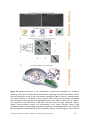
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download General principles of cellular organization in the genome
Clinical neurochemistry wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
RNA polymerase II holoenzyme wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Gene regulatory network wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Biochemical cascade wikipedia , lookup
Signal transduction wikipedia , lookup
Endogenous retrovirus wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Metalloprotein wikipedia , lookup
Transcriptional regulation wikipedia , lookup
Paracrine signalling wikipedia , lookup
Magnesium transporter wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Point mutation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Gene expression wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Expression vector wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Interactome wikipedia , lookup
Protein purification wikipedia , lookup
Proteolysis wikipedia , lookup
DISS. ETH Nr. 18904 GENERAL PRINCIPLES OF CELLULAR ORGANIZATION IN THE GENOME-REDUCED BACTERIUM MYCOPLASMA PNEUMONIAE A dissertation submitted to the SWISS FEDERAL INSTITUTE OF TECHNOLOGY ZURICH for the degree Doctor of Sciences presented by SEBASTIAN KÜHNER Dipl. Biol., Ruprecht-Karls-University Heidelberg born July 31, 1981 German citizen Accepted on the recommendation of: Prof. Dr. Ruedi Aebersold, examiner Dr. Anne-Claude Gavin, co-examiner Prof. Dr. Uwe Sauer, co-examiner 2010 Zusammenfassung Eines der Ziele naturwissenschaftlicher Forschung ist es, eine selbstreplizierende Zelle in ihrer Gesamtheit zu verstehen. Kennt man die organisatorischen Prinzipien, denen grundlegende Bestandteile der Zelle, wie Transkriptom, Proteom und Metabolom unterliegen, so ist man diesem Ziel einen bedeutenden Schritt näher. In den vergangenen Jahren haben enorme technische Fortschritte bei analytischen Hochdurchsatzverfahren die systematische Untersuchung dieser einzelnen „Ome“ ermöglicht. Dies wiederrum gibt uns heute die Gelegenheit, in einem Organismus parallel das Transkriptom und seine Komplexität, die Proteomorganisation in Proteinkomplexe sowie den Metabolismus und dessen Regulation global zu analysieren. Die vorliegende Doktorarbeit beschreibt die systematische Charakterisierung dieser zellulären Bestandteile in dem genomreduzierten Bakterium Mycoplasma pneumoniae, das zu den kleinsten sich selbstständig fortpflanzenden Lebensformen zählt. Der Fokus dieser Arbeit liegt auf der Analyse der Proteomorganisation, der Studie zu der ich, im Vergleich aller drei Teilstudien, den mit Abstand größten Teil beigetragen habe. Zur Untersuchung der grundlegenden Prinzipien bakterieller Proteomorganisation führten wir eine proteomweite, auf chromatographischer Aufreinigung sowie massenspektrometrischer Analyse von Proteinkomplexen basierende Studie durch. Die Datenanalyse identifizierte 62 homopolymere und 116 heteropolymere, lösliche Proteinkomplexe, von denen die Mehrzahl bisher uncharakterisiert ist. Etwa ein Drittel der heteropolymeren Komplexe ist an der Bildung komplexerer Formen der Proteomorganisation beteiligt. Dazu zählt die Anordnung in größere Multiproteinkomplexeinheiten, welche zum Teil die sequentiellen Schritte eines biologischen Prozesses reflektieren. Des weiteren beobachten wir, dass ein und dasselbe Protein Bestandteil mehrerer verschiedener Komplexe sein kann, was wir als Proteinmultifunktionalität interpretieren. Dies könnte eine mögliche Erklärung für die Genomreduzierung Proteinkomplexdaten von mit elektronentomographischen M. pneumoniae 484 darstellen. Proteinstrukturen, Daten liefert Kombination der elektronenmikroskopischen und zusätzliche Die strukturelle Details der Proteomorganisation dieses Bakteriums. Um die grundlegenden Prinzipien des bakteriellen Metabolismus und seiner Regulation zu verstehen, wurde ein metabolisches Netzwerk aus 129 Enzymen, die insgesamt 189 verschiedene Reaktionen katalysieren, konstruiert. Die Nutzung dieses biochemischen Atlas ermöglichte es, ein Minimalmedium, bestehend aus 19 essentiellen Bestandteilen, zu entwickeln. Das metabolische Netzwerk von M. pneumoniae hat im Vergleich zu General principles of cellular organization in Mycoplasma pneumoniae komplexeren Bakterien eine stärker lineare Topologie und enthält einen höheren Anteil an multifunktionalen Enzymen. Allgemeine Eigenschaften wie die Konzentration von Metaboliten, der zelluläre Energiehaushalt, die Anpassungsfähigkeit und die globale Genexpression sind denen anderer Bakterien ähnlich. Für die Transkriptionsanalyse wurde strangspezifische Mikrochiptechnologie „Tilingarrays“, ergänzt durch die direkte Sequenzierung der Transkripte, mit mehr als 252 Spottedarrays kombiniert. Es wurden 117 neue, meist nicht-codierende Transkripte, von denen 89 in antisense Konfiguration zu bekannten Genen liegen, detektiert. Weiterhin wurden 202 monocistronische sowie 139 polycistronische Operons identifiziert, wobei fast die Hälfte der letzteren ein stufenartiges Transkriptionsprofil aufweist. Unter variierenden Wachstumsbedingungen können sich die Operons in bis zu 447 kleinere transkriptionelle Einheiten aufteilen, was die Mannigfaltigkeit der alternativen Transkripte erklärt. Häufige antisense und alternative Transkripte sowie unterschiedliche Regulationsmechanismen verdeutlichen die hohe Dynamik dieses Transkriptoms. Die Informationen in dieser Doktorarbeit können als kleiner Teil eines Bauplans für eine minimale zelluläre Maschine angesehen werden. Die vier allgemeinen Schlußfolgerungen aus dieser Arbeit sind: 1. M. pneumoniae weist besonders in seiner Proteomorganisation und in seinem Metabolismus Aspekte der Multifunktionalität auf. 2. M. pneumoniae ist für einen nahezu minimalen Organismus, bezüglich seiner regulatorischen Prozesse, deutlich komplexer als erwartet. 3. M. pneumoniae ähnelt Eukaryoten, hinsichtlich Komplexität seines Transkriptoms, wesentlich stärker als erwartet. 4. M. pneumoniae ist auf Wirtsanpassung und nicht auf maximales Wachstum optimiert. General principles of cellular organization in Mycoplasma pneumoniae Summary One aim of research in natural sciences is to understand a self-reproducing cell in its entity. Transcriptome, proteome and metabolome are at the heart of cellular organization. To know the general principles underlying these basic functional aspects of a cell is a key part on the way to comprehensively understand cellular life. In recent years systematic analysis of the individual “omes” has been facilitated via dramatically advancing analytical high-throughput techniques. This opened opportunities to globally investigate the transcription and its complexity, the proteome organization into protein complexes as well as the metabolism and its regulation of an entire organism. In the presented PhD thesis I describe an effort to systematically characterize these features for the genome-reduced organism Mycoplasma pneumoniae, which is among the smallest self-replicating forms of life on earth. My work hereby focuses on the analysis of the proteome organization, the part, among the three investigations, to which I contributed most substantially. In order to study basic principles of bacterial proteome organization, we used tandem affinity purification followed by mass spectrometric analysis to investigate protein complexes in a proteome-wide screen. The analysis revealed 62 homomultimeric and 116 heteromultimeric soluble protein complexes, of which the majority are novel. About a third of the heteromultimeric complexes show higher levels of proteome organization, including assembly into larger, multi-protein complex entities, suggesting sequential steps in biological processes, and extensive sharing of components implying protein multifunctionality. Incorporation of structural models for 484 proteins, single particle EM and cellular electron tomograms provided supporting structural details for this proteome organization. To understand basic principles of bacterial metabolism and its regulation, a manually curated metabolic network of 129 enzymes catalyzing 189 reactions was constructed. This metabolic map allowed the design of a defined, minimal medium with 19 essential nutrients. In summary, the M. pneumoniae metabolic network has a more linear topology and contains a higher fraction of multifunctional enzymes compared to more complex bacteria; general features such as metabolite concentrations, cellular energetics, adaptability and global gene expression responses are similar though. For the transcriptome analysis we combined strand-specific tiling arrays, complemented by transcriptome sequencing, with more than 252 spotted arrays. We detected 117 previously undescribed, mostly non-coding transcripts, 89 of them in antisense configuration to known genes. We identified 202 monocistronic and 139 polycistronic operons; almost half of the latter show decaying expression in a staircase-like manner. Under various conditions, operons General principles of cellular organization in Mycoplasma pneumoniae could be divided into 447 smaller transcriptional units, resulting in many alternative transcripts. Frequent antisense transcripts, alternative transcripts, and multiple regulators per gene imply a highly dynamic transcriptome. In essence this thesis dataset provides a blueprint of the minimal cellular machinery required for life. The four general conclusions of this work are that: 1. M. pneumoniae shows aspects of multifunctionality particularly in its proteome organization and metabolism. 2. M. pneumoniae, even though being an almost minimal organism, is more complex than expected, particularly in its regulation. 3. M. pneumoniae resembles eukaryotes more than expected, particularly in its transcription. 4. M. pneumoniae is optimized for host adaptation and not for growth. General principles of cellular organization in Mycoplasma pneumoniae Abbreviations Ǻ: Ångström (1×10−10 meters) AARS: Aminoacyl tRNA synthetase AEBSF: 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride AppppA: 5',5'''-P1, P4-diadenosine tetraphosphate ATP: Adenosine triphosphate B. subtilis: Bacillus subtilis BCA: Bicinchonic acid bp: Base pairs °C: Degree Celsius CBPP: Contagious bovine pleuropneumonia c-di-AMP: cyclic di(3'→5')-adenylic acid CIRCE: Controlling inverted repeat of chaperone expression COG: Clusters of orthologous groups of proteins CRP: Catabolite regulation protein 1D: One dimensional 2D: Two dimensional Da: Dalton DNA: Deoxyribonucleic acid DSSS: Direct strand specific sequencing DTT: Dithiothreitol E. coli: Escherichia coli e.g.: For example (exempli gratia) EM: Electron microscopy et al.: And others (et aliae) FDR: False discovery rate fg: Femto gram FPInt: False positive interaction FT-MS: Fourier transform-mass spectrometry FWHM: Full-Width Half-Maximum g: Gravity or gram GC: Guanine-cytosine GE: Glycolytic enzyme GF: Gel filtration General principles of cellular organization in Mycoplasma pneumoniae GO: Gene ontology h: Hour H. pylori: Helicobacter pylori HRP: Horseradish peroxidase i.e.: That is (id est) k: Kilo kbp: Kilo base pairs KEGG: Kyoto Encyclopedia of Genes and Genomes LC: Liquid chromatography L. lactis: Lactococcus lactis LTQ: Linear trap quadrupole LTQ-FT-ICR: Linear trap quadrupole fourier transform ion cyclotron resonance µ: Micro µRPLC: Micro reversed phase liquid chromatography m: Milli M: Matrix component or Molar MALDI: Matrix assisted laser desorption ionization MALDI-TOF: Matrix assisted laser desorption ionization-time of flight Mbp: Mega base pairs MI: Multifunctionality index NCBI: National Center for Biotechnology Information mRNA: Messenger ribonucleic acid MS: Mass spectrometry MW: Molecular weight M. genitalium: Mycoplasma genitalium M. pneumoniae: Mycoplasma pneumoniae m/z: Mass to charge ratio ncRNA: Non-coding ribonucleic acid ORF: Open reading frame PBS: Phosphate buffered saline PCR: Polymerase chain reaction PDB: Protein Data Bank (p)ppGpp: Guanosine tetraphosphate (pentaphosphate) General principles of cellular organization in Mycoplasma pneumoniae pH: Negative decadal logarithm of the H+ concentration pI: Isoelectric point PMF: Peptide mass fingerprint PMSF: Phenylmethanesulphonylfluoride PPLO: Pleuro pneumonia-like organisms ppm: Parts per million PTM: Post-translational modification rRNA: Ribosomal ribonucleic acid S: Spoke component or Svedberg SA: Socio-affinity SDS-PAGE: Sodium dodecyl sulfate-polyacylamide gel electrophoresis TAP: Tandem affinity purification TAP-MS: Tandem affinity purification-mass spectrometry TCEP: Tris(2-carboxyethyl)phosphine TEV: Tobacco etch virus TF: Transcription factor TFA: Trifluoroacetic acid TPInt: True positive interaction TRIS: Tris (hydroxymethyl) aminomethane tRNA: Transfer ribonucleic acid General principles of cellular organization in Mycoplasma pneumoniae Table of Contents 1. Introduction .............................................................................................................................1 1.1. M. pneumoniae – a genome reduced model organism .....................................................1 1.1.1. History of research in M. pneumoniae..................................................................... 1 1.1.2. Pathogenicity of M. pneumoniae.............................................................................. 4 1.1.3. Establishing M. pneumoniae as a model organism for systematic studies .............. 6 1.2. Studying M. pneumoniae at different levels.....................................................................9 1.2.1. Proteome of M. pneumoniae .................................................................................... 9 1.2.2. Metabolism of M. pneumoniae ............................................................................... 10 1.2.3. Transcriptome of M. pneumoniae .......................................................................... 12 1.3. Objectives of this PhD thesis .........................................................................................13 1.4. Research articles and reviews obtained during this PhD thesis .....................................14 2. Proteome organization in a genome-reduced bacterium .......................................................15 2.1. Authorship......................................................................................................................15 2.2. Abstract ..........................................................................................................................15 2.3. Introduction ....................................................................................................................16 2.4. Materials and methods ...................................................................................................17 2.4.1. Construction of a collection of M. pneumoniae strains expressing a TAP-fusion. 17 2.4.2. TAP purification of protein complexes and mass spectrometric analysis ............. 18 2.4.3. Sample preparation for PeptideAtlas data collection ............................................ 18 2.4.4. Protein digestion for PeptideAtlas data collection ................................................ 18 2.4.5. Directed mass spectrometry for PeptideAtlas data collection ............................... 19 2.4.5. Data processing and compilation of PeptideAtlas................................................. 19 2.4.6. Peptide Mass Fingerprinting ................................................................................. 20 2.4.7. Reproducibility ....................................................................................................... 20 2.4.8. Profilogs ................................................................................................................. 20 2.4.9. Consolidated scores ............................................................................................... 22 2.4.10. Socio-affinity scoring and integration of quantitative prey profiling across the entire dataset .................................................................................................................... 26 2.4.11. Socio-affinity score benchmarking....................................................................... 29 2.4.12. Integration with predicted interactions................................................................ 29 2.4.13. Clustering ............................................................................................................. 29 2.4.14. Characterization of homomultimeric protein complexes ..................................... 30 2.4.15. Treatment of the extracts with RNase/DNase ...................................................... 30 General principles of cellular organization in Mycoplasma pneumoniae 2.4.16. Total RNA purification ......................................................................................... 30 2.4.17. Evaluating protein complexes by gel filtration chromatography and western blot .......................................................................................................................................... 31 2.4.18. Multifunctionality index ....................................................................................... 32 2.4.19. Electron microscopy and image reconstruction of selected complexes............... 33 2.4.20. Cultivation of M. pneumoniae strain B176 for electron tomography .................. 33 2.4.21. Tilt series aquisition ............................................................................................. 34 2.4.22. Image processing.................................................................................................. 34 2.4.23. Mapping of complexes in cellular tomograms ..................................................... 35 2.4.24. Quantitative Western blotting .............................................................................. 37 2.4.25. Structural bioinformatics ..................................................................................... 39 2.5. Results ............................................................................................................................40 2.5.1. Genome-wide screen for protein complexes in M. pneumoniae ............................ 40 2.5.2. Systematic detection of homomultimeric protein complexes.................................. 42 2.5.3. Characteristics of M. pneumoniae protein complexes ........................................... 43 2.5.4. Comparison of methods for estimation of proteome organization......................... 44 2.5.5. The M. pneumoniae protein complex network reveals substantial cross-talk ....... 46 2.5.6. Functional reuse and modularity of protein complexes......................................... 46 2.5.7. Structural anatomy of M. pneumoniae................................................................... 48 2.6. Conclusions ....................................................................................................................50 3. Impact of genome reduction on bacterial metabolism and its regulation .............................52 3.1. Authorship......................................................................................................................52 3.2. Abstract ..........................................................................................................................52 3.3. Introduction ....................................................................................................................53 3.4. Results ............................................................................................................................53 3.4.1 Reconstruction of the metabolic network: verification by a defined, minimal medium ............................................................................................................................. 53 3.4.2. Comparison of the metabolic network to those of more complex bacteria............ 56 3.4.3. Growth and energetics in comparison with larger bacteria. ................................. 58 3.4.4. Coordinated transcriptome dynamics along growth curve and under various conditions ......................................................................................................................... 60 3.4.5. Complex metabolic regulation despite few transcription factors .......................... 63 3.5. Conclusions ....................................................................................................................64 4. Transcriptome complexity in a genome-reduced organism ..................................................65 General principles of cellular organization in Mycoplasma pneumoniae 4.1. Authorship......................................................................................................................65 4.2. Abstract ..........................................................................................................................65 4.3. Introduction ....................................................................................................................66 4.4. Results ............................................................................................................................66 4.5. Conclusions ....................................................................................................................71 5. Summary of results................................................................................................................72 6. Conclusions ...........................................................................................................................74 7. References .............................................................................................................................77 8. Acknowledgements ...............................................................................................................87 9. Curriculum vitae....................................................................................................................89 10. Supplement..........................................................................................................................94 10.1. Table S1: List of purifications and proteins identified.................................................94 10.2. Table S2: List of high confidence interactions and corresponding socio-affinity scores...................................................................................................................................131 10.3. Table S3: List of protein complexes. .........................................................................174 10.4. Table S4: List of literature curated protein complexes. .............................................187 10.5. Table S5: List of modeled protein complexes............................................................195 10.6. Table S6: List of multifunctional proteins. ................................................................199 10.7. Table S7: List of literature curated multifunctional proteins. ....................................203 General principles of cellular organization in Mycoplasma pneumoniae 1. Introduction 1.1. M. pneumoniae – a genome reduced model organism The following subchapters will first give an overview on the historic development of research on M. pneumoniae. Secondly, its key pathogenic aspects are summarized and in a third part I provide selected arguments as to why M. pneumoniae is a suitable model organism for systematic studies. 1.1.1. History of research in M. pneumoniae The history of the term “mycoplasma”, from the Greek “mykes”, which translates to fungus and “plasma” meaning formed, dates back to the year 1889, when A. B. Frank illustrated the special relationship between plant-invading fungi or other microorganisms and their host cells (Frank 1889; Krass and Gardner 1973). The initial discovery of Mycoplasma, i.e. the first isolation of the contagious bovine pleuropneumonia (CBPP) agent, to date known as Mycoplasma mycoides subsp. mycoides, is credited to Nocard and Roux in the year 1898 (Nocard and Roux 1898; Razin, Yogev et al. 1998; Waites and Talkington 2004). Subsequently, the nomenclature pleuro pneumonia-like organisms (PPLO) was chosen for microorganisms in the genus Mycoplasma, referring to life forms similar to the causative agent of CBPP (Edward, Meiklejohn et al. 1956). It has to be noted though that the fungus like growth pattern described by the name “Mycoplasma” does only coincide with the growth characteristics of Mycoplasma mycoides. Nevertheless the name Mycoplasma was adopted for the entire genus (Razin, Yogev et al. 1998; Waites and Talkington 2004). Since the 1960s Mycoplasmas are considered as members of a bacterial class named Mollicutes. This name is derived form the Latin words “mollis” and “cutis” meaning soft skin (Razin, Yogev et al. 1998) (Figure 1). Their soft skinned properties are the consequence of the absence of a cell wall. The cellular membrane is however stiffened by the insertion of sterols. In current taxonomy the class of Mollicutes is part of the phylum tenericutes within the bacterial kingdom. It comprises 4 orders, 5 families, 8 genera and approximately 200 species (Razin, Yogev et al. 1998; Waites and Talkington 2004). General principles of cellular organization in Mycoplasma pneumoniae 1 Figure 1 M. pneumoniae amongst its bacterial relatives zoomed in from the tree of life (from http://itol.embl.de/) (Ciccarelli, Doerks et al. 2006). In blue the genome size (in number of genes) is shown next to each organism. The species highlighted in red are bacteria closely related to M. pneumoniae that are used as benchmarks within this work. Mycoplasma pneumoniae is a member of the family of Mycoplasmataceae and the order of Mycoplasmatales. M. pneumoniae was originally isolated from the sputum of a patient with primary atypical pneumoniae by Eaton, Meiklejohn and van Herick in 1944 and was hence termed “Eaton’s agent” or “primary atypical pneumonia virus” (Eaton, Meiklejohn et al. 1944; Waites and Talkington 2004). In a concerted reclassification call in 1963 several microbiological experts proposed to rename Eaton’s agent to Mycoplasma pneumoniae (Chanock 1963). They did so because M. pneumoniae growth, although being unaffected by microbial inhibitors such as thallium acetate, penicillin and amphotericin B, is sensitive to the tetracycline group of antibiotics and therefore most likely of bacterial and not viral origin (Chanock 1963). In the following years the characterization of M. pneumoniae focused mainly on its biochemical, cell biological as well as pathogenic properties. In 1966 Prescott, Sobeslavsky and colleagues used chemical and chromatographic separation techniques in two back to back studies reporting the isolation and characterization of different cellular fractions of M. pneumoniae together with the investigation of their antigenic properties (Prescott, Sobeslavsky et al. 1966; Sobeslavsky, Prescott et al. 1966). These studies General principles of cellular organization in Mycoplasma pneumoniae 2 revealed a high immunogenicity of fractionated lipids combined with protein in complexes, while lipids alone were only a weakly immunogenic (Prescott, Sobeslavsky et al. 1966; Sobeslavsky, Prescott et al. 1966). In addition to such studies the ultra structure of M. pneumoniae was investigated in detail using scanning and transmission electron microscopy (Biberfeld and Biberfeld 1970; Kammer, Pollack et al. 1970; Knudson and MacLeod 1970; Boatman and Kenny 1971; Wilson and Collier 1976). The three major messages of these studies were that i) M. pneumoniae has a highly structured, basic protein rich, electron dense rod structure at its thinner end (Tip region, attachment organelle), which is surrounded by electron-lucent cytoplasm and the cell membrane (Biberfeld and Biberfeld 1970; Wilson and Collier 1976) ii) M. pneumoniae undergoes an ordered and sequential metamorphosis during its life cycle involving spherical and filamentous forms (Kammer, Pollack et al. 1970); M. pneumoniae being pleomorph (Knudson and MacLeod 1970) iii) Nucleic acids present within M. pneumoniae appear to be excluded from the tip region and mostly located in cytoplasmic granules (Wilson and Collier 1976). An important cell biological feature that enables M. pneumoniae to survive without a cell wall is its internal cytoskeleton, which supports the integrity of the cellular membrane (Krause and Balish 2001; Krause and Balish 2004; Balish and Krause 2006). The cytoskeleton is structurally and compositionally novel in comparison to the cytoskeletons of other organisms, including other bacteria, and it is involved in the cell division process (Balish and Krause 2006). Furthermore it is associated with the terminal attachment organelle, and proves to be essential for colonization of the host as well as for gliding motility (Balish and Krause 2006). Regarding M. pneumoniae cellular motility Radestock and Bredt observed that: “The cells moved in an irregular pattern with numerous narrow bends and circles. They never changed their leading end. The average speed was relatively constant between 0.2 and 0.5 µm/s. The maximum speed was about 1.5 to 2.0 µm/s.” (Radestock and Bredt 1977). Furthermore it was observed that the gliding motility of M. pneumoniae cells required an intact energy metabolism and is susceptible to changes in pH, temperature, viscosity and various other factors (Radestock and Bredt 1977). In recent years mutant analysis revealed requirement of the protein P30 (Mpn453) in M. pneumoniae gliding motility (Hasselbring, Jordan et al. 2005); via a transposon mutagenesis approach additional genes associated with gliding motility were identified (Hasselbring, Page et al. 2006). Just in the same year it was shown that the separation of terminal organelles was impaired in a non motile mutant, indicating a requirement for gliding motility in regular cell division (Hasselbring, Jordan et al. 2006) General principles of cellular organization in Mycoplasma pneumoniae 3 With regard to understanding M. pneumoniae’s pathogenic properties two major advances have been achieved towards in the mid 1980s. First, the involvement of the P1 surface protein (Mpn141), that is located within the attachment organelle, in M. pneumoniae infection of human host cells has been highlighted (Baseman, Cole et al. 1982; Feldner, Gobel et al. 1982; Hu, Cole et al. 1982). Secondly, there was progress on the more detailed understanding of the specificity of the interaction of M. pneumoniae with human erythrocytes. Here the lab of Ten Feizi, a specialist on glycobiology and M. pneumoniae research, discovered that erythrocyte receptors for M. pneumoniae are sialylated oligosaccharides (Loomes, Uemura et al. 1984). In the late 1990s until the first decade of the 21st century M. pneumoniae research was highly driven by technical advances on genome sequencing, which leveraged research in virtually all aspects of the life sciences. In 1996 the genome of M. pneumoniae was amongst the first to be completely sequenced. This work has been directed by Richard Herrmann, an outspoken expert on M. pneumoniae biology (Himmelreich, Hilbert et al. 1996) who was also involved in a re-annotation that followed four years later (Dandekar, Huynen et al. 2000). Currently the M. pneumoniae genome contains 689 annotated genes and about 816kbp. Finally, it seem worthwhile to notice that among the human mycoplasmas M. pneumoniae is by far the best known and most carefully studied (Razin, Yogev et al. 1998; Waites and Talkington 2004). 1.1.2. Pathogenicity of M. pneumoniae Mycoplasmas in general are mucosal pathogens, which survive as parasites in close association with their hosts (Waites and Talkington 2004). M. pneumoniae in particular is a pathogenic bacterium exclusive to humans that colonizes lung epithelia (Waites and Talkington 2004). It causes a respiratory illness that mainly affects children as well as adolescents and during which cold agglutinins develop (Chanock 1963; Razin, Yogev et al. 1998; Hammerschlag 2001; Herrmann and Ruppert 2006). According to O’Donnell and colleagues “the clinical syndrome is characterized by findings that are atypical for the usual bacterial pneumonia: a lack of fever or systemic symptoms, negative cultures, unusual radiographic features, or a combination of these” (O'Donnell, Kradin et al. 2004). Hence the disease is termed “atypical pneumonia”. Also, in contrast to pneumonia caused by the more traditional pathogens Streptococcus pneumoniae or Haemophilus influenzae, it does not respond to the usual antibiotic treatment. It was already noted that M. pneumoniae growth is unaffected by a number of antibiotics and that the bacterium is sensitive to gentamycin as well as the tetracycline group of microbial inhibitors. To that end it is remarkable that the M. General principles of cellular organization in Mycoplasma pneumoniae 4 pneumoniae RNA polymerase shows resistance to rifampin, due to a point mutation, whereas other eubacteria can be successfully treated with this antibiotic. Another striking feature of M. pneumoniae is that it fulfils all four of Koch’s postulates (Rifkind, Chanock et al. 1962; Smith, Chanock et al. 1967; Clyde 1973). The postulates are criteria, formulated by Robert Koch and Friedrich Loeffler in the late 19th century, which are designed to establish a relationship between a causative microbe and a disease. The postulates are as follows: 1. The bacteria must be present in every case of the disease. 2. The bacteria must be isolated from the host with the disease and grown in pure culture. 3. The specific disease must be reproduced when a pure culture of the bacteria is inoculated into a healthy susceptible host. 4. The bacteria must be recoverable from the experimentally infected host. A prerequisite for M. pneumoniae pathogenicity is the tight connection to epithelial host cells via its attachment organelle. The attachment organelle mediating this adhesion is located in the tip structure of the cell (Figures 10 &15). Consequence of such strong adhesion is the impaired removal via the mucociliary clearance machinery of the host (Waites and Talkington 2004). Within the last decades several reports were published that included microbiological-, animalas well as human volunteer tests, which have assessed the infectious properties of M. pneumoniae (Smith, Chanock et al. 1967; Lipman, Clyde et al. 1969; Hansen, Wilson et al. 1979; Hansen, Wilson et al. 1979; Hansen, Wilson et al. 1981; Hansen, Wilson et al. 1981; Leith, Hansen et al. 1983; Franzoso, Hu et al. 1993; Franzoso, Hu et al. 1994; Catrein, Dumke et al. 2004; Dumke, Catrein et al. 2004). Lipman et al. derived pairs of virulent and attenuated or avirulent M. pneumoniae strains and compared them in an effort to elucidate the mechanisms of virulence (Lipman, Clyde et al. 1969). They found that variations in the morphology as well as its cytadsorption characteristics closely correlated with avirulence in the case of M. pneumoniae strain M129-B169 (where the 169 indicates the number of passage in broth medium: B) (Lipman, Clyde et al. 1969). In a separate investigation Hansen and colleagues report a similar strain derived from virulent M. pneumoniae M129 that is rapidly cleared from lungs of infected hamsters (Hansen, Wilson et al. 1981). Subsequent studies revealed that two proteins, a 40 and 90-kDa protein (Mpn142), which are present in cytadsorbing, pathogenic wild-type strains are absent in the non-pathogenic strain M129B176 (Hu, Collier et al. 1977; Baseman, Cole et al. 1982; Hu, Cole et al. 1982). These two General principles of cellular organization in Mycoplasma pneumoniae 5 proteins are surface exposed, localized on the terminal tip apparatus, and likely to be involved in the attachment mechanism (Franzoso, Hu et al. 1993). These two proteins are products of the mpn142 gene that encodes a membrane protein moiety, a leader signal, and a stop-transfer sequence and produces the 90- and the 40-kDa protein instead of an expected 130-kDa protein (Sperker, Hu et al. 1991; Franzoso, Hu et al. 1993). Another protein with major importance for host recognition is the adhesin P1 (Mpn141). Until today there have been numerous reports that highlight the major role of P1, which is concentrated in the tip structure and is a major component of the virulence mediating interaction of M. pneumoniae with host cells (Ruland, Wenzel et al. 1990; Layh-Schmitt and Herrmann 1992; Seto, Layh-Schmitt et al. 2001; Hasselbring, Page et al. 2006; Seybert, Herrmann et al. 2006). To conclude this subchapter on pathogenicity, I want to remark that M. pneumoniae, although being classified as an S2 (security standard) organism, is certainly not the most clinically relevant organism causing acute pneumonia. Nevertheless it is essential to always perform laboratory work according to good laboratory practices adjusted to S2 standards and with the common sense of dealing with a pathogen. Yet the pathogenic aspects have not been the major source of motivation for us to embark in a project with M. pneumoniae but rather the idea to employ it as a model system for studying a “minimal organism” as depicted in the next subchapter. 