 CO2 produced as waste 3 NADH produced for ETC 1 FADH2 produced for ETC 1 ATP formed for ENERGY Oxaloacetate reformed for cycle to repeat SO… for each pyruvate --- yields 4 NADH; 1 FADH2; 1 ATP; 3 CO2 For each glucose??? Oxidative Phosporylation • Oxidative phosphorylation is the process of extracting ATP from NADH and FADH2. • Electrons from NADH and FADH2 pass along an ETC analogous to ETC chains in photophosphorylation. • These electrons pass from one protein carrier to another along the chain, losing energy along the way • Follow with pages 172-175 Oxidative Phosporylation • Cytochromes and various other modified proteins act as carrier proteins in the chain. • One cytochrome, cytochrome c, can be used by geneticists to assess genetic relatedness • The last electron acceptor at the end of the chain is oxygen. • The ½ O2 accepts the 2 electrons and, together with H+ forms water. • NADH provides electrons that have enough energy to phosphorylate approximately 2.5 ADP to 2.5 ATP. • FADH2 produces approximately 1.5 ATP. • Follow page 173-175 p173 ATP Synthase p174 • ATP Synthase is embedded in the inner Mitochondrial membrane • H+ gradiant is formed thanks to the E.T.C. • This energy of H+ movement (due to concentration gradient) drives the cylindrical rotor of the complex protein • This spins in the knob – catalytic knob, which allow the joining of inorganic phosphate to ADP • This is where ATP is produced Fig. 9-14 INTERMEMBRANE SPACE p174 H+ Stator Rotor Internal rod Catalytic knob ADP + P i ATP MITOCHONDRIAL MATRIX ETC + Chemiosmosis • Coupled process – to ATP Synthesis • ETC and pumping of protons (H+) creates an H+ gradient across the membrane + • Chemosmosis -- Powered by the flow of H+ ions back across the membrane • ONLY occurs because of the presences of _____________ (final electron acceptor) Figure 9.15 p175 H H Intermembrane space H Protein complex of electron carriers Cyt c Q I IV III II FADH2 FAD NADH H 2 H + 1/2O2 H2O NAD ADP P i (carrying electrons from food) Mitochondria Matrix ATP synthase ATP H 1 Electron transport chain Oxidative phosphorylation 2 Chemiosmosis How Many ATP? How many ATP are made from the energy released from the breakdown of 1 glucose molecule? – – – – Glycolysis = 2 ATP and 2 NADH Pyruvate conversion = 2 NADH Acetyl CoA/Krebs = 6 NADH, 2FADH2, 2 ATP Oxidative phosphorylation = NADH produces 2.5 ATP each & FADH2 produces 1.5 ATP each (total = 28 ATP) Total ~ ~32 ATP (estimated number) per glucose molecule Figure 9.16 Electron shuttles span membrane 2 NADH Glycolysis 2 Pyruvate Glucose MITOCHONDRION 2 NADH or 2 FADH2 2 NADH Pyruvate oxidation 2 Acetyl CoA 2 ATP Maximum per glucose: CYTOSOL 6 NADH 2 FADH2 Citric acid cycle Oxidative phosphorylation: electron transport and chemiosmosis 2 ATP about 26 or 28 ATP About 30 or 32 ATP Mitochondria • The Krebs cycle and the conversion of pyruvate to acetyl CoA occur in the mitochondrial matrix (the fluid portion) • The ETC proteins are embedded in the cristae (inner folded membrane) • The cristae separate the mitochondrion into an inner compartment with matrix and an outer compartment between the cristae and outer membrane. Intermembrane Space Chemiosmotic Theory • Electrons from NADH and FADH2 lose energy as they pass along the ETC in oxidative phosphorylation. • That energy is used to phosphorylate ADP to ATP. • Chemiosmotic theory describes how that phosphorylation occurs. • The process is analogous to ATP generation in chloroplasts for Photosynthesis Anaerobic Fermentation • What if oxygen is not present? • If oxygen is not present, there is no final electron acceptor for the ETC. • If this occurs, then NADH accumulates. Once all the NAD+ has been converted to NADH, the Krebs cycle and glycolysis both stop (both need NAD+ to accept electrons). • Once this happens, no new ATP is produced and the cell dies. Anaerobic Fermentation • Anaerobic respiration (fermentation) is a method cells use to avoid death due to the lack of O2. – Alcoholic Fermentation – Lactate Fermentation Fig. 9-19 Glucose p179 Glycolysis CYTOSOL Pyruvate No O2 present: Fermentation O2 present: Aerobic cellular respiration MITOCHONDRION Ethanol or lactate Acetyl CoA Citric acid cycle Alcoholic Fermentation (p163) • Alcoholic fermentation (or just “fermentation”) occurs in plants, fungi, (yeast), and bacteria. The steps are as follows: • 1. pyruvate to acetaldehyde. For each pyruvate, 1 CO2 and 1 acetaldehyde are produced. The CO2 formed is the source of carbonation in fermented drinks. • 2. Acetaldehyde to ethanol. The important part of this step is that the energy in NADH is used to drive this reaction, releasing NAD+. Remember NAD+ is needed to run respiration. For each acetaldehyde, 1 ethanol is made and 1 NAD+ is produced. Fig. 9-18a p178 2 ADP + 2 P i Glucose 2 ATP Glycolysis 2 Pyruvate 2 NAD+ 2 Ethanol (a) Alcohol fermentation 2 NADH + 2 H+ 2 CO2 2 Acetaldehyde Alcoholic Fermentation • WHY? The goal of this pathway is the task of freeing NAD+ to allow glycolysis to continue. • Recall that in the absence of O2, all the NAD+ is bottled up in NADH. • The purpose of the fermentation pathway is to release some NAD+ for use by glycolysis. • The reward for this effort is only 2 ATP. Not much, but better than cell death. Lactate Fermentation (p163) • There is only one step in lactate fermentation. A pyruvate is converted into lactate (or lactic acid), and in the process, NADH gives up its electrons to form NAD+. • As in alcoholic fermentation, the NAD+ can now be used for glycolysis. When O2 becomes available, lactate can be broken down. • Because O2 is required to break down lactate, lactate fermentation creates an oxygen debt. Fig. 9-18b p178 2 ADP + 2 P i Glucose 2 ATP Glycolysis 2 NAD+ 2 NADH + 2 H+ 2 Pyruvate 2 Lactate (b) Lactic acid fermentation Metabolic Pathway 1/2/16 • Do other biomolecules besides Glucose go through Cellular Respiration? • Note p 180 Fig. 9-20 Proteins Carbohydrates Amino acids Sugars Glycolysis Glucose Glyceraldehyde-3- P NH3 Pyruvate Acetyl CoA Citric acid cycle Oxidative phosphorylation Fats Glycerol Fatty acids Control of Cellular Respiration (p181) • Feedback mechanisms control C.R. • Big Players are 1) ATP presence / concentration 2) Citrate presence/ concentration 3) AMP Presence / concentration • This mechanisms controls balance of a cell’s catabolic and anabolic reactions Fig. 9-21 Glucose AMP p181 Glycolysis Fructose-6-phosphate – Stimulates + Phosphofructokinase – Fructose-1,6-bisphosphate Inhibits Inhibits Pyruvate ATP Citrate Acetyl CoA Citric acid cycle Oxidative phosphorylation