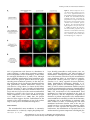
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Multiple Lines of Evidence Localize Signaling
Chloroplast DNA wikipedia , lookup
Lipid signaling wikipedia , lookup
Gene expression wikipedia , lookup
Biochemical cascade wikipedia , lookup
Paracrine signalling wikipedia , lookup
Expression vector wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Interactome wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Protein purification wikipedia , lookup
Magnesium transporter wikipedia , lookup
SNARE (protein) wikipedia , lookup
Signal transduction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Proteolysis wikipedia , lookup
Mitochondrion wikipedia , lookup
Multiple Lines of Evidence Localize Signaling, Morphology, and Lipid Biosynthesis Machinery to the Mitochondrial Outer Membrane of Arabidopsis[W][OA] Owen Duncan, Nicolas L. Taylor, Chris Carrie, Holger Eubel, Szymon Kubiszewski-Jakubiak, Botao Zhang, Reena Narsai A. Harvey Millar, and James Whelan* Australian Research Council Centre of Excellence in Plant Energy Biology (O.D., N.L.T., C.C., H.E., S.K.-J., B.Z., R.N., A.H.M., J.W.), Centre for Comparative Analysis of Biomolecular Networks (N.L.T., A.H.M.), and Centre for Computational Systems Biology (R.N.), University of Western Australia, Crawley, Western Australia 6009, Australia The composition of the mitochondrial outer membrane is notoriously difficult to deduce by orthology to other organisms, and biochemical enrichments are inevitably contaminated with the closely associated inner mitochondrial membrane and endoplasmic reticulum. In order to identify novel proteins of the outer mitochondrial membrane in Arabidopsis (Arabidopsis thaliana), we integrated a quantitative mass spectrometry analysis of highly enriched and prefractionated samples with a number of confirmatory biochemical and cell biology approaches. This approach identified 42 proteins, 27 of which were novel, more than doubling the number of confirmed outer membrane proteins in plant mitochondria and suggesting novel functions for the plant outer mitochondrial membrane. The novel components identified included proteins that affected mitochondrial morphology and/or segregation, a protein that suggests the presence of bacterial type lipid A in the outer membrane, highly stress-inducible proteins, as well as proteins necessary for embryo development and several of unknown function. Additionally, proteins previously inferred via orthology to be present in other compartments, such as an NADH: cytochrome B5 reductase required for hydroxyl fatty acid accumulation in developing seeds, were shown to be located in the outer membrane. These results also revealed novel proteins, which may have evolved to fulfill plant-specific requirements of the mitochondrial outer membrane, and provide a basis for the future functional characterization of these proteins in the context of mitochondrial intracellular interaction. Mitochondria are double membrane-bound organelles. While the inner membrane and its role in oxidative phosphorylation have been extensively studied (Eubel et al., 2004), the outer membrane is often overlooked and has been only lightly studied in plants. Much of what is known about the functions of the outer membrane has been inferred from studies carried out in yeast (Saccharomyces cerevisiae) and human (Homo sapiens). While this approach has been successful in determining the molecular identities of several key functions of the mitochondrial outer membrane, such as the protein import pore Translocase of the Outer Membrane 40-kD subunit (TOM40; Jänsch et al., 1998), and the regulators of mitochondrial distribution and morphology, mitochondrial r-type GTPases (MIRO; Yamaoka and Leaver, 2008), the lineagespecific evolution and specialization of mitochondrial * Corresponding author; e-mail [email protected]. The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: James Whelan ([email protected]). [W] The online version of this article contains Web-only data. [OA] Open Access articles can be viewed online without a subscription. www.plantphysiol.org/cgi/doi/10.1104/pp.111.183160 function has limited the applicability of much of the data gathered in other species. For example, only two of the six components making up the TOM complex in plants, TOM40 and TOM7, have been identified in plant genomes on the basis of sequence identity (Werhahn et al., 2001). Also, outer mitochondrial membrane proteins, such as OM64, which exhibits sequence similarity to a well-characterized plastidlocalized protein, would be missed or incorrectly annotated in an orthology-based approach comparing mitochondrial proteins from other organisms (Chew et al., 2004). Using GFP tagging, mitochondria have been visualized to undergo fusion, fission, and rapid movements, suggesting a dynamic interaction with components of the cytoskeleton (Sheahan et al., 2004, 2005; Logan, 2010). However, many of the specific proteins that mediate such processes remain unknown. While mitochondria do play a central role in programmed cell death in plants, it is clear that, compared with mammalian systems, different protein components mediate this process. For example, plant genomes do not appear to have genes encoding the B-cell lymphoma 2 family of proteins, which are known to mediate mitochondrial membrane permeability shifts (Reape and McCabe, 2010). The outer membrane not only acts as a barrier for molecules from the Plant PhysiologyÒ, November 2011, Vol. 157, pp. 1093–1113, www.plantphysiol.org Ó 2011 American Society of Plant Biologists. All Rights Reserved. 1093 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. cytosol to enter mitochondria, it also acts as a barrier for molecules to leave mitochondria. Thus, the outer mitochondrial membrane may also contain proteins involved in signal transduction and mediating retrograde signals from the mitochondrion to the nucleus or even from the mitochondrion to the plastid. Proteins such as Nuclear Control of ATPase (Camougrand et al., 1995) in yeast and the mammalian Mitochondrial Antiviral Signaling Protein (Koshiba et al., 2011b) are examples of such outer mitochondrial membrane signaling components identified in other species, none of which have thus far been localized in plants. The identification of proteins localized to the mitochondrial outer membrane is complicated by its relatively low protein content and its close association with the protein-rich inner membrane and endoplasmic reticulum. Furthermore, in plants, it has been shown that the inner and outer mitochondrial membranes are physically linked by a C-terminal extension of the Translocase of the Inner Membrane protein, TIM17-2, which is anchored in both membranes (Murcha et al., 2005). Similarly, in yeast, the Mitochondrial Distribution and Morphology protein, MDM10 (an ortholog of which has not been found in plant genomes), has been shown to link the mitochondrial and endoplasmic reticulum membranes (Kornmann and Walter, 2010). Attempts to isolate pure outer membrane fractions, therefore, are likely to be compromised by contamination from either of these two structures. Previous studies aimed at determining the outer membrane proteome by direct analysis of such fractions have often suffered from either limited coverage (30 proteins found, 67% estimated coverage in Neurospora crassa; Schmitt et al., 2006) or an excess of contaminants (112 proteins found, many likely contaminants, 85% estimated coverage in S. cerevisiae; Zahedi et al., 2006). Coverage estimates are calculated by summing the total numbers of proteins previously localized to the outer membrane and determining what percentage of these are independently identified in these studies. In the case of Arabidopsis (Arabidopsis thaliana) and plants in general, obtaining pure outer mitochondrial membrane fractions is further complicated by the presence of the chloroplast and a greater difficulty in obtaining sufficient quantities of mitochondria necessary to perform suborganelle proteomics on low-abundance fractions. With these challenges in mind, we developed a statistically rigorous quantitative proteomic workflow to provide a qualitative assessment of suborganelle protein location in Arabidopsis. We then coupled this with independent evaluation by biochemical and cell biology approaches (Millar et al., 2009) in order to confidently determine components of the outer mitochondrial membrane proteome of Arabidopsis. First, we enriched outer membrane vesicles from highly purified mitochondria and compared the abundance of its constituents with that in prefractionated “contaminant” samples. The proteins found to be enriched in the outer membrane fractions were confirmed or discarded as bona fide outer membrane proteins based on investigation through the literature and further experimental confirmation by GFP tagging and transient expression or by in vitro import of [35S]Metlabeled precursor proteins into purified mitochondria. By hierarchical evaluation of these data, we identified 42 mitochondrial outer membrane proteins, 27 of which are novel to this localization, for an estimated 88% coverage. The proteins identified range from plant-specific proteins with unknown functions to proteins that have putative functions in mitochondrial signaling, morphology, and defense responses. RESULTS In order to identify protein components of the mitochondrial outer membrane, we developed a workflow that used multiple lines of independent evidence to discover and confirm the subcellular localization of proteins on the mitochondrial outer membrane. The first aspect of this workflow was the selection of multiple, independent prefractionated samples for comparisons with enriched mitochondrial outer membranes in order to independently analyze the likely causes of contaminants (Fig. 1A). Furthermore, we sought a control for the protein composition of other mitochondrial membranes (IM), a control for the coenrichment of contaminating proteineous structures in the outer membrane isolation process (high-speed pellet-outer membrane [HSP-OM]), and a control for nonmembrane proteins associating with the outer membrane during the cell fraction procedures (mitoplasts). By comparing the abundance of the proteins present in each of these fractions (Fig. 1B), we were able, in effect, to subtract contaminants from the mitochondrial outer membrane fraction in a statistically rigorous fashion (Fig. 1C). It should be noted that the success or failure of this technique hinges not on the purity of the fractions analyzed but on the relative enrichment of sources of contamination in the prefractionated samples to levels greater than that observed in the mitochondrial outer membrane fraction. The result of these analyses was termed the putative outer mitochondrial membrane proteome. The putative subcellular/submitochondrial location for each of these proteins was then assessed by techniques independent of the fractionation procedure, including GFP tagging or in vitro mitochondrial protein import experiments. To rigorously define the outer mitochondrial membrane proteome, candidate proteins needed two independent pieces of evidence, such as enrichment in the outer membrane fraction as determined by mass spectrometry (MS), and confirmation by another independent approach, such as in vitro or in vivo targeting ability to the outer membrane or westernblot analysis (Millar et al., 2009). The application of these criteria led to the identification of a stringent set of 42 proteins (Fig. 1C). 