1.1.3. Establishing M. pneumoniae as a model organism for systematic studies Mollicutes are, based on 16S RNA investigations classified as, gram-positive bacteria and are generally characterized by their strikingly small genomes (Figure 1) (Fraser, Gocayne et al. 1995; Himmelreich, Hilbert et al. 1996). These genomes consist of a single circular chromosome containing 0.58 to 2.2 Mbps and are thus the smallest known genomes of freeliving organisms. Therefore Mycoplasmas have attracted much attention as blueprints of minimal cells. The M. pneumoniae genome for example encodes for 689 protein coding genes and 44 ncRNAs. M. genitalium, which could be considered as a deletion mutant of M. pneumoniae and is its phylogenetically closest relative, consists of only 485 protein coding genes (Figure 1). In comparison to the standard model bacteria E. coli and B. subtilis the M. pneumoniae genome is about 7 times smaller in terms of both base pairs and number of genes (Table 1). The same holds true for its cellular volume, which is only 10% of that of E. coli and less than 5% of that of a typical Bacillus like e.g. B. subtilis (Table 1) (Waites and Talkington 2004). Similarly, the total number of mRNA molecules in E. coli is approximately 15 times larger than in M. pneumoniae (Table 1). General principles of cellular organization in Mycoplasma pneumoniae 6 Other arguments as to why M. pneumoniae is an ideal near-minimal organism for systematic studies are its minimal enzymatic toolbox. This reduced set suggests a controllable organism complexity. For instance, the M. pneumoniae genome encodes for only one protein phosphatase and two protein kinases creating an absolutely minimal phosphorylation regulatory network. The same minimalism is evident for the current knowledge about other PTMs. Apart from phosphorylation only three other PTMs are reported, which include two variations of protein cleavage as well as modification with lipids. On a genome regulatory level there are additional arguments underlining the parsimonious way in which M. pneumoniae deals with its resources, e.g. genome annotations identified only one sigma factor (Himmelreich, Hilbert et al. 1996; Dandekar, Huynen et al. 2000; Weiner, Zimmerman et al. 2003). Feature / cell M. pneumoniae E. coli # total DNA bp 816,394 4,639,675 # ORFs 689 4,132 # Ribosomes 140 18,000 # mRNAs 230 4,000 Cell volume [L] 6.7x10-17 6.7x10-16 Protein weight [fg] 10 165 Table 1 Benchmarking M. pneumoniae against the classic model bacterium E. coli. Data from this table originates from NCBI databases and (Guell, van Noort et al. 2009; Yus, Maier et al. 2009) Apart from all positive aspects that make M. pneumoniae an ideal model organism for systematic studies there are also pitfalls to this bacterium. One major disadvantage is the elongated generation time of 8 hours. Compared to E. coli, which divides every 20 minutes this is considerably slower and makes research in M. pneumoniae more time-consuming. Additionally this could severely hamper its applicability for biotechnological and engineering purposes. A second drawback is the adherent life-style of M. pneumoniae, which enables the growth of wild-type cells only in a two dimensional fashion. In consequence the amount of cells that can be cultivated at once in comparison to bacterial suspension cultures is limited, causing additional costs for culture equipment and manpower if larger cultures are to be grown. However, the disadvantage of growing M. pneumoniae in an adherent fashion can be circumvented by using non-adherent mutants such as the M. pneumoniae strain B176. This mutant lacks parts of its attachment organelle causing the non-adherence and it has moreover been reported to be avirulent. General principles of cellular organization in Mycoplasma pneumoniae 7 Along the lines of using Mycoplasmas as a blueprint of minimal cells a team around John I. Glass, Clyde A. Hutchison, Hamilton O. Smith and Craig Venter at the J. Craig Venter Institute uses the close M. pneumoniae relative M. genitalium as a model organism for synthetic biology. In a project that started more than 15 years ago they first sequenced the M. genitalium genome (Fraser, Gocayne et al. 1995). In a following step transposon mutagenesis was globally applied to identify nonessential genes in M. pneumoniae and M. genitalium. Conversely this dataset then provides insight into the essential gene complement of the minimal organism. In their first analysis Hutchison et al. report that about 265 to 350 of the M. genitalium genes are essential under laboratory growth conditions, including about 100 proteins of unknown function (Hutchison, Peterson et al. 1999). In a second analysis of that kind Glass et al. describe 382 of the protein coding genes as essential, with 28% of them being of unknown function (Glass, Assad-Garcia et al. 2006). Just recently, the chemical synthesis of a complete M. genitalium genome was reported (Gibson, Benders et al. 2008). In another effort this team managed to perform a genome transplantation taking M. mycoides genomic DNA and transplanting it into M. capricolum cells. The successful transplants were phenotypically identical to the DNA donor cells of M. mycoides (Lartigue, Glass et al. 2007). Following this transplantation report, the subsequent step was the transplantation of Mycoplasma DNA into yeast to exploit its mechanism of homologous recombination for highly efficient genetic modifications in order to create novel M. mycoides strains (Lartigue, Vashee et al. 2009). All mentioned technological advances above could most likely also be applied to M. pneumoniae. Due to these advantageous characteristics and as M. pneumoniae is the best studied species among the Mycoplasmas M. pneumoniae appears to be a suitable model organism for systematic studies. General principles of cellular organization in Mycoplasma pneumoniae 8 1.2. Studying M. pneumoniae at different levels This subchapter introduces the state of the art of research prior to the studies that are described in chapters 2 to 4. For that reason this part of the introduction is already structured according to the research aspects of the individual studies focusing on the proteome, the metabolism and the transcriptome of M. pneumoniae. 1.2.1. Proteome of M. pneumoniae A proteome is the entire set of proteins that are expressed in an organism at a given condition and time point. In the case of M. pneumoniae, proteomic studies have been conducted for almost half a century. Initially these investigations focused mainly on one gene product at a time. With the emergence of more sophisticated analytical tools, namely the use of mass spectrometers for peptide and hence protein identification, global analysis of the M. pneumoniae proteome became more widespread. The first of these studies by Regula and coworkers characterized the proteome via a two-dimensional gels electrophoresis (Regula, Ueberle et al. 2000). Overall, 350 protein spots were analysed from various 1-D and 2-D gel electrophoresis setups using diverse pH gradients as well as two different mass spectrometers (Regula, Ueberle et al. 2000); they were assigned to 224 genes (Regula, Ueberle et al. 2000). In a follow up effort this proteome map was extended to 305 gene products identified using advanced chromatographic techniques (Ueberle, Frank et al. 2002). This new map corresponded to 44% coverage of the at that time 688 annotated genes (Ueberle, Frank et al. 2002). Two years later Jaffe and colleagues increased the proteome coverage in M. pneumoniae to 81% applying an approach called proteogenomic mapping (Jaffe, Berg et al. 2004). They mapped peptides detected in a whole-cell lysate of M. pneumoniae onto a genomic scaffold and extended these hits into ORFs bound by traditional genetic signals to generate the “proteogenomic map” (Jaffe, Berg et al. 2004). They detected 9,709 unique peptides corresponding to 557 of the now 689 predicted ORFs (This includes annotated genes with mpn numbers 1 to 688 and an additional gene called mpn528a) (Jaffe, Berg et al. 2004). Subsequent to this study by Jaffe et al. a review by Herrmann and Ruppert summarized the status of the M. pneumoniae proteome prior to the investigation described in this thesis (Herrmann and Ruppert 2006). They counted a total of 565 proteins that were experimentally identified out of 689 protein coding genes annotated (82% coverage) (Herrmann and Ruppert 2006). From the 689 protein coding genes annotated, by the NCBI via their system of COGs, General principles of cellular organization in Mycoplasma pneumoniae 9 190 do not fit any COGs, while another 16 have an unknown function and 54 have a general function prediction only (Herrmann and Ruppert 2006). This leaves 429 (of the 689) annotated genes with a functional description according to COGs. Another very important aspect of proteome research is that it benefits largely from integration of additional data such as protein-protein interactions or protein structures in order to functionally describe a cell. Regarding the systematic characterization of protein complexes and the mapping of binary protein-protein interactions major efforts have been reported for other bacteria such as Campylobacter jejuni, E. coli or Helicobater pylori amongst others (Rain, Selig et al. 2001; Butland, Peregrin-Alvarez et al. 2005; Arifuzzaman, Maeda et al. 2006; Parrish, Yu et al. 2007; Hu, Janga et al. 2009). For M. pneumoniae, a systematically derived binary proteinprotein interaction network and a global catalogue of protein complexes are yet missing. Concerning the solution of protein structures considerable effort has been undertaken by the Berkeley Structural Genomics Center to obtain a near-complete structural complement of the minimal genomes of M. genitalium and M. pneumoniae. Altogether this initiative has currently solved 90 structures from 59 targets, which are subject to various publications (Chen, Yakunin et al. 2005; Shin, Kim et al. 2006; Das, Hyun et al. 2007). Taking into account related structures from close orthologs brings the total number of M. pneumoniae proteins for which a structure or model is available to ~350 (Kim, Shin et al. 2005; Chandonia and Kim 2006). Thus the community is not that far away from a structurally solved organism. Together with additional orthogonal initiatives this will assist in adding value to data produced by classical proteomics experiments on the way to the systematic functional description of this bacterial cell. 1.2.2. Metabolism of M. pneumoniae In simple analogy to the genome of M. pneumoniae, its metabolism, the entity of all biochemical reactions, which this organism can perform autonomously, is extremely reduced and tailored towards the needs of a parasitic lifestyle closely associated with the host. Therefore M. pneumoniae has a very limited enzymatic toolbox including for instance only one protein phosphatase and two protein kinases. This might have a direct impact on the regulatory capabilities via, e.g. phosphorylation. As M. pneumoniae, like all mollicutes, misses enzymes for peptidoglycan synthesis encoded in its genome it permanently lacks a cell wall (Razin, Yogev et al. 1998; Waites and Talkington 2004). This confers pleomorphism and aggravates their taxonomical classification. General principles of cellular organization in Mycoplasma pneumoniae 10 Another consequence of the absence of a cell wall is that the maintenance of osmotic stability is especially important even though the pathogen stiffens its membrane with cholesterol insertions (Razin, Yogev et al. 1998; Waites and Talkington 2004). At yet another end of the M. pneumoniae biochemical pathways map it has been reported that purine and pyrimidine metabolism is sufficient for enabling the synthesis of RNA and DNA in this near-minimal cell based on nucleic acid precursors (Pachkov, Dandekar et al. 2007). A review of the central carbohydrate metabolism revealed that M. pneumoniae possesses all 10 reactions of Glycolysis but tricarboxylic acid cycle and cytochrome mediated electron transfer chains are however absent (Pollack, Myers et al. 2002; Waites and Talkington 2004). Besides ATP generation via glycolysis M. pneumoniae is known to be capable of lactic acid fermentation like various other bacteria (Razin, Yogev et al. 1998; Waites and Talkington 2004). This is a process of anaerobic respiration that converts sugars such as glucose, fructose, or sucrose into cellularly amenable energy via substrate phosphorylation effected by phosphoglycerate kinase (Mpn429) and pyruvate kinase (Mpn303), while the metabolic product lactic acid is formed (Waites and Talkington 2004). Other metabolic peculiarities that M. pneumoniae shares with most mollicutes lie within the way these bacteria regulate translation. For instance, M. pneumoniae uses the universal stop codon UGA as an alternative codon for tryptophan (Inamine, Ho et al. 1990). Additionally the M. pneumoniae genome sequencing revealed the absence of generic Shine-Dalgarno sequences, especially in comparison to other known, related genomes including e.g. B. subtilis (Himmelreich, Hilbert et al. 1996; Osada, Saito et al. 1999; Sakai, Imamura et al. 2001). These sequences are ribosomal binding sites to the mRNAs, generally located upstream of the start codon AUG and show a characteristic consensus sequence among many other bacterial classes. Finally, a remark to the generic growth medium so called “Hayflick medium” that has been used for M. pneumoniae research purposes in the last decades (Hayflick 1965). It provides glucose as the major carbon source feeding the energy metabolism. Hayflick medium, however is an undefined medium, as it contains horse serum. Furthermore it has not been comprehensively characterized for its minimal components. From the point of view of complete parameter control, which is prerequisite for optimal experimental reproducibility, this is certainly a weakness of Hayflick medium especially in the analysis of the biochemical properties of M. pneumoniae. General principles of cellular organization in Mycoplasma pneumoniae 11 1.2.3. Transcriptome of M. pneumoniae Besides their remarkably small sizes the Mollicutes genome’s are characterized by an extremely low GC content (23 to 40%) (Razin, Yogev et al. 1998). This holds true for the genome of M. pneumoniae (40%), too. Within the years following the completion of the M. pneumoniae genome sequence, research has focused on the control of gene expression (Weiner, Herrmann et al. 2000). From the analysis of the genomic sequences of M. pneumoniae and M. genitalium it became obvious, that major regulators of gene expression in other bacteria are missing (Fraser, Gocayne et al. 1995; Himmelreich, Plagens et al. 1997; Weiner, Herrmann et al. 2000); this includes the absence of the two component system and the Rho factor as well as the presence of a very limited number of σ factors (Himmelreich, Hilbert et al. 1996; Himmelreich, Plagens et al. 1997; Bairoch and Apweiler 2000). Interestingly, M. pneumoniae contains only one clearly identified σ factor (Mpn352), while a second putative σ factor, suggested to induce mobility in M. pneumoniae, has been proposed (Mpn626) (Bornberg-Bauer and Weiner 2002). The scarcity of σ factors in M. pneumoniae is in contrast to the at least 14 σ factors of its close relative B. subtilis (Bairoch and Apweiler 2000). Despite this apparent lack of complexity regarding its transcriptional regulation, part of the M. pneumoniae transcriptome, the entity of all RNAs (mRNA, rRNA, ncRNA) transcribed under varying conditions, has been shown to be regulated, e.g. by heat-shock, significantly increasing the expression of 47 genes including various known conserved heat shock genes including clpB (Mpn531), dnaJ (Mpn021), dnaK (Mpn434), groES (Mpn574) and lon (Mpn332) (Weiner, Zimmerman et al. 2003). Yet the transcriptome in M. pneumoniae has not been comprehensively characterized. Even though systematic gene expression studies have been reported for many other bacteria (Selinger, Cheung et al. 2000; Tjaden, Saxena et al. 2002; Reppas, Wade et al. 2006; McGrath, Lee et al. 2007; Nelson, Herron et al. 2008; Akama, Suzuki et al. 2009; ToledoArana, Dussurget et al. 2009), a complete dataset for M. pneumoniae is still missing. This limits the current understanding of the entire transcriptome in terms of its operon structures and regulation. Similarly, the number of classified ncRNAs in bacteria has recently been expanded (Vogel and Wagner 2007), however a comprehensive and unbiased repertoire is still missing and particularly underdeveloped in M. pneumoniae. To date the M. pneumoniae transcriptome consists of 689 annotated protein coding genes and 44 ncRNAs. General principles of cellular organization in Mycoplasma pneumoniae 12 1.3. Objectives of this PhD thesis Although M. pneumoniae is considered as the best known and most carefully studied species among the human mycoplasmas (Razin, Yogev et al. 1998; Waites and Talkington 2004) there are still vast parts of its biology unaddressed i.e. not understood. Consequently, current knowledge of the M. pneumoniae biology is lagging far behind that of other model organisms like E. coli or S. cerevisiae, especially considering the knowledge of tools for genetic engineering. However, as described in section 1.1.3, M. pneumoniae comes with the advantage of an extremely reduced genome, which allows genome-wide functional studies to be completed within the lifetime of a PhD thesis/student. This reduced genome size as well as the significantly reduced cellular dimensions, from which we infer a reduced organism complexity, are the main driving forces for us to globally investigate M. pneumoniae and establish it as a model organism for systematic studies on near-minimal organisms. In order to more systematically understand the general principles of cellular organization in M. pneumoniae the objectives of this PhD thesis are to elucidate: 1. its proteome organization, 2. its metabolism as well as its regulation and 3. its transcriptome complexity. Among these three projects, which are a combined effort of several research groups the focus of my work will be on the analysis of the proteome organization in M. pneumoniae. This includes the establishment of a library of M. pneumoniae strains in which all individual proteins are labeled with a TAP-tag. Using this collection I will perform a genome-wide screen for protein-protein interactions via TAP-MS to get an overview on the hetero- and homomultimeric protein complexes in this near-minimal bacterium. By integration of other datasets I want to address general questions like e.g. which molecular mechanisms must a self-sufficient organism use to still survive with such a reduced-genome. Additionally I would like to collaborate with experts on various structural biology techniques to exploit our proteinprotein interaction dataset in making the first steps towards solving the structural anatomy of a cell. General principles of cellular organization in Mycoplasma pneumoniae 13 1.4. Research articles and reviews obtained during this PhD thesis 1. Sebastian Kühner & Anne-Claude Gavin. Towards quantitative analysis of proteome dynamics. Nat Biotechnol. 2007 Mar;25(3):298-300. [Review] 2. Samuel Bader, Sebastian Kühner & Anne-Claude Gavin. Interaction networks for systems biology. FEBS Lett. 2008 Apr 9;582(8):1220-1224. Epub 2008 Feb 20. [Review] 3. Sebastian Kühner‡, Vera van Noort‡, Matthew J. Betts, Alejandra Leo-Macias, Claire Batisse, Michaela Rode, Takuji Yamada, Tobias Maier, Samuel Bader, Pedro BeltranAlvarez, Daniel Castaño-Diez, Wei-Hua Chen, Damien Devos, Marc Güell, Tomas Norambuena, Ines Racke, Vladimir Rybin, Alexander Schmidt, Eva Yus, Ruedi Aebersold, Richard Herrmann, Bettina Böttcher, Achilleas S. Frangakis, Robert B. Russell, Luis Serrano, Peer Bork, Anne-Claude Gavin. Proteome organization in a genome-reduced bacterium. Science 326, 1235-1240 (2009). 4. Marc Güell, Vera van Noort, Eva Yus, Wei-Hua Chen, Justine Leigh-Bell, Konstantinos Michalodimitrakis, Takuji Yamada, Manimozhiyan Arumugam, Tobias Doerks, Sebastian Kühner, Michaela Rode, Mikita Suyama, Sabine Schmidt, Anne-Claude Gavin, Peer Bork, Luis Serrano. Transcriptome complexity in a genome-reduced bacterium. Science 326, 1268-1271 (2009). 5. Eva Yus, Tobias Maier, Konstantinos Michalodimitrakis, Vera van Noort, Takuji Yamada, Wei-Hua Chen, Judith A.H. Wodke, Marc Güell, Sira Martínez, Ronan Bourgeois, Sebastian Kühner, Emanuele Raineri, Ivica Letunic, Olga V. Kalinina, Michaela Rode, Richard Herrmann, Ricardo Gutiérrez-Gallego, Robert B. Russell, Anne-Claude Gavin, Peer Bork, Luis Serrano. Impact of genome reduction on bacterial metabolism and its regulation. Science 326, 1263-1268 (2009). ‡ Equal contribution General principles of cellular organization in Mycoplasma pneumoniae 14 2. Proteome organization in a genome-reduced bacterium 2.1. Authorship This chapter covers the systematic investigation of the proteome organization, i.e. the analysis of protein-protein interactions and the structural anatomy, in M. pneumoniae. It represents the major part of work during my time as a predoctoral fellow at EMBL Heidelberg and ETH Zürich. This study has a shared first authorship with Vera van Noort, who contributed vast parts of the bioinformatics analysis platform and managed major parts of the data integration. Additionally there are numerous other co-authors who had their unique contributions. My main contributions were the protein biochemistry and mass spectrometry, where I performed most of the conducted experiments in this screen. I also organized and assisted in the molecular biology and cell culture parts of the project. Additionally, I contributed major parts to the data analysis and writing of the manuscripts and coordinated work between the various collaborators. This chapter represents a reformatted version of a research article and its supporting online material that has been published in Science. 2.2. Abstract The genome of Mycoplasma pneumoniae is among the smallest found in self-replicating organisms. To study the basic principles of bacterial proteome organization, we used TAPMS in a proteome-wide screen. The analysis revealed 62 homomultimeric and 116 heteromultimeric soluble protein complexes, of which the majority are novel. About a third of the heteromultimeric complexes show higher levels of proteome organization, including assembly into larger, multi-protein complex entities, suggesting sequential steps in biological processes, and extensive sharing of components implying protein multifunctionality. Incorporation of structural models for 484 proteins, single particle EM and cellular electron tomograms provided supporting structural details for this proteome organization. The dataset provides a blueprint of the minimal cellular machinery required for life. General principles of cellular organization in Mycoplasma pneumoniae 15 2.3. Introduction Biological function arises in part from the concerted actions of interacting proteins that assemble into protein complexes and networks. Protein complexes are the first level of cellular proteome organization: functional and structural units – often termed molecular machines - that participate in all major cellular processes. Complexes are also highly dynamic in the sense that their organization and composition varies in time and space (de Lichtenberg, Jensen et al. 2005), and they interact to form higher level networks; this property is central to whole-cell functioning. However, general rules concerning protein complex assembly and dynamics remain elusive. The combination of affinity purification with MS (Rigaut, Shevchenko et al. 1999) has been applied to several organisms, providing a growing repertoire of molecular machines. Genomewide screens in Saccharomyces cerevisiae (Gavin, Aloy et al. 2006; Krogan, Cagney et al. 2006; Tarassov, Messier et al. 2008), captured discrete, dynamic proteome organization, and revealed higher order assemblies with direct connections between complexes and frequent sharing of common components. To date these exhaustive analyses have been applied only in yeast. In bacteria, genome-wide yeast two-hybrid analyses have been reported (Rain, Selig et al. 2001; Parrish, Yu et al. 2007), but only a few biochemical analyses on selected sets of complexes are available (Terradot, Durnell et al. 2004; Butland, Peregrin-Alvarez et al. 2005; Arifuzzaman, Maeda et al. 2006; Hu, Janga et al. 2009). The understanding of proteome organization in these organisms concerns thus the binary interaction networks. Here we report a genome-scale analysis of protein complexes in the bacterium, Mycoplasma pneumoniae, a human pathogen that causes atypical pneumonia (Waites and Talkington 2004). This self-replicating organism has one of the smallest known genomes (689 proteinencoding genes) (Himmelreich, Hilbert et al. 1996; Dandekar, Huynen et al. 2000), making it an ideal model organism for the investigation of absolute essentiality (Glass, Assad-Garcia et al. 2006). This analysis and the integration with other consistently derived large-scale datasets provide a blueprint of the proteome organization in a minimal cell and reveal principles underlying adaptation to a reduced genome. General principles of cellular organization in Mycoplasma pneumoniae 16 2.4. Materials and methods 2.4.1. Construction of a collection of M. pneumoniae strains expressing a TAP-fusion TAP-fusions were expressed in M. pneumoniae M129 (ATTC29342 broth passage No. 31) strains. Primers were designed for all 689 ORFs in M. pneumoniae. Each ORF was amplified by PCR using cosmid DNA as template (Wenzel and Herrmann 1988; Wenzel and Herrmann 1989). PCR products were digested (NotI/ SfiI) and ligated into the pMT85-based pMT_ClpBTAP-tag vector (Zimmerman and Herrmann 2005), which carries the Staphylococcus aureus transposon Tn4001 and the TAP cassette downstream of the SfiI site (Figure 2). Transformation of M. pneumoniae was done by electroporation, following published protocols (Hedreyda, Lee et al. 1993). Following transformation and recovery, cells were plated out in modified Hayflick medium (Hayflick 1965), containing gentamycin (78 mg/l) and incubated at 37ºC as described earlier (Catrein, Herrmann et al. 2005). Cell culture was tested for expression by Western blot with a Peroxidase-Anti- Peroxidase antibody developed in rabbit (Sigma, P1291). TAPtag SfiI (616) tnp SfiI (833) NotI (854) clpB pMT clpB-TAPtag 5736 bp o-Rep-ColE1 GMR Figure 2 Generic TAP cassette vector used for M. pneumoniae transformation. After transformation, the transposase randomly integrates the construct including the gentamycin resistance into the genome. Form now on the TAP‐tagged allele of the gene of interested is expressed under control of the clpB promoter. General principles of cellular organization in Mycoplasma pneumoniae 17 2.4.2. TAP purification of protein complexes and mass spectrometric analysis Two-liter cultures were incubated in ten 300 cm2 cell culture flasks (Sarstedt) and harvested 96 h after inoculation. Cells were washed twice with ice cold PBS and centrifuged at 9,860xg. Pellets were resuspended in 2 ml lysis buffer (50 mM Tris pH 7.5, 5% glycerol, 1.5 mM MgCl2, 100 mM NaCl, 0.2% NP40, 1 mM DTT, 1 mM AEBSF, 1 mM PMSF, 1 μg/ml pepstatin A, 1 μg/ml antipain, 2 μg/ml aprotinin, 1 μg/ml leupeptin and 16 μg/ml benzamidin) and lysed mechanically using a douncer. TAP purification was done following established protocols (Rigaut, Shevchenko et al. 1999; Puig, Caspary et al. 2001). Protein samples were then analyzed by SDS-PAGE (4-12% NuPage, Invitrogen). Sample preparation for mass spectrometry was done essentially as described earlier (Shevchenko, Wilm et al. 1996; Gavin, Bosche et al. 2002). Briefly, whole gel lanes were cut into 32 equal slices; the proteins were then destained and digested (Trypsin, 12.5 mg/ml). Peptides were extracted in 1% formic acid in acetonitrile, resuspended in 0.1% TFA in water, mixed (1:1) with matrix solution (0.1% TFA in 50% acetonitrile and 50% methanol) and spotted on MALDI-TOF plates (Micromass, Waters). The MS analyses were carried out on a MALDI Micro MXTM (Waters, Milford, MA, USA). All data were acquired in positive ion mode over m/z range of 900-3,500 in reflectron mode. Peak masses were extracted from the spectra and used for protein identification (Supplementary CD). 2.4.3. Sample preparation for PeptideAtlas data collection M. pneumoniae M129 (ATTC29342 broth passage No. 31) strain cells were washed twice in ice cold PBS, harvested and pelleted by centrifugation at 9,860xg. Cells were resuspended in 100 µl denaturation buffer (100 mM ammoniumbicarbonate, 8 M urea, 0.1% RapiGest™(Waters)), sonicated for 5 minutes and spun down for 5 minutes at 20,000xg at 4ºC. A small aliquot of the supernatant was taken to determine the protein concentration using a commercially available BCA based protein assay kit (Pierce #23227). 2.4.4. Protein digestion for PeptideAtlas data collection The proteins were reduced with 5 mM TCEP for 60 minutes at 37°C and alkylated with 10 mM iodoacetamide for 30 minutes in the dark before diluting the sample with 100 mM ammoniumbicarbonate to a final urea concentration below 2 M. Proteins were digested by incubation with trypsin (1/50, w/w) overnight at 37°C. The peptides were cleaned up by C18 reversed-phase spin columns according to the manufacturer’s instructions (Harvard Apparatus). General principles of cellular organization in Mycoplasma pneumoniae 18 2.4.5. Directed mass spectrometry for PeptideAtlas data collection The setup of the μRPLC-MS system was as described previously (Schmidt, Gehlenborg et al. 2008). The hybrid LTQ-FT-ICR mass spectrometer was interfaced to a nanoelectrospray ion source (both Thermo Electron, Bremen, Germany) coupled online to a Tempo 1D-plus nanoLC (Applied Biosystems/MDS Sciex, Foster City, CA). Peptides were separated on a RP-LC column (75 μm x 15 cm) packed in-house with C18 resin (Magic C18 AQ 3 μm; Michrom BioResources, Auburn, CA, USA) using a linear gradient from 98% solvent A (98% water, 2% acetonitrile, 0.15% formic acid) and 2% solvent B (98% acetonitrile, 2% water, 0.15% formic acid) to 30% solvent B over 120 minutes at a flow rate of 0.3 μl/minutes. Each survey scan acquired in the ICR-cell at 100,000 FWHM was followed by MS/MS scans of the three most intense precursor ions in the linear ion trap with enabled dynamic exclusion for 30 seconds. Charge state screening was employed to select for ions with at least two charges and rejecting ions with undetermined charge state. The normalized collision energy was set to 32%, and one microscan was acquired for each spectrum. For directed LC-MS/MS, two data-dependent LC-MS/MS runs, the SuperHirn peak extraction and alignment algorithm (Mueller, Rinner et al. 2007) was used to extract all MS1 peaks. After removing all features for which MS2-data was obtained, the remaining 24,461 masses were divided in four lists and subsequently sequenced using directed LC-MS/MS analysis as recently specified (Schmidt, Gehlenborg et al. 2008). 2.4.5. Data processing and compilation of PeptideAtlas MS/MS spectra were searched using the SEQUEST search tool (Yates, Eng et al. 1995) against a decoy database (consisting of forward and reverse protein sequences) of the predicted proteome from M. pneumoniae strain M129 (ATTC29342), complete genome NCBI genome number NC_000912 (http://www.ncbi.nlm.nih.gov/entrez), consisting of 689 proteins as well as known contaminants such as porcine trypsin and human keratins (Non-Redundant Protein Database, National Cancer Institute Advanced Biomedical Computing Center, 2004, ftp://ftp.ncifcrf.gov/pub/nonredundant). The search was performed with semi-tryptic cleavage specificity, mass tolerance of 15 ppm, methionine oxidation as variable modification and cysteine carbamidomethylation as fixed modification. The database search results were further processed using the PeptideProphet program (Keller, Nesvizhskii et al. 2002) with decoy option. The peptide FDR was set to 1% (p=0.7). General principles of cellular organization in Mycoplasma pneumoniae 19 2.4.6. Peptide Mass Fingerprinting Mass intensity files were analyzed with MASCOT (Perkins, Pappin et al. 1999) and AlDente (Tuloup, Hernandez et al. 2003; Gasteiger, Hoogland et al. 2005) to identify proteins (Figure 3, A and B). Parameter settings were 80 ppm and 0 missed cleavages for both programs. For Aldente, shift was set at 0.20, slope at 200 and minimum number of peptides at two. For MASCOT, identifications above PMF score 45 were always taken and above 30 only if the consolidated score was at least 80 percent of the maximum consolidated score for that protein. For AlDente the same files were used for PMF against a random database. All identifications scoring lower than the random database were excluded. From these PMF identifications, proteins scoring higher than 1.5 were always taken and higher than 0.5 only if the consolidated score was at least 80 percent of the maximum consolidated score for that protein. 2.4.7. Reproducibility To assess the quality of the entire approach, we first measured the reproducibility of the overall experimental process, i.e. growth of adherent M. pneumoniae strains expressing TAPfusions, lysis of the cell pellets, TAP purification of protein complexes and mass spectrometric identification of protein complex components. For this purpose, we ran this entire procedure in duplicate for 18 strains expressing different TAP-fusions. We measure a reproducibility rate of 73% for proteins involved in the high confidence interactions, which are the ones that cluster in complexes in the dataset. This is comparable to most published studies, including that of Sowa et al. that recently reported 80% reproducibility (derived from four duplicated experiments) for high confidence interactions (Sowa, Bennett et al. 2009). We also more specifically assess the quality of the MS and protein identification strategy. For this purpose, 72 MS samples (see procedure above) were processed for MALDI-TOF analysis in duplicate; the reproducibility for protein identification is 97%. 2.4.8. Profilogs Proteins that have overlapping peptide mass profiles (we coined the term Profilogs) could lead to spurious identifications. To detect spurious identifications of this sort we use the results from the PeptideAtlas. The masses of sequenced peptides by FT-MS (PeptideAtlas) were entered into MASCOT and AlDente to identify proteins by PMF. False positives (Profilogs) are in this case identifications of proteins by PMF that were not the proteins sequenced in MS2 (PeptideAtlas). Mascot scores of false positive identifications (Profilogs) were recorded (Figure 3, C and D). For each protein identified by FT-MS, we identify all possible Profilogs as false positive identifications by PMF. In the PMF results of the TAP purifications, all General principles of cellular organization in Mycoplasma pneumoniae 20 identifications that were not the top-ranking proteins were considered as background if they are Profilogs and they were not further considered. Figure 3 Protein identification by MS. From all purifications, we processed 10,447 samples for MS. Proteins were identified using a refined approach that integrates both Mascot (Perkins, Pappin et al. 1999) and Aldente (Gasteiger, Hoogland et al. 2005) search algorithms. (A) This strategy increases the retrieval rate of true positives by 20% compared to only using Mascot. (B) The retrieval rate is calculated by comparing to known complexes. Of all the expected subunits that also belong to these known complexes, we retrieved 25%. The retrieval rate is used in the socio‐affinity scoring. (C) the PeptideAtlas was used to define profilogs; proteins that, in a MS experiment, give rise to overlapping peptide mass profiles. The masses of the sequenced peptides were used to do a PMF, if the actual sequenced protein is retrieved this is a true positive identification. If a different protein is retrieved this is a false positive identification resulting from the fact that the two proteins are profilogs. General principles of cellular organization in Mycoplasma pneumoniae 21 Black line: distribution of true positive identification scores. Red line: false positive identification scores. (D) Same as (C) however the Aldente scores are ordered to the maximum false positive score for each protein. In the screen, after using both Aldente and Mascot identifications to find significant proteins, the best‐scoring protein is always kept. Consecutive lower scoring hits are removed if a better‐scoring protein is a profilog of the lower scoring protein. 