* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hippocampal Formation
State-dependent memory wikipedia , lookup
Aging brain wikipedia , lookup
Synaptic gating wikipedia , lookup
Apical dendrite wikipedia , lookup
Development of the nervous system wikipedia , lookup
Optogenetics wikipedia , lookup
Neuroanatomy wikipedia , lookup
Subventricular zone wikipedia , lookup
Environmental enrichment wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Eyewitness memory (child testimony) wikipedia , lookup
Adult neurogenesis wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Holonomic brain theory wikipedia , lookup
Memory consolidation wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
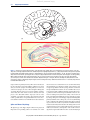
This article was originally published in the Encyclopedia of Human Behavior published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who you know, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial Spiers H.J. (2012) Hippocampal Formation. In: V.S. Ramachandran (ed.) The Encyclopedia of Human Behavior, vol. 2, pp. 297-304. Academic Press. © 2012 Elsevier Inc. All rights reserved. Author's personal copy Hippocampal Formation H J Spiers, University College London, London, UK ã 2012 Elsevier Inc. All rights reserved. Glossary Action potential A nerve impulse, comprising a brief rising and falling of a cell’s membrane potential. Episodic memory Memory for events and episodes that were personally experienced. Local field potential Local voltage changes in a brain region due to the combined electrical effects of a synchronously active population of neurons. Introduction The undulating, twisted, interlocking brain structure called the hippocampal formation has fascinated anatomists from the dawn of dissection in ancient Egypt. Over the centuries, its physical appearance has been likened to seahorses, silkworms, and the horns of a ram. The seahorse stuck, and we know it today from the Latin for seahorse: hippocampus. The hippocampal formation is composed of the hippocampus, subicular complex, and entorhinal cortex. It is unparalleled in terms of its unique circuitry and physiology. Housed within it are some of the most morphologically spectacular cells in the brain. Decoding their activity patterns has opened a window into some of the brain’s fundamental operating characteristics and uncovered the existence of a map and compass within the brain. Unlike most of the brain, new neurons are born within it during adulthood, which must strive to integrate themselves into an intricate network of existing cells. With the discovery of dense amnesia following the surgical removal of the hippocampal formation in the famous patient HM, this brain region has been firmly wedded to memory. But just what ‘type’ of memory it supports and how it functions remains contentious. This article aims to distil the key concepts surrounding the hippocampal formation into a digestible form and provide a brief overview of its anatomy, physiology, pathology, and theorized functions. A Seahorse in the Brain: Anatomy Like many of the world’s international borders, the borders of the hippocampal formation are disputed. One view, adopted in this article, is that the hippocampal formation is composed of six regions. These are the hippocampus proper, dentate gyrus, subiculum, presubiculum, parasubiculum, and entorhinal cortex. In other nomenclatures the entorhinal cortex, presubiculum, and parasubiculum are separated from the hippocampal formation and are gathered under the term ‘parahippocampal region.’ When the term ‘hippocampus’ is used it often refers to the hippocampus proper and the dentate gyrus collectively. A cross-sectional cut through the hippocampal formation reveals a snugly curled up snaking shape in which the dentate Medial Toward the midline of the brain. Neurogenesis The birth of new neurons. Pyramidal cell The main excitatory cell type in the neocortex and hippocampal formation, the cell bodies of which appear pyramidal in shape. gyrus appears to be ‘biting’ the hippocampus proper (see Figure 1). The hippocampus proper is divided into three main subdivisions: CA1, CA2, and CA3. CA stands for ‘Cornu Ammonis,’ which refers to Amun’s horns, named after the ancient Egyptian god of the hidden world whose symbol was ram’s horn. The pyramidal cells in the Cornu Ammonis occupy a single packed layer, quite unlike the neocortex where pyramidal cells are spread over several layers. By contrast the dentate gyrus contains no pyramidal cells, but is densely packed with smaller granule cells, 18 million in the human brain. In addition to these cells, a variety of interneurons are found in the different structures of the hippocampal formation. From mouse to man the hippocampus is highly homologous across all mammals. In primates, the hippocampal formation is curled inside the medial temporal lobe (Figure 1(a)), alongside the amygdala, perirhinal cortex, and parahippocampal cortex. The entorhinal cortex has been viewed as the neocortical gate-keeper, sending projections into the structure, receiving its output and communicating with other neocortical structures. The other major source of communication with the rest of the brain is the fornix, the white matter pathway connecting the hippocampal formation to various subcortical structures and providing some output to prefrontal cortex. Via