1094 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane Figure 1. Experimental strategy used to determine the outer mitochondrial membrane proteome. Three samples were analyzed to determine a putative outer membrane proteome that was subsequently evaluated by a combination of prior knowledge, GFP localization, and in vitro protein uptake experiments. A, Each sample was derived from a crude organelle preparation prepared from Arabidopsis cell culture. For the desired fraction (red, mitochondrial outer membrane), isolated mitochondria in hypoosmotic solution were subjected to mechanical disruption. The inner and outer membranes were separated by Suc gradient centrifugation. Two samples were retained from this procedure: enriched mitochondrial outer membranes and enriched mitochondrial inner membranes (intramitochondrial contaminants [blue]). Extramitochondrial contaminants (green [HSP-OM]) were enriched by subjecting the untreated crude organelle isolation to the same Suc gradient enrichment process used to enrich the mitochondrial outer membrane. B, Each of these samples was analyzed by MS, and the abundance of constituent proteins was compared. C, Statistical analysis of the data yielded a putative outer membrane proteome. This putative proteome was further refined by the use of a second quantitative technique, iTRAQ, which included an additional sample designed to eliminate matrix contaminants from the putative outer membrane. Additional evidence from previous literature was used to confirm or reject members of this refined list. Identifications for which no rigorous prior evidence existed were independently confirmed or rejected by the use of GFP localization and/or in vitro protein import experiments. Preparation of Mitochondrial Outer Membrane and Prefractionated Samples To enrich mitochondria from which outer membranes could be isolated, a two-stage separation of the organelle fraction of Arabidopsis protoplasts was carried out. This involved first obtaining a crude organelle fraction (referred to as the HSP) and then enriching mitochondria from this fraction, initially by density gradient centrifugation and subsequently by free-flow electrophoresis (FFE). The ability of FFE fractionation to separate mitochondria from other cellular contaminants, even those in close association with the mitochondria (such as the endoplasmic reticulum) on a basis independent of organelle density (i.e. surface charge) allows mitochondria to be enriched to a greater degree than single physical property isolation strategies (Fig. 2). The mitochondrial enrichment of each of these fractions was assessed by comparison of Coomassie blue staining profiles (Fig. 2A) and immunodetection (Fig. 2, B and C) of proteins found in mitochondria (TOM40), peroxisomes (3-KetoacylCoenzyme A Thiolase [KAT2]), plastids (ribulose bisphosphate carboxylase, large subunit, and lightharvesting complex B [LhcB]), and the endoplasmic reticulum (Calnexin; Fig. 2). Coomassie blue staining of proteins separated by SDS-PAGE indicated that both the gradient-fractionated and FFE-fractionated samples were enriched in mitochondria compared with the starting material, the HSP (Fig. 2A). Imagebased quantitation of band intensity following immunodetection confirmed this enrichment, as indicated by the presence of the outer membrane marker, TOM40-1, which was 2.5-fold higher following gradient fractionation and 3-fold higher after FFE (Fig. 2B). Levels of endoplasmic reticulum were assessed with Plant Physiol. Vol. 157, 2011 1095 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. Figure 2. Assessment of the enrichment of mitochondria from Arabidopsis suspension cell cultures. A, One-dimensional SDSPAGE separation of three fractions isolated at different stages of mitochondrial enrichment. HSP is the result of lysing Arabidopsis suspension cell cultures and pelleting the insoluble fraction. Percoll Grad represents mitochondrial enrichment from the HSP by application of the insoluble fraction to two serial Percoll density gradient centrifugations, preserving the fractions most enriched in mitochondria. In order to obtain our most enriched sample, the gradient-enriched mitochondrial fraction was again fractionated by surface charge utilizing FFE, with the fractions most enriched in mitochondria being retained. B, Assessment of the enrichment of mitochondrial fractions by western blot. TOM40-1 (At3g20000.1) is a mitochondrial outer membrane marker, KAT2 (At2g33150.1) is a peroxisomal marker, Calnexin (At5g61790.1 and At5g07340.1) is present in the endoplasmic reticulum, and the large subunit of ribulose bisphosphate carboxylase (RbcL; AtCg00490.1) is a soluble plastid protein. C, Assessment of plastid contamination of mitochondrial fractions by western blot. At plastids, Fraction enriched in plastids from Arabidopsis; Ps plastids, fraction enriched in plastids isolated from pea (Pisum sativum). LhcB was located on the plastid thylakoid membrane. an antibody raised to recombinant human Calreticulin (which primarily detects the membrane-bound form of this protein, Calnexin, in Arabidopsis, as evidenced by the size and solubility of the detected protein). These images indicated that endoplasmic reticulum levels were reduced 14-fold by gradient fractionation and more than 400-fold by the FFE process. Two major bands were detected in the HSP fraction (Fig. 2B) corresponding to the two Calnexin isoforms At5g07340.1 (61.4 kD) and At5g61790 (60.4 kD). Peroxisomal contamination, as indicated by the presence of KAT2, was reduced by 1.5-fold after gradient fractionation and 28-fold following FFE enrichment. Similarly, antibodies raised to the Rubisco large subunit showed a 4-fold reduction in chloroplast contamination after gradient purification and a 60-fold reduction following FFE (Fig. 2B). An additional membrane-bound plastid marker, LhcB, was readily detectable in enriched plastid samples and present in the HSP fraction but was undetectable in either of the mitochondriaenriched fractions (Fig. 2C). This high level of enrichment laid a firm foundation for the subsequent identification of extramitochondrial contaminants present in outer membrane fractions. Mitochondria were then subfractionated in order to separate the inner and outer membranes. This process is perhaps the most problematic step in the enrichment of mitochondrial outer membrane, as the inner and outer membranes are physically linked by proteins such as TIM17-2 (Murcha et al., 2005). In order to separate them, mitochondria were placed in hypoosmotic solution, swelling the highly folded inner membrane to beyond the capacity of the unfolded outer membrane. This ruptures the outer membrane, which is then dissociated from the inner membrane by several gentle strokes with a Potter-Elvehjem homogenizer. The protein-rich, dense inner membranes are separated from the less dense outer membranes by sedimentation and subsequent flotation through Suc gradients. This membrane fractionation was performed on two samples, the first being FFE-enriched mitochondria, to yield enriched mitochondrial outer membrane (Mt OM), and the second being the crude organelle enrichment HSP, to yield a contaminated outer membrane fraction (HSP-OM). Additional prefractionated samples consisting predominantly of mitochondrial inner membranes (Mt IM) and mitochondrial inner membranes and matrix (mitoplast) were prepared to allow the identification of contaminants arising from these compartments. The composition of these samples was examined by immunodetection of proteins characteristic of the endoplasmic reticulum, plastids, and the mitochondrial inner membrane (Fig. 3). Coomassie Brilliant Blue 1096 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane staining of the separated fractions revealed differing protein profiles for each of the fractionated samples (Fig. 3A). Identification of several high-abundance bands on this gel by electrospray ionization-tandem mass spectrometry (ESI-MS/MS) showed that the most abundant band in the mitochondrial outer membrane fraction consists of the previously identified mitochondrial outer membrane proteins, VoltageDependent Anion Channel1 (VDAC1), -2, -3, and -4. Identification of another high-abundance band present in the prefractionated samples but absent from the outer membrane fraction showed it to predominantly consist of a subunit of the b-subunit of ATP synthase, which is located on the inner mitochondrial membrane. Details of these identifications can be found in Supplemental Table S7. Immunodetection of the mitochondrial outer membrane marker TOM40 (At3g20000.1) in these same fractions (Fig. 3B) revealed strong enrichment of the outer membrane and corresponding depletion in the prefractionated samples. Detection of TOM40 in the mitoplast and inner membrane samples is a result of the incomplete separation of outer membrane from inner membrane during the homogenization process. Conversely, detection of the mitochondrial matrix marker Pyruvate Dehydrogenase subunit E1 (At1g01090.1), the mitochondrial inner membrane marker cytochrome c oxidase subunit 2 (COXII; AtMg00160.1), the endoplasmic reticulum membrane marker Calnexin, and the soluble peroxisome marker KAT2 revealed depletion of each of these contaminants in the outer membrane fraction and enrichment in at least one of the prefractionated samples. Detection of KAT2 in the mitoplast sample is a result of residual peroxisomal contamination of the mitochondrial enrichment procedure and can also be observed in Figure 2B. Owing to the enriched/unenriched sampling strategy used, this residual contamination is accounted for by the inclusion of HSP-OM, which is more enriched in peroxisomes and plastids than Mt OM (Fig. 2B); however, KAT2, being a soluble protein, is lost in the preparation of this membrane sample. Immunodetection of additional membrane-anchored proteins characteristic of the plastid outer envelope (TOC159; At4g02510.1) and peroxisomal membrane (PMP22; At4g04470.1) did not detect these proteins in any of the subfractionated samples. However the chloroplast membrane proteins TOC159 (data not shown) and LhcB (Fig. 2C) were detected in purified chloroplasts. Figure 3. Assessment of the enrichment of mitochondrial outer membrane and contaminants. A, One-dimensional SDS-PAGE separation of contaminant and purified outer membrane fractions. The indicated band identifications were conducted by ESI-MS/ MS, and details can be found in Supplemental Table S7. B, Western-blot assessment of the experimental fractions used for semiquantitative analysis by MS. TOM40-1 (At3g20000.1) is a mitochondrial outer membrane marker, pyruvate dehydrogenase subunit E1a (PDH E1a) is present in the mitochondrial matrix, COXII (AtMg00160.1) is on the matrix side of the inner mitochondrial membrane, Calnexin (At1g56340) is present on the endoplasmic reticulum membrane, and KAT2 (At2g33150) is a soluble peroxisomal marker. These membranes were also probed with the additional antibodies TOC159, TOC75, translocase of inner chloroplast membrane 40-kD subunit (TIC40), and peroxisomal membrane protein 22, but each of these antibodies failed to detect appropriately sized products. Plant Physiol. Vol. 157, 2011 1097 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. MS Analysis of Outer Membrane Composition The first quantitative approach undertaken involved the counting of spectra that matched individual proteins in the purified outer membrane fraction. These counts were then compared with those observed in the prefractionated samples. The first of these comparisons was designed to eliminate membraneassociated contaminants arising from other organelles/ membrane systems (Mt OM:HSP-OM). The second of these comparisons was designed to identify contaminants in the Mt OM fraction that arose from other mitochondrial compartments (Mt OM:Mt IM). These comparisons enabled a quantitative assessment of whether their constituent proteins were located on the outer mitochondrial membrane or elsewhere in the cell, allowing the assembly of a short list of likely outer membrane proteins. Each of the protein samples (Mt OM, Mt IM, and HSP-OM) was acetone precipitated, digested, and analyzed by ESI-MS/MS. A total of 751 unique Arabidopsis proteins were identified across three biological replicates of this set of samples. A false discovery rate for this data set was empirically determined by compiling the mass spectra from each of the ESI-MS/MS analyses and searching this file against a decoy (shuffled The Arabidopsis Information Resource [TAIR] 9 data set) database. These searches indicated a false discovery rate of 1.5% at the peptide level. Of the 751 proteins, 185 were identified in the outer membrane fraction (Mt OM) in more than one biological replicate (Supplemental Table S1). By modeling the numbers of spectra observed for each protein using Poisson regression, we found a total of 64 proteins that were enriched in the outer membrane fractions using the following criteria: must be detected in two or more biological replicates and have a P value of 0.13 or less for enrichment over both the HSP-OM and Mt IM samples when the spectra from all biological replicates are pooled (Supplemental Table S2). This P value was intentionally less stringent at this preliminary stage in order to maximize the ability of these analyses to discover putative mitochondrial outer membrane proteins, given that any unintentionally included contaminants would be removed in the subsequent experimental confirmation steps. The validity of this analysis was also investigated by GFP-targeting studies of several proteins falling on either side of this cutoff, which indicated that loosening the P value further would increase the number of contaminants without including more outer membrane proteins in the preliminary list (data not shown). Proteins that were only detected in the Mt OM samples, provided that they were present in more than one biological replicate, were also included on the basis that these were likely to be lower abundance mitochondrial outer membrane proteins, only detected following enrichment. This list of 64 proteins did not contain abundant mitochondrial inner membrane proteins such as the ADP/ATP carrier and was generally devoid of components of the mitochondrial respiratory chain. The 64 proteins encompassed 15 of the 17 proteins that have previously been shown to be located in the outer mitochondrial membrane in various studies in plants (Werhahn et al., 2001; Chew et al., 2004; Scott et al., 2006; Lister et al., 2007; Xu et al., 2008; Yamaoka and Leaver, 2008; Lee et al., 2009). Despite the difficulties inherent in working with plant cells, such as limited starting material, lysis complications due to the cell wall, and extra contamination arising from an additional organelle, the percentage of proteins in this preliminary list compares favorably with previously published outer mitochondrial membrane proteomes from nonplant species. We calculated 88% coverage of known outer membrane mitochondrial proteins in our preliminary data set versus 85% in S. cerevisiae (Zahedi et al., 2006) and 67% in N. crassa (Schmitt et al., 2006). This coverage was achieved with a pool of just 64 proteins versus 118 in S. cerevisiae. The two proteins that were not included in this preliminary set were TOM7 (At5g41685.1) and BIGYIN (At3g57090.1; an Arabidopsis ortholog of Fis1 in S. cerevisiae and mammals; Scott et al., 2006). Literature, MS, GFP Fluorescence, and in Vitro Import to Confirm Outer Membrane Composition Additional lines of evidence were sought until each protein present in the 64-member putative outer membrane proteome had two independent lines of evidence to confirm this location or a single high-quality line of evidence to dispute its inclusion in this list. A summary of this information can be found in Tables I and II and Supplemental Table S3. Literature Components of the TOM complex have been isolated previously from mitochondrial outer membrane preparations and identified by a variety of methods (Werhahn et al., 2001; Braun et al., 2003), contributing literature evidence for the TOM components TOM 40-1, 20-2, 20-3, 20-4, 9-1, 9-2, 5, and 6. Other members of the mitochondrial protein import apparatus, Metaxin (At2g19080.1) and OM64 (At5g09420.1), have previously been shown to be located in the outer mitochondrial membrane by GFP studies (Chew et al., 2004; Lister et al., 2007). Several members of the VDAC family, DGS1 (At5g12290.1) and ELM1 (At5g22350.1), have also been shown to be located in the mitochondrial outer membrane by GFP studies (Arimura et al., 2008; Xu et al., 2008; Lee et al., 2009; Table I). A number of proteins present in the putative outer mitochondrial membrane proteome in this study have previously been reported to be in other subcellular locations. These proteins included TIM9 (At3g46560.1) and TIM13 (At1g61570.1), which have been previously reported to be soluble proteins of the mitochondrial intermembrane space (Koehler, 2004; Lister et al., 1098 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane Table I. Proteins confirmed to be in the mitochondrial outer membrane Columns from left to right are as follows: AGI No., Arabidopsis Genome Initiative identifier (www.Arabidopsis.org); Description, common identifier; Raw Counts, tally of the total number of identifying ions summed from three biological replicates from the outer membrane sample followed by the HSP-OM and then the inner membrane sample; Ratio OM:HSP-OM, number of identifying ions identified in the outer membrane sample divided by the number found in the HSP-OM sample; P, P value applying to the magnitude of change (Supplemental Tables S3 and S4) as calculated using Poisson regression analysis; Ratio OM:IM, number of identifying ions identified in the outer membrane sample divided by the number found in the inner membrane sample; P, P value applying to the magnitude of change (Supplemental Tables S3 and S4) as calculated using Poisson regression analysis; iTRAQ, these three columns contain the ratios of reporter ions in IM/OM (inner membrane divided by outer membrane), Whole/OM (whole mitoplasts [mitochondria with outer membrane ruptured but inner membrane intact] divided by outer membrane HSP-OM/OM [HSP-OM divided by outer membrane]; values reported here are significant according to the criteria detailed in “Results”); GFP Location, interpretation of subcellular localization resulting from fluorescence microscopy (ÖMtOM refers to a sole mitochondrial outer membrane localization, while ÖMtOM++ refers to a localization on the mitochondrial outer membrane and other locations); Reference, references to previously published location information. A single protein, At5g05520.1 (SAM50-2), demonstrated enrichment in the mitochondrial outer membrane fractions but did not display specific targeting when tagged with GFP; it is included here due to evidence gathered from protease-treated mitochondria (Fig. 5). ‘ is used to indicate that the ratio cannot be calculated because a null value (0) was obtained and thus the P value cannot be calculated, as shown by ^. Raw Counts AGI No. Description Spectral Counting OM:HSPOM:IM Ratio OM:HSP-OM iTRAQ P Ratio OM:IM P GFP IM/OM Whole/OM HSP-OM/OM Location At1g04070.1 TOM9-2 3:0:0 ‘ ^ ‘ ^ At1g05270.1 41:7:0 5.9 1.50E-05 ‘ ^ At1g06530.1 At1g24267.1 At1g27390.1 TraB family protein Myosin related Unknown protein TOM20-2 53:13:0 13:3:0 6:0:0 4 4.3 ‘ 5.60E-06 0.022 ^ ‘ ‘ ‘ ^ ^ ^ At1g49410.1 TOM6 11:2:0 5.5 0.027 ‘ ^ At1g50460.1 At1g53000.1 At2g01460.1 41:22:0 53:24:16 8:0:0 1.9 2.2 ‘ 0.018 0.001 ^ ‘ 3.3 ‘ ^ 0.00003 ^ At2g19080.1 HXKL-1 KDSB ATP binding/kinase Metaxin 14:6:1 2.3 0.082 14 0.011 At2g19860.1 At2g38280.1 At2g38670.1 At3g01280.1 HXK2 FAC1 PECT1 VDAC1 92:71:25 7:2:0 55:28:0 90:50:53 1.3 3.5 2 1.8 0.101 0.118 0.004 0.001 3.7 ‘ ‘ 1.7 8.00E-09 ^ ^ 0.002 0.276 0.255 0.264 0.233 0.27 0.254 0.417 0.31 At3g11070.1 At3g20000.1 SAM50-1 TOM40-1 53:24:3 93:42:12 2.2 2.2 0.001 1.90E-05 17.7 7.7 1.30E-06 2.4E-11 0.564 0.217 0.511 0.274 0.429 0.395 At3g20040.1 At3g27080.1 HXK4 TOM20-3 13:4:0 23:0:0 3.2 ‘ 0.039 ^ ‘ ‘ ^ ^ At3g27930.1 44:13:0 3.4 0.0001 ‘ ^ 29:13:0 10:4:0 30:5:0 2.2 2.5 6 0.016 0.121 0.0002 ‘ ‘ ‘ ^ ^ ^ At3g63150.1 At4g29130.1 At5g05520.1 At5g08040.1 Unknown protein BCS-1 BCS-1-like Unknown protein MIRO2 HXK1 SAM50-2 TOM5 7:0:0 124:97:31 32:8:13 21:12:8 ‘ 1.3 4 1.75 ^ 0.07 0.0004 0.122 ‘ 4 2.5 2.6 ^ 1.2E-12 0.006 0.02 At5g09420.1 OM64 34:13:0 2.6 0.003 ‘ ^ 0.203 0.211 0.195 At5g12290.1 At5g15090.1 At5g17770.1 72:23:3 97:63:63 34:7:0 3.2 1.5 4.9 1.9E-06 0.008 0.0001 24 1.5 ‘ 7E-08 0.008 ^ 0.201 0.422 0.185 0.377 0.446 0.234 0.372 At5g22350.1 DGS1 VDAC3 NADH:cytochrome B5 reductase ELM1 17:6:0 2.8 0.028 ‘ ^ At5g27540.1 At5g40930.1 MIRO1 TOM20-4 89:55:8 8:0:0 1.6 ‘ 0.005 ^ 11.1 ‘ 7E-11 ^ At5g43970.1 TOM9-1 15:5:4 3 0.033 3.75 0.019 At3g50930.1 At3g50940.1 At3g58840.1 Reference Werhahn et al. (2001) ÖMtOM 0.285 0.462 ÖMtOM ÖMtOM Werhahn et al. (2001) Werhahn et al. (2001) 0.305 ÖMtOM ÖMtOM ÖMtOM 0.165 0.229 Lister et al. (2007) ÖMtOM ÖMtOM ÖMtOM Lee et al. (2009) ÖMtOM Werhahn et al. (2001) ÖMtOM Werhahn et al. (2001) 0.162 ÖMtOM 0.231 ÖMtOM ÖMtOM ÖMtOM 0.213 0.23 ÖMtOM ÖMtOM See legend Werhahn et al. (2001) Chew et al. (2004) Xu et al. (2008) Lee et al. (2009) ÖMtOM ÖMtOM 0.2 Arimura et al. (2008) 0.214 Werhahn et al. (2001) Werhahn et al. (2001) (Table continues on following page.) Plant Physiol. Vol. 157, 2011 1099 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. Table I. (Continued from previous page.) Raw Counts AGI No. At5g55610.1 Description Unknown protein VDAC4 Anion transport VDAC2 At5g57490.1 At5g60730.1 At5g67500.1 Outer membrane and other At5g16870.1 Aminoacyl-tRNA hydrolase At5g20520.1 WAV2 At4g35970.1 APX5 At5g39410.1 Unknown protein Spectral Counting iTRAQ GFP IM/OM Whole/OM HSP-OM/OM Location OM:HSPOM:IM Ratio OM:HSP-OM P Ratio OM:IM P 50:19:0 2.6 0.0003 ‘ ^ 80:36:37 13:5:0 122:77:64 2.2 2.6 1.6 0.00007 0.07 0.002 2.2 ‘ 1.9 0.0001 ^ 0.00003 16:6:0 2.7 0.04 ‘ ^ ÖMtOM++ 16:3:0 10:4:0 52:34:3 5.3 2.5 1.5 0.008 0.121 0.054 ‘ ‘ 17.3 ^ ^ 1.5E-06 ÖMtOM++ ÖMtOM++ ÖMtOM++ 2005). A variety of other proteins in the set of 64 are known to be proteins in the inner mitochondrial membrane: Ubiquinone cytochrome c oxidoreductase-like family protein (At3g52730.1) is a subunit of complex III (Meyer et al., 2008), F0-ATPase subunit 9 (AtMg01080.1) has been shown to be a member of complex V, and Frostbite1 (FRO1; At5g67590.1), unknown protein (At1g68680.1), and unknown protein (At4g16450.1) are subunits of complex I (Meyer et al., 2008). Isocitrate Dehydrogenase1 (IDH1; At4g35260.1) has also been shown to be present in the inner membrane/matrix fraction of mitochondria (Zhao and McAlister-Henn, 1996; Table II). MS-iTRAQ Initial inspection of the putative proteome indicated the presence of soluble contaminants arising from the mitochondrial matrix. IDH6 (At3g09810.1) and Aspartate Aminotransferase (ASP1; At2g30970.1; Table II) are two high-abundance matrix enzymes. We chose to address this source of contamination by conducting an additional quantitative proteomic study using isobaric tags. This study evaluated the abundance of proteins in four fractions: the same three as used in the spectral counting analysis with the addition of a sample composed chiefly of mitoplasts (i.e. mitochondrial matrix and inner membrane structures depleted in outer membrane). A ratio of 0.7 at P , 0.05 was selected as the cutoff on the basis that known outer membrane proteins were included, while known contaminants were excluded. The validity of this cutoff was confirmed by GFP localization of IDH6 (iTRAQ [for isobaric tags for relative and absolute quantitation] mitoplast:outer membrane ratio of 0.8, geometric SD of 1.176) and g-aminobutyrate (GABA) aminotransferase (At3g22200.1; iTRAQ mitoplast:outer membrane ratio of 0.776, geometric SD of 1.185), two proteins that fell close to but outside the cutoff and were shown to localize to the mitochondria but not to the outer membrane (Supplemental Fig. S3, I and II). Thus, the iTRAQ analysis using these criteria contributed evi- Reference ÖMtOM 0.187 0.249 0.199 Lee et al. (2009) ÖMtOM Lee et al. (2009) 0.464 dence for the removal of four proteins from the list of 64, namely ASP1, IDH6, GABA aminotransferase, and a putative NADH:cytochrome B5 reductase (At5g20080.1). GFP Tagging These exclusions left a group of 38 proteins for which additional evidence was sought in the form of GFP localization. To accurately use this method, it was first established that GFP and fluorescence microscopy were capable of distinguishing mitochondrial inner membrane or matrix proteins from those located on the outer mitochondrial membrane (Fig. 4). For these purposes, an inner membrane protein, FRO1 (At5g67590.1; Heazlewood et al., 2003), a matrix protein, IDH6 (At3g09810.1; Heazlewood et al., 2004), and a previously characterized outer membrane protein, Elongated Mitochondria1 (ELM1; At5g22350.1; Arimura et al., 2008), fused to GFP, were transiently transformed into Arabidopsis cell culture along with a mitochondrial matrix-targeted red fluorescent protein control and observed using fluorescence microscopy (Fig. 4). A clear difference was observed between the fluorescence patterns of the control proteins located in the inner membrane-FRO1 (Fig. 4A) and matrix-IDH6 (Fig. 4B) and the outer membrane-ELM1 (Fig. 4C). The inner membrane- and matrix-located controls showed filled, green, circular fluorescence patterns, whereas the outer membrane control (ELM1) displayed green ring patterns. This ring pattern can also be observed for the unknown function At5g55610.1 (Fig. 4D), which was in the set of 38 and deemed to be a positive localization of the GFP fusion protein to the mitochondrial outer membrane. This is also consistent with previous reports using GFP tagging of mitochondrial outer membrane proteins (Setoguchi et al., 2006; Lister et al., 2007). Similar ring structures corresponding to a mitochondrial outer membrane location were observed for 25 of the 38 proteins tested. Of these 25 proteins, 21 only displayed this pattern surrounding the red fluorescent protein control (Supplemental Fig. 1100 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. At5g67590.1 At3g09810.1 At3g22200.1 At5g15640.1 At4g04180.1 At1g61570.1 At1g68680.1 Mitochondrial Mitochondrial Mitochondrial Mitochondrial Mitochondrial Mitochondrial At4g00355.1 Not mitochondrial Mitochondrial At3g63520.1 Not mitochondrial At4g27760.1 At2g45870.1 Not mitochondrial At4g33360.1 At2g26240.1 Not mitochondrial Not mitochondrial At1g75200.1 Not mitochondrial Not mitochondrial At2g30970.1 At5g20080.1 AGI No. Mitochondrial Mitochondrial Location Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Unknown protein TIM13 Mitochondrial substrate carrier family protein Unknown protein IDH6 GABA aminotransferase Terpene cyclase/mutaserelated FRO1 Unknown protein FEY ASP1 NADH-cytochrome B5 reductase, putative Flavodoxin family protein Unknown protein Bestrophinlike protein CCD1 Description ^ ‘ 8:0:0 14:3:0 10:0:0 ^ ^ ‘ 7:0:0 ‘ ^ 0.00004 ‘ 3.6 17:0:4 47:13:29 0.015 ^ ‘ 5:0:0 4.7 ^ ^ 10:0:0 4:0:0 ‘ ^ ‘ ‘ 8:0:0 0.011 2 ^ ‘ 4:0:0 40:20:2 ^ ‘ 9:0:0 ^ 0.002 ‘ 3.3 0.008 P Ratio OM:HSP-OM ^ ^ ‘ ‘ ^ ^ 0.009 0.041 ^ ^ ^ ^ 3.5E-05 ^ ‘ ‘ 4.2 1.6 ‘ ‘ ‘ ‘ 20 ‘ ^ ^ ‘ ‘ 0.017 0.00015 P 2.6 7.5 Ratio OM:IM Spectral Counting 3.1 22:7:0 23:0:9 30:9:4 OM:HSP-OM: IM Raw Counts 0.557 0.341 1.229 0.713 IM/ OM 0.802 0.776 1.284 Whole/ OM iTRAQ 0.403 0.51 0.611 CON/ OM Mitochondrial Mitochondrial Mitochondrial Mitochondrial Mitochondrial Not mitochondrial Not mitochondrial Not mitochondrial Not mitochondrial Not mitochondrial Not mitochondrial Not mitochondrial GFP Location Inner membrane or matrix Inner membrane or matrix Inner membrane or matrix Inner membrane, complex I Intermembrane space Inner membrane, complex I Previous Published Location Lister et al. (2004) Meyer et al. (2008) Meyer et al. (2008) Reference (Table continues on following page.) Inner membrane or matrix In Vitro Import Result Table II. Proteins found not to be in the mitochondrial outer membrane From the initial list of 64 proteins found to be enriched in the outer membrane sample, 22 were excluded from the outer membrane proteome by one or more lines of additional evidence. Columns from left to right are as follows: Location, the subcellular compartmentalization interpretation; AGI No., Arabidopsis Genome Initiative identifier (www.Arabidopsis.org); Description, common identifier; Raw Counts, tally of the total number of identifying ions summed from three biological replicates from the outer membrane sample followed by the HSP-OM and then the inner membrane sample; Ratio OM:HSP-OM, number of identifying ions identified in the outer membrane sample divided by the number found in the HSP-OM sample; P, P value applying to the magnitude of change (Supplemental Tables S1 and S2) as calculated using Poisson regression analysis; Ratio OM:IM, number of identifying ions identified in the outer membrane sample divided by the number found in the inner membrane sample; P, P value applying to the magnitude of change (Supplemental Tables S1 and S2) as calculated using Poisson regression analysis; iTRAQ, these three columns contain the ratios of reporter ions in IM/OM (inner membrane divided by outer membrane), Whole/OM (whole mitoplasts [mitochondria with outer membrane ruptured but inner membrane intact] divided by outer membrane HSP-OM/OM [HSP-OM divided by outer membrane]; values reported here are significant according to the criteria detailed in “Results”); GFP Location, interpretation of subcellular localization resulting from fluorescence microscopy; In Vitro Import Result, mitochondrial localization of tested proteins as determined by reliance on the presence of an inner membrane electrochemical gradient; Previous Published Location, location of previously studied proteins; Reference, references to previously published location information. The final protein in this table, At4g17140.2, was found to be abundant and strongly enriched in the mitochondrial outer membrane samples; however, confirmation of localization independent of the organelle fractionation was attempted but not obtained. ‘ is used to indicate that the ratio cannot be calculated because a null value (0) was obtained and thus the P value cannot be calculated, as shown by ^. Arabidopsis Mitochondrial Outer Membrane 1101 Inner membrane, complex III Inner membrane, complex I Inner membrane, complex V Mitochondrial matrix Lister et al. (2004) Meyer et al. (2008) Klodmann et al. (2010) Meyer et al. (2008) Zhao and McAlisterHenn (1996) Intermembrane space 0.32 0.128 6.22E27 10.5 1.5E-07 2.1 220:104:21 Unknown protein AtMg01080.1 Subunit 9 of mitochondrial F0-ATPase At4g35260.1 IDH1 Mitochondrial Mitochondrial Unknown protein At4g16450.1 Mitochondrial No further evidence At4g17140.2 ^ ‘ 24:0:6 4 ^ ^ ‘ 4:0:0 ‘ ^ ^ ‘ 5:0:0 ‘ ^ ^ UQCRX-like family protein At3g52730.1 Mitochondrial 8:0:0 ‘ ‘ ^ ^ TIM9 At3g46560.1 Mitochondrial 4:0:0 ‘ ‘ P Ratio OM:IM P Ratio OM:HSP-OM Spectral Counting Raw Counts OM:HSP-OM: IM Description AGI No. Location Table II. (Continued from previous page.) 0.002 0.486 0.203 Whole/ OM IM/ OM iTRAQ CON/ OM See legend GFP Location In Vitro Import Result Previous Published Location Reference Duncan et al. S1), indicating an exclusive mitochondrial outer membrane localization, while four of the tested proteins displayed fluorescence patterns consistent with localization to the mitochondrial outer membrane but also localized to other intracellular structures, which were apparently not mitochondrial in nature (Supplemental Fig. S2). GFP analysis of the remaining 12 proteins showed that seven of these did not localize to the mitochondria (Supplemental Fig. S4), two were seen to be mitochondrial but did not display the ring structure characteristic of outer membrane proteins (At5g15640.1 and At4g04180.1; Supplemental Fig. S3, III and IV), and three proteins failed to display any GFP-related fluorescence or were inconsistent from cell to cell. Images of all of the above fluorescence patterns can be viewed in Supplemental Figures S1 to S4. In Vitro Uptake Assays The two mitochondria-localized proteins that did not display the ring structure (At5g15640.1 and At4g04180.1) were selected for in vitro protein uptake experiments. Both were seen to be protease protected when incubated with mitochondria under conditions that support protein uptake (Supplemental Fig. S3, III and IV). When valinomycin was added to the in vitro uptake assay, no protease protection was evident, indicating the requirement of membrane potential for import that is a feature of inner membrane or matrix localization (Supplemental Fig. S3, III and IV). Of the three proteins for which a consistent GFP localization could not be confirmed, two were deemed to be outer membrane proteins on the basis that other isoforms of the protein family had been investigated as part of this study and were found to be localized to the outer mitochondrial membrane. These proteins were SAM50-1 (At3g11070.1) and MIRO1 (At5g27540.1). The final protein, the unknown protein (At4g17140.2), was excluded, as no additional evidence was found for its localization beyond the quantitative MS data. The N- and C-terminal 200 amino acids of this large (471.2-kD) protein were fused to GFP but failed to show a consistent subcellular localization. Together, the MS, literature searches, and GFP analysis produced a final list of 42 plant outer membrane proteins, 27 of these being novel, doubling the number of confirmed outer membrane proteins in plant mitochondria and identifying all but two proteins that were previously known. Additional Confirmation of Novel Mitochondrial Outer Membrane Localizations In order to gain further localization confirmation for the three novel mitochondrial outer membrane proteins, 3-deoxy-manno-octulosonate cytidylyltransferase (KDSB; At1g53000.1), phosphorylethanolamine 1102 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane cytidylyltransferase (PECT1; At2g38670.1), and the sorting and assembly machinery protein SAM50 (At3g11070.1), polyclonal antibodies were raised following bacterial expression and purification of portions of these proteins. Isolated mitochondria were treated with increasing amounts of proteinase K. This treatment degrades portions of mitochondrial proteins that were accessible to the protease and preserved proteins that were protected by the lipid bilayer of the mitochondrial outer membrane. Treated mitochondria were separated by SDS-PAGE, and a number of proteins were immunodetected. TOM20-3 is a mitochondrial outer membrane protein that is exposed to the cytosol (Heins and Schmitz, 1996; Fig. 5A). This protein is rapidly degraded at low concentrations of proteinase K. Examination of KDSB (Fig. 5B) revealed a similar breakdown profile, indicating that part of this protein is exposed to the cytosol in vivo and is likely to be located on the outer mitochondrial membrane. PECT1 (Fig. 5C) was also seen to be accessible to the proteinase. Orthologs of Arabidopsis SAM50 have been observed to be localized to the outer mitochondrial membrane in S. cerevisiae (Kozjak et al., 2003). SAM50, like TOM40, is a transmembrane b-barrel protein descended from the bacterial ancestor protein OMP85 (Kozjak et al., 2003), and it appears that these two proteins are not readily accessible to the externally applied proteinase (Fig. 5, D and E). At the two highest concentrations of proteinase, both of these proteins were seen to be partially broken down, whereas the Rieske Iron Sulfur Protein (RISP; Fig. 5F), which is located on the inner mitochondrial membrane, was resistant to digestion. This indicates that SAM50, in agreement with the other data presented here, is likely Figure 4. GFP tagging of mitochondrial outer membrane proteins to confirm intraorganelle localization. Arabidopsis suspension cell culture was transiently transformed with mitochondrial red fluorescent protein (RFP) control and GFP-tagged experimental proteins. A, FRO1:GFP, inner mitochondrial membrane protein. B, IDH6: GFP, mitochondrial matrix-located protein. C, ELM1:GFP, outer mitochondrial membrane protein. D, Example of novel mitochondrial outer membrane protein displaying a similar fluorescence pattern to ELM1, identified by MS and location confirmed by fluorescence microscopy. E, GFP only. Plant Physiol. Vol. 157, 2011 1103 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. observed cells (Fig. 6A). This protein appears to be of bacterial origin (Misaki et al., 2009), is thought to be involved in the synthesis of the outer envelope lipopolysaccharide KDO2-Lipid A, and has been located in mitochondria, but not to the outer membrane, in previous studies in Arabidopsis (Heazlewood et al., 2004). PECT1 is the penultimate enzyme involved in the biosynthesis of phosphatidylethanolamine, an important membrane phospholipid. The overexpression of the outer membrane-targeted fusion protein (Fig. 6B) apparently causes mitochondria to bunch together at one pole of the cell. Overexpression of a mitochondrial outer membrane NADH:cytochrome B5 reductase (Fig. 6C) also appears to alter mitochondrial morphology when compared with the more typical mitochondrial outer membrane fluorescence pattern seen with the unknown protein At5g55610.1 (Fig. 6D). Transcriptomic Analysis of Genes Encoding Outer Membrane Proteins Reveals Patterns of Coexpression Figure 5. Immunodetection of isolated mitochondria treated with proteinase K. A, TOM20-3 is a mitochondrial outer membraneanchored protein with a large cytosolic domain and as such is vulnerable to digestion by external application of proteinase K. B and C, KDSB (B) and PECT1 (C) are novel outer membrane proteins identified in the course of this study by MS and confirmed by GFP localization. The breakdown of KDSB is similar to that of TOM20-3, indicating that it is also exposed to the cytoplasm. PECT1 is broken down by external application of proteinase K but appears to be more resistant than TOM20 and KDSB. D and E, SAM50 (D) and TOM40 (E) are b-barrel outer membrane proteins and as such are embedded in the membrane, rendering them resistant to digestion. F, RISP is found in the ubiquinol-cytochrome c reductase complex in the mitochondrial inner membrane. Its preservation indicates that the integrity of the inner membrane was maintained throughout the proteinase dilution series. to be located on the outer mitochondrial membrane in Arabidopsis and that mitochondrial integrity was not compromised by the proteinase treatment. Transient Overexpression of Outer Membrane Protein Affects Mitochondrial Morphology and Segregation An unexpected outcome of using full-length coding sequences fused to GFP was the observation that several of the GFP fusion proteins apparently altered the mitochondrial morphology in transformed cells. In the case of KDSB (At1g53000.1), highly abnormal giant mitochondria occupied much of the available space in In order to determine whether there was a relationship between colocalization and coexpression, the relative expression levels for the 38 genes that were present on the Affymetrix ATH1 microarray were analyzed during germination and across the Arabidopsis developmental microarray series (Schmid et al., 2005). Additionally, the patterns of transcript abundance for 247 genes that encode inner membrane proteins were analyzed to determine if the transcript abundance of genes that encode outer membrane proteins, or subsets of outer membrane proteins, differed from the patterns of transcript abundance for genes that encode inner membrane proteins. Data were normalized to make them comparable (see “Materials and Methods”), and expression levels are displayed relative to maximum expression (Fig. 7; Supplemental Fig. S5). Overall, it was observed that the transcript abundance of genes encoding outer and inner membrane mitochondrial proteins displayed maximum levels during seed germination, with a subset showing relatively high levels in root tissues (Supplemental Fig. S5). When all genes encoding inner membrane- and outer membrane-localized proteins were clustered together over a germination time course, it was seen that genes encoding outer membrane proteins were significantly underrepresented in cluster 2 (P , 0.05) compared with genes encoding inner membrane proteins (13% outer membrane versus 27% inner membrane; Fig. 7). Gene in cluster 2 displayed high transcript abundance in fresh harvested seeds that decreased during stratification. In contrast, 45% of genes encoding outer membrane proteins (versus 21% of inner membrane protein-encoding genes) were seen in the group of genes showing transient expression during germination in cluster 4 (Fig. 7, boxed in yellow), which represents a significant overrepresentation (P = 0.0007) of genes encoding outer membrane proteins in this cluster. Genes in cluster 4 peak in transcript abundance during the first 1104 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane Figure 6. Transient expression of several fluorescently tagged fusion proteins causes apparent alterations in mitochondrial morphology/distribution. A, KDSB. Cells transformed with this GFP-tagged protein consistently displayed fewer but larger mitochondria than was the observed norm for mitochondrial outer membrane proteins. B, PECT-1. The mitochondria bunch tightly together at one pole of the cell. C, NADH:cytochrome B5 reductase. The mitochondrial population has an abnormal size distribution with many large mitochondria. D, Unknown protein, commonly observed mitochondrial morphology for comparison. 