2.4.9. Consolidated scores MASCOT and AlDente scores of overlapping identifications were used to re-scale all the PMF scores, such that they have the same distribution. For PMF result, the maximum of the two rescaled scores was taken as the consolidated score. These consolidated scores were used in the subsequent analyses. Altogether, the set of successful redundant purifications comprises 259 purifications (Table S1) from 212 unique baits. In total 411 proteins were identified in the consolidated set of identified proteins (Table S1; Figure 4, A, B, C, D, E; and 5). General principles of cellular organization in Mycoplasma pneumoniae 22 General principles of cellular organization in Mycoplasma pneumoniae 23 Figure 4 Proteome coverage (functional & physicochemical). In (A) we represent the fraction of proteins identified per specified COG class (Tatusov, Koonin et al. 1997) in our TAP‐MS analysis (green), other proteomics analyses (Regula, Ueberle et al. 2000; Ueberle, Frank et al. 2002; Jaffe, Berg et al. 2004) including the PeptideAtlas (yellow), as well as those proteins predicted from the genome, but not yet observed (white). The M. pneumoniae genome contains 689 annotated protein coding genes, of which 564, corresponding to 82% of the genome, have been observed by MS‐based screens before our study. In the TAP‐MS analysis General principles of cellular organization in Mycoplasma pneumoniae 24 we detected 411 distinct M. pneumoniae proteins. Combining the results from the TAP‐MS screen, the PeptideAtlas as well as previous screens, a total of 622 proteins (90% of the genome) have been detected. (B) Virtual 2D gel where proteins are represented according to their size, y‐axis, and isoelectric point, x‐axis. The proteins identified in our dataset are colored red the ones not identified are seen in blue. The distribution of identified proteins covers molecular weights from 6 to 215 kDa and the pI range between 4 and 11.8. (C) The graph represents the fraction of proteins with a given molecular weight that were identified in the TAP‐MS analysis. MWs were extracted from UniProt. Proteins were grouped into different MW classes by exponential binning and subsequently counted. The plot shows that small proteins, e.g. below 20kDa, are significantly underrepresented (P = 0.000444). The R2 between the molecular weight and the fraction detected is 0.88 (correlation of 0.94). The table in panel (D) shows the five protein domains or motifs that were significantly underrepresented in the dataset. Proteins were classified according to their domain or motif composition according to SMART (Letunic, Doerks et al. 2009). The percentage of proteins with a given domain or a given motif that were identified in the TAP‐MS analysis was calculated. P‐values were calculated with the χ²‐test. (E) The graph represents the fraction of proteins with a given mRNA abundance that were identified in the TAP‐MS analysis. For abundance, we took the log2 signal of mRNA abundance on a spotted array measured under reference conditions and binned with a bin width of 0.5. Per bin, the number of proteins and the fraction detected was calculated. Low abundant proteins are underrepresented. There was no relation found between detection probability and hydrophobicity, when hydrophobicity scores were calculated using GRAVY. Figure 5 Screen saturation curve. The graph shows the cumulated number of non‐redundant proteins identified per TAP purifications as a function of increasing numbers of purifications performed. The 259 purifications are represented on the x‐axis in computationally randomized fashion, while the 411 identified proteins are depicted on the y‐axis. The average value, over ten randomization procedures, of proteins identified after a specific amount of purifications is shown in red. The maximal/minimal value, over ten randomization procedures, of proteins identified after a specific amount of purifications is shown in blue and yellow, respectively. General principles of cellular organization in Mycoplasma pneumoniae 25 2.4.10. Socio-affinity scoring and integration of quantitative prey profiling across the entire dataset The socio-affinity scoring was adapted from (Gavin, Aloy et al. 2006; Collins, Kemmeren et al. 2007). To further discriminate contaminants from cognate complexes components we also integrated systematic quantification of the preys across the entire dataset using spectral counting. Our approach is very similar to what has been reported by Sowa et al. (Sowa, Bennett et al. 2009) who originally integrated averaged spectral counts from technical duplicates to the interaction scores. Their datasets, focused on a limited set of purifications, contained only rare instances of reciprocal data (reverse tagging). In contrast, in genome-wide datasets run to saturation (Figure 5), reciprocal interactions are frequent. Thus, for each protein in the dataset, we could derive quantitative profiles across different purifications where proteins were present as bait or preys (Figure 6A). We ranked all interactions according to the quality of the MS data. This ranking is based on the PMF scores from MASCOT and AlDente, which explicitly take into account spectral counts, further normalized for length of the peptides and number of possible tryptic peptides per protein. Therefore, the PMF score represents a more accurate measure than spectral counts alone. More specifically, the approach is based on the principle that proteins present as contaminants in a given purification often have low PMF scores (i.e. they are less abundant and they are thus identified by a smaller number of peptides; they have lower spectral counts). Reciprocally, the presence of such contaminant proteins with a high PMF score in a given purification is usually indicative of a true interaction. Thus, we wanted to give a higher socioaffinity, if the PMF score was close to the maximum for that prey. We then sorted all identified preys by PMF score across different purifications (using different baits), and use the rank divided by the total number of times the prey was identified instead of the count divided by the background frequency. In this way, we ensure that preys when identified with a higher spectral count (reflected in the PMF score) receive a higher interaction score with this bait than when they are identified with a lower spectral count (usually indicative of spurious interactions). Socio-affinity (SA) is build up of the Spokes component (S) where direct bait-prey relations are counted and a Matrix component (M) where preys are counted that are seen together in a purification (equation 1). S is a summation over the scores (s) of all purifications (k) where the bait (i) was used and prey (j) was identified (equations 2 and 3) or prey (j) was not identified (equation 4). The retrieval rate (r) is estimated to be 0.25 and the probability (pijk) of finding a bait-prey interaction is estimated from a Poisson distribution (Collins, Kemmeren General principles of cellular organization in Mycoplasma pneumoniae 26 et al. 2007) where nikprey is the number of preys identified in purification k with bait i, nibait is the number of times protein i was used as bait, and fj is an estimate of the nonspecific frequency of occurrence of prey j in the dataset, where we took a value of 1 for npseudo. We computed fj as Bayesian posterior estimates based on the observed frequency of occurrence of preys in the dataset and the prior hypothesis that all preys occur non-specifically with equal frequency, for fj , we take only the prey frequency until the PMF score of the prey- identification with bait i (Figure 6A). Similarly, for the Matrix component (M), m was summed over all purifications where both prey (i) and prey (j) occur together. The value for pijk is calculated using the Poisson distribution as in equation 9 where fi and fj are computed as in equation 6, and ntotprey-prey is the total number of prey-prey pairs observed in the dataset. Equations: (1) SA = S ij + S ji + M ij (2) S ij = ∑ sijk k r + (1 − r ) pijk (3) s ijk = log10 (4) s ijk = log 10 (5) p ijk = 1 − exp(− f i f j ntotprey − prey ) pijk (1 − r )(1 − p ijk ) (1 − pijk ) = log 10 (1 − r ) n jpreyobs + n pseudo (6) fj = (7) M ijk = ∑ mijk ntotpreyobs + (n distinct preys n pseudo ) k r + (1 − r ) pijk (8) mijk = log10 (9) pijk = 1 − exp(− f i nikprey nibait ) pijk General principles of cellular organization in Mycoplasma pneumoniae 27 ∑P ∑P pc1 (10) MI = pc 2 N pc1 N pc 2 ∑ Pc1c 2 N c1c 2 Figure 6 Refined socio‐affinity scoring, benchmarking & clustering. (A) Example of PMF scores for a protein that appears either as contaminant or as cognate interactor in different purifications. On the x‐axis is the MASCOT score of all identifications of Mpn004. On the y‐ axis is the cumulative frequency of identifications of Mpn004. This protein seems to be a contaminant in many purifications (low score). However, if the bait was Mpn003 (a known interactor), Mpn004 (red arrow) is identified with a very high MASCOT score (many identified peptides). This was also the case for other interactors within known complexes. Therefore, in the spoke component of the socio‐affinity, we take for fj only the cumulative prey frequency until the PMF score of the prey‐identification with bait i. (B) The socio‐ affinity scoring was adjusted from (Gavin, Aloy et al. 2006; Collins, Kemmeren et al. 2007). For every identified prey we ranked the PMF scores. The rank is now used instead of the frequency of the prey in the socio‐affinity scoring of bait‐prey relations. On the x‐axis is the normalized, refined socio‐affinity. Accuracy was estimated for each bin of socio‐affinity scores. Accuracy was measured by the golden standard set of known complexes (Table S4). TPInts are interactions between proteins of the same complex, whereas FPInts are interactions between proteins of different complexes. Accuracy is TPInt/(TPInt+FPInt), dots. A logistic is done and the logistic curve was used to estimate for each protein‐pair, the probability of an interaction. (C) During clustering the number of proteins clustering in complexes as well as the size of the largest complex was monitored. We chose a threshold to General principles of cellular organization in Mycoplasma pneumoniae 28 define complexes such that many proteins are in complexes but still the size of the largest cluster has not exploded yet. This is depicted in c where on the x‐axis the number of proteins in complexes and on the y‐axis the number of proteins in the largest complex is shown. We chose a threshold that includes 348 proteins into the complexes of our analysis. (D) Nomenclature and definition: classification of protein complex components into cores (ellipses) and attachments (diamonds). 2.4.11. Socio-affinity score benchmarking A curated list of known complexes was inferred by homology to known complexes from the literature (Table S4). The interactions between proteins in the same complexes form the positive set. The interactions between proteins in different complexes form the negative set. Reported accuracies for function prediction are always lower boundaries as there could be true interactions also in the negative set. The protein-protein interactions were scored using socio-affinity and classified in the positive or negative set (Figure 6B). Then we performed a logistic regression on the socio-affinities and used the resulting logistic function to map the socio-affinities to interaction probabilities. 2.4.12. Integration with predicted interactions Predicted interactions were taken from STRING (COG mode) (Jensen, Kuhn et al. 2009); predictions from databases and text mining were not taken into account. For every protein pair, we now have an interaction probability from TAP and an interaction probability from STRING (Jensen, Kuhn et al. 2009). These two probabilities were integrated the same way as in STRING: P = 1- (1-p1)(1-p2). A weak score from TAP and a high score from STRING could indicate physical interactions (van Noort, Snel et al. 2007) without predicting other functional interactions. For the clustering into complexes only those protein pairs were taken that were seen together in purification at least once. From this we considered a set of 1,058 high confidence interactions for clustering into complexes (Table S2). 2.4.13. Clustering We adopted the clique percolation method from the CFinder method (Adamcsek, Palla et al. 2006). This is one of the clustering methods were proteins can belong to multiple complexes. We used k=3 to find cliques and used overlapping cliques to define the cores of the complexes. We took the high-confidence interactions (Table S2) and retrieved protein pairs that have each other as highest scoring interaction partner (stable pairs). If these stable pairs were part of a complex, they were also considered core members. If these stable pairs were not part of a complex, they were added to the complex list as complex of size two. To the core General principles of cellular organization in Mycoplasma pneumoniae 29 complexes, we added proteins with only one high-confidence interaction and call these attachment proteins. We used different cut-offs for interaction probabilities to define complexes and monitored the number of proteins clustering in complexes as well as the size of the largest complex. We chose a threshold to define complexes such that many proteins were in complexes but still the size of the largest cluster has not exploded yet (Figure 6C). Altogether the study reports 116 heteromultimeric protein complexes (Table S3). 2.4.14. Characterization of homomultimeric protein complexes The TAP-fusions were expressed from an exogenous locus and promoter. As a consequence the TAP-fusions were present together with their untagged wild type allele. It was not rare that in the same purification we could observe both TAP-tagged and untagged versions of the bait, indicating homomultimerization. We manually inspected the 1D SDS-PAGE gel and MS results for instances where the bait was present in more than one band. The bait was considered homomultimeric, if we identified it in two distinct bands corresponding to the MW of the tagged and the untagged versions. Altogether the study reports 62 homomultimeric protein complexes (Table S3). 2.4.15. Treatment of the extracts with RNase/DNase To digest DNA and RNA, some extracts were treated with RNase and DNase prior TAP. We added 3 µl of RNase (Roche) and 1 spatula tip of DNase (Roche) to a cellular pellet in lysis buffer before mechanical lysis with the douncer. TAP, SDS-PAGE and mass spectrometry were then performed as described above. The association between RpoA-TAP and the ribosome was unaffected by this DNA/RNA treatment indicating the interactions does not imply RNA or DNA (Figure 7 A and B). 2.4.16. Total RNA purification After growth, surface-attached cells are washed once with PBS at 37°C and immediately lysed in the cultivation flask by adding RLT buffer from the QiagenTM RNeasy Plus Mini Kit (Cat. Num. 74134). For cell lysis, 1.8 ml of RLT buffer in presence of 18 μl βmercaptoethanol was used per cultivation flask. RNA purification was done according to the manufacturer’s protocol. Purification of RNA in presence of RNase inhibitors is also shown in Figure 7A. General principles of cellular organization in Mycoplasma pneumoniae 30 Figure 7 The association between the RNA polymerase and the ribosome (A) Ribosomal proteins copurify with RpoA‐TAP in extracts treated with RNase and DNase. Upper panels: Coomassie‐stained SDS‐PAGE gels of RpoA‐TAP and 30S ribosomal protein RpsF‐TAP purifications. Proteins identified by MS are indicated between gel images. Lower panel: Ethidium bromide stained 1% agarose gel of total extracted RNA. Cell extracts were treated either with RNase/RNase inhibitors or RNAse/DNAse. (B) RNA polymerase and ribosomal proteins copurify in various purifications. The matrix shows the different baits used in the top of each column and the preys identified by MS in each row underneath. This underlines ability to reversely purify the RNA polymerase with ribosomal entry points and vice versa. 2.4.17. Evaluating protein complexes by gel filtration chromatography and western blot GF chromatography was performed at 10°C on a Pharmacia SMART system at a flow rate of 40 µl/minute by using a SuperoseTM 6 PC 3.2/30 column, equilibrated with lysis buffer. The chromatographic profile was monitored at 280 nm by using the µPeak monitor (Pharmacia). Volumes of 50 µl of M. pneumoniae lysates were loaded on a column and 60 µl fractions were collected and analyzed by SDS-PAGE and Western blot (Figure 8B and 14A). A peroxidase anti-peroxidase antibody (Sigma, P1291) was used to detect the TAP-fusions (alpha subunit of DNA-directed RNA polymerase: RpoA-TAP and segregation and condensation protein A: ScpA-TAP). Polyclonal antibodies produced in rabbits have been used to detect the 30S ribosomal protein S4 (RpsD), the ATP-dependent protease La (Lon) and the protein P115 homolog (P115); final detection was done using secondary antibodies coupled to HRP (Sigma). For the quantification, total band intensity was integrated with Photoshop software (Adobe) and normalized versus the highest detected peak (Figure 14A). General principles of cellular organization in Mycoplasma pneumoniae 31 Figure 8 Novelties in protein complexes. (A) Mpn266 associates with the RNA polymerase as a homodimer. On the left, M. pneumoniae RNApol electron density (~17 nm) with a fitted structure modeled on T. aquaticus RNApol (PDB code: 1l9u). The model fits apart from an extra stalk (cyan arrow), which can be explained by a 200 amino‐acids N‐terminus insertion in M. pneumoniae RpoD. On the right, interaction with an Mpn266 homodimer modeled using a template of B. subtilis SPX interacting with RpoA C‐terminus (1z3e) and a template of an E. coli RpoA C‐terminus dimer interacting with DNA (1lb2). The RpoA C‐terminus dimer is separated by a distance (48Å) similar to that observed for the RpoA N‐terminus from the polymerase fitted on the EM (43Å) (B). The cohesin‐like protein complex. The components are color coded according to their belonging to expression clusters; the heat map illustrates some of these expression patterns (Yus, Maier et al. 2009). Core components are represented by circles, attachments by diamonds. The line attribute corresponds to socio‐ affinity indices: dashed lines, 0.5–0.86; plain lines, >0.86. The blots at the bottom show co‐ elution, during gel filtration chromatography, of ScpA (59kDa), Lon (90kDa) and P115 (111kDa) in extract over‐expressing (right) or not (left) ScpA‐TAP (79kDa). N.D.: not determined. 2.4.18. Multifunctionality index For each set of two protein complexes and one protein, a multifunctionality index (MI) value was calculated (Table S6). This index reflects the tendency for the protein to be part of both complexes and corrects for the possibility that these two complexes should have been one complex, but was separated due to the chosen threshold. General principles of cellular organization in Mycoplasma pneumoniae 32 MI is calculated between each protein and each complex as above where the sums of the interaction scores (P) between the protein under consideration and the members of protein complex 1 (pc1) and protein complex 2 (pc2) are multiplied and divided by the product of the Number of members (N) in complex 1 and complex 2 (equation 10). This is divided by the sum over the interaction scores (Pc1c2) between members of complex 1 and complex 2 divided by the total number of protein pairs (N c1c2) between complex 1 and complex 2, not taking into account the protein for which the MI is being calculated (equation 10). To benchmark the results from the MI a curated list of known multifunctional proteins from the literature was used (Table S7). 2.4.19. Electron microscopy and image reconstruction of selected complexes Selected protein complexes (DNA gyrase, DNA topoisomerase, RNA polymerase) were purified according to the first step of the TAP-protocol (see above). The resulting TEV-eluate was further purified on a glycerol-gradient and fixed by cross-linking following the GRAFIX protocol (Kastner, Fischer et al. 2008). Fractions containing the bait were identified by dotblot using an antibody against calmodulin-binding domain. For electron microscopy, samples were prepared from fractions containing the bait by sandwich negative staining (Golas, Sander et al. 2003) (Figure 9). For DNA gyrase the first models were determined by random conical tilt reconstruction (Radermacher, Wagenknecht et al. 1987). The first model of the topoisomerase was determined by projection matching of class averages against the final map of the gyrase. For RNA polymerase a low-pass filtered map of the polymerase from Thermus aquaticus (1l9u) was used as first reference (Murakami, Masuda et al. 2002). Maps were further refined by iterative projection matching against the current best map using SPIDER (Frank, Radermacher et al. 1996). 2.4.20. Cultivation of M. pneumoniae strain B176 for electron tomography The M. pneumoniae B176 avirulent, noncytadsorbing strain that lacks the 40- and 90-kDa proteins, the products of the ORF6 gene (Mpn142), was used in this analysis (Ruland, Himmelreich et al. 1994). In comparison to its parental strain, the virulent M. pneumoniae M129, B176 does not grow in colonies and hence is more suitable to image individual cells by electron tomography of plunge-frozen samples. General principles of cellular organization in Mycoplasma pneumoniae 33 Figure 9 Electron microscopy particle selection. Electron microscopy and image analysis of negatively stained complexes fixed with glutaraldehyde on a glycerol density gradient. From left to right: DNA‐gyrase (Mpn003, Mpn004), RNA‐polymerase (Mpn191, Mpn352, Mpn515, Mpn516), DNA‐topoisomerase (Mpn122, Mpn123). Upper panel: Fractions of the glycerol gradient were tested by dot blot using an antibody against the calmodulin tag of the bait (fraction 1 is always at the bottom of the gradient). Arrowheads mark the fractions selected for further analysis by electron microscopy and image reconstruction. Representative micrographs of the fractions marked by arrowheads are shown below the dot blots. The samples were prepared by sandwich negative stain using 2% uranyl acetate for staining. The bottom panel shows results from the image analysis. Upper row: Selected class averages calculated by multivariate statistical analysis. The class averages match projections (central row) of the three‐dimensional image reconstructions of the respective complexes. Bottom row shows surface representations of the image reconstructions from the same direction as the projections above. The scale bars equal 10 nm. 2.4.21. Tilt series aquisition Single-axis tilt-series were collected covering an angular range from -66° to +66° with 1.5 degrees of angular increment in a Tecnai G2 Polara microscope cooled to liquid nitrogen temperature and equipped with a Gatan postcolumn GIF 2002 energy filter. Data acquisition was carried out under low-dose conditions using the UCSF tomography software (Zheng, Braunfeld et al. 2004). Images were recorded on a 2k x 2k pixel CCD camera at a defocus level between -5 and -10 μm. The pixel size at the specimen level was 0.6 nm. 2.4.22. Image processing The images were interactively aligned with respect to a common origin using 10 nm colloidal gold particles distributed in the sample as fiducial markers. The reconstructions were performed using weighted back-projection algorithm and visualized with the Amira package (Pruggnaller, Mayr et al. 2008). General principles of cellular organization in Mycoplasma pneumoniae 34 2.4.23. Mapping of complexes in cellular tomograms We scanned 26 tomograms of whole M. pneumoniae cells (with sub-pixel alignment error), with four templates: the ribosome (Gabashvili, Agrawal et al. 2000), GroEL (Forster, Pruggnaller et al. 2008), the RNA polymerase, and the structural core of the pyruvate dehydrogenase (PdhC, homomultimer) (Izard, Aevarsson et al. 1999), by means of locally normalized cross-correlation (Frangakis, Bohm et al. 2002). We have developed a classification method integrating knowledge on protein complexes networks for the detection of putative complexes inside cellular tomograms that largely overcomes the problem of template bias and significantly increases detection certainty (Figure 10). The principle here is that a tomogram is scanned with a template resembling an incomplete complex. The part of the complex, which is deliberately ignored, does not need to be structurally relevant. Complexes are identified as such, when the ignored part is recovered after the classification. Here, we illustrate the procedure on the example of the ribosome. The tomogram (Figure 10A) is scanned with a portion of the whole ribosome, in this case the large ribosomal subunit (50S). The resulting probability map (Figure 10B) contains only the probabilities that the large ribosomal subunit is located at particular positions in the electron tomogram. Most of the ribosomes will most probably have the small ribosomal subunit (the 30S) attached to the large one (Figure 10, C and D). Hence, when classifying the hits obtained for the 50S subunit, we expect to identify classes which show the ribosome as a complete entity. We expect that the sub-tomograms composing those classes will have significantly reduced false positive rates, since the additional density resembles the structure of the small ribosomal subunit increases our level of fidelity. On each cell, the locally normalized cross-correlation (Frangakis, Bohm et al. 2002) produced a list of putative particles, which were further classified using the missing wedge independent procedure (Pascual-Montano, Donate et al. 2001; Forster, Pruggnaller et al. 2008) with a network of 5x5 classification units (Figure 10E). Visual inspection of these classification units reveals the presence of extra-density which was not included in the original template. The similarity of this extra density to the 30S subunit is further asserted by normalized cross correlation and Fourier Shell Correlation. As an effective decision criterion is considered a high cross-correlation value and a Fourier Shell correlation extending into the higher frequencies. The sub-tomograms classifying in classification units with high Fourier Shell Correlation components are accounted as putative particles (Figure 10F). All other hits are rejected. General principles of cellular organization in Mycoplasma pneumoniae 35 Figure 10 Complex detection in M. pneumoniae cryoelectron tomograms by template‐ matching and protein complex‐based classification. Mapping the spatial distribution of four selected complexes in one of the cryo‐electron tomograms. (A) x‐y slice of a reconstruction. The protein density is visualized in dark intensity values. Some of the putative complexes after template matching have been highlighted with circles. (B) The same slice as in (A), in the normalized cross‐correlation map after scanning with the large ribosomal subunit. Higher cross‐correlation values are represented in the bright intensity values. High correlation values corresponding to putative complexes are highlighted with circle in (A) and (B). (C) Isosurface representation of four complexes present in the tomogram. Parts of these General principles of cellular organization in Mycoplasma pneumoniae 36 complexes only have been used as templates: the large subunit of the ribosome (yellow) only, half a RNA polymerase (purple), half the structural core of the pyruvate dehydrogenase (PdhC, homomultimer) (blue) and 10 out of the 14 apical and equatorial domains of GroEL (red). (D) The projection of the whole ribosome is visualized at the same view as the projection images in the classification units. (E) In the green overlay contour the 50S is indicated and in the purple the 30S small ribosomal subunit. The overlays are also superimposed to the classification units. (E) Protein complex‐based classification strategy using the ribosome as an example. In our methodology, the 50S subunit is used to search through the whole tomogram (in six degrees of freedom, three rotational and three translational). Sub‐Tomograms at all local maxima of the cross‐correlation map are extracted and subjected to missing wedge independent classification with KerDenSOM. A cropped region of the total output map of KerDenSOM is shown. A projection image of five out of the 25 classification units is presented. In the first row the Fourier shell correlation, visualized as the blue plot in the bottom right corner of the images, is at the high frequencies indicating high similarity of the density inside the green overlay region to the small ribosomal subunit. The Fourier shell correlation curves of all the other classification units are either close to zeros (showing no similarity) or even negative similarity (due to the inverted contrast). The visual inspection confirms this result. Hence only the sub‐tomograms classifying within the classes on the upper row are selected in this example to contain true hits for the ribosomes. The number of hits assigned to each class appears in the upper‐left corner of the classification unit. (F) Isosurface representation of the whole M. pneumoniae cell with the putative complexes mapped inside. Membrane (blue) and tip organelle (green) can also be visualized. The color mapping of the complexes is as in (C). 2.4.24. Quantitative Western blotting To determine absolute quantities of GroEL and ribosomes in M. pneumoniae, levels of RpsD and the GroEL monomer were quantified by Western blotting. M. pneumoniae recombinant RpsD and GroEL genes used as standard were cloned into the pET19b vector, and proteins carrying an N-terminal poly-histidine tag were expressed and purified from E. coli cells. Protein concentrations were determined using a commercially available BCA based protein assay kit (Pierce #23227) essentially following the instructions in the product manual. Western blotting was carried out with polyclonal antibodies against RpsD and GroEL, respectively. Western blot bands were quantified using the software “ImageJ”. Relating band intensity values to known protein amounts of the purified standards and to the experimentally determined protein per M. pneumoniae cell ratio (10 fg/cell), allowed the calculation of copies of ribosomes and GroEL particles per cell, considering the homooligomeric state of the GroEL particle (Xu, Horwich et al. 1997) (Figure 11, A and B). The value for the quantified Western blot band is divided by the slope of the standard curve and multiplied by 1e-9 to obtain the amount of RpsD and GroEL in grams per gel lane. General principles of cellular organization in Mycoplasma pneumoniae 37 Figure 11 Quantitative Western blots of GroEL and ribosomal protein S4 (RpsD) in whole cell extract (A) Top panels: 0‐100 ng affinity purified GroEL and RpsD protein was loaded next to a M. pneumoniae cell lysate and blotted with polyclonal antibodies (Antibodies: R. Herrmann). Bottom panels: Standard curves from quantified Western blots. The slope of the trend line was used to determine the amount of GroEL and RpsD protein in a M. pneumoniae cell lysates (red square). The error in quantifying the amount of GroEL in the M. pneumoniae sample is 2.1%, as judged from five measurements and 6.6% for RpsD, as judged from four measurements. (B) Estimation of the number of molecules of GroEL and RpsD proteins in a M. pneumonia cell. Determination of the protein concentration of the M. pneumoniae cell lysate (5.07 g/l) and the amount of protein per M. pneumoniae cell (10 fg/cell) allows for calculating the number of cells per gel lane, considering dilution factors and the amount of lysate loaded. Dividing the amount of RpsD and GroEL per lane by the number of cells/lane gives a value for RpsD and GroEL in grams per cell. This number is then transformed to moles by dividing it with the molecular weight of the respective proteins in g/mol. The Avogadro constant determines 1 mol to contain 6.022e23 particles. Dividing the amount of protein per cell in moles by the Avogadro constant allows the determination of molecules per cell. Since GroEL is a General principles of cellular organization in Mycoplasma pneumoniae 38 homotetradecamer, this number has to be divided by 14 to obtain a number for GroEL particles in a single cell. RpsD is believed to be in a 1:1 stochiometry with ribosomes; hence the number of RpsD molecules directly reflects the number of ribosomes in a single M. pneumoniae cell (Figure 11B). 2.4.25. Structural bioinformatics Homologous proteins were collected using PSI-BLAST (Altschul, Madden et al. 1997) with default parameters. Hidden-Markov Models were built and used to find homologues of known structures from PDB (Berman, Henrick et al. 2003) using HHMake and HHSearch modules of the HHPred suite (Soding 2005). Matches of at least 50 residues in the query and with an Evalue ≤ 0.01 were considered further. Two matches were considered to be in physical contact when they did not overlap on the target sequence and each had at least 10 residues within 5.0 Å of the other. Overlaps between multiple interfaces on a single protein were calculated as the number of such residues that they had in common, and the interfaces clustered in to groups using these overlaps and the Markov Cluster algorithm ‘MCL’ (van Dongen 2000)(Table S5). General principles of cellular organization in Mycoplasma pneumoniae 39 2.5. Results 2.5.1. Genome-wide screen for protein complexes in M. pneumoniae We adapted the TAP-MS protocol (Rigaut, Shevchenko et al. 1999)to M. pneumoniae M129 (Figure 12). We processed all 689 M. pneumoniae protein-coding genes, of which 617 were successfully cloned (90% of the genome (Dandekar, Huynen et al. 2000)). Using a transposon-based expression system, we constructed a total of 456 M. pneumoniae strains. They carry a stable genomic integration of carboxy-terminal TAP-fusions under transcriptional control of the M. pneumoniae clpB (mpn531) promoter. From this collection, all 352 individual strains expressing soluble TAP-fusions were grown to confluence in two liters of adherent culture, leading to 212 successful purifications. The components of the purified complex were separated by denaturing gel electrophoresis, and individual bands trypsin-digested and analyzed by MS (Supplementary CD). We processed a total of 10,447 MS samples and identified proteins using a new approach that integrates the Mascot (Perkins, Pappin et al. 1999) and Aldente (Gasteiger, Hoogland et al. 2005) search algorithms (Resing, Meyer-Arendt et al. 2004). This increased the identification of known complex component by ~20% compared to either method alone (Figure 3, A and B). The procedure also scores the quality of individual identifications considering all peptide profiles we observed for each protein, including our purification dataset and a PeptideAtlas, a comprehensive set of tryptic peptides (Desiere, Deutsch et al. 2006) measured with Fourier transform-MS from whole M. pneumoniae lysates (Supplementary CD). We removed protein identifications with overlapping peptide profiles (3%) (Figure 3, C and D). When applied to the entire purification dataset, this approach uncovered 411 distinct proteins from 5,899 identifications (Table S1). General principles of cellular organization in Mycoplasma pneumoniae 40 Figure 12 Synopsis of the genome‐wide screen of complexes in M. pneumoniae. The 411 proteins identified with 212 tagged proteins correspond to 60% of the annotated ORFs and 85% of the predicted soluble proteome (Figure 4). They cover all cellular functions, though low abundant, small or trans-membrane segment-containing proteins are significantly underrepresented (Figure 4). Membrane proteins purification requires separate biochemical protocols so they were not included in this screen. The proportion of new proteins identified per purification dropped asymptotically as the screen progressed, implying that the procedure was near saturation (Figure 5). This entails recurring protein complex retrieval through reverse tagging, and is important both to confirm novel interactions and to identify dynamic complexes (Gavin, Aloy et al. 2006). To define complexes in a quantitative way, we first calculated socio-affinity indices that measure the frequency with which pairs of proteins were found associated in our set of biochemical purifications (Gavin, Aloy et al. 2006). We improved the concept by integrating predicted interactions from the STRING database (Jensen, Kuhn et al. 2009) and the relative abundance of a given prey when associated with different baits (i.e. across different purifications (Sowa, Bennett et al. 2009)). We used the MS scores that measure the probability for a peptide mass fingerprint to characterize each protein based on spectral counting. A reduced score for a prey in a purification, when compared to the same prey in other purifications, reflects identifications by a smaller number of peptides (lower spectral counts) and is indicative of a spurious interaction; is therefore down-weighted (Figure 6A). We applied this new scoring scheme to the entire dataset and calculated a list of 10,083 General principles of cellular organization in Mycoplasma pneumoniae 41 interactions. A cut-off was defined at an accuracy, i.e. fraction of true interactions (von Mering, Krause et al. 2002), of more than 80%, which gave a set of 1,058 high-confidence interactions (Figure 6, B and C; see also Table S2). We also measured the overall experimental reproducibility on a set of 18 experiments that we performed twice; duplicates included growth of adherent cultures, biochemical purifications and MS analyses. For protein pairs with socio-affinity scores ≥ 0.8 the overall reproducibility is 73%; for those scoring below it is 43% (P = 10-13, χ² test). For comparison, the reproducibility calculated on the duplicated MS measurements of 72 MS samples is 97%. We then applied cluster analysis, using a procedure called clique percolation that allows proteins to be part to different complexes. We varied the clustering parameters over reasonable ranges. The best conditions in terms of coverage (see below) generated a collection of 116 heteromultimeric complexes. They are organized into densely (> one link) and loosely interconnected (one link) components we called “core” and “attachment”, respectively (Figure 6D and Table S3). Generally, M. pneumoniae proteins within complexes and cores are more often co-expressed (Guell, van Noort et al. 2009) and conserved between species than average; protein within complexes appear on average in 244 species compared to 173 for the entire proteome (median = 190). Comparison to a set of 31 known complexes, described in other species (Table S4), revealed a coverage of 61%, which is similar to results from previous screens in yeast and E. coli (coverage ~60%) (Gavin, Bosche et al. 2002; Butland, Peregrin-Alvarez et al. 2005; Gavin, Aloy et al. 2006; Krogan, Cagney et al. 2006). 