this pathway and other routes the hippocampal formation receives modulatory input from dopamine, norepinephrine (adrenaline), serotonin, and acetylcholine systems. Theories of hippocampal function were influenced by several features of its connectivity. Four important ones are: (1) information arriving at the hippocampal formation has been highly processed through various unimodal and multimodal neocortical pathways, (2) there are largely unidirectional connections between its regions, (3) there are dense projections from the dentate to CA3, and (4) there are highly self-connected network pyramidal cells in CA3. Unidirectional connectivity is unusual in the cortex, where pyramidal cells are usually characterized by dense reciprocal connections to other cells. A full characterization of connections is beyond the scope of this article (see Further Reading). The main flow of information follows four fiber pathways (see Figure 1(b)). Two arise from the entorhinal cortex. The first is the perforant path, bringing input from its layer II to the dentate and CA2/CA3, the second (less dense) is the Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 297 Author's personal copy 298 Hippocampal Formation 2 2 1 (a) Temporoammonic pathway CA1 EC Schaffer collaterals LPP III MPP CA3 II Mossy fibers Dentate gyrus Perforant pathway (b) Figure 1 Anatomy of the hippocampal formation. (a) An illustration of the medial surface of the human brain. The front of the brain is to the left. The position of the hippocampal formation in the medial temporal lobe is highlighted by darker lines, (1) hippocampal formation and (2) fornix. Reprinted from Amaral DG (1994) Hippocampal formation. In: Ramachandran V (ed.) Encyclopedia of Human Behavior, 1st edn., pp 509–515. Academic Press, with permission from Elsevier. (b) A diagram of the circuitry in the hippocampal formation. Solid arrows depict the main ‘trisynaptic’ excitatory pathway. MPP, medial perforant path; LPP, lateral perforant path. II, III refer to layers II and III of the entorhinal cortex (EC). The fornix output pathway (not shown) arises form the CA1 collaterals and subiculum. Adapted from Figure 1 in Deng W, Amoni JB, and Gage FH (2010) New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience 11: 339–350, with permission from Macmillan Publishers Ltd. temporoammonic pathway from layer III to CA1. From the dentate arises the dense ‘mossy fiber pathway’ which projects exclusively to CA3. The synapses from this pathway are referred to as ‘detonator synapses,’ because of their strength in driving CA3 cell activity. The CA3 cells project to CA1 via Schaffer collaterals, and to many other CA3 cells via recurrent collaterals. CA1 cells project to the subiculum and the deep layer neurons of the entorhinal cortex and via the fornix to other structures. The subiculum, presubiculum, and parasubiculum have interconnections with the entorhinal cortex and with various other structures. Spikes and Waves: Physiology The physiology of the hippocampal formation has played an important role in providing evidence for its function. A central idea in neuroscience is that memories are stored in the brain by the strengthening or weakening of synaptic efficacy between cells in regions responsible for memory storage. Evidence for this was first provided by Bliss and Lømo in 1973 with their discovery of long-term potentiation (LTP) in the hippocampus. LTP refers to a persistent enhancement in signal transmission between two neurons resulting from synchronous activity of the neurons. It has been traditionally studied either ‘in vitro’ (literally: ‘in glass’) by cutting a living slice from the hippocampus, stimulating fiber pathways within it and recording cells, or ‘in vivo’ by stimulating and recording in awake, behaving animals using chronic indwelling electrodes. Since its discovery, it has been studied extensively and observed in many brain structures. Mounting evidence indicates that memory storage requires LTP. Research focused on its induction, maintenance, and expression and a wealth of knowledge has been gained about the molecular cascades underlying it. Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 Author's personal copy Hippocampal Formation Our understanding of hippocampal physiology has been advanced considerably by the use of extracellular microelectrodes to record the local field potential (LFP) and individual cell activity in awake, active, and sleeping animals, typically rats or mice. The LFP is generated by local voltage changes in the brain region due to the combined electrical effects of a synchronously active population of neurons. Two predominant LFP states have been observed during awake behavior: theta state and large irregular activity. When a rat is either alert or moving, a prominent regular 4–12 Hz ‘theta rhythm,’ oscillation is observed throughout the hippocampal formation; when resting or eating the LFP is instead dominated by large irregularly activity. Several interesting aspects of theta are worth considering. Theta is modulated by the speed of running and influences the temporal pattern of pyramidal cell activity in hippocampus proper. The ability to induce LTP varies with the theta cycle, which has led to the suggestion that encoding and retrieval may be separated temporally by theta cycles. The theta oscillations are thought to