24 h of germination and decrease in abundance in young seedlings, as under these conditions germination is completed at 24 h in Arabidopsis, as the radicle has emerged (Weitbrecht et al., 2011). Thus, although genes encoding mitochondrial outer membrane proteins follow the general pattern observed for genes encoding inner membrane proteins, in that they display maximum levels of transcript abundance during seed germination, detailed time-course analysis reveals that for a large subset (i.e. cluster 4) this differs from the majority of genes encoding mitochondrial proteins. Previously, it has been shown for maize (Zea mays) and rice (Oryza sativa) that active mitochondrial biogenesis precedes the expression of respiratory chain components of the inner membrane (Logan et al., 2001; Howell et al., 2006); thus, the pattern observed here suggests that the establishment of the proteins of the outer membrane is one of the earliest steps to take place in mitochondrial biogenesis. DISCUSSION The mitochondrial outer membrane is commonly characterized as a relatively simple phospholipid bi- layer, broadly permeable to small proteins, ions, nutrients, metabolic substrates, and other products of mitochondria. Although broadly true, this simplification of the functions carried out by the mitochondrial outer membrane belies its involvement in complex, coordinated cellular processes such as apoptosis (Lindsay et al., 2011), organelle division (Kuroiwa, 2010), mitochondrial inheritance (Koshiba et al., 2011a), lipid synthesis and trafficking (Osman et al., 2011), as well as selective interactions with the complex cytosolic environment in the processes of mitochondrial protein import and biogenesis (Walther and Rapaport, 2009). The involvement of the mitochondrial outer membrane has often been incidental to the study of these processes, and the unequivocal assignment of functions or protein components to the mitochondrial outer membrane is commonly avoided in the literature, significantly due to the difficulties in gathering sufficient evidence to make a convincing case for outer membrane localization. Studies such as the one presented here have several important functions: confirmation of some expected but unproven subcellular localizations; the identification of expected, but as yet uncharacterized, protein components of biological pro- Plant Physiol. Vol. 157, 2011 1105 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. Figure 7. Coexpression of genes encoding outer mitochondrial membrane proteins. Publicly available microarrays analyzing wild-type tissues in the AtGenExpress developmental set (Schmid et al., 2005; E-AFMX-9) and during germination show the highest expression of genes encoding inner membrane and outer membrane proteins during germination (Supplemental Fig. S5). The expression of genes encoding outer membrane proteins was analyzed during germination. Har, Seeds harvested on the day of collection from wild-type plants; 0 h, seeds 15 d after ripening, prior to imbibition; S, hours in cold stratification in the dark; SL, hours into continuous light after 48 h of stratification. Expression levels were made relative to maximum expression, hierarchically clustered (Euclidean distance and average linkage clustering), and five clusters were observed (C1–C5). A group of genes showing transient expression are indicated in the yellow box. Details for each of the tissues analyzed by microarrays are shown in Supplemental Table S6. cesses; and as the basis for launching the investigation of novel, uncharacterized protein components, with the potential to lead to the assignment of new and unexpected biological functions. The analysis of the mitochondrial outer membrane in plants is especially interesting because the presence of the chloroplast in plant cells increases the complexity of the intracellular environment and possibly leads to the observed sequence divergence across kingdoms (Macasev et al., 2000) in many of the protein components of the outer membrane involved in mitochondrial protein import (Lister et al., 2007). By systematically characterizing the mitochondrial outer membrane proteome in plants, we have identified proteins putatively involved in diverse processes, including signaling, cytoplasmic streaming, protein import, protein degradation, and membrane biosynthesis. Developing a Proteomic Strategy for Characterizing the Outer Membrane Proteome Developing a strategy for characterizing the proteome of one membrane from a multicompartmented structure within the cell is challenging due to the multiple and overlapping potential sources of contamination and the probability that biochemical enrichment techniques will copurify multiple structures at the same time. This is a recurring theme in subcellular proteomics, especially in subcellular membrane proteomics (Lilley and Dunkley, 2008). Combining methods that evaluate the protein content of enriched membranes relative to contaminating structures with visual and biochemical assays such as in vitro and in vivo protein import provides a solid basis for determining subcellular and suborganelle localization. In combination, this is a highly complex and timeconsuming process to undertake for a large range of proteins. However, without it, many researchers are too often following up unexpected proteomic identifications by using genetic or functional assays, simply to discover that the false discovery rate of the proteomics was too high in the first place. Quantitative proteomic tools like spectral counting and iTRAQ offer powerful, unbiased analysis of the enrichment of known and unknown proteins between samples. However, we have found that the Exponentially Modified Protein Abundance Index and the Normalized Spectral Abundance Factor for quantita- 1106 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane tive analysis of spectral counting (Ishihama et al., 2005; Zybailov et al., 2007) and parametric analysis of mass tag abundances in iTRAQ spectra (Chong et al., 2006) are not immediately suited to assessments of enrichment. Typically, these processes were developed for the analysis of small changes of protein abundance in a backdrop where most proteins are not changing in abundance. However, in enrichment studies (including the study presented here), most proteins are changing in abundance, and significant enrichment leads to the identification of low-abundance proteins that are not detected in more crude samples. These problems affect the statistical analysis of both spectral counting and iTRAQ data, as data sets are not normally distributed in the cases of the most enriched and interesting proteins from the perspective of subcellular location. Furthermore, while the degree of enrichment might be interesting in these types of studies, the primary aim is to use quantification to give a qualitative assessment of cellular location. By using quantitative spectral counting and iTRAQ data to provide a qualitative assessment of location and coupling this to independent confirmation data for this qualitative assessment, we have developed a workflow for a confident assessment of the mitochondrial outer membrane proteome that is likely to be useful for research on any specific membrane in the cell, particularly for the analysis of membrane systems of low abundance (Fig. 1). There are three key aspects of this workflow. First, selection of multiple independent prefractionated samples for comparisons with the enriched fractions in order to independently cover likely causes of contaminants; in on our case, we sought a control for other mitochondrial membranes (Mt IM), a control for the coenrichment of contaminating structures in the Suc gradients (HSP-OM), and a control for nonmembrane proteins associating with the membrane during the cell fraction procedures (mitoplasts). Second, inclusion of proteins observed in enriched fractions and absent from prefractionated samples. Such proteins should be included despite issues related to the normality of distributions and limited spectral counts, as long as the spectra are sufficient to prove identification, as these include low-abundance proteins only found through enrichment. Third, multiple confirmation strategies that are independent of the enriched fractions are very helpful, especially those that relate to targeting of proteins in situ and/or in vitro to the location identified. In this study, we used a diverse literature of counterclaims, GFP labeling in intact cells, and in vitro protein import. In addition to this, proteinprotein interaction, in situ immunohistochemistry, and activity assays could also be included for specific studies (Millar et al., 2009). Proteins of the Mitochondrial Outer Membrane Proteome Using this workflow, 42 high-confidence identifications of outer mitochondrial membrane proteins were made (Table I). Only 16 of these proteins were previously experimentally determined to be located in the outer mitochondrial membrane in plants (Werhahn et al., 2001; Chew et al., 2004; Lister et al., 2007; Arimura et al., 2008; Xu et al., 2008; Lee et al., 2009). These 16 proteins were largely made up of members of the outer mitochondrial membrane protein import apparatus (TOM complex and associated receptors) and the VDACs, specifically, the five known import receptors (TOM 20-2, 20-3, 20-4, OM64, and Metaxin) and components of the central import pore (TOM 40, 9-1, 9-2, 5, and 6) and VDAC1 to -4. Two other proteins, DGS-1 (At5g12290.1; Xu et al., 2008) and ELM1 (At5g22350.1; Arimura et al., 2008), have also previously been shown to be present in the outer membrane. Two proteins that were expected, but not detected, in this study were TOM7 (Werhahn et al., 2001) and BIGYIN, the Arabidopsis ortholog to S. cerevisiae and human Fis1 (Scott et al., 2006). In addition to the 16 known plant outer mitochondrial membrane proteins, four other proteins were also inferred to be localized to the outer mitochondrial membrane in plants, based on experimental evidence from other species (Fransson et al., 2003; Kozjak et al., 2003). Two of these proteins are the SAM50s (At3g11070.1 and At5g05520.1), which are evolutionarily conserved across kingdoms, and orthologs of these have also been reported to be mitochondrial outer membrane proteins in N. crassa (Schmitt et al., 2006) as well as S. cerevisiae (Zahedi et al., 2006). Similarly, the location of the two MIRO-like proteins, MIRO1 (At5g27540.1) and MIRO2 (At3g63150.1), were also inferred to be located on the outer mitochondrial membrane, based on orthology with the Gem1p gene in S. cerevisiae (Frederick et al., 2004). Thus, experimentally confirming the localization of these in plants, to our knowledge for the first time, further supports a vital and evolutionarily conserved role for the SAM50s and MIROs on the outer mitochondrial membrane. Evidence supporting this crucial role can be observed in Arabidopsis knockouts of MIRO1, which have been shown to have a seed-lethal phenotype (Meinke et al., 2008; Yamaoka and Leaver, 2008), indicating that this gene is essential for seed viability and normal plant development. In addition to the aforementioned proteins, a total of 27 proteins were reported as outer mitochondrial membrane proteins in plants to our knowledge for the first time in this study. Examination of these revealed the presence of Hexokinase1 (HXK1; At4g29130.1), HXK2 (At2g19860.1), and HexokinaseLike Protein1 (HXKL1; At1g50460.1) on the mitochondrial outer membrane (Table I). The Arabidopsis hexokinases have long been reported to be associated with mitochondria, first in 1983 (Dry et al., 1983; Damari-Weissler et al., 2007) and more recently with reference to the association of glycolytic enzymes with mitochondria (Graham et al., 2007). The presence of a putative transmembrane region of 20 to 25 amino acids, high hydrophobicity at the N terminus (Supplemental Fig. S1, IV, VII, and XVII), the ability to Plant Physiol. Vol. 157, 2011 1107 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. target GFP to mitochondria, combined with its enrichment in the outer membrane samples (Table I) and a lack of detection of other glycolytic enzymes suggests that these proteins are authentic outer membrane proteins. The location of these hexokinases on the outer mitochondrial membrane in plants may be crucial for a role in the regulation of programmed cell death, with recent findings in Nicotiana benthamiana implying a role for mitochondrial hexokinases in this process (Kim et al., 2006). Another study has also proposed a role for mitochondrial hexokinases in generating ADP to support oxidative phosphorylation and minimize the limitation of respiration by restraints on ATP synthesis, thereby playing a role in antioxidant defense (da-Silva et al., 2004). Nevertheless, it appears that the presence of hexokinases on the outer mitochondrial membrane positions these proteins ideally for a functional role in signaling. Similarly, a NADH:cytochrome B5 reductase (At5g17770.1) might be expected to be in the outer membrane of mitochondria, based on the localization of its S. cerevisiae ortholog (Haucke et al., 1997). There are two NADH:cytochrome B5 reductases in Arabidopsis; thus, the identification of this protein on the outer mitochondrial membrane distinguishes it from other NADH:cytochrome B5 reductase activities in Arabidopsis, such as the one encoded by At5g20080, which has been shown to be mitochondrial but is likely to be located on the inner membrane (Heazlewood et al., 