2.5.2. Systematic detection of homomultimeric protein complexes The TAP-fusions were expressed from exogenous loci and promoter and are therefore present together with the untagged wild-type allele. It was thus common to observe both TAP-tagged and untagged versions of the bait in the same purification, which is an indication of homomultimerization (Figure 7). Careful scrutiny of the purification dataset revealed evidence for 62 homomultimeric complexes (Table S3) covering 62% of those previously seen either in M. pneumoniae or in another species by orthology (Table S4). Fourteen homomultimeric complexes were novel, and for 12 of these we could find supporting structural evidence from homologs of known structure (Berman, Henrick et al. 2003) (Table S5). An example is Mpn266, a protein of previously unknown function that we found associated to RNA polymerase (Complex 49; Table S3) as a dimer. Its binding to the polymerase is consistent with its similarity to SpxA, an RNA polymerase-binding protein that regulates transcription initiation in gram-positive bacteria (Zuber 2004; Yus, Maier et al. General principles of cellular organization in Mycoplasma pneumoniae 42 2009). Comparative modeling of structure and single particle electron microscopy (Figure 8A) (Guell, van Noort et al. 2009) show M. pneumoniae RNA-polymerase resembles that of Thermus aquaticus (Murakami, Masuda et al. 2002) with the exception of a substantially bigger stalk at the position of the sigma factor, RpoD (Mpn352), consistent with M. pneumoniae RpoD being 200 amino acids longer than its T. aquaticus ortholog (Figure 8A). The models also further support the idea that each Mpn266 in the dimer binds one of the two alpha subunits of the polymerase as do other transcription factors (Figure 8A). From the number of baits used (212) and from the effectiveness of the method in recovering known complexes (62% coverage), we estimate that as many as 47% of all soluble proteins form homomultimers in M. pneumoniae. This is in agreement with a recent analysis of more than 5,000 protein structures (Levy, Boeri Erba et al. 2008). Finally, considering both homo- and heteromultimers, almost 90% of soluble proteins were found to be part of at least one complex, a figure similar to values estimated in yeast (Gavin, Aloy et al. 2006; Krogan, Cagney et al. 2006). This further consolidates the view that exhaustive organization into complexes is a general property of proteomes in bacteria and eukaryotes. 2.5.3. Characteristics of M. pneumoniae protein complexes Overall, more than half of the identified complexes were not previously described. We also found new components in previously known complexes: the dataset contains 126 proteins with previously unknown or conflicting functional annotation. For example, complex membership identifies Mpn426, previously annotated as a P115 homolog, as the missing Smc (structural maintenance of chromosomes) DNA-binding subunit of the cohesin-like complex (Complex 40; Figure 8B and Table S3) (Yus, Maier et al. 2009). This complex also contains the ATP-dependent protease Lon (Mpn332) that binds DNA and regulates chromosome replication (Wright, Stephens et al. 1996). The observed physical association between Lon and Smc and the observation that Lon expression increases concomitant with Smc degradation at the onset of the stationary phase (Figure 8B) suggest Smc might be a target of this protease. The existence of a native complex including Lon, ScpA (Mpn300) and P115 is further supported by the observation that these three proteins co-elute during gel filtration chromatography (Figure 8B) (Yus, Maier et al. 2009). We also identified known eukaryotic complexes such as those including several GEs that have been discovered at eukaryotic plasma membrane, where they locally produce ATP (Table S4). We observed similar assemblies in M. pneumoniae (Complexes 12 and 45; Table S3) suggesting that this function is conserved in bacteria. General principles of cellular organization in Mycoplasma pneumoniae 43 2.5.4. Comparison of methods for estimation of proteome organization We overlaid the protein complex data with complementary large-scale datasets that have been previously used to deduce physical interactions (Figure 13A). Only 48% of the TAP interactions within complexes were found in any existing dataset; 359 associations were only identified by TAP-MS (Figure 13A). Even in the worst-case scenario, where we consider the upper limit of the estimated false-positives rate (20% = 100%-80% accuracy) and assume that false positives are completely excluded from the other datasets, we estimate at least 220 new true associations were identified here. Overlap with interactions inferred from genome organization or gene expression was particularly low: only 7% of the high confidence interactions are between gene products from the same operon and only 18% were consistently co-expressed (Guell, van Noort et al. 2009). This implies that temporal or conditional regulation of complex formation is analogous to that for eukaryotes, in which different components are expressed at different times (de Lichtenberg, Jensen et al. 2005). For example, the four known subunits of the RNA polymerase are in three operons and their transcription profiles correlate with two different gene expression groups along the growth curve (Guell, van Noort et al. 2009; Yus, Maier et al. 2009). With current knowledge, only a small fraction of proteome organization can be inferred from analysis of the genomes or transcriptional data making proteomics studies critical for understanding prokaryotic systems. General principles of cellular organization in Mycoplasma pneumoniae 44 Figure 13 Proteome organization is only partially reflected by other biological datasets. (A) General overlap between TAP and interactions inferred from other datasets: co‐expression, operons (Guell, van Noort et al. 2009; Yus, Maier et al. 2009), STRING (Jensen, Kuhn et al. 2009) and pathways (Kanehisa, Araki et al. 2008). Numbers refer to the interacting pairs within the different datasets. The fraction of TAP‐interactions that cluster into complexes and are covered by other datasets is given in between brackets. For TAP interacting protein pairs the cut‐off was set at 80% accuracy. Cut offs for other datasets were optimized for coverage (accuracies 40 to 100%). (B) Frequent functional cross‐talk in the protein complex dataset. All proteins within high confidence pairs were functionally annotated according to COG (Tatusov, Koonin et al. 1997). Boxed areas are colored proportionally to the number of interactions linking two functional classes. The scales represent the total (upper panel) and normalized (lower panel) number of interactions (von Mering, Krause et al. 2002). Category Q (secondary metabolites) contains only two proteins. The category most frequently linked is J (Translation) with itself; it however contains the highest number of proteins. The highest proportion of interactions is between proteins within category K (Transcription). General principles of cellular organization in Mycoplasma pneumoniae 45 2.5.5. The M. pneumoniae protein complex network reveals substantial cross-talk About a third of the heteromultimeric complexes in M. pneumoniae have extensive physical interconnections that suggest proteins participate in different cellular processes (Figure 13B). These reflect protein multifunctionality (see below) and organization into at least 35 larger assemblies, sometimes hinting at physical, possibly temporal associations of sequential steps in biological processes (Table S3). For example, we reconstituted major parts of the ribosome from the interaction screen and saw extensive cross-talk with RNA polymerase (Figure 14A). This higher level association was unaffected by RNAse and DNAse treatments suggesting protein-protein rather than protein-nucleic acid interactions were involved (Figure 7). The TAP-MS data were consistent with gel filtration results showing the RNA polymerase alphasubunit, RpoA (Mpn191), and the ribosomal protein RpsD (Mpn446) co-elute with high apparent molecular sizes (Figure 14A). These observations are further supported by the genome organization, where the rpoA gene is localized in and co-regulated with a ribosomal operon (Guell, van Noort et al. 2009). This network provides a molecular model for the coupling of transcription and translation proposed in bacteria (Gowrishankar and Harinarayanan 2004) and the direct involvement of ribosomal proteins in transcriptional regulation (Torres, Condon et al. 2001). The same assembly also includes translational initiation factors InfA (Mpn187), InfB (Mpn155) and InfC (Mpn115), which are part of the 30S initiation complex, as well as elongation factors Tuf (Mpn665) and Tsf (Mpn631), suggesting that we have captured sequential steps in a pathway running from transcription to translation. 2.5.6. Functional reuse and modularity of protein complexes Genome-wide screens in eukaryotes show that proteins often participate in more than one complex, an attribute that has been proposed to account for protein multifunctionality, pleiotropy or moonlighting (Hodgkin 1998). We defined a multifunctionality index that measures the tendency of proteins to associate with more than one complex. This is based on frequency with which pairs of proteins were found associated in our set of purifications and is insensitive to the clustering parameters. We found 156 multifunctional proteins (Table S6), covering 54% of M. pneumoniae proteins that are currently known to be multifunctional in the literature (Table S7). We also compared to a set of multifunctional enzymes that catalyze different enzymatic reactions (Yus, Maier et al. 2009) and the overlap was smaller (32%). Our analysis captured distinct mechanisms for multifunctionality that imply the combinatorial use of gene products in different contexts, for different functions. General principles of cellular organization in Mycoplasma pneumoniae 46 Figure 14 Higher level of proteome organization. (A) The RNA polymerase‐ribosome assembly. Core components are represented by circles, attachments by diamonds. The line attribute corresponds to socio‐affinity indices: dashed lines, 0.5–0.86; plain lines, > 0.86. Color code and shaded yellow circles around groups of proteins refer to individual complexes: RNA polymerase (pink), ribosome (purple) and translation elongation factor (green). The lower panel shows the ribosomal protein RpsD (23kDa) and the α subunit of the RNA polymerase, RpoA‐TAP (57kDa) co‐elute in high molecular weight fractions (MDa range) during gel filtration chromatography. (B) DNA topoisomerase (diameter ~12 nm) is a heterodimer in bacteria: ParE, (ATPase and DNA‐binding domains), and ParC (cleavage and C‐terminal domains). The interaction between ParE‐DNA‐binding and ParC‐cleavage domains was modeled using yeast topoisomerase II as a template (PDB code 2rgr) and ParE‐ATPase and ParC C‐terminal domains were modeled separately on structures of gyrase homologs (1kij & 1suu). All four domains were fitted into the EM density. Gyrase (~12 nm) is similarly split in bacteria, into GyrA/GyrB, which are paralogs of ParE/ParC, and was modeled and fitted using 1bjt as a template for the GyrB‐DNA‐binding and GyrA‐cleavage domains interaction. (C) Protein multifunctionality in M. pneumoniae illustrated with the aminoacyl‐ tRNA synthetase complexes. For example, GyrA (Mpn004) is a component of the DNA gyrase complex that introduces negative supercoils into DNA, and ParE (Mpn122), is a member of the topoisomerase IV complex which decatenates DNA (Zechiedrich and Cozzarelli 1995). Besides well documented interactions within their respective complexes (Complexes 17 and 82; Table S3), GyrA and ParE were also found to stably associate with each other (Complex 102; Table S3). Single particle electron microscopy and comparative modeling (Figure 8 and General principles of cellular organization in Mycoplasma pneumoniae 47 9) showed that DNA-topoisomerase and DNA-gyrase have related overall shapes, as expected from their functional similarity, and also supports the notion that they might be able to interchange subunits (Figure 14B). In eukaryotes ParE and ParC (Mpn123) are fused into one single polypeptide. In bacteria, the possibility for the split ParE and ParC to contribute to different complexes might represent a parsimonious way of generating functional diversity and also robustness to mutations with a set of paralogous proteins. Another example is a complex containing a cluster of five different AARSs (Complex 10; Figure 14C and Table S3). In eukaryotes and archaea, AARSs form macromolecular complexes that improve aminoacylation efficiency by channeling substrates to ribosome (Praetorius-Ibba, Hausmann et al. 2007; Kyriacou and Deutscher 2008). These assemblies also act as reservoirs of AARSs that additionally exert a range of non-canonical regulatory functions in transcription, metabolism and signaling (Park, Schimmel et al. 2008). The existence in bacteria of big multi-AARS complexes is controversial; the most recent review advocates assembly in binary complexes that are functionally involved in tRNA metabolism and editing (Hausmann and Ibba 2008). Our results suggest that higher order multi-AARS complexes might also exist in bacteria. We also found several AARSs in other complexes involved in functions as diverse as translation, transcription, DNA replication, and metabolism (Figure 14C). 2.5.7. Structural anatomy of M. pneumoniae Because of their small genome size, bacteria from the genus Mycoplasma have attracted attention as model organisms for structural genomics (Kim, Shin et al. 2005). We used these data to populate our protein complex network with structural information. Sequence similarity searches and comparative modeling provided structures for 484 M. pneumoniae proteins (70% of the genome), and 340 proteins in the network. There were also structural templates to construct models for 153 binary interactions (Figure 12) covering 29 heteromultimeric and 57 homomultimeric complexes (Table S5). These data can be used both to study particular interactions or complexes (Figure 14B and 8A) and to infer general correlations. Structural interfaces are particularly illuminating for the multifunctional proteins. When structural models are available for multiple interactions with a common protein, analysis of the interfaces can suggest whether the interactions are mutually exclusive (same binding sites), or compatible (different sites) (Kim, Lu et al. 2006). We observed that multifunctional proteins generally tend to accommodate more ligands per interacting interface (P = 0.003), consistent with the view that multifunctionality engages mutually exclusive interactions. For example, General principles of cellular organization in Mycoplasma pneumoniae 48 the protein P115 (Mpn426) has six distinct interfaces, each of which has several mutually exclusive interaction partners. Figure 15 From proteomics to the cell. By a combination of pattern recognition and classification algorithms the following TAP‐identified complexes from M. pneumoniae, matching to existing EM, X‐ray and tomogram structures (A), were placed in a whole‐cell tomogram (B): the structural core of pyruvate dehydrogenase in blue (~23 nm), the ribosome in yellow (~26 nm), RNA polymerase in purple (~17 nm), and GroEL homomultimer in red (~20 nm). Cell dimensions are ~300 nm by 700 nm. The cell‐membrane is shown in light‐blue. The rod, a prominent structure filling the space of the tip region is depicted in green. Its major structural elements are HMW2 (Mpn310) in the core and HMW3 (Mpn452) in the periphery stabilizing the rod (Seybert, Herrmann et al. 2006). The individual complexes (A) are not to scale, but they are shown to scale within the bacterial cell (B). Having assembled a repertoire of structural information, the next logical step is to map these networks and protein complexes in their native environment, the cell. For this purpose, we performed cryo-electron tomography of 26 entire M. pneumoniae cells (Seybert, Herrmann et al. 2006) (Figure 10). We used pattern recognition techniques to generate General principles of cellular organization in Mycoplasma pneumoniae 49 probability maps for complexes selected from the larger ones in M. pneumoniae (Figure 15), as they are more likely to be identified. After a thorough classification considering missing data, low signal-to-noise ratio and known spatial proximities of different sub-complexes we generated maps for the ribosome, the chaperone GroEL (Mpn573), the structural core of the pyruvate dehydrogenase (PdhC, Mpn391, homomultimer), and RNA polymerase, with a minimal number of false positives (Figure 15). These large complexes are excluded from the tip, an organelle required for the attachment to epithelial cells, illustrating that even in a simple, minimal bacteria the proteome is spatially organized (Seybert, Herrmann et al. 2006). Within the cell bodies, we could not find significant proximities or patterns among the different complexes. In contrast to E. coli that contain a compact nucleoid forming an exclusion area in the cell center (Thanbichler and Shapiro 2008), circular DNA in M. pneumoniae is apparently uniformly distributed (Seto, Layh-Schmitt et al. 2001). We estimated the average number of complexes per cell to 140 for the ribosome, 100 for GroELs, 100 for pyruvate dehydrogenase and 300 for RNA polymerase. For the ribosome and GroEL, we also quantified complex abundances by Western blotting (Figure 11). For both, the numbers derived from Western blot were in the range of those estimated from the tomograms. This adds to the emerging view that the mapping of macromolecular structures into entire cell tomograms (Al-Amoudi, Diez et al. 2007), even though still challenging, is a powerful strategy when combined with unbiased large-scale complex purification. It opens the way to more general charting of cellular networks in entire cell tomograms. 2.6. Conclusions Our genome-scale screen for soluble complexes in a bacterium provides a valuable resource for the functional annotation of many genes whose biological roles in prokaryotic or parasitic cells are elusive. The coverage of known complexes leads to an estimate of some 200 molecular machines in M. pneumoniae. The study allows estimation of unanticipated proteome complexity for an apparently minimal organism that could not be directly inferred from its genome composition and organization or from extensive transcriptional analysis. Organisms with small genomes are the most tractable for systems biology, and the biochemical dataset, proteome-wide spectra, ORFome and collection of TAP-expressing M. pneumoniae strains will provide an extremely useful resource for this community. Comparison to both more complex bacteria and to even smaller ones, such as M. genitalium with 485 annotated protein coding genes (Gibson, Benders et al. 2008), should reveal additional systemic features associated with genome streamlining. General principles of cellular organization in Mycoplasma pneumoniae 50 With protein structures available for about three quarter of its ORFs, either directly from structural genomics efforts (Kim, Shin et al. 2005) or indirectly inferred by homology, M. pneumoniae has been extensively studied. We demonstrated that we can integrate datasets of biochemically determined complexes with structural information to approximate the threedimensional organization of proteins into functional molecular machines. These models can then be mapped in entire cell tomograms, providing a three-dimensional view of cellular proteomes and interactomes (Bork and Serrano 2005); ultimately whole cell models will benefit studies of biological function and disease. General principles of cellular organization in Mycoplasma pneumoniae 51 3. Impact of genome reduction on bacterial metabolism and its regulation 3.1. Authorship This chapter covers the systematic investigation of the metabolism and its regulation in M. pneumoniae. It represents a minor part of work during my time as a predoctoral fellow at EMBL Heidelberg and ETH Zürich. This study has a first authorship by Eva Yus, who performed the minimal medium experiments, contributed to the reconstructed the metabolic network, generated expression data, did major parts of the cell culture work and majorly contributed editing manuscript and figures. Additionally there are numerous other co-authors who had their unique contributions. My main contributions were providing comparative data and reagents from the proteome analysis and participating in cell culture work. Additionally, I prepared and edited figures as well as the manuscript of this study. This chapter represents a reformatted version of a report that has been published in Science. 3.2. Abstract To understand basic principles of bacterial metabolism organization and regulation, but also the impact of genome-size thereon, we systematically studied one of the smallest bacteria, Mycoplasma pneumoniae. A manually curated metabolic network of 189 reactions catalyzed by 129 enzymes allowed the design of a defined, minimal medium with 19 essential nutrients. More than 1,300 growth curves were recorded in the presence of various nutrient concentrations. Measurements of biomass indicators, metabolites and 13 C-glucose provided information on directionality, fluxes and energetics; integration with transcription profiling enabled global analysis of metabolic regulation. Compared to more complex bacteria, the M. pneumoniae metabolic network has a more linear topology and contains a higher fraction of multifunctional enzymes; general features such as metabolite concentrations, cellular energetics, adaptability and global gene expression responses are similar though. General principles of cellular organization in Mycoplasma pneumoniae 52 3.3. Introduction Accurate representation of cellular networks by mathematical models is a central goal of integrative systems biology. For this purpose, all components and reactions of a target system should be listed and validated, and their quantitative relations should be determined and analyzed in the context of the physiology of the organism (Feist and Palsson 2008). We have selected M. pneumoniae, a human pathogen causing atypical pneumonia (Waites and Talkington 2004) as a model organism for bacterial and archebacterial systems biology. Like other mollicutes, M. pneumoniae has undergone a massive genome reduction to include only 689 protein coding genes, 231 of which have unknown function (Supplementary CD Yus et al. Table S1) (Dandekar, Huynen et al. 2000); yet it can be cultivated in vitro without helper cells (Proft and Herrmann 1994). The genome reduction of M. pneumoniae favors its suitability as a systems biology model because it largely follows genome size scaling principles (Supplementary CD Yus et al. Figure S1) (van Nimwegen 2003). We manually reconstructed and validated the metabolic network of M. pneumoniae and studied its regulation, thereby complementing analyses of the transcriptome (Guell, van Noort et al. 2009) and the proteome organization (Kuhner, van Noort et al. 2009). 3.4. Results 3.4.1 Reconstruction of the metabolic network: verification by a defined, minimal medium The metabolism of M. pneumoniae has been studied biochemically (Pollack 1997) and computationally (Pachkov, Dandekar et al. 2007). We integrated these approaches in a framework that maximized coverage and accuracy (Supplementary CD Yus et al.). To build a comprehensive metabolic network, we complemented the reactions from KEGG (www.genome.jp/kegg) with activities obtained manually from the literature and new annotations (Supplementary CD Yus et al. e.g. in Tables S1 to S5 and Figure S2) (Pollack, Williams et al. 1997). We also considered other genomic (co-occurance in one operon), sequence (homology to known enzymes) and structural information (identification of catalytic residues to ensure enzyme functionality) (Figure 16A and Supplementary CD Yus et al. Figure S2 and S3). For example, we identified an incomplete ascorbate pathway by sequence analyses and filled the gap by assigning a critical enzyme (L-ascorbate-6-phosphate lactonase, mpn497) based on sequence homology, predicted activity (metal-dependent hydrolase) and its position in the ascorbate operon (mpn492 to 497). For pathways where only one enzyme was General principles of cellular organization in Mycoplasma pneumoniae 53 missing we closed the gap by adding an unassigned reaction (e.g. transketolase activity in the pentose pathway). Putative enzymes missing conserved catalytic residues were discarded (e.g. Mpn255 and Mpn673 enzymes of the terpenoid pathway). Finally, for enzymes that could carry out more than one reaction, we removed the reactions that were decoupled from pathways and those for which the substrate was unavailable. The final result was a map without gaps, isolated reactions, or open metabolic loops (Figure 17). A number of alternative pathways interactions between pathways as well as missing enzymes still needed to be validated, and reaction directionalities had to be inferred. For this, we used two different experimental strategies. We first used the rich medium (Supplementary CD Yus et al. Figure S4) to validate the pathway functionality in various carbon sources. As expected from the map (Figure 17), all known carbon sources but mannitol supported growth to various extents (Supplementary CD Yus et al. Figure S5 and S6) (Halbedel, Hames et al. 2004). By using 13 C-glucose labeling we validated, for examples, the predicted connection between glycolysis, the pentose phosphate pathway and lipid synthesis (Supplementary CD Yus et al. Figure S3 and Table S6), and ruled out the proposed production of aspartate from pyruvate (Manolukas, Barile et al. 1988). For our second strategy we developed, based on the metabolic map, a defined medium (Figure 16A and Supplementary CD Yus et al. Table S7) with which we could validate other pathways (e.g. vitamin metabolism, Supplementary CD Yus et al. Figure S10) and reaction directionalities that could not be studied in rich medium (e.g. synthesis of uracyl and thymine nucleotides from cytosine; Supplementary CD Yus et al. Figure S7 and S8). The low number of amino acid permeases and transporters and the existence of a peptide importer (oppB-F cluster, Table S1) suggested a requirement for peptides in the medium, which we confirmed experimentally (Supplementary CD Yus et al. Figure S9). General principles of cellular organization in Mycoplasma pneumoniae 54 Figure 16 Metabolic network development and properties, and minimal medium design. (A) Schematic diagram of the process leading to M. pneumoniae metabolic network reconstruction and design of a minimal medium. (B) Comparison of M. pneumoniae metabolic network properties with the ones of other model bacteria, (C) quantification of enzyme multifuntionality among prokaryotic genomes: M. pneumoniae (red), L. lactis (yellow), B. subtilis (green) and E. coli (blue), other bacterial species (grey). We systematically tested the defined medium in more than 1,300 experiments to properly assess all the components necessary for survival. We replaced these components with simpler building blocks to obtain a defined, minimal medium that contains only 26 components (19 of which are essential; Figure 16A). This medium, as predicted from our metabolic map and comparative analysis also supports growth of Mycoplasma genitalium (Supplementary CD Yus et al. Figure S11 and S12). Based on these experiments we estimated the upper flux limits for the utilization of the various nutrients (Supplementary CD Yus et al. Figure S13). The medium implicitly validates the reconstructed metabolic map (Figure 17) which consists of 189 reactions (Supplementary CD Yus et al. Table S2): 169 are catalyzed by the products of 140 known genes, and 20 are not yet assigned to any gene (Supplementary CD Yus et al. Table S4). It includes 74 essential metabolic genes and 34 conditionally essential ones (depending on medium composition), which is in agreement with essentiality determined by transposon mutagenesis analyses (96% overlap; Supplementary CD Yus et al. Figure S14 and Table S8) (Hutchison, Peterson et al. 1999). A total of 32 enzymes (25%) are multifunctional, i.e. have more than one activity and together catalyze 91 reactions (48% of the total; Supplementary CD Yus et al. Table S3). With respect to previous genome annotations (Himmelreich, Hilbert et al. 1996; Dandekar, Huynen et al. 2000), we assigned new or refined functions to 57 metabolic genes (plus 30 non-metabolic genes, Supplementary CD Yus et al. see new annotations in Table S1). The above strategy could more generally be used to design General principles of cellular organization in Mycoplasma pneumoniae 55 media to grow anexically, hard-to-culture bacteria as was done for the recalcitrant Tropheryma whipplei (Renesto, Crapoulet et al. 2003) and might be applicable in the context of increasing metagenomics efforts. 3.4.2. Comparison of the metabolic network to those of more complex bacteria Analysis of the metabolism of M. pneumoniae reveals that it is more linear than that of larger bacteria such as B. subtilis (Figure 16B). Furthermore, M. pneumoniae has a wider metabolic network diameter (shortest biochemical pathway averaged over all pairs of substrates) although the diameter has been reported to increase with the logarithm of the network size (Zientz, Dandekar et al. 2004). The greater linearity and the wider diameter of the network suggest that it is less interconnected and contains fewer parallel paths. Thus, the M. pneumoniae network is less redundant both in terms of enzyme paralogy and in network topology. Yet, the distribution of the number of metabolites per reaction is similar to other organisms (Supplementary CD Yus et al. Figure S14). This is partly achieved by an increased fraction of multifunctional enzymes compared to that in larger bacteria, as happens in endosymbionts (Zientz, Dandekar et al. 2004). We did not find any evidence of M. pneumoniae multifunctional enzymes being more conserved than others. This suggests the larger number could be due to function acquisition not present (or detected) in their homologs. This might represent a more general mechanism expected to facilitate further genome reduction (Figure 16B and C). The increased linearity and limited redundancy in the metabolic network suggest limited robustness and adaptability to external factors (Pipe and Grimson 2008): 60% of the metabolic enzymes are essential (Glass, Assad-Garcia et al. 2006), in contrast to only 15% in E. coli (www.shigen.nig.ac.jp/ecoli/pec/index.jsp). General principles of cellular organization in Mycoplasma pneumoniae 56 Figure 17 Metabolic map of M. pneumoniae. Main metabolites are shown as boxes and enzymes and transporters as pentagons. In blue, input metabolites, in red, output products. New enzymatic activities determined in this study are displayed in yellow, enzymes catalyzing multiple reactions are bold. Essential enzymes (according to the mutagenesis study in M. genitalium) are marked with a black triangle. Minimal medium components have General principles of cellular organization in Mycoplasma pneumoniae 57 been encircled in blue. See right hand bottom legend for details, Supplementary CD Yus et al. Figure S12 and Table S2 for description of the enzymatic reactions and enzymes and Supplementary CD Yus et al. Table S25 for metabolite abbreviations. aaRS stands for aminoacyl‐tRNA synthase. 3.4.3. Growth and energetics in comparison with larger bacteria. M. pneumoniae has a relatively long duplication time (at least 8 hours) in comparison with E. coli or L. lactis (20 min), both in culture (Hasselbring, Jordan et al. 2006) and in the presence of host cells (Dallo and Baseman 2000). Slow growth in genome-reduced, parasitic bacteria has been proposed to be the result of i) less efficient enzymatic activity that is explained by the accumulation of mutations resulting from genetic drift (Smart and Thomas 1987), ii) a reduced number of rRNA operons and/or iii) other mechanisms related to the adaptation to a parasitic lifestyle. To understand the causes of slow growth it is necessary to measure the overall energetics of the metabolic network (Figure 17), as well as the changes in macromolecules (Figure 18A) and metabolites along the growth curve (Figure 18B). We used the metabolic map, the measured protein concentration (10 fg protein per cell) and the estimated turnover rates of macromolecules (~20 hours for proteins and ~7 min for mRNA; Supplementary CD Yus et al. Table S9 and Figure S15) to estimate the rate of glucose uptake required to duplicate a cell every 8 h at 18,000-24,000 glucose molecules per second (assuming that the majority of ATP is used for biomass production) (Supplementary CD Yus et al.). This figure closely matched the experimentally determined value under exponential growth: ~19,000 glucose molecules per cell per second (Figure 18C) (Supplementary CD Yus et al.). When cultures approached stationary phase (Figure 18A), the rate increased to ~45,000 glucose molecules per cell per second (Figure 18C), concomitantly with the increased transcription of many glycolytic and fermentation genes (Figure 18D and Supplementary CD Yus et al. Table S10 and S11). In both cases at least 95% of the glucose carbon was found in lactate and acetate (Figure 18B and Supplementary CD Yus et al. Figure S16), implying that the glucose is used primarily for energy production. At the fastest glucose consumption rate, assuming all ATP were devoted to biomass production, M. pneumoniae could divide about every 3 hours. However, most of the energetic parameters (i.e. concentration of glycolytic intermediates: fructose-1,6-biphosphate, glycerol-3-phosphate, phosphoenolpyruvate, glucose-6-phosphate, fructose-6-phosphate, ribose-5-phosphate and glycerone phosphate, and glucose uptake) that we measured were similar to those of larger bacteria (Supplementary CD Yus et al. Table S9), suggesting comparable enzyme efficiencies. This similarity extended to regulatory processes seen in L. lactis (Moran 2002). General principles of cellular organization in Mycoplasma pneumoniae 58 For example, as in L. lactis, we observe both a shift from mostly mixed-acid to homolactic fermentation and an acceleration of glycolysis when the medium acidifies (Figure 18A and B); the drop in O2 concentration relieves inhibition of lactate dehydrogenase (Supplementary CD Yus et al.) (Smart and Thomas 1987; Cocaign-Bousquet, Even et al. 2002). Also, the ATP yield per fermented glucose (2 to 4 ATP, depending of lactate or acetate fermentation), is the same as in L. lactis (Supplementary CD Yus et al. Table S9). Figure 18 Determination of various metabolic parameters in growing cultures. Consistently generated heterogeneous data, all derived using a rich medium, are compared using time in hours (X‐axis). (A) M. pneumoniae growth determined by monitoring the decrease in extracellular pH and the concomitant changes in the amount of protein, DNA and total RNA. (B) Determination of glucose consumption and its fermentation to lactate and acetate. (C) Changes in the number of glucose molecules imported by a cell per second and comparison with the biomass doubling time. (D) Changes in gene expression of a representative ribosomal protein (rplX), and two enzymes: ldh, and a component of the pyruvate dehydrogenase complex (pdhA), enzymes from the two fermentation branches and the relation with the shift from acetate to lactate production (ratio between acetate and lactate General principles of cellular organization in Mycoplasma pneumoniae 59 is shown in green, which can be compared to that of cells grown in the presence of oxygen in magenta). Given all the above we cannot explain the slow growth of M. pneumoniae on the basis of glycolytic efficiency or ATP yield. One of the main differences compared to fast dividing bacteria is the number of rRNA operons per genome (just one in M. pneumoniae and 6 in L. lactis) (Supplementary CD Yus et al. Figure S17); and 5 to 10 times proportionally fewer ribosomes compared to E. coli (Supplementary CD Yus et al. Table S10) (Kuhner, van Noort et al. 2009). In many bacteria the number of ribosomes correlates with the division rate (Nomura 1999). For M. pneumoniae, we see a correlation of changes in biomass duplication speed with the number of ribosomes, but not with the glycolytic rate (Figure 18C and D and Supplementary CD Yus et al. Figure S17). We thus suggest that the slow division rate of M. pneumoniae is not due to less efficient energy production but to the limit in protein biosynthesis capacity. This small parasitic bacterium does not appear to be optimized for biomass production. Instead, more complex strategies for fitness, such as suppression of growth by other microorganisms (Teusink, Wiersma et al. 2006) or optimization of interactions with host-cells might determine growth-rate in small organisms. 