reflect the combination of subthreshold membrane potentials and spiking activity, and appear to synchronize across the hippocampus to create a traveling wave across its axis. Large irregular activity has also been associated with synchronization in the bursting activity of the cells, particularly with the occurrence of sharp wave-ripples of 150–200 Hz. Evidence indicates that the ripples are generated by CA3, and during these events, brief near synchronous activity of many hippocampal neurons occurs. This reactivation of recently active cells may be important for the strengthening of connections between cells and for transfer of information to other regions. Sharp wave-ripple events are common in sleep and their disruption impedes spatial learning. In addition to studying the LFP, remarkable insights into the function of the hippocampal formation have been found 299 by decoding the activity of individual neurons during awake behavior. This is discussed in greater detail below. Maps and Compasses: Neural Coding Each of us carries in our head an internal map of our world and a compass to orient within it. These appear to be located within or near the hippocampal formation. This was discovered by implanting microwires into the hippocampal formation of rodents and recording the extracellular activity while the animal explores an environment. By continually recording the animal’s position together with the neuronal activity it is possible to map the activity of cells to the surface of the environment and to the momentary orientation of the animal within it. This approach revealed an elegant system dedicated to spatial mapping and orientation. Because of their distinctive properties, cells in different regions of the hippocampal formation have been labeled with names such as ‘place cells,’ ‘head-direction cells,’ ‘grid cells,’ and ‘border cells.’ The first to be discovered were place cells by O’Keefe and Dostrovsky in 1971. These exist in the hippocampus proper and fire action potentials (a rapid depolarization in the membrane potential) when an animal is in a particular location in the environment, but are typically silent otherwise (see Figure 2(b)). The location in an environment where a cell fires is called its place field. In a given environment, only a subset of place cells will be active, with each cell’s place field occupying a slightly different location, such that their collective, overlapping place fields carpet the whole environment. Place cells express different activity patterns in different environments, a phenomenon known as remapping. By recording a large ensemble of cells it has been possible to decode a rat’s Camera Recording system Activity High Rat on arena (a) (b) (c) Low Figure 2 Single-unit recording in the hippocampal formation. (a) A diagram of a set up used for in vivo single unit electrophysiology, courtesy of Kathryn J. Jeffery. A rat is shown in a rectangular flat arena. A cable connects the implanted microwires in the rat’s hippocampal formation to a recording system. The recording system also receives information about the location of the rat via a camera. (b, c) Top, plots of a rat’s trajectory in the arena (black lines). Overlaid red dots are the locations at which action potentials were fired by a single place cell in CA3 (b), and a single grid cell in the dorsomedial entorhinal cortex (c). Below each is shown a false color plot of the spatially smoothed firing rate of each cell, showing the Gaussian peaked fields of activity. Note that, in this example, the place cell field is large than the grid cell field, but field size can vary substantially along the dorso–ventral axis. Adapted from Figure 1 in Fyhn M, Hafting T, Treves A, Moser MB, and Moser EI (2007) Hippocampal remapping and grid realignment in entorhinal cortex. Nature 446(7132): 190–194, with permission from Macmillan Publishers Ltd; Figure 2 in Hafting T, Fyhn M, Molden S, Moser MB, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436(7052): 801–806, with permission from Macmillan Publishers Ltd. Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 Author's personal copy 300 Hippocampal Formation location in an environment to within 1 cm. Unlike the topographic maps in the visual, somatosensory, and motor cortices, the place cell map is not topographically organized within the hippocampus. Cells with place fields next to each other in an environment are not located next to each other in the hippocampus. However, the size of the place field varies from small in the dorsal hippocampus to large in the ventral hippocampus. Place cells have several interesting properties. Their response appears to be a high-level, multimodal conjunction of inputs that includes information about self-motion. They respond by remapping predominantly to changes in the boundaries, distant landmarks, and large-scale sensory aspects of the environment, such as the floor and wall colors. They can learn incidentally (without reward) over several trials to discriminate very similar environments. The cells in a number of peri-hippocampal areas such as the mammillary bodies, anterior thalamus, dorsal presubiculum, and retrosplenial cortex also produce a spatially tuned response, but it is not place related. Instead, these cells offer something akin to an internal compass by expressing activity tuned to certain head-directions in the current environment. Thus, one cell might fire maximally when an animal’s head is facing Northeast, another when facing Southeast, another Northwest, etc. Collectively the population covers all possible heading orientations. These cells are referred to as ‘head-direction cells.’ The cells can be modulated both by self-motion information (such as vestibular or motor signals) and visual information. When prominent landmarks in an environment are rotated between visits to an environment, these cells will tend to follow the rotation, with all cells rotating by the same amount. ‘Grid cells’ and ‘Border cells’ have both been discovered in the medial entorhinal cortex and more recently in other regions of the subicular complex. They are similar to place cells in that they show spatially localized patterns of activity in an environment, but they each differ from place cells in intriguing ways. Grid cells generate multiple place fields arranged in a tessellating grid-like pattern across the environment (see Figure 2(c)). If lines are drawn between all fields, their pattern appears somewhat like a sheet of graph paper imposed on the environment, but rather than graph lines being at 90 right angles forming squares, the grid lines are at 60 to each other forming triangles. Simultaneously recorded grid cells show the same orientation of their grid pattern within an environment, but may show different spacing between fields. Mirroring the dorsal ventral scaling of place field size, cells in the dorsal region have a small spacing between fields, whereas those in the ventral region have large spacing between fields. It is thought that grid cells provide inputs to place cells about the distance traveled in the environment. Border cells, referred to as boundary vector cells, likely provide inputs about the environment, and as their name suggests, they signal the location of borders in a given environment. Border cells will typically fire along, or just slightly offset to, a border placed in an orientation matching its preferred orientation, for example, Northwest. An important facet of the system is that in addition to the cells described, conjunctive cells which combine grid or place properties with head-direction tuning, exist. These cells will only fire in a given place or set of places and only when an animal is facing a particular direction. These have been found in the medial entorhinal cortex and presubiculum, but not the hippocampus proper. Cells in dentate gyrus can express spatially localized patterns of activity, but in any given environment very few cells are active. It seems humans too have place cells and grid cells. Evidence for place cells has come from recordings made from electrodes implanted in patients with drug-resistant epilepsy while they navigated a virtual reality (VR) environment. Indirect evidence for grid cells has been provided by using functional magnetic imaging (fMRI) to examine brain activity patterns during VR navigation. Because a proportion of conjunctive head-direction modulated grid cells align to the 60 triangular grid orientations it was predicted that the activity of such cells might produce a sixfold symmetry of activity patterns. This proved to be true for activity in the right entorhinal cortex, indicating that this region in humans may contain grid cells. Combining human neuroimaging and VR has provided a number of other insights into the response dynamics of the hippocampal formation. For example, people who are better navigators produce more activity in their hippocampus; activity in the right entorhinal cortex increases with the Euclidean distance to a goal, and hippocampal activity is maximal during the initial learning of a network of streets. In summary, while analyses of neural activity have taught us much about the information processed in the hippocampus, combining such work with other approaches can help uncover the functions of the hippocampal formation. Mapping, Declaring, Relating, Binding, Constructing: Theories of Function What is the function of the hippocampal formation? Does it serve a unique function or multiple functions and does it operate as part of a wider system? Undoubtedly, it does not work alone and its contributions to cognition are likely varied. A full characterization of its function will arguably require understanding the computational contributions of each subregion in its structure and their interrelations. This is a significant challenge far beyond current knowledge. However, some constraints on what it may, or may not, be involved in have been gleaned over the years, but not without considerable debate and disagreement. Historically, the hippocampal formation was linked to olfaction through a supposed strong relationship to the olfactory bulb and later tied to emotion because of its connections to other brain regions. These views are generally no longer held. However, there is evidence that the hippocampal formation forms part of a circuit involved in anxiety and this idea was developed into a theory by Gray. There is expanding interest in the role the hippocampus plays in stress disorders and depression (see section ‘Dying Cells: Pathology’). However, it was the discovery of dense amnesia following the surgical bilateral removal of the hippocampal formation in patient HM in 1953 that firmly cemented its role in memory