2004). Notably, the protein shown to be on the outer mitochondrial membrane in this study; NADH cytochrome B5 reductase (At5g17770.1), has previously been reported to be a microsomal enzyme associated with the endoplasmic reticulum, even though no direct evidence was presented to determine location (Fukuchi-Mizutani et al., 1999). A recent study has also shown that a mutation in this NADH:cytochrome B5 reductase (At5g17770.1) significantly reduced the accumulation of hydroxyl fatty acids in developing seeds by 85%, confirming an essential role for this protein in lipid metabolism (Kumar et al., 2006). NADH:cytochrome B5 reductases have been shown to catalyze the transfer of electrons from NADH to two molecules of membranebound cytochrome B5 (Strittmatter, 1965). Taken together, these findings suggest that the reactions catalyzed by both NADH:cytochrome B5 reductases are likely to occur on the mitochondrial membranes and not on the endoplasmic reticulum, as assumed previously. Novel Proteins of the Mitochondrial Outer Membrane In addition to the proteins outlined above, a number of other proteins were found to be located in the outer mitochondrial membrane that could not have been predicted by sequence orthology alone. These proteins can provide a molecular handle for processes that occur in mitochondria, where the identification of molecular components has been limited to date. A Novel b-Barrel Protein One of the proteins identified in the outer membrane proteome was a novel b-barrel protein (At3g27930.1), bringing the total number of distinct b-barrel proteins identified in the Arabidopsis mitochondrial outer membrane to four (TOM40, SAM50, VDAC, and At3g27930.1; Table I). Amino acid sequence alignments comparing this novel protein with other membrane b-barrel proteins (TOM40, SAM50, VDAC, MDM10, OMP85, and TOC75) from diverse species revealed that this b-barrel protein is only found in organisms with a green chloroplast, from algae to various plant species (data not shown). Although the function of this protein (At3g27930.1) is unknown (as annotated in TAIR 10), upon searching the literature, it was found that this gene has been identified as on the list of 437 proteins making up the predicted Arabidopsis N-myristoylome (Boisson et al., 2003). Given that N-myristoylation is a permanent modification that affects the membrane-binding properties of cytoplasmic proteins, promoting their association with membranes, it was interesting to find At3g27930.1 on this list. Taken together with the confirmed outer mitochondrial membrane localization (Table I), high expression in seed and early germination (Fig. 7), and the fact that this protein is plant specific (Plant Specific Protein Database; Gutiérrez et al., 2004), it can be speculated that this protein has a crucial and plant-specific role in the interaction between cytoplasmic proteins and the outer mitochondrial membrane, possibly in signaling or even as a novel import component. Proteins Involved in the Synthesis of Membrane Components Three proteins that were confirmed to be located to the outer mitochondrial membrane in the course of this study catalyze key steps in the synthesis of membrane components (At2g01460, At1g53000, and At2g38670). At2g01460.1 contains two uridine monophosphate/cytidine monophosphate kinase-like domains and is predicted to catalyze the conversion of uridine monophosphate/cytidine monophosphate to their diphosphate forms. It has previously been demonstrated that a nucleoside diphosphate kinase (At4g11010.1) is present in the mitochondrial intermembrane space (Sweetlove et al., 2001). Thus, the reaction of these two enzymes would yield cytidine triphosphate from a cytidine monophosphate substrate with conversion of ATP to ADP. An additional protein found to be located to the outer membrane in this study was PECT1 (At2g38670.1), which is a CTP: phosphorylethanolamine cytidyltransferase (Mizoi et al., 2006). This enzyme catalyzes the transfer of phosphoethanolamine onto cytidine triphosphate + inorganic pyrophosphate to yield cytidine diphosphoethanolamine (CDP-ethanolamine). The final enzyme required for the production of phosphatidyl- 1108 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane ethanolamine is CDP-ethanolamine phosphotransferase, the location of which is not yet characterized in Arabidopsis but is found in the endoplasmic reticulum in S. cerevisiae (Jelsema and Morré, 1978). Interestingly, despite the fact that PECT1 is an intermediate in this pathway, it has been shown that when this gene is knocked out in Arabidopsis, a seed-lethal phenotype is observed (Mizoi et al., 2006; Meinke et al., 2008). Thus, it appears that this role for PECT1 on the outer mitochondrial membrane is essential for seed viability and development in Arabidopsis. In the course of confirming these proteins as bona fide outer membrane proteins, full-length coding sequences were fused to the GFP coding sequence and expressed using the 35S cauliflower mosaic virus promoter, resulting in transient overexpression of the experimental protein. Interestingly, in the case of PECT1, this resulted in abnormally tight bunching of the mitochondria at one pole of the cell as opposed to the regular evenly distributed morphology (Supplemental Fig. S1, IX), suggesting that PECT1 is not only essential for catalyzing the reaction yielding CDP-ethanolamine but that increasing the abundance of this protein or perhaps the flux of its reaction can lead to abnormal phospholipid composition of the outer mitochondrial membrane or association of mitochondria. Also with a proposed role in membrane synthesis, At1g53000.1 encodes 3-deoxy-D-manno-2octulosonic acid (Kdo) transferase, which catalyzes the transfer of Kdo onto cytidine triphosphate to form CMP-Kdo (Séveno et al., 2010). This protein is orthologous to the Escherichia coli protein KDSB, which participates in the synthesis of KDO2-Lipid A, an essential component of the gram-negative bacterial cell wall (Opiyo et al., 2010). Basic local alignment searching showed that orthologous genes to each of the essential members of the bacterial KDO2-Lipid A biosynthesis pathway are present in the Arabidopsis genome and are predicted to be targeted to mitochondria (Raetz and Whitfield, 2002; Heazlewood et al., 2007). Taken together with the histochemical evidence for the presence of Lipid A in eukaryotic organisms (Armstrong et al., 2006), the evidence that overexpression of this protein produced giant mitochondria (Fig. 4A) suggests the presence of KDO2-Lipid A or a related compound in the outer membrane of mitochondria. domain in common and lacks the BCS domain. Interestingly, it differs from the AAA-ATPase proteases that are located on the inner membrane and displays closer similarity to another AAA-ATPase, which has been shown to be a calcium-binding protein whose function is still unclear but is dual localized to both mitochondria and chloroplasts (Bussemer et al., 2009). Given that BCS1 was seen to be stably expressed over normal development (Fig. 7) but has been shown to be among the most stress-inducible transcripts encoding mitochondrial proteins (Van Aken et al., 2009), it may have a more specific role in the repair of proteins following a wide variety of stresses (Xu et al., 2011). AAA-Type ATPases CONCLUSION Two related proteins containing ATPases associated with diverse cellular activities (AAA)-type ATPase domains were identified on the outer membrane, At3g50930.1 and At3g50940.1, which are 67% identical at the amino acid level and appear to be the result of a recent duplication in the Arabidopsis genome. Although the protein encoded by At3g50940 is annotated as encoding a ubiquinol-cytochrome c reductase synthesis (BCS)-like protein, closer examination reveals that it only contains the AAA-ATPase While we have identified 42 proteins located at the mitochondrial interface with the cell cytoplasm in plants, there are likely to be some membrane proteins that have not been identified in this study. Dualtargeted or multitargeted proteins would be excluded by the strategy undertaken, as they would be detected in the contaminant fractions. The cell culture system used may not have components that may be present in photosynthetic cells, or they may be present at levels below the level of detection. No addi- Proteins Involved in Signaling and Catabolic Processes In addition to the stress-inducible AAA-type ATPases discussed above, a number of other proteins identified in this study may have significant roles in signaling. Hexokinases and MIRO have been shown to have roles in signaling, with hexokinase and hexokinase-like proteins shown to have roles in regulating reactive oxygen species levels and plant growth, respectively (Karve and Moore, 2009; BolouriMoghaddam et al., 2010), while MIRO proteins have been observed in regulating mitochondrial transport and morphology (Yamaoka and Leaver, 2008; Reis et al., 2009). Another protein that may be linked to signaling is a TraB family protein (At1g05270.1), which plays a role in the conjugative transfer of plasmids in bacteria (An and Clewell, 1994); however, its role in eukaryotic cells is still unknown. Nevertheless, the identification of this protein implies a conserved role for it, both on bacterial membranes and evidently on the mitochondrial outer membrane in plants. Another well-conserved protein is Embryonic Factor1 (FAC1; At2g38280.1), for which the subcellular location has long been discussed (Han et al., 2006), but in this study we report an outer mitochondrial membrane localization for this protein, on the basis of both proteomic and GFP evidence. Interestingly, like PECT1 and MIRO1, it has been observed that plants have a seed-lethal phenotype when FAC1 is knocked out (Xu et al., 2005; Meinke et al., 2008), indicating a vital role for these outer mitochondrial membrane proteins in Arabidopsis development. Plant Physiol. Vol. 157, 2011 1109 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. tional components of the SAM complex of the outer membrane were identified that might be expected to be present, in comparison with the SAM complex from yeast. Nevertheless, 27 proteins were experimentally determined to be located on the outer mitochondrial membrane, to our knowledge for the first time in plants, and only one of these (At5g60730.1) has been consistently predicted to be mitochondrial (Heazlewood et al., 2007). Of the other 15 proteins, some had counterparts in S. cerevisiae or mammalian systems and some have been previously shown to be associated with the mitochondrial outer membrane. However, many can only be identified as on the outer mitochondrial membrane based on direct experimental analysis, as they are members of larger families that have a variety of locations in the cell. Several proteins that were identified had no functional annotations. For these, the discovery of outer membrane localization provides, to our knowledge, the first functionally related information for the proteins encoded at these loci. Examination of the full list of proteins identified (Table I) reveals that plant outer membrane functions exist beyond their well-established roles in protein and small molecule import. Importantly, we discovered that plant mitochondrial homologs were not identified for a range of proteins known to be located in, and to function in, the outer membrane in other organisms. Examples include the B-cell lymphoma 2-like proteins, which play roles in cell death in mammalian systems (Lindsay et al., 2011), the GTP protein b2 subunit, which plays a role in regulating mitochondrial fusion in mammalian systems (Zhang et al., 2010), Mim1, which plays a role in protein import in fungi (Stefan Dimmer and Rapaport, 2010), and a peripheral-type benzodiazepine receptor that mediates cholesterol transport in mammalian cells but is located in the secretory pathway in Arabidopsis (Vanhee et al., 2011). Although we were unable to conclusively rule out the existence of these proteins on the mitochondrial outer membrane (expression of these proteins may be tissue specific or of low abundance), it is likely that at least some of the proteins that we identified with no known functions may fulfill similar roles. These findings, together with the identification of five plant-specific genes (Plant Specific Protein Database; Gutiérrez et al., 2004), including a novel b-barrel protein (At3g27930.1) and a protein of unknown function (At5g55610.1), suggests that despite the conserved presence of mitochondria in eukaryotes, several constituents of the outer mitochondrial membrane have diverged and specialized in different species. MATERIALS AND METHODS Purification of Mitochondria from Arabidopsis Suspension Cell Culture Protoplasts were prepared from 6-d-old Arabidopsis (Arabidopsis thaliana) suspension cell cultures. Typically, 500 g fresh weight of cells was collected by filtration and incubated for 3 h at 21°C with shaking at 30 rpm in 1 L of 0.4 M mannitol, 3.5 mM MES, pH 5.7, with 0.4% (w/v) cellulase and 0.05% (w/v) pectolyase (Yakult). Protoplasts were collected by centrifugation at 800g for 10 min, resuspended in 500 mL of 0.4 M Suc, 50 mM Tris, 3 mM EDTA, 20 mM Cys, 0.1% (w/v) bovine serum albumin (BSA), pH 7.5, and HCl and ruptured in a Potter-Elvehjem homogenizer. Cell debris was removed by centrifugation at 2,500g