3.4.4. Coordinated transcriptome dynamics along growth curve and under various conditions It has been suggested that genome-reduced organisms have limited adaptability to external factors (Cocaign-Bousquet, Even et al. 2002). To determine the capacity of M. pneumoniae to respond to environmental changes we performed three types of experiments. First, we followed the changes in gene expression from the exponential growth phase to the stationary phase (Figure 19A). Analysis of changes in gene expression (validated by tiling arrays and quantitative PCR; Supplementary CD Yus et al. Figure S18) at different points along the growth curve showed that a large part of the transcriptome can be grouped into four timedependent expression clusters (Figure 19A and Supplementary CD Yus et al. Figure. S19 and S20, Table S11 and S12). These clusters can be regarded as two pairs of anti-correlating patterns, indicating a complex regulation. Subsequent analysis by mass spectrometry for a subset of enzymes showed correlation between changes in mRNA and protein abundance (Supplementary CD Yus et al. Figure S18 and Table S10). For example, the production of lactate by lactate dehydrogenase (Mpn674|Ldh) revealed the close temporal coordination of General principles of cellular organization in Mycoplasma pneumoniae 60 gene and protein expression, and metabolite turnover (Figure 18B and D and Supplementary CD Yus et al. Table S10). Second, we analyzed the response of M. pneumoniae to specific individual metabolic perturbations encountered as the population grows, such as low pH, accumulation of fermentation end-products, and sugar and amino acid starvation, as well as to more complex stimuli like entry into the stationary phase (Figure 19 B to D and Supplementary CD Yus et al. Tables S13 to S16),. We found coordinated changes in gene expression specific to each condition (Figure 19B and Supplementary CD Yus et al. Figure S21). For example, we see a general inhibition of transcription and translation upon glucose deprivation, and an increase of ATP proton pump genes at pH 6.5 (Figure 19B and C). Induction of the stringent response (a global response to the absence of amino acids) results in upregulation of peptide and amino acid transporters (Figure 19D). Also a specific repression of the Thr-tRNA synthetase gene (mpn553) (Supplementary CD Yus et al. Table S17), which is a core component of a tRNA synthetase complex (Kuhner, van Noort et al. 2009), suggests its possible regulatory role in complex assembly and therefore, in regulation of translation. Interestingly, we found some common responses to multiple stresses. Some were known as was the down-regulation of ribosomal proteins or peptide importers, common to all stresses. Others, like the up-regulation of ldh and glycerol-3-P dehydrogenase (mpn051), were unexpected suggesting additional functions for these proteins during stress (Figure 19B). Third, we adapted the cells by serial passage (15 passages) to efficient growth in other carbon sources (fructose, mannose, and glycerol) (Supplementary CD Yus et al. Tables S18 to S20). Fructose adaptation resulted in over-expression of fruA and fruK (>3 Log2), and mannose-adapted cells over-expressed the mannitol importer (>5 Log2) (Supplementary CD Yus et al. Tables S19 and S20). Thus M. pneumoniae shows surprising adaptation capability similar to that reported for E. coli (Oxman, Alon et al. 2008). The coordinated changes in gene expression along the growth curve, the specific responses to many various metabolic perturbations and the adaptability of the cells to various carbon sources indicate that M. pneumoniae retains some robustness and adaptability despite its extreme genome reduction. General principles of cellular organization in Mycoplasma pneumoniae 61 Figure 19 Regulation of metabolism. (A) Representative plot of the four main gene co‐ expression clusters identified along the growth curve, named after the main functional classes of the genes involved. (B) Upper panel: overlap between changes in gene expression under various stresses, i.e.: lactate (80 mM buffered lactate), low pH (pH 6.5), glucose or amino acid starvation (stringent response) and the entry into stationary phase. Lower panel: heatmap of the genes found to be commonly up‐ or down regulated under stress and growth inhibition. (C) GO functional classification of genes significantly regulated during exponential growth and glucose deprivation in M. pneumoniae (Mpn) or L. lactis (Lla). The average of the significant changes within each category is depicted. C Energy production and conversion, D Cell division and chromosome partitioning, E Amino acid transport and metabolism, F Nucleotide transport and metabolism, G Carbohydrate transport and metabolism, H Coenzyme metabolism, I Lipid metabolism, J Translation, ribosomal structure and biogenesis, K Transcription, L DNA replication, recombination and repair, M Cell envelope biogenesis, outer membrane, O Posttranslational modification, protein turnover, chaperones, P Inorganic ion transport and metabolism, R General function, T Signal transduction mechanisms. (D) Stringent response expression pattern was compared with that of L. lactis and B. subtilis (Bsu). The average of the significant changes upon stringent response induction (with norvaline) is shown. (E) Venn diagrams showing the overlap in M. pneumoniae and L. lactis of ortholog genes under various metabolic conditions. High P‐value General principles of cellular organization in Mycoplasma pneumoniae 62 in the case of the stringent response indicates that it is not statistically significant. (F) Upper panel: growth curve of cells growing in minimal medium plus increasing amounts of glycerol. Lower panel: glucose titration in Hayflick is shown for comparison. 3.4.5. Complex metabolic regulation despite few transcription factors Compared to more complex bacteria, M. pneumoniae lacks the majority of TFs regulating metabolic genes factors (such as the CRP), major sigma factors and other regulators (Goebel and Lory 2006). Gene assignment based on sequence analysis (Supplementary CD Yus et al. Table S1), in some cases validated by co-purification with the RNA polymerase complex (e.g. mpn266|spxA) (Kuhner, van Noort et al. 2009), revealed four transcription factors (mpn239|gntR; mpn329|fur; mpn124|hrcA), the general sigma 70 (mpn352|sigA) factor, two putative sigma-like factors (mpn626|sigD; mpn424|ylxM) and a putative DNA-binding protein (mpn241|whiA) (see Supplementary CD Yus et al. Table S1 and Figure S2). Despite this apparently reduced gene regulatory toolbox, both environmental stresses (Guell, van Noort et al. 2009) and metabolic insults induced complex, specific transcriptional responses; comparison with more complex bacteria showed similarities, but also some specific differences in regulation of gene expression (Supplementary CD Yus et al. Table S21 to S23). For example, we observed an increase in mRNA and protein expression of glycolytic enzymes concomitant with the increase of glycolytic rate upon medium acidification (Figure 18C and D and Supplementary CD Yus et al. Figure S20 and Table S15), very similar to what has been described in L. lactis cultures (Even, Lindley et al. 2003). Response to glucose starvation was also similar to that of L. lactis (Figure 19C and E and Supplementary CD Yus et al. Table S21) (Even, Lindley et al. 2002). Part of the stringent response, e.g. induction of peptide and amino acid transporters and down-regulation of carbohydrate catabolism (Potrykus and Cashel 2008), was conserved in M. pneumoniae (Supplementary CD Yus et al. Table S22); other mechanisms such as the repression of ribosomal protein operons or rRNA synthesis were not observed (Figure 19D and Supplementary CD Yus et al. Figure S22). This is in agreement with the proposed involvement of the RNA polymerase omega subunit (missing in M. pneumoniae) in sensing guanosine pentaphosphate/tetraphosphate [(p)ppGpp] and thus arresting rRNA biosynthesis (Vrentas, Gaal et al. 2005). We believe it is unlikely that the conserved responses, and the specific differences in regulation, can be caused only by combinations of the few TFs that regulate operons and suboperons, even if one includes regulation by antisense RNA (Guell, van Noort et al. 2009). The presence of genes for synthesis or degradation of a number of chemical messengers, such as (p)ppGpp (mpn397|spoT), AppppA (mpn273|hit1), or c-di-AMP (mpn244|disA) General principles of cellular organization in Mycoplasma pneumoniae 63 (Supplementary CD Yus et al. see Table S1 and Figure S2) (Su, Hutchison et al. 2007), implies that signaling mechanisms has been preserved despite genome reduction. For example, over-expression of the spoT gene that regulates (p)ppGpp levels (Potrykus and Cashel 2008) results in significant changes in gene expression, mainly related to the stringent response (Supplementary CD Yus et al. Table S24). The presence of genes coding for a Ser/Thr phosphatase (mpn247|ptc1) and two protein kinases (Ser/Thr/Tyr kinase: mpn248|prkC, mpn223|hrpK, an HPr kinase) and the differential phosphorylation of key metabolic enzymes under various growth conditions (Su, Hutchison et al. 2007) suggest posttranslational control. Also metabolites like glycerol regulate gene expression at the base of the fermentation branches in M. pneumoniae (Halbedel, Eilers et al. 2007), as well as glucose import (Halbedel, Hames et al. 2007). This explains why glycerol is essential in the minimal medium in a concentration-independent manner (Figure 19F). 3.5. Conclusions Our results suggest that complex metabolic regulation can be achieved in a streamlined genome despite the absence of the respective TFs, probably by a combination of transcriptional regulators, post-translational modifications and small molecules including chemical messengers and metabolites. Taken together, our newly established M. pneumoniae resource, containing a manually annotated metabolic map, full annotations, reactome, consistently measured growth curves and gene expression profiles corresponding to an extensive list of metabolites, should facilitate integrative systems biology studies at a high resolution. Comparison to more complex bacteria revealed systemic features associated with genome streamlining, which should be examined in other small bacteria. Despite its apparently simplicity, we have shown that M. pneumoniae shows metabolic responses and adaptation similar to more complex bacteria, providing hints that other, unknown regulatory mechanisms might exist. General principles of cellular organization in Mycoplasma pneumoniae 64 4. Transcriptome complexity in a genome-reduced organism 4.1. Authorship This chapter covers the systematic investigation of the transcriptome and its complexity in M. pneumoniae. It represents a minor part of work during my time as a predoctoral fellow at EMBL Heidelberg and ETH Zürich. This study has a first authorship by Marc Güell, who performed most of the tiling and microarray experiments, identified new transcripts, defined operons and suboperons, contributed to the correlation analysis, did major parts of the cell culture and mainly contributed to editing manuscript and figures. Additionally there are several other co-authors who had their substantial contributions. My main contributions were providing comparative data and reagents from the proteome organization analysis and participating in cell culture work. Additionally, I edited figures as well as the manuscript of this study. This chapter represents a reformatted version of a report that has been published in Science. 4.2. Abstract To study basic principles of transcriptome organization in bacteria, we analyzed one of the smallest self-replicating organisms, Mycoplasma pneumoniae. We combined strand-specific tiling arrays, complemented by transcriptome sequencing, with more than 252 spotted arrays. We detected 117 previously un-described, mostly non-coding transcripts, 89 of them in antisense configuration to known genes. We identified 341 operons, of which 139 are polycistronic; almost half of the latter show decaying expression in a staircase-like manner. Under various conditions, operons could be divided into 447 smaller transcriptional units, resulting in many alternative transcripts. Frequent antisense transcripts, alternative transcripts, and multiple regulators per gene imply a highly dynamic transcriptome, more similar to that of eukaryotes than previously thought. General principles of cellular organization in Mycoplasma pneumoniae 65 4.3. Introduction Although large-scale gene expression studies have been reported for various bacteria (Selinger, Cheung et al. 2000; Tjaden, Saxena et al. 2002; Reppas, Wade et al. 2006; McGrath, Lee et al. 2007; Nelson, Herron et al. 2008; Akama, Suzuki et al. 2009; ToledoArana, Dussurget et al. 2009), comprehensive strand-specific data sets are still missing, limiting our understanding of operon structure and regulation. Similarly, the number of classified non-coding RNAs in bacteria has recently been expanded (Vogel and Wagner 2007), but a complete and unbiased repertoire is still not available. To obtain a blueprint of bacterial transcription, we combined the robustness and versatility of spotted arrays (62 independent conditions and 252 array experiments) (Supplementary CD Güell et al.), the superior resolution of strand specific tiling arrays (Figure 20A) (designed after genome resequencing, Supplementary CD Güell et al. Table S1) and the mapping capacity of RNA deep sequencing (DSSS) (Fig 20A and Supplementary CD Güell et al. Figure S1) in one of the smallest bacteria that can live outside a host cell, M. pneumoniae, with annotated 689 protein coding genes and 44 non coding RNAs (ncRNAs). 4.4. Results Considering DSSS under reference conditions (Supplementary CD Güell et al.) and 43 tiling arrays from four time series (growth-curve, heat shock, DNA damage and cell cycle arrest) (Supplementary CD Güell et al. Table S8), we observed the expression of all genes. Using a segmentation algorithm for the tiling arrays (Huber, Toedling et al. 2006), we identified additional 117 regions with no previous annotation (Supplementary CD Güell et al. Table S2). These regions were further confirmed by DSSS (Figure 20B and Supplementary CD Güell et al. Figure S1) and in four cases by quantitative polymerase chain reaction (Supplementary CD Güell et al. Table S3). Sequence similarity with known proteins revealed the presence of two new protein-coding genes, a pseudogene, one N-terminal truncation, and five 5’-extensions of known genes (Supplementary CD Güell et al. Table S2). The remaining 108 transcripts are probably regulatory rather than structural RNAs, because comparison of their predicted secondary structures with the ones of coding genes does not show any substantial difference (Supplementary CD Güell et al.). Eighty-nine of them are antisense with respect to previously annotated genes. Out of the non-overlapping ones, two of them (NEW87 and NEW8) are General principles of cellular organization in Mycoplasma pneumoniae 66 conserved in M. genitalium and could be involved in DNA replication and repair, and in peptide transport, respectively (Supplementary CD Güell et al. Figure S3 to S5). In total, 13% of the coding genes are covered by antisense; this is twice more than in yeast (7%) (Xu, Wei et al. 2009), and about half of what was reported for plants (22.2%) (Wang, Gaasterland et al. 2005; Henz, Cumbie et al. 2007), or humans (22.6%) (Ge, Wu et al. 2006). Antisense transcripts may affect expression of the overlapping functional sense transcripts through several mechanisms (Lapidot and Pilpel 2006): double-stranded RNA-dependent mechanisms require coexpression with their target (Brantl and Wagner 1994), whereas transcriptional interference rather implies mutual exclusion of sense and antisense transcripts (Brantl 2007; Andre, Even et al. 2008). In M. pneumoniae, we observed a predominance of double-stranded RNA mechanism as in mammals (Katayama, Tomaru et al. 2005) (47% positive correlation versus 2% negative correlation). In addition, we detected a reduced expression level of genes targeted by antisense transcripts, as reported in some prokaryotes (Brantl 2007) (Supplementary CD Güell et al. Figure S6). Figure 20 Transcriptome feature in the reference condition. (A) The first operon in the genome on the forward strand has a staircase behavior, meaning that the consecutive genes have lower and steady expression levels. (B) Example of an anti‐sense RNA transcript. (C) Analysis of staircase operons. Left: all reference operons subdivided by the number of protein coding genes they contain. Right: all reference operons subdivided by their staircase behavior (see bottom graphs). (D) Left: overlap of operon starts and single gene starts with previously identified ‐10 promoter sequence motifs in M. pneumoniae (Weiner, Herrmann et al. 2000) and predicted promoters based on hexamers. Right, Overlap of operon ends and single gene ends with predicted transcription termination hairpins. General principles of cellular organization in Mycoplasma pneumoniae 67 We identified operon boundaries through sharp transcription changes in the tiling reference condition by using local convolution methods (Figure 20A) (Supplementary CD Güell et al.) (Hooper, Boue et al. 2007). More than 90% of the operons (139 polycistronic and 202 monocistronic operons; Supplementary CD Güell et al. Table S4) were well supported by DSSS reads (DSSS alone was not sufficient to unambiguously characterize operons; Supplementary CD Güell et al. Figure S2). Most polycistronic operons contain two or three genes (Figure 20C and Supplementary CD Güell et al. Figure S7, Table S4); the largest one is the ribosomal operon containing 20 genes. For the majority of operons, we observed a canonical or slightly altered version of a standard sigma 70 promoter region (Supplementary CD Güell et al. Figure S8), with transcription starts located within 60 bp (Supplementary CD Güell et al. Figure S9) upstream of the translation start (McGrath, Lee et al. 2007). In contrast to previous suggestions (Washio, Sasayama et al. 1998), we observe, as proposed by others (de Hoon, Makita et al. 2005), a preferential use of termination hairpins for tight regulation of gene expression (Figure 20A and D and Supplementary CD Güell et al. Table S5). Moreover, we found that almost half of the consecutive genes within polycistronic operons show a decay behaviour (Figure 20A and Supplementary CD Güell et al. Figure S1), indicating that such ‘staircase’-like expression is a widespread phenomenon in bacteria (Supplementary CD Güell et al.). Analysis of the 43 tiling arrays and integration with 252 spotted arrays representing 173 independent conditions, some of them from time-series, revealed context-dependent modulation of operon structures involving repression or activation of operon internal genes, as well as of genes located at the beginning, or end (Figure 21A and B and Supplementary CD Güell et al. Figure S10 and Table S5). In some cases this modulation can be assigned to specific environmental changes. Down regulation of the four first genes of the ftsZ operon involved in initiation of cell division corresponds to entry into stationary phase (Figure 21B, lower panel). Increase in expression of arginine fermentation genes (arcA, arcI, arcC) (Figure 21B) in stationary phase could be a mechanism to cope with acidification (Budin-Verneuila, Maguin et al. 2004). We found formal evidence for a total of 447 transcriptional units (336 monocistronic and 111 polycistronic), implying a high rate of alternative transcripts (42%) in this bacterium in the conditions studied, similar to that in eukaryotes (40%, although still under debate) (Boue, Letunic et al. 2003) and archea (40% in H. salinarum) (Koide, Reiss et al. 2009). Interestingly we found that genes that are split into different suboperons tend to belong to different functional categories (Supplementary CD Güell et al.). Thus, although General principles of cellular organization in Mycoplasma pneumoniae 68 genome reduction leads to longer operons accommodating genes with different functions (Yus, Maier et al. 2009), the latter can still retain internal transcription and termination sites under certain conditions. The high frequency of alternative transcripts of M. pneumoniae genes hints at a situation similar to that in eukaryotes where many factors contribute to the regulation of gene expression. To further support this hypothesis, we used gene expression clustering under the 62 distinct conditions (Supplementary CD Güell et al. Table S7) to identify groups of coexpressed genes and their possible common regulatory motifs. Using a correlation cut-off of 0.65 we identified 94 co-expression groups (Supplementary CD Güell et al. Table S6 and Figure S11), encompassing 416 genes. Thirty of the clusters contained genes from more than two operons. Of these, 14 share a unique sequence motif in their upstream region and another 8 have a unique combination of motifs (Supplementary CD Güell et al. Figure S12), which might drive the co-expression (e.g. 4 of the 14 motifs are found at splitting sites inside operons). This is exemplified by the five heat shock induced genes containing a regulatory CIRCE element (Chang, Chen et al. 2008) (Figure 21C). Not all of them clustered together indicating at least another regulatory element. Similarly, over expression of a transcription factor (mpn329; Fur, ferric uptake regulator) reveals a common motif in all genes significantly changing expression, although they belong to different co expression clusters (Supplementary CD Güell et al. Figure S13 and Table S6). General principles of cellular organization in Mycoplasma pneumoniae 69 Figure 21 Alternative operon structure. The continuous lines in A and B indicate expression level measured with tiling arrays. (A), Alternative transcripts discovery pipeline . Reference operon 001 is splitted into 3 suboperons. (Top) Tiling and DSSS under reference conditions. (Middle) Specific expression changes for genes dnaA and xdj1 involved in DNA repair and replication. (Bottom) The coexpression matrices correspond to the final conditional operon splitting by 252 arrays. (B) Two examples of conditional operons are presented. (Top) Specific induction of the middle genes in the operon 126 when the cells reach stationary phase. (Bottom) Repression of the first 4 genes of the operon 129 involved in cell division when the cells reach stationary phase. (C), Example of heat shock induced genes sharing the known CIRCE element. The calculated consensus sequence is represented below. General principles of cellular organization in Mycoplasma pneumoniae 70 4.5. Conclusions Our work revealed an unanticipated complexity in the transcriptome of a genomereduced bacterium. This complexity cannot be explained by the presence of 8 predicted TFs (Yus, Maier et al. 2009). Furthermore, the fact that the proteome organization is not explainable by the genome organization (Kuhner, van Noort et al. 2009) indicates the existence of other regulatory processes. The surprisingly frequent expression heterogeneity within operons, the change of operon structures leading to alternative transcripts in response to environmental perturbations and the frequency of antisense RNA which might explain some of these expression changes suggest that transcriptional regulation in bacteria resemble that of eukaryotes more than previously thought. General principles of cellular organization in Mycoplasma pneumoniae 71 5. Summary of results The systematic investigation of cellular organization on different levels and the integration of this acquired information into other orthogonal datasets can leverage the creation of biological knowledge. This was one of the main tasks and is one of the major results of this thesis, which aims at describing general principles of cellular organization in the genome-reduced bacterium M. pneumoniae. Altogether there are three major studies described in this thesis which aim at the characterization of the: 1. proteome organization, 2. metabolism and its regulation, 3. transcriptome and its complexity in M. pneumoniae. In chapter 2, I describe the results of the investigation of the proteome organization in M. pneumoniae. The analysis revealed 62 homomultimeric and 116 heteromultimeric soluble protein complexes, of which the majority are novel. About a third of the heteromultimeric complexes show higher levels of proteome organization, including assembly into larger, multi-protein complex entities, suggesting sequential steps in biological processes, and extensive sharing of components implying protein multifunctionality. Incorporation of structural models for 484 proteins, single particle EM and cellular electron tomograms provided supporting structural details for this proteome organization. The dataset provides a blueprint of the minimal cellular machinery required for life. Chapter 3 outlines the results of the investigation of the metabolism and its regulation in M. pneumoniae. In order to understand basic principles of its metabolic organization and regulation, but also the impact of genome-size thereon a manually curated metabolic network of 189 reactions catalyzed by 129 enzymes was created. This allowed the design of a defined, minimal medium with 19 essential nutrients. More than 1,300 growth curves were recorded in the presence of various nutrient concentrations. Measurements of biomass indicators, metabolites and 13 C-glucose provided information on directionality, fluxes and energetics. The integration of transcriptional profiles enabled global analysis of metabolic regulation. Compared to more complex bacteria, the M. pneumoniae metabolic network has a more linear topology and contains a higher fraction of multifunctional enzymes; general features such as metabolite concentrations, cellular energetics, adaptability and global gene expression responses are similar though. General principles of cellular organization in Mycoplasma pneumoniae 72 Chapter 4 highlights the results of the investigation of the transcriptome complexity in M. pneumoniae. Here, we combined strand-specific tiling arrays, complemented by transcriptome sequencing, with more than 252 spotted arrays. We detected 117 previously un-described, mostly non-coding transcripts, 89 of them in antisense configuration to known genes. We identified 341 operons, of which 139 are polycistronic; almost half of the latter show decaying expression in a staircase-like manner. Under various conditions, operons could be divided into 447 smaller transcriptional units, resulting in many alternative transcripts. Frequent antisense transcripts, alternative transcripts, and multiple regulators per gene imply a highly dynamic transcriptome, more similar to that of eukaryotes than previously thought. In a more conceptual summary there are four major messages, which the three studies, of which my PhD thesis is part of, have produced: 1. M. pneumoniae shows aspects of multifunctionality particularly in its proteome organization and metabolism. 2. M. pneumoniae, even though being an almost minimal organism, is more complex than expected, particularly in its regulation. 3. M. pneumoniae resembles eukaryotes more than expected, particularly in its transcription. 4. M. pneumoniae is optimized for host adaptation and not for growth. General principles of cellular organization in Mycoplasma pneumoniae 73 6. Conclusions The work on the proteome organization, the metabolism and its regulation as well as on the transcriptional complexity in M. pneumoniae provided in this thesis adds to previous research and will certainly strengthen the interest in this genome-reduced organism (Ochman and Raghavan 2009). Furthermore, it will help to establish M. pneumoniae as a near-minimal model organism (Glass, Hutchison et al. 2009). As such this work will provide a useful ground for further systematic biological investigations in M. pneumoniae as well as related species and maybe even for its use in synthetic biology (Marshall 2009). In the following paragraphs I will therefore briefly express my personal view on i) the next possible directions of research in M. pneumoniae, ii) medically relevant aspects to be addressed, iii) necessary steps to make M. pneumoniae a candidate for biotechnological use as well as iv) possible biotechnological and synthetic biological applications of minimal cells. There are various scientific challenges and next steps of systematic research in M. pneumoniae focusing again on the basic understanding of this organism. In analogy to work performed in Leptospira interrogans useful insights could be derived from measuring proteome-wide cellular protein concentrations in M. pneumoniae as well as half-life of proteins and RNA (Malmstrom, Beck et al. 2009). In the best case scenario this would be performed investigating various relevant growth conditions. Similarly the protein interactions should also be charted in various conditions and along different stages of the cell cycle of M. pneumoniae. Along these lines it is also essential to get a better understanding of the global use of PTMs in these near-minimal cells. Currently only two types of protein cleavage, protein phosphorylation as well as protein acetylation are reported while others remain to be discovered (Herrmann and Ruppert 2006). Another rather neglected aspect is the membrane biology in M. pneumoniae and here especially the membrane proteome, which currently consists of about 100 proteins many of which are only vaguely annotated. At this end comprehensive mapping of the membrane proteome would further advance the understanding of M. pneumoniae. In addition to that the topology of all membrane proteins should also be looked at. Experiments using MS/MS analysis for membrane protein identification could be validated by orthogonal approaches e.g. immunogold labelling of membrane proteins and detection via EM, which could moreover add spatial information on the distribution patterns of membrane proteins. The relative or even absolute quantification via heavy isotope labelling would yet add another level of detail and could also help assess false positive hits from a nonGeneral principles of cellular organization in Mycoplasma pneumoniae 74 quantitative mapping approach. Finally the combination of these results and the integration of additional datasets like the ones provided in this thesis could help elucidate regulatory patterns of the extracellular membrane proteome in single- or multi-conditional analysis. Eventually these combined datasets could then be used for entire cell modelling in order to better understand the M. pneumoniae lifestyle and growth behaviour. From a therapeutic point of view an intensified investigation of the growth on host cells and a more thorough understanding of the pathogenicity of M. pneumoniae are the most urgent questions. Although numerous advanced have been made in the last 25 years in the elucidation of the attachment process and the proteins as well as oligosaccharides involved (chapters 1.1.1 & 1.1.2.) there still many regulatory details not understood. These ask for an in detail investigation of the attachment process to host cells as well as its regulation and a more systematic charting of the host-pathogen interaction network. Inhibitors with antibacterial function could be derived from exhaustive insights into the involved target molecules at various stages of the attachment process. These could be M. pneumoniae specific or in a best case scenario also amenable for other pathogens with a similarly regulated host cell recognition process. In order to make M. pneumoniae available for biotechnological applications there are a few limitations to tackle (chapter 1.1.3). Among them is the rather long generation time of 8h which severely slows down the growth potential of this pathogen and probably reflects its adaptive lifestyle to the host, which should not be killed. Trying to accelerate the M. pneumoniae generation time to 2h or less via genetic modifications seems a necessary prerequisite for industrial use. To that end it also seems required to use a non-adherent M. pneumoniae M129 strain like, e.g. B169/176 (Lipman, Clyde et al. 1969). This strain comes with the advantage of being able to grow in a three-dimensional fashion batch mode so that the biomass production can be increased. Additionally this strain appears to be avirulent and could possibly be reclassified to S1 security level, which would dramatically decrease the growth costs for a weight unit of cells. Another aspect that needs to be addressed is the growth medium. In this thesis a minimal medium for M. pneumoniae growth has been proposed (Yus, Maier et al. 2009). It seems worthwhile to check whether the defined minimal medium or the defined non-minimal medium are optimal also for long term batch culture growth of a modified bacterium that has the desired growth characteristics. From a genetic manipulation point of view the above proposed modifications seem to be achievable, especially considering the progress that has been accomplished by the synthetic biology & General principles of cellular organization in Mycoplasma pneumoniae 75 bioenergy group at the J. Craig Venter Institute (see chapter 1.1.3. for details) (Lartigue, Glass et al. 2007; Gibson, Benders et al. 2008; Lartigue, Vashee et al. 2009). Finally a short glimpse at potential applications for a controllable minimal organism in biotechnology and synthetic biology as reviewed (Serrano 2007). Such an organism could be used as a source for growing initial compounds for chemical synthesis, which are either impossible to produce in a chemical way or their chemical synthesis is simply uneconomic. Feasibility of such an approach was e.g. demonstrated for the production of terpenoid compounds in E. coli and S. cerevisiae, which have been used as precursors in the synthesis of antimalarial drugs (Martin, Pitera et al. 2003; Lindahl, Olsson et al. 2006; Ro, Paradise et al. 2006; Serrano 2007). Controllable minimal cells could also be exploited as a source for energy supply and research towards that aim appears to accelerate (Marshall 2009). Even more than for pure chemical synthesis, controllable cellular systems seem to be predestined for the production of a wide range of biologics, a market that is still growing according to a recent analysis (Aggarwal 2009). The above discussed points clearly show that there are still many questions of M. pneumoniae biology unanswered. Therefore future projects will help to further understand this nearminimal organism. The exact social benefit for the support of academic research towards that direction cannot be exactly estimated; however the odds are not too long that further investigations turn out to be extremely valuable. General principles of cellular organization in Mycoplasma pneumoniae 76 7. References Adamcsek, B., G. Palla, et al. (2006). "CFinder: locating cliques and overlapping modules in biological networks." Bioinformatics 22(8): 1021-3. Aggarwal, S. (2009). "What's fueling the biotech engine--2008." Nat Biotechnol 27(11): 98793. Akama, T., K. Suzuki, et al. (2009). "Whole-genome tiling array analysis of Mycobacterium leprae RNA reveals high expression of pseudogenes and noncoding regions." J Bacteriol 191(10): 3321-7. Al-Amoudi, A., D. C. Diez, et al. (2007). "The molecular architecture of cadherins in native epidermal desmosomes." Nature 450(7171): 832-7. Altschul, S. F., T. L. Madden, et al. (1997). "Gapped BLAST and PSI-BLAST: a new generation of protein database search programs." Nucleic Acids Res 25(17): 3389-402. Andre, G., S. Even, et al. (2008). "S-box and T-box riboswitches and antisense RNA control a sulfur metabolic operon of Clostridium acetobutylicum." Nucleic Acids Res 36(18): 5955-69. Arifuzzaman, M., M. Maeda, et al. (2006). "Large-scale identification of protein-protein interaction of Escherichia coli K-12." Genome Res 16(5): 686-91. Bairoch, A. and R. Apweiler (2000). "The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000." Nucleic Acids Res 28(1): 45-8. Balish, M. F. and D. C. Krause (2006). "Mycoplasmas: a distinct cytoskeleton for wall-less bacteria." J Mol Microbiol Biotechnol 11(3-5): 244-55. Baseman, J. B., R. M. Cole, et al. (1982). "Molecular basis for cytadsorption of Mycoplasma pneumoniae." J Bacteriol 151(3): 1514-22. Berman, H., K. Henrick, et al. (2003). "Announcing the worldwide Protein Data Bank." Nat Struct Biol 10(12): 980. Biberfeld, G. and P. Biberfeld (1970). "Ultrastructural features of Mycoplasma pneumoniae." J Bacteriol 102(3): 855-61. Boatman, E. S. and G. E. Kenny (1971). "Morphology and ultrastructure of Mycoplasma pneumoniae spherules." J Bacteriol 106(3): 1005-15. Bork, P. and L. Serrano (2005). "Towards cellular systems in 4D." Cell 121(4): 507-9. Bornberg-Bauer, E. and J. Weiner, 3rd (2002). "A putative transcription factor inducing mobility in Mycoplasma pneumoniae." Microbiology 148(Pt 12): 3764-5. Boue, S., I. Letunic, et al. (2003). "Alternative splicing and evolution." Bioessays 25(11): 1031-4. Brantl, S. (2007). "Regulatory mechanisms employed by cis-encoded antisense RNAs." Curr Opin Microbiol 10(2): 102-9. Brantl, S. and E. G. Wagner (1994). "Antisense RNA-mediated transcriptional attenuation occurs faster than stable antisense/target RNA pairing: an in vitro study of plasmid pIP501." Embo J 13(15): 3599-607. Budin-Verneuila, A., E. Maguin, et al. (2004). Lait 84: 8. Butland, G., J. M. Peregrin-Alvarez, et al. (2005). "Interaction network containing conserved and essential protein complexes in Escherichia coli." Nature 433(7025): 531-7. Catrein, I., R. Dumke, et al. (2004). "Cross-complementation between the products of the genes P1 and ORF6 of Mycoplasma pneumoniae subtypes 1 and 2." Microbiology 150(Pt 12): 3989-4000. Catrein, I., R. Herrmann, et al. (2005). "Experimental proof for a signal peptidase I like activity in Mycoplasma pneumoniae, but absence of a gene encoding a conserved bacterial type I SPase." Febs J 272(11): 2892-900. General principles of cellular organization in Mycoplasma pneumoniae 77 Chandonia, J. M. and S. H. Kim (2006). "Structural proteomics of minimal organisms: conservation of protein fold usage and evolutionary implications." BMC Struct Biol 6: 7. Chang, L. J., W. H. Chen, et al. (2008). "Mycoplasmas regulate the expression of heat-shock protein genes through CIRCE-HrcA interactions." Biochem Biophys Res Commun 367(1): 213-8. Chanock, R. M. (1963). "Mycoplasma pneumoniae: proposed nomenclature for atypical pneumonia organism (Eaton agent)." Science 140: 662. Chen, S., A. F. Yakunin, et al. (2005). "Structural and functional characterization of a 5,10methenyltetrahydrofolate synthetase from Mycoplasma pneumoniae (GI: 13508087)." Proteins 61(2): 433-43. Ciccarelli, F. D., T. Doerks, et al. (2006). "Toward automatic reconstruction of a highly resolved tree of life." Science 311(5765): 1283-7. Clyde, W. A., Jr. (1973). "Models of Mycoplasma pneumoniae infection." J Infect Dis 127: Suppl:S69-72. Cocaign-Bousquet, M., S. Even, et al. (2002). "Anaerobic sugar catabolism in Lactococcus lactis: genetic regulation and enzyme control over pathway flux." Appl Microbiol Biotechnol 60(1-2): 24-32. Collins, S. R., P. Kemmeren, et al. (2007). "Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae." Mol Cell Proteomics 6(3): 439-50. Dallo, S. F. and J. B. Baseman (2000). "Intracellular DNA replication and long-term survival of pathogenic mycoplasmas." Microb Pathog 29(5): 301-9. Dandekar, T., M. Huynen, et al. (2000). "Re-annotating the Mycoplasma pneumoniae genome sequence: adding value, function and reading frames." Nucleic Acids Res 28(17): 3278-88. Das, D., H. Hyun, et al. (2007). "Crystal structure of a novel single-stranded DNA binding protein from Mycoplasma pneumoniae." Proteins 67(3): 776-82. de Hoon, M. J., Y. Makita, et al. (2005). "Prediction of transcriptional terminators in Bacillus subtilis and related species." PLoS Comput Biol 1(3): e25. de Lichtenberg, U., L. J. Jensen, et al. (2005). "Dynamic complex formation during the yeast cell cycle." Science 307(5710): 724-7. Desiere, F., E. W. Deutsch, et al. (2006). "The PeptideAtlas project." Nucleic Acids Res 34(Database issue): D655-8. Dumke, R., I. Catrein, et al. (2004). "Preference, adaptation and survival of Mycoplasma pneumoniae subtypes in an animal model." Int J Med Microbiol 294(2-3): 149-55. Eaton, M. D., G. Meiklejohn, et al. (1944). "Studies on the Etiology of Primary Atypical Pneumonia : a Filterable Agent Transmissible to Cotton Rats, Hamsters, and Chick Embryos." J Exp Med 79(6): 649-668. Edward, D., G. Meiklejohn, et al. (1956). "The classification and nomenclature of organisms of the pleuropneumonia group." J. Gen Microbiol. 