processing. Patient HM, now known as Henry Molaison, was reported (along with several other cases) in a seminal paper by Scoville and Milner in 1957. He underwent the surgery for the treatment of drug-resistant epilepsy (see section ‘Dying Cells: Pathology’). The surgery removed the hippocampal formation, Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 Author's personal copy Hippocampal Formation parahippocampal gyrus and amygdala. From the time he awoke from the surgery until the time of his death in 2008 he was unable to remember any new events he experienced – a severe anterograde amnesia. He also suffered a temporally graded retrograde amnesia, such that he could not remember events or information from a period just before the surgery, but the further back in time he was probed, the better he was able to recall memories. This gradient of retrograde amnesia has been observed across a large number of amnesic cases. Despite HM’s dense amnesia he (and other similar patients) showed a remarkable capacity for short-term memory, procedural memory and priming (the speeded response to a repeated stimulus). This led to the argument that the hippocampus is necessary for a ‘special type’ of memory. While numerous theories have been proposed over the past 60 years, this article will highlight six current and influential theories. The focus of many of these theories has been on the role played by the hippocampus, as opposed to the hippocampal formation more broadly. These theories are: 1. Cognitive map: Hippocampus stores a map of the environment supporting flexible navigation and spatial memory retrieval, and in humans, remembering events. 2. Declarative memory: Hippocampal formation is part of a medial temporal lobe system which supports memory for facts and events for a limited time period. 3. Relational memory: The hippocampus is necessary for storing and representing flexible relationships between stimuli disjointed over space and/or time. 4. Multiple trace: The hippocampus is permanently needed to support rich detailed episodic memory, but not semantic memory. 5. Episodic memory (binding – what/where/when): The hippocampal formation is necessary to bind together information about what happened, where and when, and later retrieving this memory for such episodes. It plays a time-limited role in representing these memories. 6. Construction: The hippocampal formation is part of a system supporting the reconstruction of the past or construction of possible future events. Cognitive map theory was developed by O’Keefe and Nadel. It argues that the hippocampus forms a flexible cognitive map of the environment to support the recall of what is located or happened in different places and navigation to unseen goals. This theory evolved primarily from the idea of a cognitive map put forward by Tolman in the 1940s, together with an analysis of the effect of lesions to the hippocampus and the discovery of place cells. An important aspect of the cognitive map is that information is learnt rapidly and incidentally, that is, without explicit reward. A criticism leveled at the theory is that in humans the hippocampus appears to be in need of remembering nonspatial information, and that spatial information is just one type of information stored by the hippocampus. However, it was proposed that the map may store information about what objects are located in different places and that for humans the map may have evolved with the addition of linguistic and temporal inputs to support an episodic memory system. One aspect of cognitive map theory that is disputed is that it puts no time limit on the involvement of the hippocampus. Thus, once a map of an environment is 301 formed in the hippocampus, the brain is dependent on the hippocampus indefinitely for the retrieval of the information in the map or the episodic memory. Declarative theory, proposed by Cohen and Squire, and developed by Squire thereafter, contrasts with cognitive map theory by arguing that: (1) the hippocampus has a time-limited role in memory retrieval and (2) the hippocampal formation is part of a unitary medial temporal lobe memory system (which includes the perirhinal and parahippocampal cortices) for the conscious encoding and memory for facts and events. The term ‘declarative’ is used because both memory for facts (semantic memory) and events (episodic memory) can be declared as either true or false, for example, ‘Socrates was a man,’ ‘I ate eggs for breakfast today.’ A central idea in the theory is that the declarative memory system is distinct from several nondeclarative memory systems, which are responsible for procedural memory, priming, conditioning, and stimulus adaption. Information in these systems is not veridical. For example, knowing how to move your body to ride a bicycle (a form of procedural memory) cannot be said to be true or false. Unlike other theories, declarative theory makes no specific claims about the contribution of different medial temporal lobe regions to memory function. Based on the temporal gradient of amnesia, declarative theory argues for a time-limited role in long-term memory. Building on a model by Marr, initially declarative memories are stored in the connections within medial temporal lobe and between it and the neocortex. Gradually, over time a ‘systems-level consolidation’ occurs such that