for 5 min, and the organellar fraction (supernatant) was pelleted at 20,000g for 20 min. A portion (1/16th) of this material was removed and stored at 4°C for preparation of the HSP and HSP-OM samples. The remaining organellar fraction was layered onto discontinuous Percoll gradients consisting of 18%/25%/40% (v/v) Percoll in 0.3 M mannitol, 10 mM TES, pH 7.5, and centrifuged at 40,000g for 40 min. The 25%/40% (v/v) interphase was removed and added to 300 mL of 0.3 M Suc, 10 mM TES, and 0.2% (w/v) BSA, pH 7.5 (Suc wash buffer), and centrifuged at 20,000g for 15 min. Subsequently, the supernatant was removed and the pellet was layered onto 40% (v/v) Percoll in Suc wash buffer and centrifuged at 40,000g for 40 min. The gradient-purified mitochondria were removed and washed twice in Suc wash buffer. A total of 500 mg of this sample was removed and stored at 4°C for later analysis. Typically, 100 to 150 mg of mitochondrial protein (Bradford assay) was recovered. The gradient-purified mitochondria were further purified by FFE based on a method defined previously with several alterations (Eubel et al., 2007). The gradient-purified mitochondria were first dispersed by passing them back and forth between two 3-mL syringes through a 10-mm membrane filter (Bio-Rad) and then passed through a 5-mm nylon syringe filter prior to introduction to the FFE separation chamber. EDTA was excluded from the FFE separation medium. FFE-purified mitochondria were collected by centrifugation at 20,000g for 10 min and washed in Suc wash medium for 10 min. Purification of Mitochondrial Outer Membranes and Generation of Contaminant Samples Outer membranes were purified according to a previously published protocol (Werhahn et al., 2001). In addition to the mitochondrial outer membranes, a “contaminant outer membrane” sample was generated by treating the HSP collected from the protoplast disruption step in a similar manner to the FFE-purified mitochondria. A second contaminant sample primarily consisting of mitoplasts was taken from the 32%/60% (w/v) interphase of the first Suc gradient. An inner membrane fraction was prepared by diluting this in 11 mL of Suc wash medium before freezing/ thawing five times and pelleting at 504,000g for 1 h in a fixed-angle rotor. An additional contaminant sample consisting of whole mitoplasts removed from the same 32%/60% interphase but not subjected to the freezing/ thawing procedure was included in the iTRAQ experiment. Western-Blot Analysis Ten micrograms of membrane fractions and 20 mg of organelle fractions per lane were separated by 14% (w/v) SDS-PAGE. Proteins were transferred onto nitrocellulose membranes. Primary antibodies were as follows: TOM40-1 (At3g20000.1) at 1:5,000 for 1 h at 21°C and developed according to the manufacturer’s instructions (Roche; Carrie et al., 2009); KAT2 antibody (Germain et al., 2001) was used at 1:1,000 for 1 h at 21°C; Rubisco large subunit and Calreticulin antibodies were obtained from Abcam and used at 1:500 for 18 h at 4°C; E1a antibody was obtained from Tom Elthon (University of Nebraska) and used at 1:2,000 for 1 h at 21°C; COXII antibody was obtained from Agrisera and used at 1:5,000 for 1 h at 21°C. Antibodies to TOM20-3 (Lister et al., 2007), KDSB, SAM50, PECT1, and RISP were created by purifying 63His-tagged recombinant proteins from Escherichia coli expression using Profinia-based immobilized metal affinity chromatography (Bio-Rad). The purified proteins were used to generate rabbit polyclonal antibodies. Proteinase K Titrations A total of 250 mg of isolated mitochondria was resuspended in 0, 2, 8, 32, and 64 mg mL21 proteinase K (Sigma-Aldrich) in 2.5 mL of 0.4 M Suc, 50 mM Tris, 3 mM EDTA, 0.1% (w/v) BSA, pH 7.5, and HCl. Samples were incubated 1110 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane on ice for 30 min before dilution in 30 mL of 0.4 M Suc, 50 mM Tris, 3 mM EDTA, 0.1% (w/v) BSA, pH 7.5, and HCl containing 1 mmol of Pefabloc SC (Roche). Samples were centrifuged at 20,000g for 15 min, resuspended in Laemmli sample buffer, and separated by SDS-PAGE. Sequence data from this article can be found in the GenBank/EMBL data libraries under the accession numbers listed in Supplemental Table S8. Supplemental Data MS The following materials are available in the online version of this article. Label-Free Analysis and Statistical Assessment Supplemental Figure S1. Members of the mitochondrial outer membrane proteome, as shown by fluorescence microscopy of transiently transformed Arabidopsis cell culture or onion (Allium cepa) cells with fulllength coding sequences fused to GFP. Fifty micrograms of outer membrane, HSP-OM, and inner membrane protein were individually digested overnight with trypsin (10:1), 10 mM ammonium bicarbonate, and 20% (v/v) acetonitrile. Insoluble material was removed by centrifugation at 20,000g for 5 min. Samples were then dried down in a vacuum centrifuge and analyzed on an Agilent 6510 Q-TOF mass spectrometer according to a modification of the methods reported by Eubel et al. (2008), as outlined in detail in Supplemental Materials and Methods S1. Spectra are available via the ProteomeCommons Tranche Project hash: 3y16ynJWUCAiKGSfZNprN/paRI32nQA9XM0asqZItzPwnf2jEB/ zsnOAoSxno14kymUwnJbylKkk4cA2sGdLN52sAQ0AAAAAAAAHqg = =. Peptide count data were compiled from three biological replicates each of the outer membrane, HSP-OM, and mitochondrial inner membrane samples, as outlined in detail in Supplemental Materials and Methods S1. iTRAQ Labeling and Data and Statistical Analysis Supplemental Figure S2. Members of the mitochondrial outer membrane proteome that are also found in other subcellular locations, as shown by fluorescence microscopy of transiently transformed Arabidopsis cell culture or onion cells with full-length coding sequences fused to GFP. Supplemental Figure S3. Members of the putative mitochondrial outer membrane proteome that have been shown to be mitochondrial but not outer membrane by fluorescence microscopy of transiently transformed Arabidopsis cell culture or onion cells with full-length coding sequences fused to GFP. Supplemental Figure S4. Members of the putative mitochondrial outer membrane proteome that have been shown not to be mitochondrial by fluorescence microscopy of transiently transformed Arabidopsis cell culture or onion cells with full-length coding sequences fused to GFP. A total of 100 mg of four samples (outer membrane [Mt OM], contaminant outer membrane [HSP-OM], mitoplast, and inner membrane [IM]), each created by pooling three biological replicates, were analyzed according to a modification of the method reported previously (Shingaki-Wells et al., 2011) as outlined in Supplemental Materials and Methods S1. Quantitation was carried out using an in-house quantitation method that carried out protein identifications and quantitation on isobaric tags of mass 114 to 117 at the peptide level. Ratios for individual peptide matches were obtained from peptides meeting the minimum criteria outlined above and were then combined to determine ratios for protein hits using a weighted average. The in-house method of identification and quantitation, outlier removal, minimum number of required peptides, and definitions of statistical confidence internals are outlined in detail in Supplemental Materials and Methods S1. Supplemental Figure S5. Coexpression of genes encoding outer mitochondrial membrane proteins. In Vitro Imports Supplemental Table S5. Details of the germination microarrays and developmental tissue set (Schmid et al., 2005; E-AFMX-9). In vitro import of putative outer membrane proteins into isolated mitochondria was performed as described previously (Lister et al., 2007). Data are provided in Supplemental Figure S3, II, III, and IV. Supplemental Table S6. Details of the genes defined as encoding inner mitochondrial membrane proteins. Supplemental Table S1. List of the 185 proteins detected in two or more of the outer membrane-enriched biological replicates (www.Arabidopsis. org). Supplemental Table S2. List of the 64 members of the putative outer membrane proteome with evidence for identification and enrichment in the outer membrane fraction (www.Arabidopsis.org). Supplemental Table S3. Summary of the location evidence gathered in the course of this research. Supplemental Table S4. Summary of data gathered from iTRAQ analysis of the outer membrane-enriched and three prefractionation samples. Supplemental Table S7. Details of polypeptide identifications resulting from the in-gel digestion of bands excised from the gel pictured in Figure 3A GFP Analysis Full-length coding sequences (with the exception of At4g17140.2) of putative outer membrane proteins were cloned into the Gateway cassette cloning system according to the manufacturer’s instructions (Invitrogen; http://invitrogen.com/). Coding sequences were transferred into GFP vectors and cotransformed into Arabidopsis suspension cell culture with a red fluorescent protein control by particle bombardment as outlined previously (Carrie et al., 2009). Data are provided in Supplemental Figures S1 to S4. Supplemental Table S8. Sequence data. Supplemental Materials and Methods S1. Detailed MS methods. Received July 9, 2011; accepted August 31, 2011; published September 6, 2011. LITERATURE CITED Arabidopsis Microarray Analysis The Arabidopsis AtGenExpress developmental data set was downloaded as CEL files (E-AFMX-9) from the MIAME ArrayExpress database (http:// www.ebi.ac.uk/arrayexpress/). These CEL files, in addition to the 30 CEL files that analyzed expression during germination, were imported and quantile normalized together to enable comparability across these arrays using Partek Genomics Suite version 6.5. Details for each tissue are shown in Supplemental Table S5. Once normalized, expression values were made relative to maximum expression and hierarchically clustered on Euclidean distance using average linkage clustering. Note that four genes, At1g24267, At3g11070, At3g50940, and At5g22350, were not represented on the ATH1 microarray. This method of normalization and clustering has been used effectively before to examine coexpression (Narsai et al., 2010). An FY, Clewell DB (1994) Characterization of the determinant (traB) encoding sex pheromone shutdown by the hemolysin/bacteriocin plasmid pAD1 in Enterococcus faecalis. Plasmid 31: 215–221 Arimura S, Fujimoto M, Doniwa Y, Kadoya N, Nakazono M, Sakamoto W, Tsutsumi N (2008) Arabidopsis ELONGATED MITOCHONDRIA1 is required for localization of DYNAMIN-RELATED PROTEIN3A to mitochondrial fission sites. Plant Cell 20: 1555–1566 Armstrong MT, Theg SM, Braun N, Wainwright N, Pardy RL, Armstrong PB (2006) Histochemical evidence for lipid A (endotoxin) in eukaryote chloroplasts. FASEB J 20: 2145–2146 Boisson B, Giglione C, Meinnel T (2003) Unexpected protein families including cell defense components feature in the N-myristoylome of a higher eukaryote. J Biol Chem 278: 43418–43429 Bolouri-Moghaddam MR, Le Roy K, Xiang L, Rolland F, Van den Ende W Plant Physiol. Vol. 157, 2011 1111 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Duncan et al. (2010) Sugar signalling and antioxidant network connections in plant cells. FEBS J 277: 2022–2037 Braun HP, Werhahn W, Jansch L (2003) Identification of novel subunits of the TOM complex from Arabidopsis thaliana. Plant Physiol Biochem 41: 407–416 Bussemer J, Vothknecht UC, Chigri F (2009) Calcium regulation in endosymbiotic organelles of plants. Plant Signal Behav 4: 805–808 Camougrand N, Pélissier P, Velours G, Guérin M (1995) NCA2, a second nuclear gene required for the control of mitochondrial synthesis of subunits 6 and 8 of ATP synthase in Saccharomyces cerevisiae. J Mol Biol 247: 588–596 Carrie C, Kühn K, Murcha MW, Duncan O, Small ID, O’Toole N, Whelan J (2009) Approaches to defining dual-targeted proteins in Arabidopsis. Plant J 57: 1128–1139 Chew O, Lister R, Qbadou S, Heazlewood JL, Soll J, Schleiff E, Millar AH, Whelan J (2004) A plant outer mitochondrial membrane protein with high amino acid sequence identity to a chloroplast protein import receptor. FEBS Lett 557: 109–114 Chong PK, Gan CS, Pham TK, Wright PC (2006) Isobaric tags for relative and absolute quantitation (iTRAQ) reproducibility: implication of multiple injections. J Proteome Res 5: 1232–1240 Damari-Weissler H, Ginzburg A, Gidoni D, Mett A, Krassovskaya I, Weber AP, Belausov E, Granot D (2007) Spinach SoHXK1 is a mitochondria-associated hexokinase. Planta 226: 1053–1058 da-Silva WS, Gómez-Puyou A, de Gómez-Puyou MT, Moreno-Sanchez R, De Felice FG, de Meis L, Oliveira MF, Galina A (2004) Mitochondrial bound hexokinase activity as a preventive antioxidant defense: steadystate ADP formation as a regulatory mechanism of membrane potential and reactive oxygen species generation in mitochondria. J Biol Chem 279: 39846–39855 Dry I, Nash D, Wishich JT (1983) The mitochondrial localization of hexokinase in pea leaves. Planta 158: 152–156 Eubel H, Heinemeyer J, Sunderhaus S, Braun HP (2004) Respiratory chain supercomplexes in plant mitochondria. Plant Physiol Biochem 42: 937–942 Eubel H, Lee CP, Kuo J, Meyer EH, Taylor NL, Millar AH (2007) Free-flow electrophoresis for purification of plant mitochondria by surface charge. Plant J 52: 583–594 Eubel H, Meyer EH, Taylor NL, Bussell JD, O’Toole N, Heazlewood JL, Castleden I, Small ID, Smith SM, Millar