14: 197-207. Even, S., N. D. Lindley, et al. (2003). "Transcriptional, translational and metabolic regulation of glycolysis in Lactococcus lactis subsp. cremoris MG 1363 grown in continuous acidic cultures." Microbiology 149(Pt 7): 1935-44. Even, S., N. D. Lindley, et al. (2002). "Dynamic response of catabolic pathways to autoacidification in Lactococcus lactis: transcript profiling and stability in relation to metabolic and energetic constraints." Mol Microbiol 45(4): 1143-52. Feist, A. M. and B. O. Palsson (2008). "The growing scope of applications of genome-scale metabolic reconstructions using Escherichia coli." Nat Biotechnol 26(6): 659-67. Feldner, J., U. Gobel, et al. (1982). "Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody." Nature 298(5876): 765-7. General principles of cellular organization in Mycoplasma pneumoniae 78 Forster, F., S. Pruggnaller, et al. (2008). "Classification of cryo-electron sub-tomograms using constrained correlation." J Struct Biol 161(3): 276-86. Frangakis, A. S., J. Bohm, et al. (2002). "Identification of macromolecular complexes in cryoelectron tomograms of phantom cells." Proc Natl Acad Sci U S A 99(22): 141538. Frank, B. (1889). "Über die Pilzsymbiose der Leguminosen." Ber. Deut. Bot. Ges. 7: 332-346. Frank, J., M. Radermacher, et al. (1996). "SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields." J Struct Biol 116(1): 190-9. Franzoso, G., P. C. Hu, et al. (1993). "The immunodominant 90-kilodalton protein is localized on the terminal tip structure of Mycoplasma pneumoniae." Infect Immun 61(4): 152330. Franzoso, G., P. C. Hu, et al. (1994). "Immunoblot analyses of chimpanzee sera after infection and after immunization and challenge with Mycoplasma pneumoniae." Infect Immun 62(3): 1008-14. Fraser, C. M., J. D. Gocayne, et al. (1995). "The minimal gene complement of Mycoplasma genitalium." Science 270(5235): 397-403. Gabashvili, I. S., R. K. Agrawal, et al. (2000). "Solution structure of the E. coli 70S ribosome at 11.5 A resolution." Cell 100(5): 537-49. Gasteiger, E., C. Hoogland, et al. (2005). Protein Identification and Analysis Tools on the ExPASy Server, Humana Press. Gavin, A. C., P. Aloy, et al. (2006). "Proteome survey reveals modularity of the yeast cell machinery." Nature 440(7084): 631-6. Gavin, A. C., M. Bosche, et al. (2002). "Functional organization of the yeast proteome by systematic analysis of protein complexes." Nature 415(6868): 141-7. Ge, X., Q. Wu, et al. (2006). "A large quantity of novel human antisense transcripts detected by LongSAGE." Bioinformatics 22(20): 2475-9. Gibson, D. G., G. A. Benders, et al. (2008). "Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome." Science 319(5867): 1215-20. Glass, J. I., N. Assad-Garcia, et al. (2006). "Essential genes of a minimal bacterium." Proc Natl Acad Sci U S A 103(2): 425-30. Glass, J. I., C. A. Hutchison, 3rd, et al. (2009). "A systems biology tour de force for a nearminimal bacterium." Mol Syst Biol 5: 330. Goebel, W. and S. Lory (2006). "Prokaryotic cell regulation." Curr. Opin. Microbiol. 9: 123. Golas, M. M., B. Sander, et al. (2003). "Molecular architecture of the multiprotein splicing factor SF3b." Science 300(5621): 980-4. Gowrishankar, J. and R. Harinarayanan (2004). "Why is transcription coupled to translation in bacteria?" Mol Microbiol 54(3): 598-603. Guell, M., V. van Noort, et al. (2009). "Transcriptome complexity in a genome-reduced bacterium." Science 326(5957): 1268-71. Halbedel, S., H. Eilers, et al. (2007). "Transcription in Mycoplasma pneumoniae: analysis of the promoters of the ackA and ldh genes." J Mol Biol 371(3): 596-607. Halbedel, S., C. Hames, et al. (2004). "In vivo activity of enzymatic and regulatory components of the phosphoenolpyruvate:sugar phosphotransferase system in Mycoplasma pneumoniae." J Bacteriol 186(23): 7936-43. Halbedel, S., C. Hames, et al. (2007). "Regulation of carbon metabolism in the mollicutes and its relation to virulence." J Mol Microbiol Biotechnol 12(1-2): 147-54. Hammerschlag, M. R. (2001). "Mycoplasma pneumoniae infections." Curr Opin Infect Dis 14(2): 181-6. Hansen, E. J., R. M. Wilson, et al. (1979). "Isolation of mutants of Mycoplasma pneumoniae defective in hemadsorption." Infect Immun 23(3): 903-6. General principles of cellular organization in Mycoplasma pneumoniae 79 Hansen, E. J., R. M. Wilson, et al. (1979). "Two-dimensional gel electrophoretic comparison of proteins from virulent and avirulent strains of Mycoplasma pneumoniae." Infect Immun 24(2): 468-75. Hansen, E. J., R. M. Wilson, et al. (1981). "Hemadsorption and virulence of Mycoplasma pneumoniae." Adv Exp Med Biol 134: 241-51. Hansen, E. J., R. M. Wilson, et al. (1981). "Characterization of hemadsorption-negative mutants of Mycoplasma pneumoniae." Infect Immun 32(1): 127-36. Hasselbring, B. M., J. L. Jordan, et al. (2005). "Mutant analysis reveals a specific requirement for protein P30 in Mycoplasma pneumoniae gliding motility." J Bacteriol 187(18): 6281-9. Hasselbring, B. M., J. L. Jordan, et al. (2006). "Terminal organelle development in the cell wall-less bacterium Mycoplasma pneumoniae." Proc Natl Acad Sci U S A 103(44): 16478-83. Hasselbring, B. M., C. A. Page, et al. (2006). "Transposon mutagenesis identifies genes associated with Mycoplasma pneumoniae gliding motility." J Bacteriol 188(17): 633545. Hausmann, C. D. and M. Ibba (2008). "Aminoacyl-tRNA synthetase complexes: molecular multitasking revealed." FEMS Microbiol Rev 32(4): 705-21. Hayflick, L. (1965). "Tissue cultures and mycoplasmas." Tex Rep Biol Med 23: Suppl 1:285+. Hedreyda, C. T., K. K. Lee, et al. (1993). "Transformation of Mycoplasma pneumoniae with Tn4001 by electroporation." Plasmid 30(2): 170-5. Henz, S. R., J. S. Cumbie, et al. (2007). "Distinct expression patterns of natural antisense transcripts in Arabidopsis." Plant Physiol 144(3): 1247-55. Herrmann, R. and T. Ruppert (2006). "Proteome of Mycoplasma pneumoniae." Methods Biochem Anal 49: 39-56. Himmelreich, R., H. Hilbert, et al. (1996). "Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae." Nucleic Acids Res 24(22): 4420-49. Himmelreich, R., H. Plagens, et al. (1997). "Comparative analysis of the genomes of the bacteria Mycoplasma pneumoniae and Mycoplasma genitalium." Nucleic Acids Res 25(4): 701-12. Hodgkin, J. (1998). "Seven types of pleiotropy." Int J Dev Biol 42(3): 501-5. Hooper, S. D., S. Boue, et al. (2007). "Identification of tightly regulated groups of genes during Drosophila melanogaster embryogenesis." Mol Syst Biol 3: 72. Hu, P., S. C. Janga, et al. (2009). "Global functional atlas of Escherichia coli encompassing previously uncharacterized proteins." PLoS Biol 7(4): e96. Hu, P. C., R. M. Cole, et al. (1982). "Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle." Science 216(4543): 313-5. Hu, P. C., A. M. Collier, et al. (1977). "Surface parasitism by Mycoplasma pneumoniae of respiratory epithelium." J Exp Med 145(5): 1328-43. Huber, W., J. Toedling, et al. (2006). "Transcript mapping with high-density oligonucleotide tiling arrays." Bioinformatics 22(16): 1963-70. Hutchison, C. A., S. N. Peterson, et al. (1999). "Global transposon mutagenesis and a minimal Mycoplasma genome." Science 286(5447): 2165-9. Inamine, J. M., K. C. Ho, et al. (1990). "Evidence that UGA is read as a tryptophan codon rather than as a stop codon by Mycoplasma pneumoniae, Mycoplasma genitalium, and Mycoplasma gallisepticum." J Bacteriol 172(1): 504-6. Izard, T., A. Aevarsson, et al. (1999). "Principles of quasi-equivalence and Euclidean geometry govern the assembly of cubic and dodecahedral cores of pyruvate dehydrogenase complexes." Proc Natl Acad Sci U S A 96(4): 1240-5. General principles of cellular organization in Mycoplasma pneumoniae 80 Jaffe, J. D., H. C. Berg, et al. (2004). "Proteogenomic mapping as a complementary method to perform genome annotation." Proteomics 4(1): 59-77. Jensen, L. J., M. Kuhn, et al. (2009). "STRING 8--a global view on proteins and their functional interactions in 630 organisms." Nucleic Acids Res 37(Database issue): D412-6. Kammer, G. M., J. D. Pollack, et al. (1970). "Scanning-beam electron microscopy of Mycoplasma pneumoniae." J Bacteriol 104(1): 499-502. Kanehisa, M., M. Araki, et al. (2008). "KEGG for linking genomes to life and the environment." Nucleic Acids Res 36(Database issue): D480-4. Kastner, B., N. Fischer, et al. (2008). "GraFix: sample preparation for single-particle electron cryomicroscopy." Nat Methods 5(1): 53-5. Katayama, S., Y. Tomaru, et al. (2005). "Antisense transcription in the mammalian transcriptome." Science 309(5740): 1564-6. Keller, A., A. I. Nesvizhskii, et al. (2002). "Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search." Anal Chem 74(20): 5383-92. Kim, P. M., L. J. Lu, et al. (2006). "Relating three-dimensional structures to protein networks provides evolutionary insights." Science 314(5807): 1938-41. Kim, S. H., D. H. Shin, et al. (2005). "Structural genomics of minimal organisms and protein fold space." J Struct Funct Genomics 6(2-3): 63-70. Knudson, D. L. and R. MacLeod (1970). "Mycoplasma pneumoniae and Mycoplasma salivarium: electron microscopy of colony growth in agar." J Bacteriol 101(2): 609-17. Koide, T., D. J. Reiss, et al. (2009). "Prevalence of transcription promoters within archaeal operons and coding sequences." Mol Syst Biol 5: 285. Krass, C. and M. Gardner (1973). "Etimology of the term Mycoplasma." International Journal of Systematic Bacteriology 23(1): 62-64. Krause, D. C. and M. F. Balish (2001). "Structure, function, and assembly of the terminal organelle of Mycoplasma pneumoniae." FEMS Microbiol Lett 198(1): 1-7. Krause, D. C. and M. F. Balish (2004). "Cellular engineering in a minimal microbe: structure and assembly of the terminal organelle of Mycoplasma pneumoniae." Mol Microbiol 51(4): 917-24. Krogan, N. J., G. Cagney, et al. (2006). "Global landscape of protein complexes in the yeast Saccharomyces cerevisiae." Nature 440(7084): 637-43. Kuhner, S., V. van Noort, et al. (2009). "Proteome organization in a genome-reduced bacterium." Science 326(5957): 1235-40. Kyriacou, S. V. and M. P. Deutscher (2008). "An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth." Mol Cell 29(4): 419-27. Lapidot, M. and Y. Pilpel (2006). "Genome-wide natural antisense transcription: coupling its regulation to its different regulatory mechanisms." EMBO Rep 7(12): 1216-22. Lartigue, C., J. I. Glass, et al. (2007). "Genome transplantation in bacteria: changing one species to another." Science 317(5838): 632-8. Lartigue, C., S. Vashee, et al. (2009). "Creating bacterial strains from genomes that have been cloned and engineered in yeast." Science 325(5948): 1693-6. Layh-Schmitt, G. and R. Herrmann (1992). "Localization and biochemical characterization of the ORF6 gene product of the Mycoplasma pneumoniae P1 operon." Infect Immun 60(7): 2906-13. Leith, D. K., E. J. Hansen, et al. (1983). "Hemadsorption and virulence are separable properties of Mycoplasma pneumoniae." Infect Immun 39(2): 844-50. Letunic, I., T. Doerks, et al. (2009). "SMART 6: recent updates and new developments." Nucleic Acids Res 37(Database issue): D229-32. General principles of cellular organization in Mycoplasma pneumoniae 81 Levy, E. D., E. Boeri Erba, et al. (2008). "Assembly reflects evolution of protein complexes." Nature 453(7199): 1262-5. Lindahl, A. L., M. E. Olsson, et al. (2006). "Production of the artemisinin precursor amorpha4,11-diene by engineered Saccharomyces cerevisiae." Biotechnol Lett 28(8): 571-80. Lipman, R. P., W. A. Clyde, Jr., et al. (1969). "Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains." J Bacteriol 100(2): 1037-43. Loomes, L. M., K. Uemura, et al. (1984). "Erythrocyte receptors for Mycoplasma pneumoniae are sialylated oligosaccharides of Ii antigen type." Nature 307(5951): 560-3. Malmstrom, J., M. Beck, et al. (2009). "Proteome-wide cellular protein concentrations of the human pathogen Leptospira interrogans." Nature 460(7256): 762-5. Manolukas, J. T., M. F. Barile, et al. (1988). "Presence of anaplerotic reactions and transamination, and the absence of the tricarboxylic acid cycle in mollicutes." J Gen Microbiol 134(3): 791-800. Marshall, A. (2009). "The sorcerer of synthetic genomes." Nat Biotechnol 27(12): 1121-4. Martin, V. J., D. J. Pitera, et al. (2003). "Engineering a mevalonate pathway in Escherichia coli for production of terpenoids." Nat Biotechnol 21(7): 796-802. McGrath, P. T., H. Lee, et al. (2007). "High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons." Nat Biotechnol 25(5): 58492. Moran, N. A. (2002). "Microbial minimalism: genome reduction in bacterial pathogens." Cell 108(5): 583-6. Mueller, L. N., O. Rinner, et al. (2007). "SuperHirn - a novel tool for high resolution LC-MSbased peptide/protein profiling." Proteomics 7(19): 3470-80. Murakami, K. S., S. Masuda, et al. (2002). "Structural basis of transcription initiation: RNA polymerase holoenzyme at 4 A resolution." Science 296(5571): 1280-4. Nelson, C. M., M. J. Herron, et al. (2008). "Whole genome transcription profiling of Anaplasma phagocytophilum in human and tick host cells by tiling array analysis." BMC Genomics 9: 364. Nocard, E. and E. Roux (1898). "Le microbe de la peripneumonie." Ann. Inst. Pasteur (Paris) 12: 240-262. Nomura, M. (1999). "Regulation of ribosome biosynthesis in Escherichia coli and Saccharomyces cerevisiae: diversity and common principles." J Bacteriol 181(22): 6857-64. O'Donnell, W. J., R. L. Kradin, et al. (2004). "Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 39-2004. A 52-year-old woman with recurrent episodes of atypical pneumonia." N Engl J Med 351(26): 2741-9. Ochman, H. and R. Raghavan (2009). "Systems biology. Excavating the functional landscape of bacterial cells." Science 326(5957): 1200-1. Osada, Y., R. Saito, et al. (1999). "Analysis of base-pairing potentials between 16S rRNA and 5' UTR for translation initiation in various prokaryotes." Bioinformatics 15(7-8): 57881. Oxman, E., U. Alon, et al. (2008). "Defined order of evolutionary adaptations: experimental evidence." Evolution 62(7): 1547-54. Pachkov, M., T. Dandekar, et al. (2007). "Use of pathway analysis and genome context methods for functional genomics of Mycoplasma pneumoniae nucleotide metabolism." Gene 396(2): 215-25. Park, S. G., P. Schimmel, et al. (2008). "Aminoacyl tRNA synthetases and their connections to disease." Proc Natl Acad Sci U S A 105(32): 11043-9. Parrish, J. R., J. Yu, et al. (2007). "A proteome-wide protein interaction map for Campylobacter jejuni." Genome Biol 8(7): R130. General principles of cellular organization in Mycoplasma pneumoniae 82 Pascual-Montano, A., L. E. Donate, et al. (2001). "A novel neural network technique for analysis and classification of EM single-particle images." J Struct Biol 133(2-3): 23345. Perkins, D. N., D. J. Pappin, et al. (1999). "Probability-based protein identification by searching sequence databases using mass spectrometry data." Electrophoresis 20(18): 3551-67. Pipe, L. Z. and M. J. Grimson (2008). "Spatial-temporal modelling of bacterial colony growth on solid media." Mol Biosyst 4(3): 192-8. Pollack, J. D. (1997). "Mycoplasma genes: a case for reflective annotation." Trends Microbiol 5(10): 413-9. Pollack, J. D., M. A. Myers, et al. (2002). "Suspected utility of enzymes with multiple activities in the small genome Mycoplasma species: the replacement of the missing "household" nucleoside diphosphate kinase gene and activity by glycolytic kinases." Omics 6(3): 247-58. Pollack, J. D., M. V. Williams, et al. (1997). "The comparative metabolism of the mollicutes (Mycoplasmas): the utility for taxonomic classification and the relationship of putative gene annotation and phylogeny to enzymatic function in the smallest free-living cells." Crit Rev Microbiol 23(4): 269-354. Potrykus, K. and M. Cashel (2008). "(p)ppGpp: still magical?" Annu Rev Microbiol 62: 3551. Praetorius-Ibba, M., C. D. Hausmann, et al. (2007). "Functional association between three archaeal aminoacyl-tRNA synthetases." J Biol Chem 282(6): 3680-7. Prescott, B., O. Sobeslavsky, et al. (1966). "Isolation and characterization of fractions of Mycoplasma pneumoniae. I. Chemical and chromatographic separation." J Bacteriol 91(6): 2117-25. Proft, T. and R. Herrmann (1994). "Identification and characterization of hitherto unknown Mycoplasma pneumoniae proteins." Mol Microbiol 13(2): 337-48. Pruggnaller, S., M. Mayr, et al. (2008). "A visualization and segmentation toolbox for electron microscopy." J Struct Biol 164(1): 161-5. Puig, O., F. Caspary, et al. (2001). "The tandem affinity purification (TAP) method: a general procedure of protein complex purification." Methods 24(3): 218-29. Radermacher, M., T. Wagenknecht, et al. (1987). "Three-dimensional reconstruction from a single-exposure, random conical tilt series applied to the 50S ribosomal subunit of Escherichia coli." J Microsc 146(Pt 2): 113-36. Radestock, U. and W. Bredt (1977). "Motility of Mycoplasma pneumoniae." J Bacteriol 129(3): 1495-501. Rain, J. C., L. Selig, et al. (2001). "The protein-protein interaction map of Helicobacter pylori." Nature 409(6817): 211-5. Razin, S., D. Yogev, et al. (1998). "Molecular biology and pathogenicity of mycoplasmas." Microbiol Mol Biol Rev 62(4): 1094-156. Regula, J. T., B. Ueberle, et al. (2000). "Towards a two-dimensional proteome map of Mycoplasma pneumoniae." Electrophoresis 21(17): 3765-80. Renesto, P., N. Crapoulet, et al. (2003). "Genome-based design of a cell-free culture medium for Tropheryma whipplei." Lancet 362(9382): 447-9. Reppas, N. B., J. T. Wade, et al. (2006). "The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting." Mol Cell 24(5): 74757. Resing, K. A., K. Meyer-Arendt, et al. (2004). "Improving reproducibility and sensitivity in identifying human proteins by shotgun proteomics." Anal Chem 76(13): 3556-68. General principles of cellular organization in Mycoplasma pneumoniae 83 Rifkind, D., R. Chanock, et al. (1962). "Ear involvement (myringitis) and primary atypical pneumonia following inoculation of volunteers with Eaton agent." Am Rev Respir Dis 85: 479-89. Rigaut, G., A. Shevchenko, et al. (1999). "A generic protein purification method for protein complex characterization and proteome exploration." Nat Biotechnol 17(10): 1030-2. Ro, D. K., E. M. Paradise, et al. (2006). "Production of the antimalarial drug precursor artemisinic acid in engineered yeast." Nature 440(7086): 940-3. Ruland, K., R. Himmelreich, et al. (1994). "Sequence divergence in the ORF6 gene of Mycoplasma pneumonia." J Bacteriol 176(17): 5202-9. Ruland, K., R. Wenzel, et al. (1990). "Analysis of three different repeated DNA elements present in the P1 operon of Mycoplasma pneumoniae: size, number and distribution on the genome." Nucleic Acids Res 18(21): 6311-7. Sakai, H., C. Imamura, et al. (2001). "Correlation between Shine--Dalgarno sequence conservation and codon usage of bacterial genes." J Mol Evol 52(2): 164-70. Schmidt, A., N. Gehlenborg, et al. (2008). "An integrated, directed mass spectrometric approach for in-depth characterization of complex peptide mixtures." Mol Cell Proteomics 7(11): 2138-50. Selinger, D. W., K. J. Cheung, et al. (2000). "RNA expression analysis using a 30 base pair resolution Escherichia coli genome array." Nat Biotechnol 18(12): 1262-8. Serrano, L. (2007). "Synthetic biology: promises and challenges." Mol Syst Biol 3: 158. Seto, S., G. Layh-Schmitt, et al. (2001). "Visualization of the attachment organelle and cytadherence proteins of Mycoplasma pneumoniae by immunofluorescence microscopy." J Bacteriol 183(5): 1621-30. Seybert, A., R. Herrmann, et al. (2006). "Structural analysis of Mycoplasma pneumoniae by cryo-electron tomography." J Struct Biol 156(2): 342-54. Shevchenko, A., M. Wilm, et al. (1996). "Mass spectrometric sequencing of proteins silverstained polyacrylamide gels." Anal Chem 68(5): 850-8. Shin, D. H., J. S. Kim, et al. (2006). "Crystal structure of the DUF16 domain of MPN010 from Mycoplasma pneumoniae." Protein Sci 15(4): 921-8. Smart, J. B. and T. D. Thomas (1987). "Effect of Oxygen on Lactose Metabolism in Lactic Streptococci." Appl Environ Microbiol 53(3): 533-541. Smith, C. B., R. M. Chanock, et al. (1967). "Mycoplasma pneumoniae infections in volunteers." Ann N Y Acad Sci 143(1): 471-83. Sobeslavsky, O., B. Prescott, et al. (1966). "Isolation and characterization of fractions of Mycoplasma pneumoniae. II. Antigenicity and immunogenicity." J Bacteriol 91(6): 2126-38. Soding, J. (2005). "Protein homology detection by HMM-HMM comparison." Bioinformatics 21(7): 951-60. Sowa, M. E., E. J. Bennett, et al. (2009). "Defining the human deubiquitinating enzyme interaction landscape." Cell 138(2): 389-403. Sperker, B., P. Hu, et al. (1991). "Identification of gene products of the P1 operon of Mycoplasma pneumoniae." Mol Microbiol 5(2): 299-306. Su, H. C., C. A. Hutchison, 3rd, et al. (2007). "Mapping phosphoproteins in Mycoplasma genitalium and Mycoplasma pneumoniae." BMC Microbiol 7: 63. Tarassov, K., V. Messier, et al. (2008). "An in vivo map of the yeast protein interactome." Science 320(5882): 1465-70. Tatusov, R. L., E. V. Koonin, et al. (1997). "A genomic perspective on protein families." Science 278(5338): 631-7. Terradot, L., N. Durnell, et al. (2004). "Biochemical characterization of protein complexes from the Helicobacter pylori protein interaction map: strategies for complex formation General principles of cellular organization in Mycoplasma pneumoniae 84 and evidence for novel interactions within type IV secretion systems." Mol Cell Proteomics 3(8): 809-19. Teusink, B., A. Wiersma, et al. (2006). "Analysis of growth of Lactobacillus plantarum WCFS1 on a complex medium using a genome-scale metabolic model." J Biol Chem 281(52): 40041-8. Thanbichler, M. and L. Shapiro (2008). "Getting organized--how bacterial cells move proteins and DNA." Nat Rev Microbiol 6(1): 28-40. Tjaden, B., R. M. Saxena, et al. (2002). "Transcriptome analysis of Escherichia coli using high-density oligonucleotide probe arrays." Nucleic Acids Res 30(17): 3732-8. Toledo-Arana, A., O. Dussurget, et al. (2009). "The Listeria transcriptional landscape from saprophytism to virulence." Nature 459(7249): 950-6. Torres, M., C. Condon, et al. (2001). "Ribosomal protein S4 is a transcription factor with properties remarkably similar to NusA, a protein involved in both non-ribosomal and ribosomal RNA antitermination." Embo J 20(14): 3811-20. Tuloup, M., C. Hernandez, et al. (2003). Aldente and BioGraph: An improved peptide mass fingerprinting protein identification environment. Swiss Proteomics Society 2003 Congress: Understanding Biological Systems through Proteomics. Basel, Switzerland, FontisMedia. Ueberle, B., R. Frank, et al. (2002). "The proteome of the bacterium Mycoplasma pneumoniae: comparing predicted open reading frames to identified gene products." Proteomics 2(6): 754-64. van Dongen, S. (2000). Graph Clustering by Flow Simulation. Utrecht, University of Utrecht. PhD. van Nimwegen, E. (2003). "Scaling laws in the functional content of genomes." Trends Genet 19(9): 479-84. van Noort, V., B. Snel, et al. (2007). "Exploration of the omics evidence landscape: adding qualitative labels to predicted protein-protein interactions." Genome Biol 8(9): R197. Vogel, J. and E. G. Wagner (2007). "Target identification of small noncoding RNAs in bacteria." Curr Opin Microbiol 10(3): 262-70. von Mering, C., R. Krause, et al. (2002). "Comparative assessment of large-scale data sets of protein-protein interactions." Nature 417(6887): 399-403. Vrentas, C. E., T. Gaal, et al. (2005). "Response of RNA polymerase to ppGpp: requirement for the omega subunit and relief of this requirement by DksA." Genes Dev 19(19): 2378-87. Waites, K. B. and D. F. Talkington (2004). "Mycoplasma pneumoniae and its role as a human pathogen." Clin Microbiol Rev 17(4): 697-728, table of contents. Wang, X. J., T. Gaasterland, et al. (2005). "Genome-wide prediction and identification of cisnatural antisense transcripts in Arabidopsis thaliana." Genome Biol 6(4): R30. Washio, T., J. Sasayama, et al. (1998). "Analysis of complete genomes suggests that many prokaryotes do not rely on hairpin formation in transcription termination." Nucleic Acids Res 26(23): 5456-63. Weiner, J., 3rd, R. Herrmann, et al. (2000). "Transcription in Mycoplasma pneumoniae." Nucleic Acids Res 28(22): 4488-96. Weiner, J., 3rd, C. U. Zimmerman, et al. (2003). "Transcription profiles of the bacterium Mycoplasma pneumoniae grown at different temperatures." Nucleic Acids Res 31(21): 6306-20. Wenzel, R. and R. Herrmann (1988). "Physical mapping of the Mycoplasma pneumoniae genome." Nucleic Acids Res 16(17): 8323-36. Wenzel, R. and R. Herrmann (1989). "Cloning of the complete Mycoplasma pneumoniae genome." Nucleic Acids Res 17(17): 7029-43. General principles of cellular organization in Mycoplasma pneumoniae 85 Wilson, M. H. and A. M. Collier (1976). "Ultrastructural study of Mycoplasma pneumoniae in organ culture." J Bacteriol 125(1): 332-9. Wright, R., C. Stephens, et al. (1996). "Caulobacter Lon protease has a critical role in cellcycle control of DNA methylation." Genes Dev 10(12): 1532-42. Xu, Z., A. L. Horwich, et al. (1997). "The crystal structure of the asymmetric GroEL-GroES(ADP)7 chaperonin complex." Nature 388(6644): 741-50. Xu, Z., W. Wei, et al. (2009). "Bidirectional promoters generate pervasive transcription in yeast." Nature 457(7232): 1033-7. Yates, J. R., 3rd, J. K. Eng, et al. (1995). "Method to correlate tandem mass spectra of modified peptides to amino acid sequences in the protein database." Anal Chem 67(8): 1426-36. Yus, E., T. Maier, et al. (2009). "Impact of genome reduction on bacterial metabolism and its regulation." Science 326(5957): 1263-8. Zechiedrich, E. L. and N. R. Cozzarelli (1995). "Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli." Genes Dev 9(22): 2859-69. Zheng, Q. S., M. B. Braunfeld, et al. (2004). "An improved strategy for automated electron microscopic tomography." J Struct Biol 147(2): 91-101. Zientz, E., T. Dandekar, et al. (2004). "Metabolic interdependence of obligate intracellular bacteria and their insect hosts." Microbiol Mol Biol Rev 68(4): 745-70. Zimmerman, C. U. and R. Herrmann (2005). "Synthesis of a small, cysteine-rich, 29 amino acids long peptide in Mycoplasma pneumoniae." FEMS Microbiol Lett 253(2): 31521. Zuber, P. (2004). "Spx-RNA polymerase interaction and global transcriptional control during oxidative stress." J Bacteriol 186(7): 1911-8. General principles of cellular organization in Mycoplasma pneumoniae 86 8. Acknowledgements To say “Thank you” to many great individuals who helped me in one way or another over the past few years and in some cases life-long is not a compulsory task here but is something I do with the greatest pleasure and gratitude towards all my supporters, friends and family. First and most important for the successful completion of my PhD thesis I want to thank my supervisor at EMBL Heidelberg Dr. Anne-Claude Gavin, who was always extremely motivated, incredibly supportive and a great person to work with. I want to thank you AnneClaude for the great time I had in the lab, the invaluable lessons I learned form you as well as the great degrees of freedom I had during my work and the confidence you always had and have in our fascinating projects. Secondly, I would like to thank Prof. Dr. Ruedi Aebersold for giving me the opportunity and the support to perform my PhD thesis as an external PhD student in his laboratory at ETH Zürich and for the reliably great input during our meetings. I am especially thankful to you Ruedi for the very warm welcome in Zürich and all your open mindedness that I will also always forward in life. In order to perform a lot of high quality work it is of priceless to be in a lab with a great atmosphere, where interesting and very smart personalities from various countries gather to address interesting research questions. I found this working atmosphere in our lab at EMBL and enjoyed spending lots of time with all of you. I especially enjoyed speaking different languages, though mostly our beloved English, having so many diverse cultural backgrounds around and I took pleasure from the fact that all of you are great characters. Therefore I want to thank all lab members for the very good time I had. This includes: Dr. Anne-Claude Gavin, Michaela Rode, Dr. Pedro Beltran, Ines Racké, Emma Dalton, Christian Matetzki, Jamie Trott, Jelena Gvozdenovic-Jeremic, Carmen Gurrieri Aguilar, Tomás Norambuena, Samuel Bader, Dr. Sebastian Charbonnier, Dr. Stefan Bonn, Dr. Oriol Gallego, Evelyn Sawa, Tatjana Schneidt, Dr. Emmanuel Poilpré, Dr. Kenji Maeda, Dr. Arun Kumar, Dr. Emmanuel Saliba, Dr. Jan Seebacher, Lisa Lirussi and Mattia Poletto. For the scientific input to the projects mentioned within this PhD thesis I am also grateful to Dr. Matthias Wilm, Dr. Sven Fraterman, Frank Thommen, Emma Dalton, Michael Schulz, Evely Sawa, Dr. Meikel Diepholz, Elisabeth Pirkl, Dr. Anja Seybert, all the members of the Gavin group as well as the Bork group, Dr. Thomas Franz, Dr. Jeroen Krijgsveld, Stefan Leicht, Sabrina Rüggeberg, Kristina Dzeyk from the EMBL Proteomics core facility and Dr. Vladimir Benes, Sabine Schmidt, Jos de Graaf, Tomi Bähr-Ivacevic and Jens Stolte from the EMBL Genomics Core facility for expert help and discussion as well as all authors of the General principles of cellular organization in Mycoplasma pneumoniae 87 studies. Especially, I also want to thank Prof. Dr. Richard Herrmann, who is the groups external Mycoplasma pneumoniae expert and who shares all his knowledge and materials with us, for his great support with manuscripts, intensive suggestions for improvements of this thesis as well as for countless valuable discussions. For the record, the protein-protein interaction data set has been submitted to the International Molecular Exchange Consortium (http://imex.sf.net) through IntAct (pmid is 17145710; identifier is IM-11644). The electron microscopy maps have been submitted to the Electron Microscopy Data Bank (www.ebi.ac.uk/pdbe-srv/emsearch/) (identification codes EMD1637, EMD-1638, and EMD-1639). At EMBL everybody knows about the great advice you get from your TAC (Thesis advisory committee) members. Therefore I would like to thank the members of my TAC for all their efforts: So thanks to Dr. Lars Steinmetz, Dr. Toby Gibson, Dr. Anne-Claude Gavin & Prof. Dr. Ruedi Aebersold. Furthermore I would like to thank all the people at our administration at EMBL Heidelberg and ETH Zürich for their great support. This includes in particular, Tamara Kantzos-Marinkovic, Nelly van der Jagt González, Milanka Stojkovic, Dr. Helke Hillebrand and Dr. Lars Steinmetz from EMBL Heidelberg and Dominic Dähler and the entire Doktoratsadministration from ETH Zürich. To spend a lot of time at EMBL means to spend less time elsewhere. For being so patient when I came late to countless occasions e.g. Fussballmittwoch, poker nights, occasional cooking sessions etc. and for being wonderful friends I want to thank: Alexander, Sebastian, Christoph, Jochen, Jens, Thiemo, Mathias, Philipp, Jochen, Kathrin, Nina, Alicja, Katharina, Patricia, Petra, Matthias, Tim, Boris, Stefan, David, Patrick, Christian, Christoph, Felix, Niklas, Tillman, Tobias & Evelyn. I am looking forward to the next years! Finally, I want to thank my parents Ingrid and Heinz Werner, my sister Julia, my grand and great grand parents and all family members for their continuous and enduring support. General principles of cellular organization in Mycoplasma pneumoniae 88 9. Curriculum vitae Sebastian Kühner, Dipl. Biol. & cand. oec. Address: Phone: E-Mail: Date of Birth: Place of Birth: Nationality: Martial Status: Kirschenstraße 49 68519 Viernheim 0179/4767031 [email protected] July 31st 1981 Heidelberg German Single Studies & PhD thesis October 2006 February 2010 Predoctoral fellow in biochemistry at the European Molecular Biology Laboratory, Heidelberg & ETH Zürich with Dr. Anne-Claude Gavin PhD thesis title: „General principles of cellular organization in the genome-reduced bacterium Mycoplasma pneumoniae” Since October 2005 Second degree studies in economics at Ruprecht-Karls-Universität Heidelberg, prediploma exam passed in winter semester 2007, presumable end of studies summer semester 2010. October 2001 July 2006 Studies of biochemistry & molecular biology in Heidelberg & Oxford, UK as an Erasmus fellow, Diploma grade average: 1,0 Diploma thesis on: „ATP induced Myosin unbinding of Actin“ Internships & working experience Since August 2008 Project member & share holder of Klein, Kühner, Tussing & Woertge GbR a company organizing an entire intranet relaunch for a german subsidiary of an international electronic- & industrial multicorporate enterprise August 2008 November 2008 Project leader & share holder of Doganov, Ksienzyk, Kühner, Lang & Ritter GbR a company that analyzed mechanical engineering and construction markets for a medium sized investment good producer July 2006 October 2006 Bayer AG, Bayer Business Services GmbH, business consulting segment with Dr. Alexander Moscho. Analysis, calculation and successful presentation of a business case for partial restructuring of the information technology landscape at all corporations of Bayer AG November 2005 June 2006 Interdisciplinary Center of Scientific Computing Heidelberg, Computational Biochemistry Group of Dr. Stefan Fischer, Diploma thesis in computational biochemistry investigating conformational transitions between protein crystal structures using simulation techniques General principles of cellular organization in Mycoplasma pneumoniae 89 April 2005 June 2005 BASF AG Ludwigshafen, Agricultural products unit in Limburgerhof Germany, Laboratory of Dr. B. Navé, In vitro investigation of the molecular mode of action of fungicides targeting agriculturally relevant pathogenic fungi July 2004 January 2005 Biochemistry Center Heidelberg, Dr. Elisabeth Davioud Charvet. In vitro characterization of glutathione-reductase inhibitors as potent anti-malarial drugs. January 2004 July 2004 Plant Cell Biology & Microscopy Group Prof. Dr. C. Hawes Oxford Brookes University: Investigation of the dynamics of protein localization in the membrane of the endoplasmic reticulum October 2002 March 2005 Ruprecht-Karls-Universität Heidelberg, teaching tutorials in inorganic and biological chemistry for molecular cell biology students Since 1996 Working experience in parental owned business in staffing, logistics, financial and budgetary planning as well as accounting School education 1992 - 2001 Albertus-Magnus-Secondary school, Viernheim A-Levels / Majors: Maths and Chemistry, Grade: 1,4 1999 Brother Rice High School, Bloomfield Hills, Michigan U.S.A. Language skills German: English: French: Chinese: Latin: native language Fluent communication and written skills written and spoken on an advanced level basic communication skills in mandarin good translation and reading abilities (Latinum) Awards & Honours July 2009 Finn Wold Travel award from the U.S. Protein Society for the 23rd annual protein society symposium in Boston, MA, U.S.A. July 2008 Travel award from the German science foundation (DFG) for an oral presentation at the HUPO conference in Amsterdam, Netherlands 2008 July 2008 Hoffmann la Roche travel award for an oral presentation at the HUPO conference in Amsterdam, Netherlands 2008 (declined) Since 2008 E-fellows scholar October 2007 Awarded with the diploma & master prize of the German Society of biochemistry & molecular biology (GBM) for the best thesis at the Ruprecht-Karls University Heidelberg in 2006/2007 General principles of cellular organization in Mycoplasma pneumoniae Since 2007 “Capstone” Fellow in the mentoring programme of McKinsey & Company Inc. Since October 2006 Predoctoral fellow at the European molecular biology laboratory in Heidelberg, Germany enrolled as PhD student at ETH Zürich Since 2006 “Join the Best” Scholar in the fellow program of MLP AG January 2004 July 2004 Erasmus fellow for studies and research at Oxford Brookes University, Oxford UK Memberships & additional qualifications Since 2006 Member of consulting society GalileiConsult e.V. of the University of Heidelberg, department of information technology Since 2004 Member of the Oxford Union debating society, member of the German Society of Biochemistry and Molecular Biology (GBM) and their international associations FEBS and IUBMB Since 2004 Member of the Initiative Wertpapier Heidelberg (IWH), the capital market and stock exchange society of the University of Heidelberg 2002-2006 Member of the Biological Union of the Ruprecht-Karls University Heidelberg, involved in consultancy, administration and organising tutorials Since 1999 Treasurer and active organizer of summer camps and trips of the Catholic Youth Society St. Hildegard, Viernheim Since 1999 Full and clean driving licence, various motorboat and sailing licences Publications 1. Runions J., Brach T., Kühner S. & Hawes C. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane; Journal of Experimental Botany, 2006 57(1):43-50. 2. Bauer H., Fritz-Wolf K., Winzer A., Kühner S., Little S., Yardley V., Verzin H., Palfey B., Schirmer R. H. & Davioud-Charvet E. A Fluoro Analogue of the Menadione Derivative 6-[2'-(3'-Methyl)-1',4'-naphthoquinolyl]hexanoic Acid Is a Suicide Substrate of Glutathione Reductase. Crystal Structure of the Alkylated Human Enzyme; J. Am. Chem. Soc., 128 (33), 10784 -10794, 2006. 3. Kühner S. ATP induced Myosin unbinding of Actin. Diploma Thesis in Computational Biochemistry at the Ruprecht-Karls-Universität Heidelberg 2006. download available: http://www.embl-heidelberg.de/~kuehner/publications/thesis.pdf 4. Kühner S. & Gavin A.-C.. Towards quantitative analysis of proteome dynamics, Nature Biotechnology, 2007, 25, 298-300. 5. Bader S., Kühner S. & Gavin A.-C. Interaction networks for systems biology. FEBS Lett. 2008, 582(8):1220-1224. General principles of cellular organization in Mycoplasma pneumoniae 91 6. Kühner S., van Noort V., ..., Aebersold R., ..., Gavin AC. Proteome organization in a genome-reduced bacterium. Science, 2009, 326, 1235-1240. 7. Güell M., ..., Kühner S., ..., Gavin A.C., ..., Serrano L. Transcriptome complexity in a genome-reduced bacterium. Science, 2009, 326, 1268-1271. 8. Yus E., ..., Kühner S., ..., Gavin A.C., ..., Serrano L. Impact of genome reduction on bacterial metabolism and its regulation. Science, 2009, 326, 1263-1268. 9. Kühner S., & Fischer S. Structural Mechanism of ATP-triggered Myosin release from Actin in muscle contraction. “Manuscript in preparation.” Invited Talks, Poster presentations, workshops & teaching November 2009 11th EMBL International PhD symposium Heidelberg – talk November 2009 EMBL Biochemical PhD student course - teaching September 2009 EMBL PhD student retreat Marseille/France - talk July 2009 23rd Protein Society Meeting Boston/U.S. – poster presentation March 2009 MBTI Workshop “Power of Personality” Berlin November 2008 „Capstone Communication Skills Workshop“ Düsseldorf September 2008 EMBL PhD student retreat Lisbon, Portugal - talk September 2008 CSHL/WT Network Biology meeting Hinxton/Cambridge, UK poster presentation August 2008 HUPO annual meeting Amsterdam, Netherlands- talk Juli 2008 Proteomics course ETH Zürich, Schweiz March 2008 Computational proteomics workshop Schloss Dagstuhl, Saarland November 2007 Organization of the 9th EMBL International PhD symposium “Patterns in Biology” in Heidelberg November 2007 EMBL Biochemical PhD student practical - teaching October 2007 EBI Bioinformatics course in Hinxton/Cambridge, UK September 2007 EMBL PhD Student retreat Barcelona, Spain - talk September 2007 Biomolecular mass spectrometry course in Utrecht, Netherlands October 2006 December 2006 EMBL International PhD Program starting course in Heidelberg and Grenoble, France Hobbies Tennis, Soccer, Cooking & Eating, Literature, Skiing, Sailing etc. General principles of cellular organization in Mycoplasma pneumoniae 92 References Dr. Anne-Claude Gavin, Head of Gavin Group, European Molecular Biology Laboratory , 69117 Heidelberg; Phone: +49 (0)6221 387-8816 E-Mail: [email protected] Dr. Alexander Moscho, Head of Business Consulting, Bayer Business Services GmbH, 51368 Leverkusen; Phone: +49 (0)214 30 71070 E-Mail: [email protected] Dr. Elisabeth Davioud-Charvet, Antiparasitic Drug Design, Biochemie Zentrum Heidelberg, 69120 Heidelberg; Phone: +49 (0)6221 544188 E-Mail: [email protected] General principles of cellular organization in Mycoplasma pneumoniae 93 10. Supplement 10.1. Table S1: List of purifications and proteins identified. In total 259 successful purifications were performed using 212 distinct TAP-fusion proteins. i) Is the list of TAP purifications. The first column gives the purification ID. The second and third columns provide the annotated systematic and protein names of the TAP-protein used as entry point, respectively. The last column gives the list of prey proteins that co-purify with the bait. ii) Is the list of proteins identified in the PeptideAtlas by FT-MS/MS. The first column provides the annotated systematic name of each identified protein. The second column gives the protein name. i) List of purifications Bait Bait Purification (systematic (protein ID name) name) EMBL001 Mpn025 Preys Fba Mpn025 Mpn434 Mpn067 Mpn573 Mpn118 Mpn034 Mpn003 EMBL002 Mpn025 Fba Mpn025 Mpn390 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn365 Mpn034 Mpn141 Mpn669 Mpn210 Mpn434 Mpn573 Mpn207 Mpn515 Mpn426 Mpn546 Mpn608 Mpn191 Mpn020 Mpn252 Mpn452 Mpn621 Mpn004 EMBL003 Mpn567 P200 Mpn210 Mpn434 Mpn095 Mpn567 Mpn390 Mpn295 Mpn003 Mpn393 Mpn004 Mpn390 Mpn230 Mpn516 Mpn394 Mpn440 Mpn354 Mpn258 Mpn658 Mpn228 Mpn556 Mpn166 Mpn573 Mpn515 Mpn686 Mpn191 Mpn020 Mpn452 Mpn621 Mpn004 Mpn619 Mpn303 Mpn179 Mpn430 Mpn665 Mpn261 Mpn685 Mpn044 Mpn574 Mpn019 Mpn660 Mpn210 Mpn434 Mpn226 Mpn280 Mpn426 Mpn050 Mpn189 EMBL004 Mpn230 RpsR EMBL005 Mpn051 Mpn051 Mpn210 Mpn627 Mpn361 Mpn573 Mpn051 Mpn042 Mpn034 EMBL006 Mpn516 RpoB Mpn434 Mpn352 Mpn191 Mpn020 Mpn516 Mpn228 Mpn515 General principles of cellular organization in Mycoplasma pneumoniae 94 EMBL007 Mpn516 RpoB Mpn328 Mpn352 Mpn516 Mpn665 Mpn171 Mpn330 Mpn571 Mpn228 Mpn210 Mpn226 Mpn024 Mpn515 Mpn673 Mpn239 Mpn191 Mpn020 Mpn004 EMBL008 Mpn379 PolA Mpn210 Mpn621 EMBL009 Mpn379 PolA Mpn684 Mpn390 Mpn665 Mpn261 Mpn379 Mpn258 Mpn141 Mpn210 Mpn434 Mpn573 Mpn310 Mpn207 Mpn032 Mpn515 Mpn426 Mpn050 Mpn020 Mpn621 Mpn004 EMBL010 Mpn006 Tmk Mpn372 Mpn210 Mpn134 Mpn434 Mpn390 Mpn280 Mpn516 Mpn665 Mpn515 Mpn006 Mpn191 Mpn258 Mpn452 Mpn621 EMBL011 Mpn006 Tmk Mpn210 Mpn434 Mpn204 Mpn390 Mpn118 Mpn665 Mpn426 Mpn006 Mpn034 Mpn040 Mpn452 Mpn213 EMBL012 Mpn006 Tmk Mpn134 Mpn025 Mpn619 Mpn390 Mpn303 Mpn118 Mpn516 Mpn430 Mpn197 Mpn665 Mpn394 Mpn261 Mpn378 Mpn006 Mpn393 Mpn258 Mpn141 Mpn210 Mpn553 Mpn434 Mpn322 Mpn573 Mpn280 Mpn515 Mpn191 Mpn452 Mpn106 Mpn621 Mpn004 EMBL013 Mpn416 P29 Mpn623 Mpn434 Mpn656 Mpn674 Mpn665 Mpn108 Mpn314 Mpn392 Mpn416 Mpn393 Mpn034 Mpn337 EMBL014 Mpn320 ThyA Mpn563 Mpn210 Mpn434 Mpn573 Mpn324 Mpn516 Mpn515 Mpn426 Mpn320 EMBL015 Mpn320 ThyA Mpn284 Mpn434 Mpn320 Mpn573 Mpn555 Mpn076 Mpn032 EMBL016 Mpn493 UlaD Mpn596 Mpn434 Mpn555 Mpn674 Mpn665 Mpn314 Mpn392 Mpn034 General principles of cellular organization in Mycoplasma pneumoniae 95 EMBL017 Mpn327 RpmA Mpn390 Mpn516 Mpn430 Mpn261 Mpn615 Mpn658 Mpn574 Mpn228 Mpn165 Mpn322 Mpn434 Mpn166 Mpn226 Mpn676 Mpn032 Mpn314 Mpn239 Mpn549 Mpn081 Mpn621 EMBL018 Mpn228 RpsF Mpn352 Mpn230 Mpn516 Mpn103 Mpn518 Mpn169 Mpn115 Mpn228 Mpn553 Mpn616 Mpn515 Mpn178 Mpn020 Mpn191 Mpn621 Mpn004 Mpn446 Mpn480 Mpn179 Mpn295 Mpn520 Mpn665 Mpn171 Mpn182 Mpn465 Mpn685 Mpn660 Mpn035 Mpn210 Mpn434 Mpn226 Mpn622 Mpn280 Mpn239 Mpn189 Mpn252 Mpn561 EMBL019 Mpn389 LplA Mpn389 Mpn434 Mpn295 EMBL020 Mpn553 ThrS Mpn210 Mpn553 Mpn204 Mpn573 Mpn595 Mpn034 EMBL021 Mpn322 NrdF Mpn210 Mpn434 Mpn322 Mpn573 Mpn390 Mpn419 Mpn261 Mpn034 Mpn365 Mpn004 EMBL022 Mpn322 NrdF Mpn454 Mpn210 Mpn434 Mpn322 Mpn059 Mpn324 EMBL023 Mpn550 ThiI Mpn210 Mpn434 Mpn550 Mpn573 Mpn295 Mpn003 Mpn515 EMBL024 Mpn573 GroL Mpn062 Mpn392 Mpn573 Mpn264 Mpn194 Mpn430 Mpn008 Mpn269 Mpn430 Mpn315 Mpn665 Mpn309 Mpn365 Mpn019 Mpn228 Mpn210 Mpn434 Mpn324 Mpn495 Mpn515 Mpn426 Mpn050 Mpn345 Mpn004 Mpn621 EMBL025 Mpn495 UlaB EMBL026 Mpn668 Mpn668 Mpn563 Mpn391 Mpn203 Mpn514 Mpn118 Mpn665 Mpn668 General principles of cellular organization in Mycoplasma pneumoniae 96 Mpn111 Mpn390 Mpn340 Mpn303 Mpn555 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn658 Mpn574 Mpn669 Mpn210 Mpn434 Mpn573 Mpn032 Mpn111 Mpn314 Mpn515 Mpn111 Mpn050 Mpn547 Mpn621 Mpn004 EMBL028 Mpn663 Mpn671 Mpn390 Mpn342 Mpn516 Mpn430 Mpn003 Mpn536 Mpn438 Mpn246 Mpn034 Mpn210 Mpn185 Mpn434 Mpn573 Mpn515 Mpn452 Mpn663 Mpn621 Mpn004 EMBL029 Mpn686 DnaA EMBL030 Mpn509 Mpn210 Mpn434 Mpn390 Mpn516 Mpn430 Mpn295 Mpn003 Mpn665 Mpn509 Mpn281 Mpn034 Mpn599 Mpn509 Mpn452 EMBL031 Mpn079 FruK EMBL032 Mpn013 Mpn390 Mpn665 Mpn258 Mpn210 Mpn556 Mpn434 Mpn553 Mpn280 Mpn013 Mpn207 Mpn346 Mpn515 Mpn426 Mpn050 Mpn452 Mpn004 Mpn621 EMBL033 Mpn075 Mpn210 Mpn075 Mpn434 Mpn322 Mpn573 Mpn324 Mpn665 Mpn261 Mpn075 Mpn426 Mpn020 Mpn258 Mpn228 Mpn399 EMBL034 Mpn328 Nfo Mpn525 Mpn300 Mpn328 Mpn567 Mpn390 Mpn042 Mpn516 Mpn665 Mpn261 Mpn258 Mpn574 Mpn141 Mpn210 Mpn434 Mpn226 Mpn573 Mpn192 Mpn388 Mpn110 Mpn515 Mpn314 Mpn426 Mpn050 Mpn621 Mpn004 EMBL035 Mpn328 Nfo Mpn210 Mpn434 Mpn328 Mpn573 Mpn118 Mpn516 Mpn515 Mpn034 Mpn072 RbfA Mpn390 Mpn555 Mpn156 Mpn516 Mpn430 Mpn665 Mpn261 Mpn033 Mpn034 Mpn228 Mpn210 Mpn434 Mpn226 Mpn063 Mpn515 Mpn593 Mpn191 Mpn621 Mpn004 EMBL027 EMBL036 Mpn156 Mpn210 Mpn434 Mpn686 Mpn516 Mpn452 Mpn376 Mpn141 Mpn434 Mpn062 Mpn573 Mpn390 Mpn295 Mpn665 Mpn079 General principles of cellular organization in Mycoplasma pneumoniae 97 EMBL037 Mpn269 Mpn390 Mpn555 Mpn516 Mpn430 Mpn315 Mpn665 Mpn536 Mpn436 Mpn261 Mpn115 Mpn574 Mpn072 Mpn210 Mpn434 Mpn550 Mpn573 Mpn524 Mpn515 Mpn050 Mpn020 Mpn345 Mpn452 Mpn621 Mpn536 RuvB EMBL038 Mpn002 Mpn397 Mpn134 Mpn303 Mpn352 Mpn516 Mpn430 Mpn295 Mpn665 Mpn261 Mpn002 Mpn034 Mpn165 Mpn210 Mpn553 Mpn434 Mpn573 Mpn002 Mpn280 Mpn310 Mpn674 Mpn515 Mpn050 Mpn191 Mpn621 Mpn004 EMBL039 Mpn638 Mpn638 Mpn434 EMBL040 Mpn638 Mpn210 Mpn434 Mpn366 Mpn295 Mpn474 Mpn518 Mpn273 Mpn638 Mpn638 Mpn004 Mpn072 EMBL041 Mpn393 PdhA Mpn210 Mpn434 Mpn280 Mpn516 Mpn665 Mpn662 Mpn515 Mpn426 Mpn392 Mpn050 Mpn393 Mpn020 Mpn258 Mpn621 Mpn004 EMBL042 Mpn246 Def Mpn434 Mpn573 Mpn390 Mpn246 Mpn155 Mpn245 EMBL043 Mpn168 RplB Mpn210 Mpn434 Mpn390 Mpn315 Mpn295 Mpn665 Mpn261 Mpn207 Mpn060 Mpn252 Mpn621 Mpn004 Mpn275 Mpn596 Mpn568 Mpn390 Mpn118 Mpn520 Mpn516 Mpn430 Mpn315 Mpn665 Mpn394 Mpn261 Mpn275 Mpn556 Mpn210 Mpn434 Mpn676 Mpn275 Mpn036 Mpn515 Mpn392 Mpn050 Mpn621 Mpn004 EMBL045 Mpn172 RplP Mpn642 Mpn390 Mpn430 Mpn211 Mpn103 Mpn665 Mpn261 Mpn658 Mpn228 Mpn210 Mpn418 Mpn434 Mpn166 Mpn063 Mpn032 Mpn426 Mpn050 Mpn020 Mpn252 Mpn452 Mpn621 EMBL046 Mpn268 Mpn062 Mpn671 Mpn390 Mpn516 Mpn665 Mpn261 Mpn379 Mpn259 Mpn268 Mpn258 Mpn210 Mpn322 Mpn434 Mpn268 Mpn207 Mpn252 Mpn452 EMBL044 General principles of cellular organization in Mycoplasma pneumoniae 98 EMBL047 Mpn268 Mpn210 Mpn434 Mpn268 Mpn390 Mpn555 Mpn295 Mpn003 Mpn665 Mpn268 Mpn261 Mpn314 Mpn426 Mpn489 Mpn034 Mpn040 Mpn452 EMBL048 Mpn192 RplQ EMBL049 Mpn559 Mpn210 Mpn434 Mpn516 Mpn430 Mpn665 Mpn436 Mpn640 Mpn559 Mpn559 Mpn426 Mpn514 Mpn452 Mpn072 EMBL050 Mpn472 Mpn210 Mpn434 Mpn429 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn472 Mpn472 Mpn317 Mpn426 Mpn034 Mpn452 EMBL051 Mpn479 AzoR EMBL052 Mpn461 Mpn461 Mpn434 Mpn390 Mpn034 Mpn295 Mpn476 Mpn253 Mpn434 Mpn034 Mpn665 Mpn108 Mpn390 Mpn479 Mpn040 EMBL053 Mpn208 RpsB Mpn390 Mpn555 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn169 Mpn115 Mpn034 Mpn658 Mpn228 Mpn210 Mpn434 Mpn226 Mpn622 Mpn674 Mpn515 Mpn314 Mpn426 Mpn239 Mpn050 Mpn191 Mpn208 Mpn621 Mpn004 EMBL054 Mpn558 GidB Mpn558 Mpn434 Mpn573 Mpn390 Mpn280 Mpn562 Mpn059 Mpn452 Mpn621 EMBL055 Mpn117 RplT Mpn210 Mpn434 Mpn269 Mpn303 Mpn180 Mpn430 Mpn665 Mpn050 Mpn222 Mpn034 Mpn658 Mpn574 Mpn452 Mpn650 Mpn621 Mpn004 EMBL056 Mpn117 RplT Mpn165 Mpn423 Mpn390 Mpn436 Mpn515 Mpn034 Mpn191 Mpn658 Mpn189 Mpn228 Mpn332 Mpn167 General principles of cellular organization in Mycoplasma pneumoniae 99 EMBL057 Mpn390 PdhD Mpn210 Mpn390 EMBL058 Mpn245 Gmk Mpn434 EMBL059 Mpn419 AlaS Mpn434 Mpn011 Mpn573 Mpn118 Mpn555 Mpn516 Mpn419 Mpn003 Mpn582 EMBL060 Mpn100 Mpn210 Mpn434 Mpn390 Mpn118 Mpn295 Mpn100 Mpn547 Mpn034 Mpn100 Mpn509 Mpn650 Mpn004 EMBL061 Mpn108 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn108 Mpn529 Mpn210 Mpn434 Mpn124 Mpn524 Mpn346 Mpn515 Mpn426 Mpn452 Mpn646 Mpn108 Mpn004 Mpn621 EMBL062 Mpn108 Mpn210 Mpn434 Mpn619 Mpn155 Mpn430 Mpn665 Mpn640 Mpn032 Mpn108 Mpn034 Mpn004 Mpn072 EMBL063 Mpn471 Mpn390 Mpn555 Mpn516 Mpn430 Mpn261 Mpn115 Mpn228 Mpn210 Mpn462 Mpn434 Mpn207 Mpn294 Mpn515 Mpn020 Mpn252 Mpn004 RpmG1 Mpn621 EMBL064 Mpn631 Tsf EMBL065 Mpn521 Mpn521 Mpn573 EMBL066 Mpn023 MetG Mpn631 Mpn434 Mpn295 Mpn665 Mpn025 Mpn619 Mpn390 Mpn303 Mpn118 Mpn516 Mpn430 Mpn401 Mpn315 Mpn665 Mpn261 Mpn354 Mpn638 Mpn393 Mpn023 Mpn141 Mpn061 Mpn210 Mpn434 Mpn322 Mpn007 Mpn280 Mpn324 Mpn515 Mpn032 Mpn426 Mpn347 Mpn191 Mpn106 Mpn621 Mpn004 General principles of cellular organization in Mycoplasma pneumoniae 100 EMBL067 Mpn001 DnaN Mpn434 Mpn001 EMBL068 Mpn001 DnaN Mpn434 Mpn001 Mpn516 Mpn621 EMBL069 Mpn064 DeoA Mpn210 Mpn033 Mpn434 Mpn064 Efp Mpn555 Mpn516 Mpn430 Mpn295 Mpn665 Mpn261 Mpn321 Mpn393 Mpn258 Mpn029 Mpn586 Mpn210 Mpn322 Mpn434 Mpn573 Mpn280 Mpn207 Mpn314 Mpn515 Mpn621 Mpn004 ParE Mpn402 Mpn390 Mpn516 Mpn665 Mpn436 Mpn006 Mpn060 Mpn351 Mpn122 Mpn141 Mpn210 Mpn434 Mpn424 Mpn674 Mpn426 Mpn123 Mpn392 Mpn050 Mpn266 Mpn191 Mpn452 Mpn252 Mpn387 Mpn621 Mpn282 Mpn062 Mpn516 Mpn430 Mpn665 Mpn262 Mpn044 Mpn034 Mpn658 Mpn122 Mpn210 Mpn434 Mpn573 Mpn515 Mpn050 Mpn549 Mpn191 Mpn020 Mpn345 Mpn621 Mpn004 EMBL070 EMBL071 Mpn029 Mpn122 EMBL072 Mpn122 ParE EMBL073 Mpn563 Mpn563 Mpn210 Mpn434 Mpn486 Mpn573 Mpn390 Mpn251 Mpn665 Mpn563 Mpn006 Mpn125 Mpn034 Mpn658 Mpn650 Mpn399 EMBL074 Mpn165 RplC Mpn390 Mpn430 Mpn261 Mpn228 Mpn141 Mpn165 Mpn210 Mpn475 Mpn434 Mpn166 Mpn226 Mpn487 Mpn207 Mpn515 Mpn426 Mpn050 Mpn191 Mpn020 Mpn252 Mpn413 Mpn452 Mpn621 Mpn004 EMBL075 Mpn251 Rpe Mpn434 Mpn390 Mpn555 Mpn315 Mpn251 Mpn674 Mpn665 Mpn261 Mpn640 Mpn034 Mpn040 Mpn660 EMBL076 Mpn223 HprK Mpn341 Mpn434 Mpn573 Mpn044 Mpn555 Mpn658 Mpn223 General principles of cellular organization in Mycoplasma pneumoniae 101 EMBL077 Mpn067 Mpn067 Mpn210 Mpn434 Mpn067 Mpn550 Mpn504 Mpn516 Mpn248 Mpn261 EMBL078 Mpn067 Mpn210 Mpn434 Mpn595 Mpn545 Mpn516 Mpn003 Mpn261 Mpn008 Mpn067 Mpn515 Mpn426 Mpn067 Mpn452 Mpn678 EMBL079 Mpn067 Mpn430 Mpn315 Mpn665 Mpn261 Mpn258 Mpn210 Mpn322 Mpn434 Mpn067 Mpn573 Mpn324 Mpn426 Mpn472 Mpn067 Mpn050 Mpn191 Mpn020 EMBL080 Mpn392 PdhB EMBL081 Mpn266 Mpn352 Mpn516 Mpn665 Mpn261 Mpn247 Mpn228 Mpn210 Mpn669 Mpn434 Mpn280 Mpn515 Mpn673 Mpn266 Mpn191 Mpn020 Mpn004 Mpn266 Mpn621 EMBL082 Mpn266 Mpn434 Mpn226 Mpn352 Mpn516 Mpn665 Mpn515 Mpn266 Mpn020 Mpn266 Mpn191 Mpn658 Mpn509 Mpn621 Mpn004 EMBL083 Mpn427 Mpn210 Mpn434 Mpn573 Mpn516 Mpn419 Mpn003 Mpn034 Mpn427 Mpn427 Mpn004 Mpn020 Mpn025 Mpn062 Mpn352 Mpn516 Mpn394 Mpn354 Mpn386 Mpn258 Mpn141 Mpn669 Mpn553 Mpn322 Mpn573 Mpn598 Mpn515 Mpn392 Mpn020 Mpn191 Mpn106 Mpn153 Mpn621 Mpn004 Mpn014 Mpn303 Mpn315 Mpn430 Mpn520 Mpn665 Mpn261 Mpn393 Mpn034 Mpn061 Mpn020 Mpn210 Mpn434 Mpn280 Mpn324 Mpn688 EMBL085 Mpn020 Mpn390 Mpn555 Mpn352 Mpn264 Mpn516 Mpn003 Mpn665 Mpn394 Mpn261 Mpn571 Mpn228 Mpn476 Mpn072 Mpn210 Mpn429 Mpn434 Mpn226 Mpn592 Mpn674 Mpn515 Mpn032 Mpn314 Mpn673 Mpn191 Mpn020 Mpn020 Mpn509 Mpn004 EMBL086 Mpn191 RpoA EMBL084 Mpn434 Mpn392 Mpn306 Mpn393 Mpn437 Mpn352 Mpn191 Mpn516 Mpn515 General principles of cellular organization in Mycoplasma pneumoniae 102 EMBL087 Mpn191 RpoA Mpn118 Mpn352 Mpn516 Mpn443 Mpn171 Mpn228 Mpn210 Mpn265 Mpn434 Mpn226 Mpn640 Mpn362 Mpn515 Mpn673 Mpn239 Mpn191 Mpn020 Mpn621 Mpn004 EMBL088 Mpn106 PheT Mpn210 Mpn434 Mpn322 Mpn516 Mpn295 Mpn665 Mpn261 Mpn034 Mpn106 Mpn141 PheT Mpn269 Mpn390 Mpn470 Mpn516 Mpn430 Mpn295 Mpn665 Mpn394 Mpn261 Mpn141 Mpn165 Mpn434 Mpn573 Mpn324 Mpn515 Mpn608 Mpn191 Mpn466 Mpn106 Mpn621 RpsL Mpn390 Mpn516 Mpn430 Mpn100 Mpn665 Mpn034 Mpn228 Mpn277 Mpn210 Mpn434 Mpn280 Mpn515 Mpn173 Mpn050 Mpn191 Mpn040 Mpn345 Mpn452 Mpn621 Mpn004 EMBL089 EMBL090 Mpn106 Mpn225 EMBL091 Mpn397 SpoT Mpn397 Mpn062 Mpn390 Mpn142 Mpn555 Mpn430 Mpn665 Mpn171 Mpn297 Mpn354 Mpn441 Mpn021 Mpn434 Mpn573 Mpn559 Mpn538 Mpn546 Mpn547 Mpn079 EMBL092 Mpn300 ScpA Mpn300 Mpn210 Mpn434 Mpn423 Mpn573 Mpn390 Mpn211 Mpn665 Mpn551 Mpn032 Mpn301 Mpn426 Mpn508 Mpn040 RplI Mpn231 Mpn390 Mpn303 Mpn118 Mpn516 Mpn430 Mpn154 Mpn665 Mpn261 Mpn365 Mpn658 Mpn574 Mpn228 Mpn210 Mpn434 Mpn063 Mpn515 Mpn426 Mpn173 Mpn050 Mpn020 Mpn452 Mpn621 EMBL093 Mpn231 EMBL094 Mpn231 RplI Mpn231 Mpn352 Mpn516 Mpn665 Mpn365 Mpn034 Mpn658 Mpn574 Mpn210 Mpn434 Mpn166 Mpn515 Mpn640 Mpn020 Mpn252 Mpn452 Mpn004 EMBL095 Mpn303 Pyk Mpn303 Mpn191 Mpn430 EMBL096 Mpn118 Mpn563 Mpn298 Mpn269 Mpn390 Mpn155 Mpn124 Mpn430 Mpn346 Mpn118 Mpn041 Mpn050 Mpn331 Mpn400 General principles of cellular organization in Mycoplasma pneumoniae 103 EMBL097 Mpn470 PepP EMBL098 Mpn381 Mpn381 Mpn434 Mpn573 Mpn656 Mpn324 Mpn034 Mpn381 Mpn665 EMBL099 Mpn295 Mpn295 Mpn434 EMBL100 Mpn120 GrpE Mpn210 Mpn434 Mpn390 Mpn430 Mpn295 Mpn120 Mpn032 Mpn452 Mpn621 Mpn004 Mpn171 RpsC Mpn558 Mpn390 Mpn555 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn309 Mpn297 Mpn169 Mpn034 Mpn574 Mpn228 Mpn165 Mpn210 Mpn265 Mpn434 Mpn166 Mpn226 Mpn573 Mpn280 Mpn380 Mpn008 Mpn515 Mpn426 Mpn191 Mpn020 Mpn621 Mpn004 EMBL102 Mpn330 Mpn303 Mpn352 Mpn516 Mpn315 Mpn665 Mpn330 Mpn115 Mpn258 Mpn658 Mpn574 Mpn228 Mpn141 Mpn660 Mpn210 Mpn434 Mpn226 Mpn063 Mpn622 Mpn280 Mpn380 Mpn515 Mpn032 Mpn294 Mpn426 Mpn330 Mpn445 Mpn050 Mpn191 Mpn020 Mpn252 Mpn621 Mpn004 EMBL103 Mpn677 Mpn677 Mpn434 Mpn677 Mpn573 Mpn034 Mpn658 Mpn223 Mpn665 EMBL104 Mpn351 Mpn351 Mpn351 Mpn034 Mpn009 Mpn619 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn141 Mpn210 Mpn434 Mpn573 Mpn310 Mpn380 Mpn515 Mpn426 Mpn191 Mpn452 Mpn009 Mpn004 Mpn009 Mpn619 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn009 Mpn141 Mpn210 Mpn434 Mpn573 Mpn515 Mpn426 Mpn050 Mpn191 Mpn452 Mpn009 Mpn004 EMBL101 EMBL105 EMBL106 Mpn210 Mpn434 Mpn470 Mpn004 General principles of cellular organization in Mycoplasma pneumoniae 104 EMBL107 Mpn365 Mpn365 Mpn210 Mpn434 Mpn615 Mpn343 Mpn365 Mpn040 Mpn034 Mpn665 EMBL108 Mpn278 Glf Mpn210 Mpn434 Mpn573 Mpn555 Mpn324 Mpn430 Mpn665 Mpn314 Mpn050 Mpn278 Mpn542 Mpn228 Mpn621 Mpn004 EMBL109 Mpn061 Ffh Mpn390 Mpn516 Mpn665 Mpn261 Mpn600 Mpn258 Mpn228 Mpn210 Mpn434 Mpn226 Mpn280 Mpn032 Mpn515 Mpn191 Mpn004 Mpn621 EMBL110 Mpn618 DnaX Mpn684 Mpn516 Mpn430 Mpn665 Mpn465 Mpn169 Mpn685 Mpn258 Mpn658 Mpn141 Mpn618 Mpn322 Mpn434 Mpn573 Mpn280 Mpn515 Mpn032 Mpn317 Mpn426 Mpn514 Mpn191 Mpn020 Mpn252 Mpn452 Mpn153 EMBL111 Mpn429 Pgk Mpn210 Mpn434 Mpn429 EMBL112 Mpn185 Adk Mpn210 Mpn434 Mpn185 Mpn034 EMBL113 Mpn595 RpiB Mpn269 Mpn595 Mpn295 EMBL114 Mpn324 NrdE Mpn434 Mpn322 Mpn573 Mpn390 Mpn555 Mpn324 Mpn516 Mpn430 Mpn003 Mpn665 Mpn515 Mpn050 Mpn222 Mpn621 EMBL115 Mpn007 Mpn618 Mpn434 Mpn573 Mpn007 Mpn665 Mpn032 Mpn351 Mpn034 Mpn007 Mpn040 Mpn629 EMBL116 Mpn007 Mpn618 Mpn210 Mpn434 Mpn280 Mpn007 Mpn516 Mpn430 Mpn665 Mpn007 Mpn515 Mpn258 Mpn621 General principles of cellular organization in Mycoplasma pneumoniae 105 EMBL117 Mpn007 Mpn303 Mpn516 Mpn430 Mpn665 Mpn171 Mpn440 Mpn246 Mpn258 Mpn034 Mpn148 Mpn618 Mpn556 Mpn210 Mpn434 Mpn011 Mpn573 Mpn007 Mpn140 Mpn207 Mpn598 Mpn515 Mpn392 Mpn311 Mpn466 Mpn007 Mpn020 Mpn450 Mpn452 Mpn153 Mpn621 Mpn004 EMBL118 Mpn545 Rnc Mpn434 EMBL119 Mpn257 GalE Mpn210 Mpn497 Mpn434 Mpn089 Mpn269 Mpn390 Mpn118 Mpn656 Mpn516 Mpn257 Mpn207 Mpn034 Mpn040 Mpn621 Mpn004 Mpn329 Mpn099 Mpn390 Mpn303 Mpn516 Mpn430 Mpn665 Mpn261 Mpn440 Mpn609 Mpn258 Mpn658 Mpn228 Mpn210 Mpn434 Mpn573 Mpn324 Mpn207 Mpn329 Mpn515 Mpn314 Mpn050 Mpn191 Mpn020 Mpn452 Mpn329 Mpn252 Mpn621 Mpn004 Mpn317 FtsZ Mpn062 Mpn390 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn258 Mpn141 Mpn228 Mpn210 Mpn434 Mpn322 Mpn573 Mpn066 Mpn515 Mpn032 Mpn317 Mpn426 Mpn546 Mpn191 Mpn020 Mpn621 Mpn004 Mpn062 Mpn118 Mpn555 Mpn516 Mpn430 Mpn003 Mpn665 Mpn361 Mpn685 Mpn258 Mpn210 Mpn434 Mpn071 Mpn573 Mpn008 Mpn032 Mpn515 Mpn317 Mpn426 Mpn273 Mpn020 EMBL120 EMBL121 EMBL122 Mpn317 FtsZ EMBL123 Mpn483 Mpn210 Mpn434 Mpn573 Mpn430 Mpn665 Mpn515 Mpn483 Mpn609 Mpn483 Mpn050 Mpn258 Mpn141 Mpn004 EMBL124 Mpn483 Mpn210 Mpn497 Mpn434 Mpn306 Mpn390 Mpn430 Mpn665 Mpn515 Mpn483 Mpn032 Mpn027 Mpn483 Mpn452 EMBL125 Mpn158 RibF Mpn292 Mpn210 Mpn434 Mpn573 Mpn390 Mpn516 Mpn003 Mpn665 Mpn515 Mpn426 Mpn292 Mpn094 Mpn547 Mpn034 Mpn106 Mpn221 Mpn305 Mpn292 Mpn678 EMBL126 Mpn158 Mpn390 Mpn295 Mpn665 General principles of cellular organization in Mycoplasma pneumoniae 106 Mpn273 Mpn210 Mpn434 Mpn390 Mpn261 Mpn207 Mpn515 Mpn273 Mpn050 Mpn273 Mpn040 Mpn658 Mpn452 Mpn153 Mpn621 Mpn004 EMBL128 Mpn526 Mpn340 Mpn516 Mpn003 Mpn141 Mpn228 Mpn210 Mpn434 Mpn592 Mpn573 Mpn116 Mpn324 Mpn257 Mpn674 Mpn346 Mpn515 Mpn347 Mpn526 Mpn254 Mpn526 Mpn232 Mpn004 EMBL129 Mpn387 Mpn387 Mpn434 Mpn390 Mpn387 Mpn293 EMBL130 Mpn221 Pth Mpn434 Mpn226 Mpn340 Mpn430 Mpn665 Mpn649 Mpn034 Mpn658 Mpn221 Mpn228 Mpn004 Mpn621 Mpn390 Mpn516 Mpn430 Mpn574 Mpn228 Mpn434 Mpn226 Mpn063 Mpn280 Mpn207 Mpn032 Mpn515 Mpn239 Mpn191 Mpn020 Mpn452 Mpn621 Mpn004 EMBL127 EMBL131 Mpn541 RpsT EMBL132 Mpn089 Mpn089 Mpn210 Mpn497 Mpn434 Mpn089 Mpn573 Mpn003 Mpn674 Mpn665 EMBL133 Mpn269 Mpn210 Mpn434 Mpn684 Mpn269 Mpn573 Mpn207 Mpn114 Mpn341 Mpn269 Mpn262 Mpn050 Mpn547 Mpn258 Mpn241 Mpn004 Mpn277 EMBL134 Mpn568 Era EMBL135 Mpn307 Mpn210 Mpn434 Mpn063 Mpn516 Mpn430 Mpn207 Mpn515 Mpn346 Mpn307 Mpn472 Mpn040 Mpn621 Mpn004 EMBL136 Mpn307 Mpn307 Mpn027 Mpn434 Mpn034 Mpn665 Mpn210 Mpn434 Mpn573 Mpn568 Mpn390 Mpn665 Mpn640 Mpn663 Mpn034 General principles of cellular organization in Mycoplasma pneumoniae 107 EMBL137 Mpn628 GpmI Mpn218 Mpn210 Mpn390 Mpn516 Mpn430 Mpn628 Mpn027 Mpn350 Mpn191 Mpn599 Mpn252 EMBL138 Mpn378 DnaE Mpn434 Mpn353 Mpn573 Mpn118 Mpn003 Mpn665 Mpn551 Mpn108 Mpn378 Mpn034 Mpn141 EMBL139 Mpn533 AckA Mpn210 Mpn533 Mpn434 Mpn516 EMBL140 Mpn321 FolA Mpn210 Mpn434 Mpn573 Mpn390 Mpn555 Mpn516 Mpn430 Mpn457 Mpn207 Mpn321 Mpn050 Mpn034 Mpn252 Mpn621 Mpn004 EMBL141 Mpn361 PrfA Mpn210 Mpn434 Mpn361 Mpn034 Mpn419 Mpn003 EMBL142 Mpn499 Mpn210 Mpn645 Mpn434 Mpn674 Mpn665 Mpn314 Mpn200 Mpn392 Mpn499 Mpn514 Mpn499 Mpn034 Mpn367 Mpn187 InfA Mpn099 Mpn352 Mpn516 Mpn665 Mpn536 Mpn171 Mpn416 Mpn685 Mpn034 Mpn187 Mpn660 Mpn078 Mpn210 Mpn429 Mpn434 Mpn226 Mpn515 Mpn559 Mpn191 Mpn189 Mpn252 Mpn621 Mpn004 EMBL143 EMBL144 Mpn155 InfB Mpn179 Mpn042 Mpn516 Mpn171 Mpn685 Mpn571 Mpn115 Mpn034 Mpn658 Mpn228 Mpn226 Mpn155 Mpn515 Mpn616 Mpn191 Mpn189 Mpn621 Mpn167 Mpn004 EMBL145 Mpn124 HrcA Mpn210 Mpn434 Mpn573 Mpn390 Mpn124 Mpn516 Mpn515 Mpn452 Mpn621 Mpn004 EMBL146 Mpn229 Ssb Mpn034 Mpn229 Mpn665 General principles of cellular organization in Mycoplasma pneumoniae 108 EMBL147 Mpn487 Csd EMBL148 Mpn349 Mpn210 Mpn434 Mpn573 Mpn063 Mpn656 Mpn555 Mpn665 Mpn349 Mpn349 Mpn044 Mpn034 EMBL149 Mpn220 RplA Mpn390 Mpn516 Mpn430 Mpn315 Mpn103 Mpn665 Mpn261 Mpn115 Mpn258 Mpn034 Mpn658 Mpn574 Mpn228 Mpn210 Mpn434 Mpn226 Mpn063 Mpn357 Mpn280 Mpn310 Mpn515 Mpn294 Mpn426 Mpn050 Mpn191 Mpn020 Mpn452 Mpn621 Mpn004 EMBL150 Mpn081 Mpn081 Mpn434 Mpn573 Mpn295 Mpn081 Mpn665 Mpn515 Mpn505 EMBL151 Mpn446 Mpn210 Mpn434 Mpn390 Mpn034 Mpn436 Mpn399 RpsD Mpn390 Mpn118 Mpn295 Mpn261 Mpn456 Mpn258 Mpn228 Mpn210 Mpn434 Mpn524 Mpn487 Mpn294 Mpn426 Mpn222 Mpn050 Mpn020 Mpn252 Mpn621 Mpn004 Mpn353 Mpn142 Mpn118 Mpn555 Mpn516 Mpn003 Mpn665 Mpn551 Mpn378 Mpn034 Mpn258 Mpn141 Mpn434 Mpn083 Mpn686 Mpn040 Mpn582 EMBL152 Mpn353 DnaG EMBL153 Mpn340 Mpn340 Mpn005 Mpn521 Mpn340 Mpn034 Mpn674 Mpn108 Mpn042 Mpn390 Mpn042 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn354 Mpn115 Mpn258 Mpn574 Mpn046 Mpn228 Mpn210 Mpn434 Mpn063 Mpn207 Mpn515 Mpn294 Mpn426 Mpn445 Mpn050 Mpn040 Mpn252 Mpn042 Mpn621 Mpn004 EMBL155 Mpn126 Mpn390 Mpn303 Mpn126 Mpn516 Mpn430 Mpn665 Mpn394 Mpn365 Mpn034 Mpn658 Mpn574 Mpn019 Mpn210 Mpn434 Mpn573 Mpn398 Mpn126 Mpn515 Mpn050 Mpn040 Mpn452 Mpn621 Mpn004 EMBL156 Mpn126 Mpn210 Mpn434 Mpn269 Mpn573 Mpn390 Mpn126 Mpn516 Mpn665 Mpn126 Mpn580 Mpn346 Mpn034 EMBL154 General principles of cellular organization in Mycoplasma pneumoniae 109 EMBL157 Mpn430 GapA Mpn390 Mpn516 Mpn430 Mpn665 Mpn379 Mpn258 Mpn019 Mpn141 Mpn210 Mpn434 Mpn280 Mpn207 Mpn515 Mpn426 Mpn546 Mpn050 Mpn020 Mpn252 Mpn621 Mpn004 EMBL158 Mpn430 GapA Mpn210 Mpn434 Mpn619 Mpn390 Mpn555 Mpn516 Mpn430 Mpn683 Mpn426 Mpn361 Mpn542 Mpn034 Mpn452 Mpn072 EMBL159 Mpn401 GreA Mpn210 Mpn434 Mpn063 Mpn352 Mpn516 Mpn401 Mpn665 Mpn515 Mpn673 Mpn321 Mpn350 Mpn191 Mpn621 Mpn004 EMBL160 Mpn003 GyrB Mpn210 Mpn434 Mpn486 Mpn516 Mpn003 Mpn020 Mpn004 Mpn621 EMBL161 Mpn003 GyrB Mpn210 Mpn434 Mpn486 Mpn573 Mpn390 Mpn280 Mpn003 Mpn665 Mpn426 Mpn677 Mpn020 Mpn258 Mpn004 EMBL162 Mpn665 Tuf Mpn434 Mpn137 Mpn658 Mpn665 EMBL163 Mpn177 RplE Mpn623 Mpn434 Mpn573 Mpn665 EMBL164 Mpn033 Upp Mpn210 Mpn133 Mpn434 Mpn155 Mpn295 Mpn665 Mpn346 Mpn635 Mpn016 Mpn072 Mpn390 Mpn516 Mpn430 Mpn315 Mpn295 Mpn665 Mpn261 Mpn456 Mpn034 Mpn133 Mpn210 Mpn434 Mpn155 Mpn515 Mpn346 Mpn426 Mpn635 Mpn050 Mpn191 Mpn020 Mpn016 Mpn621 Mpn004 EMBL165 Mpn033 Upp EMBL166 Mpn297 Mpn297 Mpn297 Mpn210 Mpn434 Mpn042 Mpn034 General principles of cellular organization in Mycoplasma pneumoniae 110 EMBL167 Mpn606 Eno Mpn210 Mpn612 Mpn434 Mpn390 Mpn118 Mpn516 Mpn003 Mpn606 Mpn629 Mpn316 Mpn452 Mpn569 EMBL168 Mpn235 Ung Mpn210 Mpn434 Mpn390 Mpn014 Mpn674 Mpn515 Mpn222 Mpn235 Mpn034 Mpn678 EMBL169 Mpn494 UlaC Mpn390 Mpn555 Mpn352 Mpn516 Mpn430 Mpn665 Mpn261 Mpn494 Mpn210 Mpn434 Mpn314 Mpn515 Mpn426 Mpn050 Mpn191 Mpn452 Mpn621 Mpn004 EMBL170 Mpn574 GroS Mpn434 Mpn226 Mpn535 Mpn352 Mpn280 Mpn516 Mpn024 Mpn665 Mpn515 Mpn191 Mpn658 Mpn228 Mpn621 EMBL171 Mpn653 MtlF Mpn210 Mpn434 Mpn042 Mpn516 Mpn295 Mpn665 Mpn551 Mpn653 EMBL172 Mpn544 Mpn544 Mpn210 Mpn544 Mpn434 Mpn361 Mpn390 Mpn295 Mpn003 Mpn665 EMBL173 Mpn539 RplL Mpn480 Mpn516 Mpn430 Mpn003 Mpn665 Mpn551 Mpn261 Mpn065 Mpn658 Mpn210 Mpn434 Mpn643 Mpn207 Mpn515 Mpn426 Mpn073 Mpn191 Mpn020 Mpn621 Mpn004 EMBL174 Mpn066 ManB Mpn434 Mpn118 Mpn066 Mpn042 Mpn295 Mpn674 Mpn564 Mpn394 Mpn048 Mpn365 EMBL175 Mpn280 Mpn430 Mpn103 Mpn665 Mpn261 Mpn562 Mpn278 Mpn228 Mpn434 Mpn280 Mpn573 Mpn280 Mpn239 Mpn549 Mpn191 Mpn686 Mpn345 Mpn621 EMBL176 Mpn492 UlaE Mpn210 Mpn434 Mpn573 Mpn390 Mpn283 Mpn665 Mpn436 Mpn492 Mpn034 Mpn277 General principles of cellular organization in Mycoplasma pneumoniae 111 EMBL177 Mpn602 AtpF Mpn665 Mpn261 Mpn600 Mpn258 Mpn072 Mpn210 Mpn434 Mpn207 Mpn515 Mpn598 Mpn426 Mpn020 Mpn452 Mpn153 Mpn004 Mpn621 EMBL178 Mpn073 Prs Mpn210 Mpn434 Mpn573 Mpn404 Mpn674 Mpn515 Mpn073 Mpn034 Mpn004 EMBL179 Mpn050 GlpK Mpn210 Mpn434 Mpn322 Mpn324 Mpn516 Mpn419 Mpn295 Mpn665 Mpn261 Mpn050 Mpn266 Mpn050 GlpK Mpn062 Mpn269 Mpn390 Mpn555 Mpn516 Mpn430 Mpn665 Mpn141 Mpn210 Mpn434 Mpn032 Mpn515 Mpn426 Mpn483 Mpn050 Mpn020 Mpn452 Mpn079 EMBL181 Mpn107 Mpn390 Mpn555 Mpn516 Mpn665 Mpn261 Mpn379 Mpn258 Mpn556 Mpn210 Mpn434 Mpn573 Mpn207 Mpn032 Mpn515 Mpn426 Mpn444 Mpn107 Mpn107 Mpn050 Mpn608 Mpn020 Mpn452 Mpn621 Mpn004 EMBL182 Mpn107 Mpn434 Mpn573 Mpn118 Mpn555 Mpn003 Mpn665 Mpn032 Mpn426 Mpn107 Mpn107 Mpn141 EMBL183 Mpn222 TilS Mpn623 Mpn434 Mpn222 Mpn516 Mpn430 Mpn665 Mpn621 Mpn515 Mpn004 EMBL180 EMBL184 Mpn323 NrdI Mpn407 Mpn390 Mpn118 Mpn516 Mpn430 Mpn261 Mpn034 Mpn072 Mpn210 Mpn322 Mpn434 Mpn573 Mpn515 Mpn323 Mpn050 Mpn452 Mpn621 Mpn004 EMBL185 Mpn395 Apt Mpn434 Mpn514 Mpn388 Mpn042 Mpn001 Mpn395 Mpn367 Mpn295 Mpn195 EMBL186 Mpn652 MtlD Mpn210 Mpn434 Mpn573 Mpn516 Mpn515 Mpn032 Mpn426 Mpn191 Mpn652 Mpn228 Mpn004 General principles of cellular organization in Mycoplasma pneumoniae 112 Mpn383 Mpn210 Mpn434 Mpn656 Mpn674 Mpn665 Mpn314 Mpn533 Mpn034 Mpn383 Mpn383 EMBL188 Mpn167 RplW Mpn390 Mpn516 Mpn665 Mpn261 Mpn428 Mpn115 Mpn034 Mpn658 Mpn574 Mpn228 Mpn399 Mpn165 Mpn210 Mpn434 Mpn226 Mpn063 Mpn515 Mpn426 Mpn191 Mpn020 Mpn621 Mpn167 Mpn004 EMBL189 Mpn105 PheS Mpn210 Mpn105 Mpn434 Mpn322 Mpn062 Mpn573 Mpn568 Mpn516 Mpn665 Mpn515 Mpn472 Mpn426 Mpn247 Mpn020 Mpn106 Mpn141 EMBL190 Mpn105 PheS Mpn105 Mpn434 Mpn573 Mpn555 Mpn516 Mpn106 Mpn329 Mpn141 Mpn515 EMBL191 Mpn504 Mpn504 Mpn434 Mpn555 Mpn331 Mpn295 Mpn674 Mpn665 EMBL192 Mpn180 RplF EMBL193 Mpn555 Mpn390 Mpn555 Mpn516 Mpn665 Mpn261 Mpn141 Mpn210 Mpn434 Mpn555 Mpn324 Mpn515 Mpn426 Mpn020 Mpn452 Mpn153 Mpn652 Mpn004 EMBL194 Mpn555 Mpn555 Mpn210 Mpn434 Mpn555 Mpn324 Mpn665 Mpn261 Mpn072 EMBL195 Mpn531 ClpB Mpn434 Mpn686 Mpn531 Mpn280 Mpn258 Mpn516 Mpn621 ClpB Mpn531 Mpn352 Mpn516 Mpn665 Mpn182 Mpn171 Mpn571 Mpn228 Mpn434 Mpn226 Mpn515 Mpn616 Mpn640 Mpn673 Mpn239 Mpn191 Mpn020 Mpn189 EMBL187 EMBL196 Mpn531 Mpn434 Mpn180 Mpn658 General principles of cellular organization in Mycoplasma pneumoniae 113 EMBL197 Mpn283 Mpn470 Mpn352 Mpn283 Mpn516 Mpn430 Mpn315 Mpn665 Mpn394 Mpn261 Mpn354 Mpn258 Mpn556 Mpn210 Mpn434 Mpn007 Mpn380 Mpn283 Mpn515 Mpn050 Mpn191 Mpn020 Mpn621 Mpn004 EMBL198 Mpn283 Mpn283 Mpn210 Mpn434 Mpn118 Mpn643 Mpn283 Mpn216 Mpn003 Mpn665 EMBL199 Mpn517 Mpn517 Mpn434 Mpn226 Mpn517 EMBL200 Mpn551 Mpn210 Mpn434 Mpn573 Mpn295 Mpn003 Mpn551 Mpn683 Mpn059 Mpn551 Mpn034 Mpn452 EMBL201 Mpn562 NadE EMBL202 Mpn247 Mpn210 Mpn434 Mpn429 Mpn619 Mpn390 Mpn665 Mpn426 Mpn247 Mpn247 Mpn094 Mpn461 Mpn004 EMBL203 Mpn169 RpsS Mpn269 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn171 Mpn363 Mpn341 Mpn571 Mpn115 Mpn228 Mpn210 Mpn434 Mpn226 Mpn280 Mpn515 Mpn426 Mpn191 Mpn020 Mpn452 Mpn621 Mpn004 EMBL204 Mpn658 RplS Mpn573 Mpn571 Mpn034 Mpn665 Mpn536 RplS Mpn568 Mpn516 Mpn034 Mpn658 Mpn574 Mpn228 Mpn078 Mpn165 Mpn210 Mpn434 Mpn226 Mpn515 Mpn292 Mpn222 Mpn020 Mpn547 Mpn252 Mpn189 Mpn004 Pip Mpn062 Mpn619 Mpn303 Mpn516 Mpn430 Mpn665 Mpn261 Mpn321 Mpn393 Mpn023 Mpn365 Mpn034 Mpn022 Mpn141 Mpn210 Mpn322 Mpn573 Mpn388 Mpn324 Mpn310 Mpn598 Mpn515 Mpn472 Mpn426 Mpn050 Mpn191 Mpn452 Mpn106 Mpn621 Mpn004 EMBL205 EMBL206 Mpn658 Mpn022 Mpn426 Mpn210 Mpn562 Mpn434 Mpn573 Mpn516 Mpn515 Mpn097 General principles of cellular organization in Mycoplasma pneumoniae 114 EMBL207 Mpn556 ArgS Mpn556 Mpn361 Mpn073 Mpn665 EMBL208 Mpn265 TrpS Mpn300 Mpn390 Mpn555 Mpn516 Mpn430 Mpn665 Mpn320 Mpn210 Mpn265 Mpn322 Mpn434 Mpn573 Mpn207 Mpn644 Mpn515 Mpn191 Mpn020 Mpn452 Mpn621 Mpn004 EMBL209 Mpn265 TrpS Mpn265 Mpn434 Mpn573 Mpn118 Mpn003 Mpn665 EMBL210 Mpn063 DeoC Mpn210 Mpn434 Mpn063 Mpn047 Mpn555 Mpn516 Mpn430 Mpn665 Mpn515 Mpn426 Mpn392 Mpn191 Mpn658 Mpn621 Mpn004 EMBL211 Mpn656 Mpn516 Mpn665 Mpn536 Mpn034 Mpn658 Mpn210 Mpn434 Mpn573 Mpn674 Mpn515 Mpn426 Mpn392 Mpn050 Mpn663 Mpn452 Mpn243 Mpn656 Mpn004 Mpn621 EMBL212 Mpn557 GidA Mpn210 Mpn434 Mpn573 Mpn390 Mpn155 Mpn118 Mpn557 Mpn042 Mpn516 Mpn295 Mpn003 Mpn665 Mpn515 Mpn621 EMBL213 Mpn219 RplK Mpn555 Mpn516 Mpn430 Mpn665 Mpn394 Mpn261 Mpn606 Mpn533 Mpn574 Mpn141 Mpn228 Mpn078 Mpn556 Mpn210 Mpn389 Mpn289 Mpn434 Mpn573 Mpn324 Mpn674 Mpn668 Mpn314 Mpn426 Mpn020 Mpn479 Mpn621 Mpn004 EMBL214 Mpn598 AtpD Mpn602 Mpn516 Mpn598 EMBL215 Mpn032 Mpn556 Mpn434 Mpn573 Mpn555 Mpn516 Mpn665 Mpn515 Mpn032 Mpn032 Mpn191 Mpn574 Mpn621 EMBL216 Mpn314 MraZ Mpn210 Mpn434 Mpn390 Mpn516 Mpn430 Mpn339 Mpn314 Mpn032 Mpn521 Mpn034 Mpn040 Mpn633 Mpn621 Mpn004 General principles of cellular organization in Mycoplasma pneumoniae 115 EMBL217 Mpn109 Mpn596 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn309 Mpn169 Mpn115 Mpn658 Mpn574 Mpn210 Mpn434 Mpn550 Mpn578 Mpn032 Mpn314 Mpn515 Mpn426 Mpn444 Mpn635 Mpn109 Mpn073 Mpn050 Mpn109 Mpn191 Mpn020 Mpn621 Mpn004 EMBL218 Mpn109 Mpn109 Mpn210 Mpn434 Mpn118 Mpn109 Mpn034 Mpn516 EMBL219 Mpn360 RpmE Mpn390 Mpn531 Mpn516 Mpn430 Mpn401 Mpn307 Mpn261 Mpn177 Mpn297 Mpn365 Mpn658 Mpn228 Mpn669 Mpn210 Mpn434 Mpn590 Mpn226 Mpn063 Mpn280 Mpn207 Mpn329 Mpn515 Mpn426 Mpn050 Mpn020 Mpn452 Mpn252 Mpn621 EMBL220 Mpn332 Lon Mpn269 Mpn390 Mpn555 Mpn516 Mpn443 Mpn430 Mpn665 Mpn606 Mpn210 Mpn434 Mpn207 Mpn515 Mpn040 Mpn452 Mpn332 Mpn004 Mpn621 EMBL221 Mpn332 Lon Mpn210 Mpn434 Mpn390 Mpn034 Mpn665 EMBL222 Mpn560 Mpn560 Mpn560 Mpn434 Mpn573 Mpn555 Mpn480 Mpn118 Mpn516 Mpn295 Mpn003 Mpn665 Mpn551 Mpn034 Mpn228 Mpn210 Mpn434 Mpn226 Mpn573 Mpn032 Mpn515 Mpn454 Mpn004 EMBL223 Mpn480 ValS EMBL224 Mpn342 Mpn210 Mpn089 Mpn507 Mpn390 Mpn342 Mpn295 Mpn003 Mpn551 Mpn342 Mpn361 Mpn034 EMBL225 Mpn182 RpsE Mpn516 Mpn295 Mpn182 Mpn171 Mpn518 Mpn571 Mpn441 Mpn228 Mpn210 Mpn434 Mpn226 Mpn573 Mpn032 Mpn515 Mpn547 Mpn191 Mpn020 Mpn189 Mpn252 Mpn621 Mpn004 EMBL226 Mpn662 MsrB Mpn434 Mpn555 Mpn537 Mpn516 Mpn665 Mpn662 General principles of cellular organization in Mycoplasma pneumoniae 116 EMBL227 Mpn540 RpmF Mpn390 Mpn470 Mpn051 Mpn516 Mpn430 Mpn315 Mpn665 Mpn261 Mpn571 Mpn115 Mpn658 Mpn228 Mpn210 Mpn434 Mpn226 Mpn535 Mpn515 Mpn032 Mpn294 Mpn426 Mpn191 Mpn020 Mpn621 Mpn004 EMBL228 Mpn391 PdhC Mpn391 EMBL229 Mpn060 MetK Mpn048 Mpn434 Mpn060 Mpn034 EMBL230 Mpn508 Mpn508 Mpn426 Mpn210 Mpn434 Mpn034 Mpn516 Mpn452 Mpn430 Mpn665 EMBL231 Mpn034 PolC Mpn336 Mpn210 Mpn434 Mpn390 Mpn324 Mpn034 Mpn516 Mpn261 Mpn207 Mpn004 EMBL232 Mpn034 PolC Mpn210 Mpn612 Mpn434 Mpn573 Mpn280 Mpn516 Mpn008 Mpn145 Mpn620 Mpn677 Mpn514 Mpn526 Mpn034 Mpn088 Mpn621 PolC Mpn218 Mpn269 Mpn352 Mpn516 Mpn609 Mpn258 Mpn228 Mpn141 Mpn553 Mpn598 Mpn515 Mpn358 Mpn238 Mpn250 Mpn392 Mpn611 Mpn452 Mpn572 Mpn153 Mpn004 Mpn671 Mpn303 Mpn470 Mpn197 Mpn295 Mpn315 Mpn430 Mpn261 Mpn665 Mpn393 Mpn034 Mpn210 Mpn372 Mpn434 Mpn207 EMBL233 Mpn034 EMBL234 Mpn034 PolC Mpn645 Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn034 Mpn258 Mpn210 Mpn434 Mpn573 Mpn280 Mpn207 Mpn515 Mpn426 Mpn546 Mpn050 Mpn490 Mpn191 Mpn020 Mpn621 Mpn004 EMBL235 Mpn331 Tig Mpn210 Mpn165 Mpn434 Mpn209 Mpn516 Mpn003 Mpn665 Mpn158 Mpn331 Mpn452 Mpn228 Mpn004 EMBL236 Mpn302 PfkA Mpn210 Mpn434 Mpn034 Mpn302 Mpn665 Mpn644 Mpn097 General principles of cellular organization in Mycoplasma pneumoniae 117 EMBL237 Mpn263 TrxA Mpn321 Mpn034 Mpn452 Mpn263 Mpn314 EMBL238 Mpn188 RpmJ Mpn434 Mpn390 Mpn665 EMBL239 Mpn072 Mpn210 Mpn434 Mpn042 Mpn516 Mpn295 Mpn177 Mpn515 Mpn059 Mpn072 Mpn191 Mpn599 Mpn004 Mpn072 Mpn226 RpsG Mpn390 Mpn555 Mpn516 Mpn430 Mpn003 Mpn665 Mpn428 Mpn034 Mpn658 Mpn228 Mpn210 Mpn434 Mpn226 Mpn324 Mpn294 Mpn314 Mpn452 Mpn621 Mpn004 EMBL241 Mpn110 Mpn062 Mpn390 Mpn555 Mpn516 Mpn430 Mpn419 Mpn315 Mpn665 Mpn261 Mpn297 Mpn169 Mpn115 Mpn034 Mpn574 Mpn660 Mpn210 Mpn434 Mpn226 Mpn524 Mpn110 Mpn280 Mpn515 Mpn426 Mpn239 Mpn110 Mpn050 Mpn191 Mpn020 Mpn452 Mpn252 Mpn621 Mpn004 EMBL242 Mpn267 PpnK Mpn210 Mpn434 Mpn573 Mpn280 Mpn264 Mpn516 Mpn267 Mpn515 Mpn050 Mpn621 EMBL243 Mpn674 Ldh Mpn210 Mpn434 Mpn516 Mpn674 Mpn621 EMBL244 Mpn380 MutM Mpn434 Mpn555 Mpn295 Mpn665 Mpn380 EMBL240 EMBL245 Mpn008 TrmE Mpn390 Mpn516 Mpn430 Mpn665 Mpn261 Mpn258 Mpn228 Mpn210 Mpn322 Mpn434 Mpn553 Mpn573 Mpn008 Mpn515 Mpn317 Mpn050 Mpn191 Mpn547 EMBL246 Mpn008 TrmE Mpn210 Mpn434 Mpn573 Mpn516 Mpn665 Mpn261 Mpn008 Mpn050 General principles of cellular organization in Mycoplasma pneumoniae 118 Mpn434 Mpn226 Mpn390 Mpn430 Mpn295 Mpn665 Mpn174 Mpn386 Mpn020 Mpn191 Mpn228 EMBL247 Mpn174 RpsQ EMBL248 Mpn362 Mpn362 Mpn434 Mpn573 Mpn555 Mpn516 Mpn665 Mpn108 Mpn362 EMBL249 Mpn121 Mpn210 Mpn434 Mpn366 Mpn390 Mpn135 Mpn430 Mpn346 Mpn121 Mpn121 Mpn050 Mpn034 EMBL250 Mpn377 Mpn377 Mpn377 Mpn434 Mpn014 Mpn034 EMBL251 Mpn490 RecA Mpn210 Mpn434 Mpn402 Mpn490 Mpn034 Mpn252 Mpn079 Mpn621 EMBL252 Mpn490 RecA Mpn284 Mpn210 Mpn480 Mpn434 Mpn573 Mpn003 Mpn490 Mpn034 Mpn452 EMBL253 Mpn420 Mpn210 Mpn434 Mpn573 Mpn555 Mpn516 Mpn419 Mpn394 Mpn668 Mpn420 Mpn515 Mpn314 Mpn420 Mpn034 EMBL254 Mpn232 DnaB Mpn210 Mpn434 Mpn390 Mpn665 Mpn415 Mpn207 Mpn232 Mpn258 Mpn141 Mpn241 EMBL255 Mpn232 DnaB Mpn298 Mpn210 Mpn390 Mpn118 Mpn295 Mpn108 Mpn521 Mpn526 Mpn232 Mpn034 Mpn452 EMBL256 Mpn311 Mpn210 Mpn434 Mpn204 Mpn095 Mpn390 Mpn324 Mpn280 Mpn516 Mpn311 Mpn430 Mpn665 Mpn261 Mpn621 Mpn004 General principles of cellular organization in Mycoplasma pneumoniae 119 EMBL257 Mpn176 RplX Mpn210 Mpn434 Mpn665 Mpn615 Mpn220 Mpn685 Mpn034 Mpn658 Mpn189 Mpn091 Mpn228 Mpn004 Mpn097 EMBL258 Mpn189 RpsM Mpn434 Mpn629 Mpn034 Mpn228 Mpn316 Mpn390 Mpn555 Mpn516 Mpn430 Mpn665 Mpn261 Mpn440 Mpn258 Mpn141 Mpn556 Mpn210 Mpn553 Mpn434 Mpn573 Mpn192 Mpn636 Mpn329 Mpn515 Mpn546 Mpn549 Mpn050 Mpn191 Mpn252 Mpn316 Mpn316 Mpn452 Mpn621 EMBL259 ii) Proteins identified in the PeptideAtlas by FT-MS/MS Systematic name Protein name Mpn001 DnaN Mpn002 Mpn002 Mpn003 GyrB Mpn004 GyrA Mpn005 SerS Mpn006 Tmk Mpn007 Mpn007 Mpn008 TrmE Mpn009 Mpn009 Mpn011 Mpn011 Mpn012 Mpn012 Mpn013 Mpn013 Mpn015 Mpn015 Mpn016 Mpn016 Mpn017 FolD Mpn018 Mpn018 Mpn019 Mpn019 Mpn020 Mpn020 Mpn021 DnaJ Mpn022 Pip Mpn023 MetG Mpn024 RpoE Mpn025 Fba Mpn026 Mpn026 Mpn027 Mpn027 Mpn029 Efp Mpn030 Mpn030 Mpn031 Mpn031 Mpn033 Upp Mpn034 PolC Mpn035 Mpn035 General principles of cellular organization in Mycoplasma pneumoniae 120 Mpn036 Mpn043 Mpn044 Mpn045 Mpn046 Mpn047 Mpn050 Mpn051 Mpn052 Mpn053 Mpn055 Mpn058 Mpn059 Mpn060 Mpn061 Mpn062 Mpn063 Mpn064 Mpn065 Mpn066 Mpn067 Mpn068 Mpn070 Mpn071 Mpn072 Mpn073 Mpn075 Mpn076 Mpn077 Mpn078 Mpn079 Mpn080 Mpn081 Mpn082 Mpn083 Mpn084 Mpn090 Mpn099 Mpn105 Mpn106 Mpn109 Mpn115 Mpn116 Mpn117 Mpn118 Mpn119 Mpn120 Mpn121 Mpn122 Mpn123 Mpn036 GlpF Tdk HisS AspS Mpn047 GlpK Mpn051 Mpn052 PtsH PotA Mpn058 Gcp MetK Ffh DeoD DeoC DeoA Cdd ManB Mpn067 Mpn068 Mpn070 Mpn071 Mpn072 Prs Mpn075 Mpn076 Mpn077 FruA FruK Mpn080 Mpn081 Tkt Mpn083 Mpn084 Mpn090 Mpn099 PheS PheT Mpn109 InfC RpmI RplT Mpn118 Mpn119 GrpE Mpn121 ParE ParC General principles of cellular organization in Mycoplasma pneumoniae 121 Mpn124 Mpn125 Mpn126 Mpn131 Mpn132 Mpn133 Mpn134 Mpn135 Mpn136 Mpn140 Mpn141 Mpn142 Mpn144 Mpn149 Mpn152 Mpn153 Mpn154 