connections form and strengthen between neocortical regions and eventually the neocortex rather than the hippocampus is needed to recall the memories. This view has also been referred to as the standard model of memory consolidation. Multiple trace theory (MTT), proposed by Moscovitch and Nadel disputes the idea that the medial temporal lobe is a unitary long-term memory system in which all information is consolidated in the neocortex. MTT agrees with the standard view with regard to semantic information, but argues that the rich, vivid reexperiencing of events or episodes always relies on the hippocampal region. This is based predominately on the observation that amnesic patients with medial temporal lobe damage typically report few episodic memories from any time point before their lesion and those they do are rarely vivid and detailed. This view is disputed by declarative memory proponents who argue amnesics can recall detailed episodic memories from time points in their remote past. Relational theory, proposed by Cohen and Eichenbaum, builds on the idea of a declarative memory system and a time-limited role in memory. It argues that the specific role of the hippocampus is in processing the relationships between stimuli disjointed in space or time and that other regions of the medial temporal lobe are involved in processing information about items or contexts. In this view, the hippocampal formation can be used to compare and contrast information held in the system to extract relationships and apply this information flexibly to new situations. The focus on processing relationships and flexibility is somewhat similar to the concept of a cognitive map, since a map is a very good example of this. However, the key difference is that in relational theory space is viewed as only one of the many types of relationship that can be coded by the hippocampal formation. Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 Author's personal copy 302 Hippocampal Formation The episodic memory theory has multiple proponents and has evolved from a range of findings. According to this view, the main purpose of the hippocampal formation is to encode and store episodic (or episodic-like) memories for weeks, months, or possibly years. Episodic-like is used to refer to the fact that while animals can show something like episodic memory, it may be qualitatively different from the sense of mental time travel that accompanies episodic retrieval in humans. This view is similar to the MTT view, with the exception that episodic memories are consolidated in the neocortex. Evidence that hippocampal formation might be especially crucial for episodic, but not semantic memory came from the study by Vargha-Khadem and colleagues in 1997 of several patients with amnesia due to bilateral hippocampal damage that occurred in childhood. They all had profound episodic memory problems. Despite this they had all gained a remarkable amount of semantic knowledge. This was surprising because amnesics with damage in adulthood do not show such levels of semantic learning. This led to the view that the neocortex may be able to slowly incrementally learn about information, and that the hippocampus normally facilitates this process by providing information stored in episodic memory. Like many other cases of amnesia due to relatively selective damage within the hippocampal formation, children were unable to recall past events, but were unimpaired in their capacity to recognize previously seen stimuli on some recognition tests. This and results from lesion studies in primates and rats led to the view, proposed by Aggleton and Brown, that the hippocampus supports the episodic or episodic-like abilities, and that the perirhinal cortex imbues the brain with the capacity to determine whether a stimulus is familiar or not. Evidence for the latter comes from lesions and single unit recording of perirhinal neurons which signal whether a stimulus has been encountered before or not. In addition, some models argue that the parahippocampal cortex is important for representing context. These ‘binding into context’ models argue that the role of the hippocampus is to bind together perirhinal object representations with parahippocampal background context representation, allowing a rich reexperiencing of the whole event when information is later recalled. The idea of vivid recollection of the past emphasized by MTT, has led several researchers recently to argue that the hippocampal formation does more than store and retrieve memories; it reconstructs the past and even constructs possible future events. The ‘construction’ theory is relatively new and based predominantly on neuroimaging and neuropsychological observations. It argues that the hippocampal formation is part of a ‘core network’ required to support episodic memory and navigation, and also imagination. Increased activity in the hippocampus has been observed during periods when subjects were thinking about the future and amnesic patients with hippocampal damage has been found to struggle to describe new, imagined situations. Such patients appear to be able to imagine individual objects and colors, suggesting that their problem lies in creating a rich and coherent mental construction of a scene. This view also accords with other evidence indicating that the hippocampus is needed to solve odd-one-out or working memory tasks, which involve holding a mental representation of a room or scene in working memory. Thus, it has been argued that a function of the hippocampal formation is to construct rapid online mental representations of scenes and places. This view offers an alternative explanation for why the hippocampus may be critical for remembering rich, vivid events from all time periods of one’s life in that they need to be reconstructed. It would seem hard to imagine that constructing future scenarios is a function of the rodent or other nonhuman animal hippocampi. However, recent evidence suggests that hippocampal place cells can show activity that might be akin to ‘simulating possible future paths.’ During some sharp-wave-ripples (see section ‘Spikes and Waves: Physiology’), a set of cells can briefly fire action potentials in the order that they would be activated if an animal ran along a path in the environment. While this ‘sequence replay and preplay’ might be related to future thinking, an alternative possibility is that during evolution humans adapted a spatial memory system to support not only the reconstruction of episodic memories, but also the construction of fictitious events and places. Undoubtedly, pinning the theory on the tail and body of the hippocampal formation will continue for decades to come. The study of hippocampal formation has, and will continue to be, influenced by research conducted with patients who have suffered some form of pathological damage to the hippocampus. Such pathological states are considered in the next section. Dying Cells: Pathology The hippocampal formation appears to be particularly prone to disruption from a variety of causes. In this section, the effects of dementia, stress, epilepsy, and schizophrenia are considered. Before we consider these various aberrant states, it is worth considering the healthy aging. Analysis of the human brain has revealed mixed results as to whether cell loss occurs substantially in the hippocampus. In rats, healthy aging is not associated with significant cell loss, but rather with disrupted synaptic plasticity, such that LTP is short lasting. Place cells (see section ‘Maps and Compasses: Neural Coding’) in aged rats show reduced remapping responses to changes in the environment, suggesting that these cells are less capable of detecting environment novelty. The place cells can also fail to reestablish old patterns of activity in familiar environments, suggesting that they are not capable of detecting a previously visited place with the same accuracy. Common causes of substantial cell damage in the hippocampal formation include Alzheimer’s dementia, ischemia/ hypoxia (loss of oxygen supplied by the blood), trauma, hypoglycemia (low blood glucose), and epilepsy. Hippocampal damage is also associated with rare conditions such as herpes encephalitis, where an acute infection from the herpes simplex virus spreads through the hippocampus, amygdala, and associated structures. Due to the relative sparing of other brain structures that can occur in this disease and in cases of hypoxia, patients with these pathologies have proved highly important for the study of hippocampal amnesia. Epilepsy and Alzheimer’s disease are among the most common of neurological diseases and are both strongly associated with the dysfunction of the hippocampal formation. Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 Author's personal copy Hippocampal Formation Alzheimer’s disease is a hugely debilitating disease that typically manifests as an inability to acquire new memories and disorientation. It has been associated with cell loss, originating in the entorhinal cortex and spreading to the rest of the hippocampal formation and beyond as the disease progresses. Epilepsy is commonly linked to hippocampal pathology, in the form of hippocampal sclerosis. It is unknown whether the seizures arising in epilepsy are the result of hippocampal damage or whether they are the cause of the damage. In cases of hippocampal sclerosis, evidence indicates that the seizures originate from the hippocampus. Links have been made between its unique anatomy and the origin of the seizures. Increased connectivity, neurogenesis, loss of inhibitory inputs and increased excitatory drive, among other factors, have all been suggested to contribute to the development of epilepsy. Stress can also have a damaging affect on the hippocampal formation. Stress produces glucocorticoids which then bind to the dense glucocorticoid receptors inside the hippocampal formation. They have been found to inhibit neurogenesis (see section ‘New Birth: Adult Neurogenesis and Morphological Change’), alter gene expression and reduce the excitability of some classes of cell, and prune the dendrites of CA3 pyramidal cells. Patients suffering posttraumatic stress disorder and who have experienced extreme stress over long time periods can show hippocampal atrophy. Schizophrenia is another disease associated with hippocampal damage in the form of reductions of its volume. It has been argued this may be the cause of the long-term memory problems many patients show with the disease. Because of the lack of cell damage markers at autopsy and the presence of abnormal cytoarchitecture, established prenatally, it has been suggested that the changes are likely the result of altered developmental