AH (2008) Novel proteins, putative membrane transporters, and an integrated metabolic network are revealed by quantitative proteomic analysis of Arabidopsis cell culture peroxisomes. Plant Physiol 148: 1809–1829 Fransson A, Ruusala A, Aspenström P (2003) Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem 278: 6495–6502 Frederick RL, McCaffery JM, Cunningham KW, Okamoto K, Shaw JM (2004) Yeast Miro GTPase, Gem1p, regulates mitochondrial morphology via a novel pathway. J Cell Biol 167: 87–98 Fukuchi-Mizutani M, Mizutani M, Tanaka Y, Kusumi T, Ohta D (1999) Microsomal electron transfer in higher plants: cloning and heterologous expression of NADH-cytochrome b5 reductase from Arabidopsis. Plant Physiol 119: 353–362 Germain V, Rylott EL, Larson TR, Sherson SM, Bechtold N, Carde JP, Bryce JH, Graham IA, Smith SM (2001) Requirement for 3-ketoacylCoA thiolase-2 in peroxisome development, fatty acid beta-oxidation and breakdown of triacylglycerol in lipid bodies of Arabidopsis seedlings. Plant J 28: 1–12 Graham JW, Williams TC, Morgan M, Fernie AR, Ratcliffe RG, Sweetlove LJ (2007) Glycolytic enzymes associate dynamically with mitochondria in response to respiratory demand and support substrate channeling. Plant Cell 19: 3723–3738 Gutiérrez RA, Larson MD, Wilkerson C (2004) The plant-specific database: classification of Arabidopsis proteins based on their phylogenetic profile. Plant Physiol 135: 1888–1892 Han BW, Bingman CA, Mahnke DK, Bannen RM, Bednarek SY, Sabina RL, Phillips GN Jr (2006) Membrane association, mechanism of action, and structure of Arabidopsis embryonic factor 1 (FAC1). J Biol Chem 281: 14939–14947 Haucke V, Ocana CS, Hönlinger A, Tokatlidis K, Pfanner N, Schatz G (1997) Analysis of the sorting signals directing NADH-cytochrome b5 reductase to two locations within yeast mitochondria. Mol Cell Biol 17: 4024–4032 Heazlewood JL, Howell KA, Millar AH (2003) Mitochondrial complex I from Arabidopsis and rice: orthologs of mammalian and fungal components coupled with plant-specific subunits. Biochim Biophys Acta 1604: 159–169 Heazlewood JL, Tonti-Filippini JS, Gout AM, Day DA, Whelan J, Millar AH (2004) Experimental analysis of the Arabidopsis mitochondrial proteome highlights signaling and regulatory components, provides assessment of targeting prediction programs, and indicates plantspecific mitochondrial proteins. Plant Cell 16: 241–256 Heazlewood JL, Verboom RE, Tonti-Filippini J, Small I, Millar AH (2007) SUBA: the Arabidopsis Subcellular Database. Nucleic Acids Res 35: D213–D218 Heins L, Schmitz UK (1996) A receptor for protein import into potato mitochondria. Plant J 9: 829–839 Howell KA, Millar AH, Whelan J (2006) Ordered assembly of mitochondria during rice germination begins with pro-mitochondrial structures rich in components of the protein import apparatus. Plant Mol Biol 60: 201–223 Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M (2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 4: 1265–1272 Jänsch L, Kruft V, Schmitz UK, Braun HP (1998) Unique composition of the preprotein translocase of the outer mitochondrial membrane from plants. J Biol Chem 273: 17251–17257 Jelsema CL, Morré DJ (1978) Distribution of phospholipid biosynthetic enzymes among cell components of rat liver. J Biol Chem 253: 7960–7971 Karve A, Moore BD (2009) Function of Arabidopsis hexokinase-like1 as a negative regulator of plant growth. J Exp Bot 60: 4137–4149 Kim M, Lim JH, Ahn CS, Park K, Kim GT, Kim WT, Pai HS (2006) Mitochondria-associated hexokinases play a role in the control of programmed cell death in Nicotiana benthamiana. Plant Cell 18: 2341–2355 Klodmann J, Sunderhaus S, Nimtz M, Jansch L, Braun HP. (2010) Internal architecture of mitochondrial complex I from Arabidopsis thaliana. Plant Cell 22: 797–810 Koehler CM (2004) The small Tim proteins and the twin Cx3C motif. Trends Biochem Sci 29: 1–4 Kornmann B, Walter P (2010) ERMES-mediated ER-mitochondria contacts: molecular hubs for the regulation of mitochondrial biology. J Cell Sci 123: 1389–1393 Koshiba T, Holman HA, Kubara K, Yasukawa K, Kawabata S, Okamoto K, MacFarlane J, Shaw JM (2011a) Structure-function analysis of the yeast mitochondrial Rho GTPase, Gem1p: implications for mitochondrial inheritance. J Biol Chem 286: 354–362 Koshiba T, Yasukawa K, Yanagi Y, Kawabata S (2011b) Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci Signal 4: ra7 Kozjak V, Wiedemann N, Milenkovic D, Lohaus C, Meyer HE, Guiard B, Meisinger C, Pfanner N (2003) An essential role of Sam50 in the protein sorting and assembly machinery of the mitochondrial outer membrane. J Biol Chem 278: 48520–48523 Kumar R, Wallis JG, Skidmore C, Browse J (2006) A mutation in Arabidopsis cytochrome b5 reductase identified by high-throughput screening differentially affects hydroxylation and desaturation. Plant J 48: 920–932 Kuroiwa T (2010) Mechanisms of organelle division and inheritance and their implications regarding the origin of eukaryotic cells. Proc Jpn Acad Ser B Phys Biol Sci 86: 455–471 Lee SM, Hoang MH, Han HJ, Kim HS, Lee K, Kim KE, Kim DH, Lee SY, Chung WS (2009) Pathogen inducible voltage-dependent anion channel (AtVDAC) isoforms are localized to mitochondria membrane in Arabidopsis. Mol Cells 27: 321–327 Lilley KS, Dunkley TP (2008) Determination of genuine residents of plant endomembrane organelles using isotope tagging and multivariate statistics. Methods Mol Biol 432: 373–387 Lindsay J, Esposti MD, Gilmore AP (2011) Bcl-2 proteins and mitochondria: specificity in membrane targeting for death. Biochim Biophys Acta 1813: 532–539 Lister R, Carrie C, Duncan O, Ho LH, Howell KA, Murcha MW, Whelan J (2007) Functional definition of outer membrane proteins involved in preprotein import into mitochondria. Plant Cell 19: 3739–3759 Lister R, Chew O, Lee MN, Heazlewood JL, Clifton R, Parker KL, Millar 1112 Plant Physiol. Vol. 157, 2011 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved. Arabidopsis Mitochondrial Outer Membrane AH, Whelan J (2004) A transcriptomic and proteomic characterization of the Arabidopsis mitochondrial protein import apparatus and its response to mitochondrial dysfunction. Plant Physiol 134: 777–789 Lister R, Hulett JM, Lithgow T, Whelan J (2005) Protein import into mitochondria: origins and functions today (review). Mol Membr Biol 22: 87–100 Logan DC (2010) Mitochondrial fusion, division and positioning in plants. Biochem Soc Trans 38: 789–795 Logan DC, Millar AH, Sweetlove LJ, Hill SA, Leaver CJ (2001) Mitochondrial biogenesis during germination in maize embryos. Plant Physiol 125: 662–672 Macasev D, Newbigin E, Whelan J, Lithgow T (2000) How do plant mitochondria avoid importing chloroplast proteins? Components of the import apparatus Tom20 and Tom22 from Arabidopsis differ from their fungal counterparts. Plant Physiol 123: 811–816 Meinke D, Muralla R, Sweeney C, Dickerman A (2008) Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci 13: 483–491 Meyer EH, Taylor NL, Millar AH (2008) Resolving and identifying protein components of plant mitochondrial respiratory complexes using three dimensions of gel electrophoresis. J Proteome Res 7: 786–794 Millar AH, Carrie C, Pogson B, Whelan J (2009) Exploring the functionlocation nexus: using multiple lines of evidence in defining the subcellular location of plant proteins. Plant Cell 21: 1625–1631 Misaki R, Kajiura H, Fujii K, Fujiyama K, Seki T (2009) Cloning and characterization of cytidine monophosphate-3-deoxy-D-manno-octulosonate synthetase from Arabidopsis thaliana. J Biosci Bioeng 108: 527–529 Mizoi J, Nakamura M, Nishida I (2006) Defects in CTP:PHOSPHORYLETHANOLAMINE CYTIDYLYLTRANSFERASE affect embryonic and postembryonic development in Arabidopsis. Plant Cell 18: 3370–3385 Murcha MW, Elhafez D, Millar AH, Whelan J (2005) The C-terminal region of TIM17 links the outer and inner mitochondrial membranes in Arabidopsis and is essential for protein import. J Biol Chem 280: 16476–16483 Narsai R, Ivanova A, Ng S, Whelan J (2010) Defining reference genes in Oryza sativa using organ, development, biotic and abiotic transcriptome datasets. BMC Plant Biol 10: 56 Opiyo SO, Pardy RL, Moriyama H, Moriyama EN (2010) Evolution of the Kdo2-lipid A biosynthesis in bacteria. BMC Evol Biol 10: 362 Osman C, Voelker DR, Langer T (2011) Making heads or tails of phospholipids in mitochondria. J Cell Biol 192: 7–16 Raetz CR, Whitfield C (2002) Lipopolysaccharide endotoxins. Annu Rev Biochem 71: 635–700 Reape TJ, McCabe PF (2010) Apoptotic-like regulation of programmed cell death in plants. Apoptosis 15: 249–256 Reis K, Fransson A, Aspenström P (2009) The Miro GTPases: at the heart of the mitochondrial transport machinery. FEBS Lett 583: 1391–1398 Schmid M, Davison TS, Henz SR, Pape UJ, Demar M, Vingron M, Schölkopf B, Weigel D, Lohmann JU (2005) A gene expression map of Arabidopsis thaliana development. Nat Genet 37: 501–506 Schmitt S, Prokisch H, Schlunck T, Camp DG II, Ahting U, Waizenegger T, Scharfe C, Meitinger T, Imhof A, Neupert W, et al (2006) Proteome analysis of mitochondrial outer membrane from Neurospora crassa. Proteomics 6: 72–80 Scott I, Tobin AK, Logan DC (2006) BIGYIN, an orthologue of human and yeast FIS1 genes functions in the control of mitochondrial size and number in Arabidopsis thaliana. J Exp Bot 57: 1275–1280 Setoguchi K, Otera H, Mihara K (2006) Cytosolic factor- and TOMindependent import of C-tail-anchored mitochondrial outer membrane proteins. EMBO J 25: 5635–5647 Séveno M, Séveno-Carpentier E, Voxeur A, Menu-Bouaouiche L, Rihouey C, Delmas F, Chevalier C, Driouich A, Lerouge P (2010) Characterization of a putative 3-deoxy-D-manno-2-octulosonic acid (Kdo) transferase gene from Arabidopsis thaliana. Glycobiology 20: 617–628 Sheahan MB, McCurdy DW, Rose RJ (2005) Mitochondria as a connected population: ensuring continuity of the mitochondrial genome during plant cell dedifferentiation through massive mitochondrial fusion. Plant J 44: 744–755 Sheahan MB, Rose RJ, McCurdy DW (2004) Organelle inheritance in plant cell division: the actin cytoskeleton is required for unbiased inheritance of chloroplasts, mitochondria and endoplasmic reticulum in dividing protoplasts. Plant J 37: 379–390 Shingaki-Wells RN, Huang S, Taylor NL, Carroll AJ, Zhou W, Millar AH (2011) Differential molecular responses of rice and wheat coleoptiles to anoxia reveal novel metabolic adaptations in amino acid metabolism for tissue tolerance. Plant Physiol 156: 1706–1724 Stefan Dimmer K, Rapaport D (2010) The enigmatic role of Mim1 in mitochondrial biogenesis. Eur J Cell Biol 89: 212–215 Strittmatter P (1965) The reaction sequence in electron transfer in the reduced nicotinamide adenine dinucleotide-cytochrome b5 reductase system. J Biol Chem 240: 4481–4487 Sweetlove LJ, Mowday B, Hebestreit HF, Leaver CJ, Millar AH (2001) Nucleoside diphosphate kinase III is localized to the inter-membrane space in plant mitochondria. FEBS Lett 508: 272–276 Van Aken O, Zhang B, Carrie C, Uggalla V, Paynter E, Giraud E, Whelan J (2009) Defining the mitochondrial stress response in Arabidopsis thaliana. Mol Plant 2: 1310–1324 Vanhee C, Guillon S, Masquelier D, Degand H, Deleu M, Morsomme P, Batoko H (2011) A TSPO-related protein localizes to the early secretory pathway in Arabidopsis, but is targeted to mitochondria when expressed in yeast. J Exp Bot 62: 497–508 Walther DM, Rapaport D (2009) Biogenesis of mitochondrial outer membrane proteins. Biochim Biophys Acta 1793: 42–51 Weitbrecht K, Müller K, Leubner-Metzger G (2011) First off the mark: early seed germination. J Exp Bot 62: 3289–3309 Werhahn W, Niemeyer A, Jänsch L, Kruft V, Schmitz UK, Braun H (2001) Purification and characterization of the preprotein translocase of the outer mitochondrial membrane from Arabidopsis: identification of multiple forms of TOM20. Plant Physiol 125: 943–954 Xu C, Moellering ER, Fan J, Benning C (2008) Mutation of a mitochondrial outer membrane protein affects chloroplast lipid biosynthesis. Plant J 54: 163–175 Xu J, Zhang HY, Xie CH, Xue HW, Dijkhuis P, Liu CM (2005) EMBRYONIC FACTOR 1 encodes an AMP deaminase and is essential for the zygote to embryo transition in Arabidopsis. Plant J 42: 743–756 Xu S, Peng G, Wang Y, Fang S, Karbowski M (2011) The AAA-ATPase p97 is essential for outer mitochondrial membrane protein turnover. Mol Biol Cell 22: 291–300 Yamaoka S, Leaver CJ (2008) EMB2473/MIRO1, an Arabidopsis Miro GTPase, is required for embryogenesis and influences mitochondrial morphology in pollen. Plant Cell 20: 589–601 Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schönfisch B, Guiard B, Pfanner N, Meisinger C (2006) Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol Biol Cell 17: 1436–1450 Zhang J, Liu W, Liu J, Xiao W, Liu L, Jiang C, Sun X, Liu P, Zhu Y, Zhang C, et al (2010) G-protein b2 subunit interacts with mitofusin 1 to regulate mitochondrial fusion. Nat Commun 1: 101 Zhao WN, McAlister-Henn L (1996) Assembly and function of a cytosolic form of NADH-specific isocitrate dehydrogenase in yeast. J Biol Chem 271: 10347–10352 Zybailov BL, Florens L, Washburn MP (2007) Quantitative shotgun proteomics using a protease with broad specificity and normalized spectral abundance factors. Mol Biosyst 3: 354–360 Plant Physiol. Vol. 157, 2011 1113 Downloaded from on June 18, 2017 - Published by www.plantphysiol.org Copyright © 2011 American Society of Plant Biologists. All rights reserved.