processes rather than due to damage. Also, altered dopamine levels, central to the syndrome, may be partly caused by the reduction of hippocampal formation input. New Birth: Adult Neurogenesis and Morphological Change While the hippocampal formation seems particularly fragile, prone to decay and disease, it is also one of the few brain regions where the birth of new neurons (neurogenesis) occurs in adulthood occurs. From the time of the renowned neuroanatomist Ramon y Cajal, a central tenet in the brain was that no new neurons grow in adulthood. It took many decades before this view was overturned and the growth of new neurons was identified in two brain areas, the olfactory bulb and part of the hippocampal formation: the dentate gyrus. This neurogenesis may help support new learning and memory, possibly by reducing interference, increased capacity, and tagging memories with temporal information. Despite initial mixed results, this research is continuing to weigh in favor of neurogenesis being important for memory. Does size matter? It would appear to for the hippocampal formation. In several nonhuman species, its size varies depending on the demands placed on spatial memory. Furthermore, in some species the volume may change as a function of 303 the seasonal demands. In humans, some jobs place greater demands on spatial memory than others. An extreme case of this is the licensed London taxi driver, who must learn the labyrinth of London’s (UK) 25 000 streets in order to obtain a license, and navigate to hundreds of destinations on a daily basis. By examining magnetic resonance imaging scans of London taxi driver brains, Maguire and colleagues found greater gray matter volume in the posterior hippocampi and reduced gray matter volume in their anterior hippocampi compared with an age-matched control group. In addition, the longer taxi drivers had navigated in London the greater the posterior gray matter volume and the more reduced the anterior gray matter. Thus, suggesting that healthy adult humans have the capacity to change the structure of their hippocampus when accruing knowledge of complex environments. The same changes do not appear to occur in London bus drivers who were matched for driving experience, indicating that this change is unlikely to be driven by stress, driving experience, or daily selfmotion. Whether the change in structure is related to neurogenesis, changes in cell morphology, or other factors remains to be explored in future research. Summary From the ancient Egyptian era to present day, people have written about the hippocampal formation and pondered over its anatomy and function. In the last 40 years, our knowledge has leapt forward. Crucial discoveries have been made in understanding its unique circuitry, physiology, neural code, and functional properties. This has been made possible by the development of a wide range of technical innovations and a creative approach to experimentation. There is a general consensus that the hippocampal formation is crucial for episodic memory, but how best to characterize its involvement in this and other functions remains an issue to be resolved. See also: Amnesia and the Brain; Episodic Memory; Memory, Neural Substrates; Memory; Our Cognitive Map; Spatial Orientation. Further Reading Aggleton JP and Brown MW (2006) Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences 10(10): 456–463. Andersen P, Morris RGM, Amaral DG, Bliss T, and O’Keefe J (2007) The Hippocampus Book. Oxford: Oxford University Press. Buzsaki G (2006) Rhythms of the Brain. Oxford: Oxford University Press. Deng W, Amoni JB, and Gage FH (2010) New neurons and new memories: How does adult hippocampal neurogenesis affect learning and memory? Nature Reviews Neuroscience 11: 339–350. Eichenbaum H and Cohen NJ (2001) From Conditioning to Conscious Recollection: Memory Systems in the Brain. Oxford: Oxford University Press. Hassabis D and Maguire EA (2007) Deconstructing episodic memory with construction. Trends in Cognitive Sciences 11(7): 299–306. Jeffery KJ (2008) Self-localisation and the entorhinal–hippocampal system. Current Opinion in Neurobiology 17: 1–8. Moscovitch M, Nadel L, Winocur G, Gilboa A, and Rosenbaum RS (2006) The cognitive neuroscience of remote episodic, semantic and spatial memory. Current Opinion in Neurobiology 16(2): 179–190. Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304 Author's personal copy 304 Hippocampal Formation O’Keefe J and Nadel L (1978) The Hippocampus as a Cognitive Map. Oxford: Oxford University Press. Spiers HJ, Maguire EA, and Burgess N (2001) Hippocampal amnesia. Neurocase 7(5): 357–382. Squire LR (2004) Memory systems in the brain: A brief history and current perspective. Neurobiology of Learning and Memory 82: 171–177. Van Strien NM, Cappaert NL, and Witter MP (2009) The anatomy of memory: An interactive overview of the parahippocampal–hippocampal network. Nature Reviews Neuroscience 10(4): 272–282. Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, and Mishkin M (1997) Differential effects of early hippocampal pathology on episodic and semantic memory. Science 18(277(5324)): 376–380. Relevant Websites http://www.cognitivemap.net – The Hippocampus as a Cognitive Map. http://www.ucl.ac.uk/spierslab – Webpage for Dr Hugo Spiers. Encyclopedia of Human Behavior, Second Edition (2012), vol. 2, pp. 297-304