Mpn155 Mpn156 Mpn157 Mpn158 Mpn159 Mpn160 Mpn161 Mpn162 Mpn163 Mpn164 Mpn165 Mpn166 Mpn167 Mpn168 Mpn169 Mpn170 Mpn171 Mpn172 Mpn173 Mpn174 Mpn175 Mpn176 Mpn177 Mpn178 Mpn179 Mpn180 Mpn181 Mpn182 Mpn183 Mpn184 Mpn185 Mpn186 Mpn187 HrcA UvrC Mpn126 Mpn131 Mpn132 Mpn133 Mpn134 Mpn135 Mpn136 Mpn140 MgpA Mpn142 Mpn144 Mpn149 Mpn152 Mpn153 NusA InfB RbfA Mpn157 RibF Mpn159 Mpn160 Mpn161 Mpn162 Mpn163 RpsJ RplC RplD RplW RplB RpsS RplV RpsC RplP RpmC RpsQ RplN RplX RplE RpsZ RpsH RplF RplR RpsE RplO SecY Adk Map InfA General principles of cellular organization in Mycoplasma pneumoniae 122 Mpn188 Mpn189 Mpn190 Mpn191 Mpn192 Mpn193 Mpn194 Mpn195 Mpn196 Mpn197 Mpn199 Mpn200 Mpn202 Mpn205 Mpn207 Mpn208 Mpn209 Mpn210 Mpn211 Mpn213 Mpn214 Mpn215 Mpn216 Mpn217 Mpn218 Mpn219 Mpn220 Mpn221 Mpn222 Mpn223 Mpn224 Mpn225 Mpn226 Mpn227 Mpn228 Mpn229 Mpn230 Mpn231 Mpn232 Mpn233 Mpn235 Mpn236 Mpn237 Mpn238 Mpn239 Mpn240 Mpn241 Mpn243 Mpn244 Mpn245 RpmJ RpsM RpsK RpoA RplQ CbiO1 CbiO2 Mpn195 TruA PepF Mpn199 Mpn200 Mpn202 Mpn205 PtsG RpsB PacL SecA UvrB Mpn213 Mpn214 OppB OppC OppD OppF RplK RplA Pth TilS HprK Lgt RpsL RpsG FusA RpsF Ssb RpsR RplI DnaB Mpn233 Ung Mpn236 GatA GatB Mpn239 TrxB Mpn241 RnR Mpn244 Gmk General principles of cellular organization in Mycoplasma pneumoniae 123 Mpn246 Mpn247 Mpn248 Mpn250 Mpn251 Mpn252 Mpn255 Mpn256 Mpn257 Mpn258 Mpn259 Mpn260 Mpn261 Mpn262 Mpn263 Mpn264 Mpn265 Mpn266 Mpn267 Mpn268 Mpn269 Mpn271 Mpn273 Mpn275 Mpn276 Mpn277 Mpn278 Mpn279 Mpn280 Mpn284 Mpn286 Mpn288 Mpn291 Mpn292 Mpn293 Mpn294 Mpn295 Mpn296 Mpn297 Mpn298 Mpn299 Mpn300 Mpn301 Mpn302 Mpn303 Mpn307 Mpn308 Mpn309 Mpn310 Mpn311 Def Mpn247 Mpn248 Pgi Rpe AsnS Mpn255 Mpn256 GalE Mpn258 Mpn259 Mpn260 TopA Mpn262 TrxA Mpn264 TrpS Mpn266 PpnK Mpn268 Mpn269 Mpn271 Mpn273 Mpn275 Mpn276 LysS Glf LepA Mpn280 Mpn284 Mpn286 Mpn288 Mpn291 Mpn292 LspA Mpn294 Mpn295 RpsU Mpn297 AcpS PlsC ScpA ScpB PfkA Pyk Mpn307 Mpn308 P65 Hmw2 Mpn311 General principles of cellular organization in Mycoplasma pneumoniae 124 Mpn312 Mpn314 Mpn315 Mpn316 Mpn317 Mpn318 Mpn319 Mpn320 Mpn321 Mpn322 Mpn323 Mpn324 Mpn325 Mpn326 Mpn327 Mpn328 Mpn329 Mpn330 Mpn331 Mpn332 Mpn336 Mpn337 Mpn338 Mpn339 Mpn340 Mpn342 Mpn343 Mpn346 Mpn349 Mpn352 Mpn353 Mpn354 Mpn355 Mpn356 Mpn357 Mpn358 Mpn359 Mpn360 Mpn361 Mpn362 Mpn366 Mpn370 Mpn372 Mpn376 Mpn378 Mpn379 Mpn380 Mpn381 Mpn383 Mpn384 Mpn312 MraZ MraW Mpn316 FtsZ Mpn318 Mpn319 ThyA FolA NrdF NrdI NrdE RplU Mpn326 RpmA Nfo Mpn329 Mpn330 Tig Lon Mpn336 Mpn337 Mpn338 Mpn339 Mpn340 Mpn342 Mpn343 Mpn346 Mpn349 RpoD DnaG GlyQS Mpn355 CysS LigA Mpn358 Mpn359 RpmE PrfA Mpn362 Mpn366 Mpn370 Mpn372 Mpn376 DnaE PolA MutM Mpn381 Mpn383 LeuS General principles of cellular organization in Mycoplasma pneumoniae 125 Mpn386 Mpn387 Mpn389 Mpn390 Mpn391 Mpn392 Mpn393 Mpn394 Mpn395 Mpn396 Mpn397 Mpn398 Mpn399 Mpn400 Mpn401 Mpn402 Mpn406 Mpn407 Mpn408 Mpn409 Mpn415 Mpn419 Mpn420 Mpn421 Mpn422 Mpn423 Mpn425 Mpn426 Mpn427 Mpn428 Mpn429 Mpn430 Mpn432 Mpn434 Mpn436 Mpn438 Mpn443 Mpn444 Mpn445 Mpn446 Mpn447 Mpn449 Mpn452 Mpn453 Mpn454 Mpn456 Mpn459 Mpn460 Mpn461 Mpn469 Mpn386 Mpn387 LplA PdhD PdhC PdhB PdhA Nox Apt Mpn396 SpoT Mpn398 Mpn399 Mpn400 GreA ProS Mpn406 Mpn407 Mpn408 Mpn409 P37 AlaS Mpn420 Mpn421 TrmU Mpn423 FtsY P115 Mpn427 Pta Pgk GapA Mpn432 DnaK Mpn436 Mpn438 Mpn443 Mpn444 Mpn445 RpsD Hmw1 Mpn449 Hmw3 P30 Mpn454 Mpn456 Mpn459 Mpn460 Mpn461 Mpn469 General principles of cellular organization in Mycoplasma pneumoniae 126 Mpn470 Mpn471 Mpn472 Mpn473 Mpn474 Mpn475 Mpn476 Mpn477 Mpn478 Mpn479 Mpn480 Mpn481 Mpn483 Mpn487 Mpn488 Mpn489 Mpn490 Mpn491 Mpn492 Mpn493 Mpn494 Mpn496 Mpn497 Mpn499 Mpn500 Mpn505 Mpn506 Mpn509 Mpn512 Mpn514 Mpn515 Mpn516 Mpn517 Mpn518 Mpn519 Mpn520 Mpn521 Mpn522 Mpn523 Mpn524 Mpn525 Mpn526 Mpn528 Mpn529 Mpn530 Mpn531 Mpn532 Mpn533 Mpn538 Mpn539 PepP RpmG1 Mpn472 Mpn473 Mpn474 EngA Cmk Mpn477 Mpn478 AzoR ValS EngB Mpn483 Csd Mpn488 Mpn489 RecA Mpn491 UlaE UlaD UlaC UlaA Mpn497 Mpn499 Mpn500 Mpn505 Mpn506 Mpn509 Mpn512 Mpn514 RpoC RpoB Mpn517 Mpn518 Mpn519 IleS Mpn521 TrmB Mpn523 Mpn524 Mpn525 Mpn526 Ppa Mpn529 Mpn530 ClpB Mpn532 AckA RplJ RplL General principles of cellular organization in Mycoplasma pneumoniae 127 Mpn540 Mpn541 Mpn542 Mpn543 Mpn544 Mpn545 Mpn546 Mpn547 Mpn548 Mpn549 Mpn550 Mpn551 Mpn552 Mpn553 Mpn554 Mpn555 Mpn556 Mpn557 Mpn558 Mpn560 Mpn561 Mpn562 Mpn563 Mpn564 Mpn566 Mpn567 Mpn568 Mpn572 Mpn573 Mpn574 Mpn576 Mpn578 Mpn591 Mpn592 Mpn593 Mpn595 Mpn597 Mpn598 Mpn599 Mpn600 Mpn601 Mpn602 Mpn603 Mpn604 Mpn606 Mpn607 Mpn608 Mpn609 Mpn610 Mpn611 RpmF RpsT Mpn542 Fmt Mpn544 Rnc PlsX Mpn547 Mpn548 Mpn549 ThiI Mpn551 Mpn552 ThrS Mpn554 Mpn555 ArgS GidA GidB Mpn560 Udk NadE Mpn563 Adh Mpn566 P200 Era PepA GroL GroS GlyA Mpn578 Mpn591 Mpn592 Mpn593 RpiB AtpC AtpD AtpG AtpA AtpH AtpF AtpE AtpB Eno MsrA Mpn608 PstB PstA Mpn611 General principles of cellular organization in Mycoplasma pneumoniae 128 Mpn612 Mpn616 Mpn617 Mpn618 Mpn619 Mpn620 Mpn621 Mpn622 Mpn623 Mpn624 Mpn625 Mpn627 Mpn628 Mpn629 Mpn630 Mpn631 Mpn632 Mpn636 Mpn638 Mpn639 Mpn641 Mpn642 Mpn643 Mpn652 Mpn653 Mpn655 Mpn658 Mpn659 Mpn660 Mpn661 Mpn662 Mpn663 Mpn664 Mpn665 Mpn666 Mpn667 Mpn668 Mpn669 Mpn670 Mpn671 Mpn672 Mpn673 Mpn674 Mpn677 Mpn678 Mpn679 Mpn680 Mpn682 Mpn683 Mpn684 Mpn612 RpsI RplM DnaX UvrA Mpn620 Mpn621 RpsO Mpn623 RpmB Mpn625 PtsI GpmI TpiA Mpn630 Tsf PyrH Frr Mpn638 Mpn639 Mpn641 Mpn642 Mpn643 MtlD MtlF Mpn655 RplS TrmD RpsP Mpn661 MsrB Mpn663 Mpn664 Tuf Mpn666 GalU Mpn668 TyrS Mpn670 FtsH Hpt Mpn673 Ldh Mpn677 GltX KsgA OxaA RpmH Mpn683 Mpn684 General principles of cellular organization in Mycoplasma pneumoniae 129 Mpn685 Mpn686 Mpn687 Mpn688 Mpn685 DnaA Mpn687 Mpn688 General principles of cellular organization in Mycoplasma pneumoniae 130 10.2. Table S2: List of high confidence interactions and corresponding socio-affinity scores. In total 2,116 redundant high confidence interactions, i.e. 1,058 non-redundant interactions, are reported in this list. The first two columns give the proteins involved in these interactions. The third column provides the socio-affinity score attributed to this interaction. Protein 1 Mpn230 Mpn230 Mpn230 Mpn230 Mpn230 Mpn516 Mpn516 Mpn322 Mpn573 Mpn178 Mpn178 Mpn178 Mpn178 Mpn178 Mpn178 Mpn023 Mpn122 Mpn122 Mpn392 Mpn106 Mpn106 Mpn171 Mpn171 Mpn171 Mpn171 Mpn171 Mpn622 Mpn622 Mpn622 Mpn622 Mpn622 Mpn622 Mpn622 Mpn622 Mpn622 Mpn207 Mpn089 Mpn378 Mpn515 Mpn515 Protein 2 Mpn178 Mpn228 Mpn660 Mpn179 Mpn616 Mpn515 Mpn191 Mpn324 Mpn574 Mpn230 Mpn171 Mpn446 Mpn182 Mpn179 Mpn169 Mpn106 Mpn004 Mpn123 Mpn393 Mpn023 Mpn105 Mpn178 Mpn182 Mpn189 Mpn169 Mpn226 Mpn446 Mpn182 Mpn228 Mpn660 Mpn208 Mpn179 Mpn169 Mpn616 Mpn226 Mpn268 Mpn342 Mpn353 Mpn516 Mpn191 Socio-affinity score x1000 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 General principles of cellular organization in Mycoplasma pneumoniae 131 Mpn611 Mpn446 Mpn446 Mpn446 Mpn446 Mpn446 Mpn446 Mpn446 Mpn446 Mpn446 Mpn003 Mpn602 Mpn602 Mpn167 Mpn167 Mpn166 Mpn166 Mpn598 Mpn598 Mpn182 Mpn182 Mpn182 Mpn182 Mpn182 Mpn182 Mpn182 Mpn182 Mpn182 Mpn182 Mpn189 Mpn189 Mpn189 Mpn189 Mpn189 Mpn189 Mpn189 Mpn352 Mpn600 Mpn600 Mpn609 Mpn228 Mpn228 Mpn228 Mpn228 Mpn228 Mpn228 Mpn004 Mpn004 Mpn393 Mpn172 Mpn609 Mpn178 Mpn622 Mpn182 Mpn189 Mpn228 Mpn179 Mpn169 Mpn616 Mpn226 Mpn004 Mpn598 Mpn600 Mpn165 Mpn179 Mpn172 Mpn165 Mpn602 Mpn600 Mpn178 Mpn171 Mpn622 Mpn446 Mpn189 Mpn228 Mpn179 Mpn169 Mpn616 Mpn226 Mpn171 Mpn446 Mpn182 Mpn179 Mpn169 Mpn616 Mpn226 Mpn191 Mpn602 Mpn598 Mpn611 Mpn230 Mpn622 Mpn446 Mpn182 Mpn660 Mpn616 Mpn122 Mpn003 Mpn392 Mpn166 General principles of cellular organization in Mycoplasma pneumoniae 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 132 Mpn660 Mpn660 Mpn660 Mpn660 Mpn268 Mpn123 Mpn208 Mpn208 Mpn208 Mpn165 Mpn165 Mpn165 Mpn191 Mpn191 Mpn191 Mpn507 Mpn618 Mpn618 Mpn007 Mpn324 Mpn450 Mpn353 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn179 Mpn574 Mpn105 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn169 Mpn616 Mpn616 Mpn616 Mpn616 Mpn230 Mpn622 Mpn228 Mpn179 Mpn207 Mpn122 Mpn622 Mpn169 Mpn226 Mpn167 Mpn166 Mpn169 Mpn516 Mpn515 Mpn352 Mpn342 Mpn007 Mpn450 Mpn618 Mpn322 Mpn618 Mpn378 Mpn230 Mpn178 Mpn622 Mpn446 Mpn167 Mpn182 Mpn189 Mpn660 Mpn169 Mpn616 Mpn226 Mpn573 Mpn106 Mpn178 Mpn171 Mpn622 Mpn446 Mpn182 Mpn189 Mpn208 Mpn165 Mpn179 Mpn616 Mpn226 Mpn230 Mpn622 Mpn446 Mpn182 General principles of cellular organization in Mycoplasma pneumoniae 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 133 Mpn616 Mpn616 Mpn616 Mpn616 Mpn342 Mpn342 Mpn226 Mpn226 Mpn226 Mpn226 Mpn226 Mpn226 Mpn226 Mpn226 Mpn226 Mpn174 Mpn230 Mpn141 Mpn106 Mpn171 Mpn171 Mpn171 Mpn446 Mpn434 Mpn434 Mpn166 Mpn166 Mpn189 Mpn189 Mpn327 Mpn228 Mpn660 Mpn660 Mpn165 Mpn165 Mpn120 Mpn220 Mpn179 Mpn658 Mpn658 Mpn021 Mpn616 Mpn616 Mpn226 Mpn230 Mpn230 Mpn178 Mpn178 Mpn178 Mpn106 Mpn189 Mpn228 Mpn179 Mpn169 Mpn089 Mpn507 Mpn171 Mpn622 Mpn446 Mpn182 Mpn189 Mpn208 Mpn179 Mpn169 Mpn174 Mpn226 Mpn189 Mpn106 Mpn141 Mpn166 Mpn165 Mpn179 Mpn660 Mpn120 Mpn021 Mpn171 Mpn327 Mpn230 Mpn228 Mpn166 Mpn189 Mpn446 Mpn616 Mpn171 Mpn658 Mpn434 Mpn658 Mpn171 Mpn165 Mpn220 Mpn434 Mpn660 Mpn226 Mpn616 Mpn622 Mpn446 Mpn622 Mpn616 Mpn226 Mpn678 General principles of cellular organization in Mycoplasma pneumoniae 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 999 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 998 997 997 997 997 997 997 134 Mpn171 Mpn171 Mpn171 Mpn622 Mpn622 Mpn538 Mpn446 Mpn446 Mpn167 Mpn167 Mpn678 Mpn182 Mpn189 Mpn660 Mpn660 Mpn631 Mpn429 Mpn430 Mpn665 Mpn180 Mpn658 Mpn616 Mpn226 Mpn226 Mpn230 Mpn516 Mpn178 Mpn003 Mpn182 Mpn182 Mpn034 Mpn490 Mpn189 Mpn352 Mpn327 Mpn228 Mpn228 Mpn228 Mpn208 Mpn165 Mpn191 Mpn007 Mpn450 Mpn179 Mpn486 Mpn658 Mpn226 Mpn226 Mpn638 Mpn171 Mpn538 Mpn446 Mpn167 Mpn230 Mpn178 Mpn171 Mpn230 Mpn171 Mpn171 Mpn189 Mpn106 Mpn660 Mpn167 Mpn182 Mpn226 Mpn665 Mpn430 Mpn429 Mpn631 Mpn658 Mpn180 Mpn178 Mpn178 Mpn660 Mpn182 Mpn352 Mpn189 Mpn486 Mpn230 Mpn191 Mpn490 Mpn034 Mpn178 Mpn516 Mpn658 Mpn208 Mpn179 Mpn226 Mpn228 Mpn226 Mpn182 Mpn450 Mpn007 Mpn228 Mpn003 Mpn327 Mpn228 Mpn165 Mpn347 Mpn616 General principles of cellular organization in Mycoplasma pneumoniae 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 997 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 996 995 995 135 Mpn347 Mpn089 Mpn177 Mpn258 Mpn166 Mpn166 Mpn182 Mpn176 Mpn327 Mpn518 Mpn165 Mpn259 Mpn497 Mpn220 Mpn179 Mpn169 Mpn658 Mpn616 Mpn230 Mpn606 Mpn025 Mpn320 Mpn133 Mpn115 Mpn300 Mpn629 Mpn430 Mpn169 Mpn016 Mpn226 Mpn230 Mpn392 Mpn166 Mpn390 Mpn115 Mpn658 Mpn230 Mpn178 Mpn177 Mpn176 Mpn189 Mpn228 Mpn228 Mpn402 Mpn169 Mpn060 Mpn226 Mpn226 Mpn516 Mpn020 Mpn638 Mpn497 Mpn658 Mpn259 Mpn179 Mpn169 Mpn518 Mpn220 Mpn165 Mpn182 Mpn327 Mpn258 Mpn089 Mpn176 Mpn166 Mpn166 Mpn177 Mpn171 Mpn169 Mpn629 Mpn430 Mpn300 Mpn016 Mpn226 Mpn320 Mpn606 Mpn025 Mpn230 Mpn133 Mpn115 Mpn115 Mpn390 Mpn658 Mpn392 Mpn230 Mpn166 Mpn226 Mpn228 Mpn226 Mpn189 Mpn176 Mpn178 Mpn169 Mpn060 Mpn228 Mpn402 Mpn230 Mpn177 Mpn020 Mpn516 General principles of cellular organization in Mycoplasma pneumoniae 995 995 995 995 995 995 995 995 995 995 995 995 995 995 995 995 995 995 994 994 994 994 994 994 994 994 994 994 994 994 993 993 993 993 993 993 992 992 992 992 992 992 992 992 992 992 992 992 991 991 136 Mpn177 Mpn115 Mpn191 Mpn220 Mpn169 Mpn226 Mpn171 Mpn515 Mpn352 Mpn191 Mpn192 Mpn166 Mpn426 Mpn191 Mpn300 Mpn226 Mpn166 Mpn191 Mpn178 Mpn329 Mpn434 Mpn551 Mpn079 Mpn172 Mpn660 Mpn133 Mpn062 Mpn440 Mpn353 Mpn635 Mpn635 Mpn531 Mpn658 Mpn016 Mpn322 Mpn306 Mpn392 Mpn106 Mpn387 Mpn293 Mpn191 Mpn137 Mpn179 Mpn665 Mpn167 Mpn226 Mpn165 Mpn191 Mpn232 Mpn225 Mpn191 Mpn169 Mpn177 Mpn226 Mpn115 Mpn220 Mpn191 Mpn352 Mpn515 Mpn171 Mpn191 Mpn226 Mpn300 Mpn192 Mpn426 Mpn166 Mpn191 Mpn166 Mpn660 Mpn440 Mpn531 Mpn353 Mpn062 Mpn658 Mpn178 Mpn635 Mpn079 Mpn329 Mpn551 Mpn133 Mpn016 Mpn434 Mpn172 Mpn635 Mpn106 Mpn392 Mpn306 Mpn322 Mpn293 Mpn387 Mpn179 Mpn665 Mpn191 Mpn137 Mpn226 Mpn167 Mpn191 Mpn165 Mpn526 Mpn173 General principles of cellular organization in Mycoplasma pneumoniae 991 991 991 991 991 991 990 990 990 990 989 989 989 989 989 989 988 988 986 986 986 986 986 986 986 986 986 986 986 986 986 986 986 986 985 985 985 985 985 985 985 985 985 985 984 984 983 983 982 982 137 Mpn173 Mpn526 Mpn298 Mpn118 Mpn446 Mpn191 Mpn246 Mpn365 Mpn189 Mpn245 Mpn191 Mpn345 Mpn002 Mpn023 Mpn434 Mpn619 Mpn537 Mpn033 Mpn033 Mpn189 Mpn133 Mpn220 Mpn016 Mpn662 Mpn192 Mpn253 Mpn005 Mpn340 Mpn228 Mpn231 Mpn177 Mpn167 Mpn623 Mpn191 Mpn573 Mpn192 Mpn171 Mpn009 Mpn606 Mpn228 Mpn292 Mpn430 Mpn547 Mpn226 Mpn516 Mpn064 Mpn033 Mpn678 Mpn189 Mpn660 Mpn225 Mpn232 Mpn118 Mpn298 Mpn191 Mpn446 Mpn245 Mpn345 Mpn191 Mpn246 Mpn189 Mpn365 Mpn434 Mpn619 Mpn002 Mpn023 Mpn662 Mpn133 Mpn016 Mpn220 Mpn033 Mpn189 Mpn033 Mpn537 Mpn253 Mpn192 Mpn340 Mpn005 Mpn231 Mpn228 Mpn623 Mpn191 Mpn177 Mpn167 Mpn009 Mpn226 Mpn228 Mpn573 Mpn430 Mpn171 Mpn547 Mpn606 Mpn292 Mpn192 Mpn678 Mpn033 Mpn064 Mpn516 Mpn660 Mpn189 General principles of cellular organization in Mycoplasma pneumoniae 982 982 981 981 981 981 980 980 980 980 980 980 979 979 979 979 978 978 978 978 978 978 978 978 977 977 977 977 977 977 976 976 976 976 975 975 975 975 975 975 975 975 975 975 974 974 974 974 974 974 138 Mpn179 Mpn685 Mpn171 Mpn187 Mpn340 Mpn166 Mpn189 Mpn521 Mpn434 Mpn598 Mpn476 Mpn189 Mpn352 Mpn153 Mpn461 Mpn165 Mpn573 Mpn573 Mpn194 Mpn008 Mpn023 Mpn182 Mpn393 Mpn571 Mpn061 Mpn025 Mpn660 Mpn361 Mpn169 Mpn556 Mpn051 Mpn668 Mpn341 Mpn376 Mpn203 Mpn014 Mpn228 Mpn686 Mpn223 Mpn048 Mpn627 Mpn505 Mpn644 Mpn081 Mpn060 Mpn302 Mpn174 Mpn377 Mpn515 Mpn261 Mpn685 Mpn179 Mpn187 Mpn171 Mpn521 Mpn189 Mpn166 Mpn340 Mpn352 Mpn153 Mpn461 Mpn165 Mpn434 Mpn598 Mpn476 Mpn189 Mpn194 Mpn008 Mpn573 Mpn573 Mpn393 Mpn571 Mpn023 Mpn182 Mpn025 Mpn061 Mpn169 Mpn556 Mpn660 Mpn361 Mpn627 Mpn203 Mpn223 Mpn686 Mpn668 Mpn377 Mpn174 Mpn376 Mpn341 Mpn060 Mpn051 Mpn081 Mpn302 Mpn505 Mpn048 Mpn644 Mpn228 Mpn014 Mpn261 Mpn515 General principles of cellular organization in Mycoplasma pneumoniae 974 974 973 973 973 973 973 973 972 972 972 972 972 972 972 972 971 971 971 971 969 969 969 969 968 968 967 967 967 967 966 966 966 966 966 966 966 966 966 966 966 966 966 966 966 966 966 966 965 965 139 Mpn341 Mpn622 Mpn221 Mpn269 Mpn189 Mpn228 Mpn020 Mpn572 Mpn515 Mpn611 Mpn191 Mpn169 Mpn573 Mpn573 Mpn668 Mpn178 Mpn392 Mpn391 Mpn191 Mpn665 Mpn006 Mpn178 Mpn572 Mpn561 Mpn378 Mpn538 Mpn222 Mpn551 Mpn250 Mpn623 Mpn663 Mpn536 Mpn665 Mpn021 Mpn615 Mpn365 Mpn365 Mpn343 Mpn097 Mpn562 Mpn573 Mpn606 Mpn434 Mpn264 Mpn665 Mpn539 Mpn014 Mpn066 Mpn167 Mpn564 Mpn269 Mpn189 Mpn228 Mpn341 Mpn622 Mpn221 Mpn515 Mpn611 Mpn020 Mpn572 Mpn169 Mpn191 Mpn392 Mpn665 Mpn391 Mpn191 Mpn573 Mpn668 Mpn178 Mpn573 Mpn665 Mpn561 Mpn250 Mpn178 Mpn551 Mpn021 Mpn623 Mpn378 Mpn572 Mpn222 Mpn536 Mpn663 Mpn006 Mpn538 Mpn365 Mpn615 Mpn343 Mpn365 Mpn562 Mpn097 Mpn264 Mpn434 Mpn606 Mpn573 Mpn539 Mpn665 Mpn352 Mpn564 Mpn616 Mpn066 General principles of cellular organization in Mycoplasma pneumoniae 963 963 963 963 963 963 962 962 962 962 962 962 961 961 961 961 961 961 961 961 960 960 960 960 960 960 960 960 960 960 960 960 960 960 959 959 959 959 959 959 958 958 958 958 958 958 957 957 957 957 140 Mpn352 Mpn660 Mpn658 Mpn616 Mpn176 Mpn658 Mpn014 Mpn235 Mpn573 Mpn167 Mpn025 Mpn115 Mpn191 Mpn191 Mpn226 Mpn174 Mpn191 Mpn220 Mpn424 Mpn424 Mpn238 Mpn238 Mpn238 Mpn238 Mpn178 Mpn419 Mpn341 Mpn088 Mpn088 Mpn358 Mpn358 Mpn358 Mpn358 Mpn020 Mpn572 Mpn572 Mpn035 Mpn035 Mpn145 Mpn145 Mpn561 Mpn339 Mpn611 Mpn611 Mpn611 Mpn282 Mpn282 Mpn140 Mpn140 Mpn395 Mpn014 Mpn658 Mpn660 Mpn167 Mpn658 Mpn176 Mpn235 Mpn014 Mpn025 Mpn115 Mpn573 Mpn167 Mpn226 Mpn174 Mpn191 Mpn191 Mpn220 Mpn191 Mpn282 Mpn123 Mpn358 Mpn572 Mpn611 Mpn250 Mpn035 Mpn582 Mpn020 Mpn145 Mpn620 Mpn238 Mpn572 Mpn611 Mpn250 Mpn341 Mpn238 Mpn358 Mpn178 Mpn561 Mpn088 Mpn620 Mpn035 Mpn633 Mpn238 Mpn358 Mpn250 Mpn424 Mpn123 Mpn450 Mpn148 Mpn195 General principles of cellular organization in Mycoplasma pneumoniae 957 957 957 957 956 956 955 955 954 954 954 954 954 954 954 954 953 953 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 141 Mpn646 Mpn642 Mpn404 Mpn633 Mpn529 Mpn041 Mpn250 Mpn250 Mpn250 Mpn418 Mpn189 Mpn400 Mpn254 Mpn123 Mpn123 Mpn475 Mpn582 Mpn450 Mpn450 Mpn629 Mpn073 Mpn073 Mpn556 Mpn620 Mpn620 Mpn413 Mpn148 Mpn148 Mpn195 Mpn116 Mpn192 Mpn665 Mpn516 Mpn027 Mpn033 Mpn033 Mpn346 Mpn125 Mpn563 Mpn628 Mpn635 Mpn226 Mpn230 Mpn393 Mpn390 Mpn685 Mpn251 Mpn606 Mpn125 Mpn569 Mpn529 Mpn418 Mpn073 Mpn339 Mpn646 Mpn400 Mpn238 Mpn358 Mpn611 Mpn642 Mpn629 Mpn041 Mpn116 Mpn424 Mpn282 Mpn413 Mpn419 Mpn140 Mpn148 Mpn189 Mpn404 Mpn556 Mpn073 Mpn088 Mpn145 Mpn475 Mpn140 Mpn450 Mpn395 Mpn254 Mpn665 Mpn192 Mpn226 Mpn628 Mpn346 Mpn635 Mpn033 Mpn563 Mpn125 Mpn027 Mpn033 Mpn516 Mpn685 Mpn390 Mpn393 Mpn230 Mpn125 Mpn569 Mpn251 Mpn606 General principles of cellular organization in Mycoplasma pneumoniae 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 952 950 950 949 949 949 949 949 949 949 949 949 949 948 948 948 948 947 947 947 947 142 Mpn135 Mpn187 Mpn187 Mpn235 Mpn066 Mpn492 Mpn492 Mpn551 Mpn366 Mpn678 Mpn189 Mpn436 Mpn277 Mpn191 Mpn300 Mpn211 Mpn048 Mpn059 Mpn665 Mpn574 Mpn032 Mpn226 Mpn121 Mpn121 Mpn232 Mpn116 Mpn246 Mpn257 Mpn257 Mpn561 Mpn155 Mpn446 Mpn418 Mpn254 Mpn211 Mpn158 Mpn209 Mpn116 Mpn006 Mpn134 Mpn171 Mpn622 Mpn320 Mpn076 Mpn573 Mpn367 Mpn426 Mpn514 Mpn100 Mpn228 Mpn121 Mpn189 Mpn191 Mpn678 Mpn048 Mpn436 Mpn277 Mpn059 Mpn121 Mpn235 Mpn187 Mpn492 Mpn492 Mpn187 Mpn211 Mpn300 Mpn066 Mpn551 Mpn226 Mpn032 Mpn574 Mpn665 Mpn135 Mpn366 Mpn116 Mpn232 Mpn155 Mpn254 Mpn116 Mpn446 Mpn246 Mpn561 Mpn211 Mpn257 Mpn418 Mpn209 Mpn158 Mpn257 Mpn134 Mpn006 Mpn622 Mpn171 Mpn076 Mpn320 Mpn426 Mpn514 Mpn573 Mpn367 Mpn650 Mpn225 General principles of cellular organization in Mycoplasma pneumoniae 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 946 945 945 944 944 944 944 944 944 944 944 944 944 944 944 943 943 942 942 941 941 940 940 940 940 939 939 143 Mpn225 Mpn650 Mpn303 Mpn269 Mpn301 Mpn258 Mpn025 Mpn300 Mpn009 Mpn619 Mpn232 Mpn254 Mpn573 Mpn321 Mpn103 Mpn352 Mpn673 Mpn280 Mpn020 Mpn061 Mpn561 Mpn437 Mpn490 Mpn553 Mpn663 Mpn191 Mpn568 Mpn280 Mpn567 Mpn230 Mpn230 Mpn424 Mpn479 Mpn141 Mpn035 Mpn035 Mpn185 Mpn350 Mpn561 Mpn387 Mpn387 Mpn387 Mpn221 Mpn378 Mpn515 Mpn282 Mpn446 Mpn203 Mpn401 Mpn301 Mpn228 Mpn100 Mpn025 Mpn258 Mpn300 Mpn269 Mpn303 Mpn301 Mpn619 Mpn009 Mpn254 Mpn232 Mpn321 Mpn573 Mpn280 Mpn673 Mpn352 Mpn103 Mpn490 Mpn280 Mpn553 Mpn191 Mpn020 Mpn561 Mpn568 Mpn437 Mpn663 Mpn061 Mpn525 Mpn035 Mpn561 Mpn387 Mpn289 Mpn378 Mpn230 Mpn446 Mpn438 Mpn401 Mpn230 Mpn424 Mpn282 Mpn123 Mpn305 Mpn141 Mpn539 Mpn387 Mpn035 Mpn391 Mpn350 Mpn508 General principles of cellular organization in Mycoplasma pneumoniae 939 939 937 937 937 937 937 937 936 936 936 936 935 935 935 935 935 935 934 934 934 934 934 934 934 934 934 934 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 144 Mpn140 Mpn504 Mpn438 Mpn438 Mpn391 Mpn508 Mpn311 Mpn311 Mpn311 Mpn311 Mpn316 Mpn389 Mpn248 Mpn123 Mpn569 Mpn095 Mpn091 Mpn220 Mpn450 Mpn305 Mpn525 Mpn525 Mpn539 Mpn289 Mpn289 Mpn342 Mpn148 Mpn110 Mpn416 Mpn251 Mpn221 Mpn301 Mpn367 Mpn211 Mpn209 Mpn200 Mpn499 Mpn499 Mpn337 Mpn640 Mpn649 Mpn331 Mpn354 Mpn061 Mpn402 Mpn252 Mpn317 Mpn685 Mpn269 Mpn241 Mpn311 Mpn248 Mpn185 Mpn342 Mpn203 Mpn301 Mpn140 Mpn095 Mpn450 Mpn148 Mpn569 Mpn289 Mpn504 Mpn387 Mpn316 Mpn311 Mpn220 Mpn091 Mpn311 Mpn221 Mpn567 Mpn110 Mpn515 Mpn479 Mpn389 Mpn438 Mpn311 Mpn525 Mpn337 Mpn640 Mpn649 Mpn211 Mpn499 Mpn301 Mpn331 Mpn499 Mpn367 Mpn200 Mpn416 Mpn251 Mpn221 Mpn209 Mpn061 Mpn354 Mpn252 Mpn402 Mpn685 Mpn317 Mpn241 Mpn269 General principles of cellular organization in Mycoplasma pneumoniae 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 933 932 932 932 932 932 932 932 932 932 932 932 932 932 932 931 931 931 931 930 930 929 929 145 Mpn379 Mpn210 Mpn379 Mpn020 Mpn020 Mpn261 Mpn397 Mpn021 Mpn509 Mpn509 Mpn315 Mpn218 Mpn572 Mpn118 Mpn118 Mpn097 Mpn041 Mpn400 Mpn281 Mpn536 Mpn225 Mpn599 Mpn665 Mpn658 Mpn314 Mpn302 Mpn006 Mpn549 Mpn452 Mpn232 Mpn228 Mpn665 Mpn280 Mpn219 Mpn516 Mpn219 Mpn573 Mpn020 Mpn265 Mpn189 Mpn390 Mpn191 Mpn225 Mpn220 Mpn665 Mpn685 Mpn315 Mpn611 Mpn197 Mpn317 Mpn210 Mpn379 Mpn020 Mpn379 Mpn261 Mpn020 Mpn021 Mpn397 Mpn281 Mpn599 Mpn314 Mpn572 Mpn218 Mpn041 Mpn400 Mpn302 Mpn118 Mpn118 Mpn509 Mpn658 Mpn665 Mpn509 Mpn225 Mpn536 Mpn315 Mpn097 Mpn452 Mpn280 Mpn006 Mpn228 Mpn232 Mpn219 Mpn549 Mpn665 Mpn219 Mpn516 Mpn390 Mpn265 Mpn020 Mpn685 Mpn573 Mpn225 Mpn191 Mpn665 Mpn220 Mpn189 Mpn317 Mpn197 Mpn611 Mpn315 General principles of cellular organization in Mycoplasma pneumoniae 928 928 927 927 926 926 926 926 925 925 925 925 925 925 925 925 925 925 925 925 925 925 925 925 925 925 923 923 923 923 923 923 923 923 922 922 921 921 921 921 921 921 921 921 921 921 920 920 920 920 146 Mpn238 Mpn306 Mpn192 Mpn218 Mpn636 Mpn497 Mpn294 Mpn399 Mpn118 Mpn677 Mpn009 Mpn339 Mpn633 Mpn557 Mpn445 Mpn261 Mpn423 Mpn311 Mpn176 Mpn563 Mpn223 Mpn300 Mpn465 Mpn091 Mpn024 Mpn574 Mpn204 Mpn314 Mpn314 Mpn685 Mpn155 Mpn155 Mpn155 Mpn103 Mpn228 Mpn133 Mpn465 Mpn158 Mpn179 Mpn169 Mpn016 Mpn331 Mpn168 Mpn118 Mpn172 Mpn191 Mpn173 Mpn353 Mpn665 Mpn665 Mpn218 Mpn497 Mpn636 Mpn238 Mpn192 Mpn306 Mpn445 Mpn563 Mpn557 Mpn223 Mpn261 Mpn314 Mpn314 Mpn118 Mpn294 Mpn009 Mpn300 Mpn204 Mpn091 Mpn399 Mpn677 Mpn423 Mpn685 Mpn176 Mpn574 Mpn024 Mpn311 Mpn339 Mpn633 Mpn465 Mpn133 Mpn179 Mpn016 Mpn228 Mpn103 Mpn155 Mpn169 Mpn331 Mpn155 Mpn465 Mpn155 Mpn158 Mpn665 Mpn353 Mpn665 Mpn173 Mpn191 Mpn118 Mpn168 Mpn172 General principles of cellular organization in Mycoplasma pneumoniae 919 919 919 919 919 919 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 918 917 917 917 917 917 917 917 917 917 917 917 917 916 916 916 916 916 916 916 916 147 Mpn354 Mpn322 Mpn023 Mpn454 Mpn461 Mpn247 Mpn218 Mpn218 Mpn572 Mpn611 Mpn688 Mpn250 Mpn197 Mpn386 Mpn280 Mpn562 Mpn424 Mpn154 Mpn310 Mpn426 Mpn173 Mpn402 Mpn516 Mpn243 Mpn310 Mpn688 Mpn022 Mpn598 Mpn663 Mpn225 Mpn457 Mpn269 Mpn269 Mpn262 Mpn321 Mpn063 Mpn176 Mpn665 Mpn114 Mpn047 Mpn182 Mpn665 Mpn516 Mpn428 Mpn539 Mpn226 Mpn538 Mpn179 Mpn665 Mpn665 Mpn023 Mpn454 Mpn354 Mpn322 Mpn247 Mpn461 Mpn611 Mpn250 Mpn197 Mpn218 Mpn386 Mpn218 Mpn572 Mpn688 Mpn562 Mpn280 Mpn402 Mpn173 Mpn426 Mpn310 Mpn154 Mpn424 Mpn225 Mpn663 Mpn022 Mpn598 Mpn310 Mpn688 Mpn243 Mpn516 Mpn321 Mpn262 Mpn114 Mpn269 Mpn457 Mpn047 Mpn665 Mpn176 Mpn269 Mpn063 Mpn665 Mpn182 Mpn539 Mpn226 Mpn516 Mpn428 Mpn665 Mpn665 Mpn538 Mpn179 General principles of cellular organization in Mycoplasma pneumoniae 915 915 915 915 915 915 914 914 914 914 914 914 914 914 914 914 913 913 913 913 913 913 912 912 912 912 912 912 912 912 911 911 911 911 911 911 911 911 911 911 910 910 909 909 909 909 908 908 908 908 148 Mpn567 Mpn479 Mpn001 Mpn185 Mpn307 Mpn155 Mpn515 Mpn167 Mpn590 Mpn590 Mpn389 Mpn165 Mpn220 Mpn665 Mpn531 Mpn342 Mpn195 Mpn110 Mpn177 Mpn553 Mpn115 Mpn618 Mpn685 Mpn072 Mpn516 Mpn118 Mpn563 Mpn220 Mpn671 Mpn246 Mpn611 Mpn553 Mpn450 Mpn204 Mpn106 Mpn303 Mpn025 Mpn430 Mpn243 Mpn328 Mpn656 Mpn330 Mpn665 Mpn174 Mpn020 Mpn020 Mpn177 Mpn434 Mpn264 Mpn191 Mpn110 Mpn389 Mpn195 Mpn342 Mpn590 Mpn167 Mpn220 Mpn155 Mpn307 Mpn531 Mpn479 Mpn665 Mpn515 Mpn165 Mpn590 Mpn185 Mpn001 Mpn567 Mpn072 Mpn115 Mpn553 Mpn685 Mpn618 Mpn177 Mpn220 Mpn563 Mpn118 Mpn516 Mpn611 Mpn450 Mpn671 Mpn204 Mpn246 Mpn553 Mpn025 Mpn430 Mpn106 Mpn303 Mpn656 Mpn330 Mpn243 Mpn328 Mpn174 Mpn665 Mpn434 Mpn264 Mpn665 Mpn020 Mpn020 Mpn665 General principles of cellular organization in Mycoplasma pneumoniae 907 907 907 907 907 907 907 907 907 907 907 907 907 907 907 907 907 907 906 906 906 906 906 906 905 905 905 905 904 904 904 904 904 904 903 903 903 903 901 901 901 901 901 901 900 900 900 900 900 900 149 Mpn665 Mpn665 Mpn006 Mpn515 Mpn182 Mpn490 Mpn390 Mpn402 Mpn567 Mpn011 Mpn083 Mpn419 Mpn321 Mpn177 Mpn434 Mpn094 Mpn395 Mpn395 Mpn367 Mpn142 Mpn388 Mpn025 Mpn095 Mpn292 Mpn292 Mpn669 Mpn305 Mpn353 Mpn353 Mpn263 Mpn178 Mpn171 Mpn320 Mpn665 Mpn665 Mpn284 Mpn612 Mpn612 Mpn612 Mpn612 Mpn298 Mpn298 Mpn011 Mpn011 Mpn011 Mpn135 Mpn238 Mpn238 Mpn178 Mpn083 Mpn177 Mpn191 Mpn390 Mpn182 Mpn515 Mpn402 Mpn006 Mpn490 Mpn095 Mpn419 Mpn353 Mpn011 Mpn263 Mpn434 Mpn177 Mpn292 Mpn367 Mpn388 Mpn395 Mpn353 Mpn395 Mpn669 Mpn567 Mpn094 Mpn305 Mpn025 Mpn292 Mpn083 Mpn142 Mpn321 Mpn665 Mpn665 Mpn284 Mpn178 Mpn171 Mpn320 Mpn088 Mpn145 Mpn569 Mpn620 Mpn041 Mpn400 Mpn140 Mpn450 Mpn148 Mpn366 Mpn197 Mpn372 Mpn465 Mpn142 General principles of cellular organization in Mycoplasma pneumoniae 900 900 899 899 899 899 899 899 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 898 897 897 897 897 897 897 896 896 896 896 896 896 896 896 896 896 896 896 896 896 150 Mpn083 Mpn578 Mpn676 Mpn218 Mpn046 Mpn088 Mpn358 Mpn358 Mpn358 Mpn572 Mpn035 Mpn347 Mpn347 Mpn145 Mpn561 Mpn262 Mpn592 Mpn592 Mpn538 Mpn538 Mpn611 Mpn282 Mpn301 Mpn643 Mpn643 Mpn140 Mpn140 Mpn094 Mpn367 Mpn367 Mpn642 Mpn142 Mpn142 Mpn142 Mpn366 Mpn366 Mpn564 Mpn041 Mpn250 Mpn250 Mpn445 Mpn197 Mpn197 Mpn197 Mpn441 Mpn441 Mpn372 Mpn372 Mpn372 Mpn372 Mpn582 Mpn444 Mpn036 Mpn358 Mpn445 Mpn612 Mpn218 Mpn197 Mpn372 Mpn372 Mpn465 Mpn254 Mpn116 Mpn612 Mpn465 Mpn114 Mpn254 Mpn116 Mpn142 Mpn441 Mpn372 Mpn402 Mpn423 Mpn065 Mpn216 Mpn011 Mpn466 Mpn305 Mpn200 Mpn195 Mpn211 Mpn083 Mpn538 Mpn021 Mpn135 Mpn474 Mpn048 Mpn298 Mpn197 Mpn372 Mpn046 Mpn238 Mpn358 Mpn250 Mpn538 Mpn021 Mpn238 Mpn358 Mpn572 Mpn611 General principles of cellular organization in Mycoplasma pneumoniae 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 151 Mpn372 Mpn423 Mpn400 Mpn254 Mpn254 Mpn065 Mpn474 Mpn123 Mpn241 Mpn241 Mpn415 Mpn211 Mpn048 Mpn465 Mpn465 Mpn465 Mpn569 Mpn582 Mpn200 Mpn200 Mpn076 Mpn444 Mpn450 Mpn450 Mpn305 Mpn665 Mpn665 Mpn114 Mpn114 Mpn466 Mpn466 Mpn466 Mpn402 Mpn402 Mpn645 Mpn169 Mpn021 Mpn021 Mpn036 Mpn620 Mpn216 Mpn284 Mpn148 Mpn148 Mpn195 Mpn188 Mpn116 Mpn116 Mpn167 Mpn166 Mpn250 Mpn301 Mpn298 Mpn347 Mpn592 Mpn643 Mpn366 Mpn402 Mpn415 Mpn114 Mpn241 Mpn642 Mpn564 Mpn178 Mpn035 Mpn561 Mpn612 Mpn083 Mpn367 Mpn645 Mpn284 Mpn578 Mpn011 Mpn466 Mpn094 Mpn169 Mpn188 Mpn262 Mpn241 Mpn140 Mpn450 Mpn148 Mpn282 Mpn123 Mpn200 Mpn665 Mpn142 Mpn441 Mpn676 Mpn612 Mpn643 Mpn076 Mpn011 Mpn466 Mpn367 Mpn665 Mpn347 Mpn592 Mpn665 Mpn665 General principles of cellular organization in Mycoplasma pneumoniae 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 896 895 895 152 Mpn665 Mpn665 Mpn515 Mpn441 Mpn225 Mpn547 Mpn573 Mpn606 Mpn418 Mpn172 Mpn178 Mpn561 Mpn538 Mpn434 Mpn386 Mpn520 Mpn520 Mpn174 Mpn595 Mpn462 Mpn553 Mpn471 Mpn231 Mpn407 Mpn665 Mpn574 Mpn323 Mpn180 Mpn191 Mpn443 Mpn516 Mpn416 Mpn154 Mpn067 Mpn515 Mpn555 Mpn623 Mpn324 Mpn434 Mpn394 Mpn622 Mpn508 Mpn115 Mpn300 Mpn567 Mpn424 Mpn351 Mpn365 Mpn155 Mpn515 Mpn167 Mpn166 Mpn225 Mpn547 Mpn515 Mpn441 Mpn606 Mpn573 Mpn172 Mpn418 Mpn520 Mpn520 Mpn434 Mpn538 Mpn174 Mpn178 Mpn561 Mpn386 Mpn553 Mpn471 Mpn595 Mpn462 Mpn574 Mpn323 Mpn180 Mpn231 Mpn407 Mpn665 Mpn443 Mpn191 Mpn067 Mpn623 Mpn515 Mpn516 Mpn154 Mpn324 Mpn416 Mpn555 Mpn394 Mpn434 Mpn115 Mpn300 Mpn622 Mpn508 Mpn390 Mpn351 Mpn424 Mpn040 Mpn515 Mpn155 General principles of cellular organization in Mycoplasma pneumoniae 895 895 894 894 894 894 893 893 893 893 892 892 892 892 892 892 892 892 891 891 891 891 891 891 891 891 891 891 890 890 889 889 889 889 889 889 889 889 888 888 887 887 887 887 886 886 886 886 886 886 153 Mpn390 Mpn040 Mpn516 Mpn003 Mpn189 Mpn686 Mpn179 Mpn665 Mpn156 Mpn156 Mpn479 Mpn541 Mpn538 Mpn446 Mpn033 Mpn683 Mpn551 Mpn438 Mpn663 Mpn593 Mpn397 Mpn569 Mpn040 Mpn629 Mpn226 Mpn252 Mpn424 Mpn246 Mpn266 Mpn622 Mpn257 Mpn347 Mpn561 Mpn515 Mpn140 Mpn352 Mpn060 Mpn226 Mpn516 Mpn178 Mpn061 Mpn434 Mpn688 Mpn362 Mpn430 Mpn443 Mpn428 Mpn187 Mpn167 Mpn656 Mpn567 Mpn365 Mpn179 Mpn686 Mpn665 Mpn003 Mpn516 Mpn189 Mpn033 Mpn593 Mpn040 Mpn226 Mpn397 Mpn252 Mpn156 Mpn551 Mpn683 Mpn663 Mpn438 Mpn156 Mpn538 Mpn629 Mpn479 Mpn569 Mpn541 Mpn446 Mpn060 Mpn140 Mpn352 Mpn561 Mpn347 Mpn257 Mpn622 Mpn226 Mpn246 Mpn266 Mpn424 Mpn515 Mpn178 Mpn516 Mpn688 Mpn430 Mpn061 Mpn443 Mpn434 Mpn362 Mpn167 Mpn660 Mpn428 Mpn381 General principles of cellular organization in Mycoplasma pneumoniae 886 886 885 885 885 885 885 885 884 884 884 884 884 884 884 884 884 884 884 884 884 884 884 884 884 884 883 883 883 883 883 883 883 883 883 883 883 883 882 882 882 882 882 882 882 882 881 881 881 881 154 Mpn660 Mpn165 Mpn475 Mpn381 Mpn516 Mpn154 Mpn067 Mpn020 Mpn009 Mpn515 Mpn210 Mpn008 Mpn025 Mpn248 Mpn007 Mpn317 Mpn574 Mpn535 Mpn434 Mpn194 Mpn264 Mpn247 Mpn322 Mpn671 Mpn106 Mpn118 Mpn185 Mpn257 Mpn378 Mpn656 Mpn426 Mpn553 Mpn173 Mpn665 Mpn283 Mpn216 Mpn524 Mpn572 Mpn347 Mpn592 Mpn446 Mpn434 Mpn265 Mpn362 Mpn609 Mpn254 Mpn429 Mpn526 Mpn526 Mpn526 Mpn187 Mpn475 Mpn165 Mpn656 Mpn154 Mpn516 Mpn248 Mpn007 Mpn210 Mpn025 Mpn009 Mpn317 Mpn515 Mpn067 Mpn020 Mpn008 Mpn535 Mpn574 Mpn247 Mpn264 Mpn194 Mpn434 Mpn426 Mpn185 Mpn553 Mpn378 Mpn671 Mpn656 Mpn118 Mpn257 Mpn322 Mpn106 Mpn665 Mpn173 Mpn216 Mpn283 Mpn446 Mpn609 Mpn526 Mpn526 Mpn524 Mpn429 Mpn362 Mpn265 Mpn572 Mpn526 Mpn434 Mpn347 Mpn592 Mpn254 General principles of cellular organization in Mycoplasma pneumoniae 881 881 881 881 880 880 880 880 880 880 880 880 880 880 880 880 880 880 879 879 879 879 878 878 878 878 878 878 878 878 878 878 878 878 878 878 877 877 877 877 877 877 877 877 877 877 877 877 877 877 155 Mpn526 Mpn116 Mpn002 Mpn553 Mpn002 Mpn660 Mpn191 Mpn685 Mpn020 Mpn106 Mpn257 Mpn232 Mpn153 Mpn669 Mpn182 Mpn008 Mpn558 Mpn252 Mpn668 Mpn479 Mpn022 Mpn619 Mpn586 Mpn676 Mpn218 Mpn218 Mpn001 Mpn029 Mpn350 Mpn434 Mpn643 Mpn094 Mpn395 Mpn656 Mpn197 Mpn182 Mpn441 Mpn327 Mpn065 Mpn558 Mpn059 Mpn628 Mpn628 Mpn124 Mpn349 Mpn539 Mpn539 Mpn247 Mpn671 Mpn419 Mpn116 Mpn526 Mpn553 Mpn002 Mpn191 Mpn685 Mpn002 Mpn660 Mpn153 Mpn669 Mpn232 Mpn257 Mpn020 Mpn106 Mpn252 Mpn558 Mpn008 Mpn182 Mpn479 Mpn668 Mpn619 Mpn022 Mpn029 Mpn327 Mpn197 Mpn628 Mpn395 Mpn586 Mpn628 Mpn124 Mpn539 Mpn247 Mpn001 Mpn349 Mpn218 Mpn441 Mpn182 Mpn676 Mpn539 Mpn059 Mpn558 Mpn218 Mpn350 Mpn434 Mpn656 Mpn643 Mpn065 Mpn094 Mpn259 Mpn361 General principles of cellular organization in Mycoplasma pneumoniae 877 877 876 876 875 875 875 875 873 873 873 873 873 873 872 872 872 872 871 871 871 871 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 870 869 869 156 Mpn341 Mpn259 Mpn361 Mpn114 Mpn516 Mpn549 Mpn020 Mpn171 Mpn561 Mpn346 Mpn346 Mpn540 Mpn636 Mpn133 Mpn191 Mpn535 Mpn616 Mpn016 Mpn171 Mpn265 Mpn446 Mpn665 Mpn573 Mpn275 Mpn003 Mpn544 Mpn642 Mpn172 Mpn021 Mpn036 Mpn616 Mpn685 Mpn354 Mpn668 Mpn434 Mpn420 Mpn189 Mpn115 Mpn434 Mpn025 Mpn393 Mpn220 Mpn354 Mpn483 Mpn497 Mpn520 Mpn567 Mpn573 Mpn187 Mpn553 Mpn114 Mpn671 Mpn419 Mpn341 Mpn171 Mpn636 Mpn191 Mpn516 Mpn616 Mpn133 Mpn016 Mpn535 Mpn549 Mpn346 Mpn020 Mpn540 Mpn561 Mpn346 Mpn265 Mpn171 Mpn665 Mpn446 Mpn021 Mpn036 Mpn544 Mpn003 Mpn172 Mpn642 Mpn573 Mpn275 Mpn685 Mpn616 Mpn434 Mpn420 Mpn354 Mpn668 Mpn115 Mpn189 Mpn220 Mpn393 Mpn025 Mpn434 Mpn520 Mpn497 Mpn483 Mpn354 Mpn295 Mpn553 Mpn226 Mpn573 General principles of cellular organization in Mycoplasma pneumoniae 869 869 869 869 868 868 868 868 868 868 868 868 868 868 868 868 868 868 867 867 865 865 864 864 864 864 864 864 864 864 864 864 863 863 863 863 863 863 862 862 862 862 861 861 861 861 860 860 860 860 157 Mpn295 Mpn226 Mpn322 Mpn269 Mpn555 Mpn362 Mpn277 Mpn108 Mpn223 Mpn323 Mpn612 Mpn230 Mpn051 Mpn354 Mpn416 Mpn011 Mpn668 Mpn246 Mpn246 Mpn192 Mpn083 Mpn676 Mpn559 Mpn559 Mpn100 Mpn088 Mpn088 Mpn099 Mpn677 Mpn677 Mpn677 Mpn351 Mpn351 Mpn035 Mpn257 Mpn145 Mpn145 Mpn561 Mpn387 Mpn221 Mpn089 Mpn378 Mpn533 Mpn071 Mpn592 Mpn487 Mpn487 Mpn538 Mpn282 Mpn282 Mpn567 Mpn187 Mpn323 Mpn277 Mpn223 Mpn108 Mpn269 Mpn362 Mpn555 Mpn322 Mpn316 Mpn465 Mpn535 Mpn252 Mpn099 Mpn311 Mpn289 Mpn438 Mpn148 Mpn525 Mpn378 Mpn081 Mpn538 Mpn021 Mpn173 Mpn677 Mpn526 Mpn416 Mpn088 Mpn145 Mpn620 Mpn282 Mpn123 Mpn182 Mpn592 Mpn677 Mpn526 Mpn182 Mpn402 Mpn094 Mpn507 Mpn083 Mpn289 Mpn273 Mpn257 Mpn475 Mpn413 Mpn559 Mpn351 Mpn060 General principles of cellular organization in Mycoplasma pneumoniae 860 860 859 859 859 859 859 859 859 859 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 158 Mpn446 Mpn446 Mpn401 Mpn177 Mpn606 Mpn094 Mpn094 Mpn646 Mpn529 Mpn041 Mpn041 Mpn480 Mpn182 Mpn182 Mpn438 Mpn508 Mpn508 Mpn423 Mpn590 Mpn590 Mpn311 Mpn311 Mpn316 Mpn400 Mpn400 Mpn474 Mpn123 Mpn123 Mpn461 Mpn475 Mpn300 Mpn507 Mpn211 Mpn465 Mpn465 Mpn330 Mpn173 Mpn273 Mpn273 Mpn526 Mpn526 Mpn526 Mpn124 Mpn124 Mpn124 Mpn124 Mpn024 Mpn081 Mpn525 Mpn525 Mpn480 Mpn465 Mpn590 Mpn590 Mpn289 Mpn221 Mpn461 Mpn124 Mpn124 Mpn124 Mpn331 Mpn446 Mpn035 Mpn561 Mpn246 Mpn423 Mpn211 Mpn508 Mpn401 Mpn177 Mpn011 Mpn466 Mpn612 Mpn124 Mpn331 Mpn273 Mpn351 Mpn060 Mpn094 Mpn487 Mpn525 Mpn089 Mpn508 Mpn230 Mpn446 Mpn024 Mpn100 Mpn071 Mpn474 Mpn088 Mpn145 Mpn620 Mpn646 Mpn529 Mpn041 Mpn400 Mpn330 Mpn676 Mpn192 Mpn300 General principles of cellular organization in Mycoplasma pneumoniae 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 159 Mpn535 Mpn466 Mpn402 Mpn021 Mpn289 Mpn289 Mpn289 Mpn620 Mpn620 Mpn413 Mpn060 Mpn060 Mpn331 Mpn331 Mpn148 Mpn252 Mpn612 Mpn516 Mpn559 Mpn099 Mpn307 Mpn187 Mpn340 Mpn606 Mpn078 Mpn674 Mpn674 Mpn165 Mpn292 Mpn599 Mpn499 Mpn499 Mpn640 Mpn645 Mpn531 Mpn413 Mpn072 Mpn514 Mpn533 Mpn197 Mpn389 Mpn553 Mpn652 Mpn032 Mpn516 Mpn182 Mpn264 Mpn267 Mpn303 Mpn434 Mpn051 Mpn311 Mpn387 Mpn559 Mpn668 Mpn533 Mpn606 Mpn677 Mpn526 Mpn487 Mpn282 Mpn123 Mpn041 Mpn400 Mpn246 Mpn354 Mpn606 Mpn674 Mpn640 Mpn187 Mpn531 Mpn099 Mpn674 Mpn612 Mpn292 Mpn516 Mpn340 Mpn413 Mpn078 Mpn072 Mpn645 Mpn514 Mpn559 Mpn499 Mpn307 Mpn165 Mpn599 Mpn499 Mpn389 Mpn553 Mpn533 Mpn197 Mpn032 Mpn652 Mpn182 Mpn516 Mpn267 Mpn264 Mpn434 Mpn303 General principles of cellular organization in Mycoplasma pneumoniae 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 858 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 857 856 856 856 856 856 856 854 854 854 854 852 852 160 Mpn208 Mpn191 Mpn516 Mpn002 Mpn002 Mpn002 Mpn446 Mpn452 Mpn261 Mpn686 Mpn363 Mpn165 Mpn571 Mpn169 Mpn169 Mpn520 Mpn428 Mpn354 Mpn298 Mpn322 Mpn322 Mpn424 Mpn549 Mpn549 Mpn549 Mpn621 Mpn621 Mpn621 Mpn621 Mpn621 Mpn002 Mpn246 Mpn294 Mpn294 Mpn218 Mpn023 Mpn023 Mpn141 Mpn251 Mpn392 Mpn020 Mpn020 Mpn020 Mpn303 Mpn303 Mpn009 Mpn185 Mpn622 Mpn350 Mpn221 Mpn191 Mpn208 Mpn002 Mpn516 Mpn261 Mpn165 Mpn520 Mpn686 Mpn002 Mpn452 Mpn169 Mpn002 Mpn169 Mpn363 Mpn571 Mpn446 Mpn430 Mpn352 Mpn295 Mpn321 Mpn320 Mpn426 Mpn555 Mpn553 Mpn556 Mpn622 Mpn619 Mpn618 Mpn616 Mpn620 Mpn004 Mpn258 Mpn303 Mpn295 Mpn228 Mpn022 Mpn025 Mpn142 Mpn261 Mpn394 Mpn025 Mpn024 Mpn019 Mpn294 Mpn295 Mpn004 Mpn663 Mpn621 Mpn352 Mpn226 General principles of cellular organization in Mycoplasma pneumoniae 852 852 851 851 851 851 851 851 851 851 851 851 851 851 851 851 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 161 Mpn262 Mpn321 Mpn321 Mpn003 Mpn033 Mpn434 Mpn066 Mpn222 Mpn222 Mpn142 Mpn555 Mpn555 Mpn555 Mpn555 Mpn555 Mpn551 Mpn258 Mpn258 Mpn258 Mpn258 Mpn258 Mpn022 Mpn656 Mpn656 Mpn656 Mpn250 Mpn250 Mpn452 Mpn619 Mpn684 Mpn197 Mpn261 Mpn261 Mpn261 Mpn261 Mpn261 Mpn261 Mpn261 Mpn261 Mpn034 Mpn034 Mpn210 Mpn210 Mpn210 Mpn423 Mpn008 Mpn008 Mpn426 Mpn426 Mpn025 Mpn258 Mpn322 Mpn324 Mpn008 Mpn034 Mpn436 Mpn062 Mpn228 Mpn226 Mpn141 Mpn549 Mpn551 Mpn553 Mpn550 Mpn556 Mpn555 Mpn246 Mpn262 Mpn250 Mpn261 Mpn252 Mpn023 Mpn663 Mpn665 Mpn658 Mpn258 Mpn261 Mpn450 Mpn621 Mpn685 Mpn191 Mpn251 Mpn258 Mpn250 Mpn264 Mpn248 Mpn259 Mpn247 Mpn252 Mpn033 Mpn032 Mpn208 Mpn211 Mpn209 Mpn426 Mpn003 Mpn004 Mpn424 Mpn423 Mpn023 General principles of cellular organization in Mycoplasma pneumoniae 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 162 Mpn025 Mpn352 Mpn352 Mpn264 Mpn320 Mpn320 Mpn228 Mpn228 Mpn228 Mpn228 Mpn553 Mpn553 Mpn553 Mpn550 Mpn663 Mpn663 Mpn663 Mpn663 Mpn686 Mpn004 Mpn004 Mpn004 Mpn004 Mpn248 Mpn436 Mpn393 Mpn660 Mpn208 Mpn062 Mpn191 Mpn295 Mpn295 Mpn295 Mpn211 Mpn259 Mpn259 Mpn618 Mpn007 Mpn324 Mpn324 Mpn209 Mpn024 Mpn546 Mpn220 Mpn450 Mpn430 Mpn665 Mpn665 Mpn665 Mpn665 Mpn020 Mpn354 Mpn350 Mpn261 Mpn322 Mpn324 Mpn218 Mpn222 Mpn220 Mpn219 Mpn549 Mpn555 Mpn556 Mpn555 Mpn185 Mpn656 Mpn665 Mpn658 Mpn685 Mpn002 Mpn009 Mpn008 Mpn007 Mpn261 Mpn434 Mpn394 Mpn665 Mpn210 Mpn066 Mpn197 Mpn298 Mpn294 Mpn303 Mpn210 Mpn261 Mpn252 Mpn621 Mpn004 Mpn321 Mpn320 Mpn210 Mpn020 Mpn547 Mpn228 Mpn452 Mpn428 Mpn656 Mpn663 Mpn660 Mpn658 General principles of cellular organization in Mycoplasma pneumoniae 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 163 Mpn665 Mpn019 Mpn547 Mpn394 Mpn394 Mpn247 Mpn658 Mpn658 Mpn658 Mpn556 Mpn556 Mpn556 Mpn219 Mpn032 Mpn616 Mpn620 Mpn662 Mpn685 Mpn685 Mpn226 Mpn226 Mpn252 Mpn252 Mpn252 Mpn424 Mpn266 Mpn434 Mpn434 Mpn674 Mpn191 Mpn023 Mpn303 Mpn155 Mpn033 Mpn440 Mpn556 Mpn472 Mpn207 Mpn602 Mpn542 Mpn429 Mpn430 Mpn426 Mpn317 Mpn354 Mpn487 Mpn515 Mpn222 Mpn007 Mpn169 Mpn662 Mpn020 Mpn546 Mpn392 Mpn393 Mpn261 Mpn656 Mpn663 Mpn665 Mpn549 Mpn555 Mpn553 Mpn228 Mpn034 Mpn621 Mpn621 Mpn665 Mpn684 Mpn686 Mpn221 Mpn222 Mpn258 Mpn261 Mpn259 Mpn266 Mpn424 Mpn674 Mpn191 Mpn434 Mpn434 Mpn303 Mpn023 Mpn033 Mpn155 Mpn556 Mpn440 Mpn429 Mpn602 Mpn207 Mpn430 Mpn472 Mpn542 Mpn317 Mpn426 Mpn007 Mpn222 Mpn169 Mpn487 Mpn354 Mpn515 General principles of cellular organization in Mycoplasma pneumoniae 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 850 849 849 849 849 849 849 848 848 848 848 848 848 847 847 847 847 847 847 846 846 845 845 845 845 845 845 164 Mpn168 Mpn294 Mpn399 Mpn533 Mpn487 Mpn044 Mpn383 Mpn517 Mpn596 Mpn684 Mpn540 Mpn493 Mpn223 Mpn618 Mpn618 Mpn060 Mpn226 Mpn252 Mpn638 Mpn365 Mpn297 Mpn078 Mpn066 Mpn504 Mpn232 Mpn389 Mpn474 Mpn415 Mpn397 Mpn331 Mpn416 Mpn573 Mpn238 Mpn238 Mpn178 Mpn671 Mpn671 Mpn671 Mpn671 Mpn671 Mpn578 Mpn578 Mpn578 Mpn341 Mpn615 Mpn615 Mpn358 Mpn358 Mpn266 Mpn572 Mpn060 Mpn540 Mpn487 Mpn383 Mpn399 Mpn223 Mpn533 Mpn226 Mpn493 Mpn618 Mpn294 Mpn596 Mpn044 Mpn684 Mpn252 Mpn168 Mpn517 Mpn618 Mpn474 Mpn066 Mpn397 Mpn389 Mpn365 Mpn331 Mpn415 Mpn078 Mpn638 Mpn232 Mpn297 Mpn504 Mpn656 Mpn663 Mpn671 Mpn609 Mpn518 Mpn238 Mpn358 Mpn572 Mpn250 Mpn438 Mpn596 Mpn309 Mpn635 Mpn363 Mpn343 Mpn091 Mpn671 Mpn609 Mpn123 Mpn671 General principles of cellular organization in Mycoplasma pneumoniae 844 844 844 844 844 844 844 844 844 844 844 844 844 844 844 844 844 844 843 843 843 843 843 843 843 843 843 843 843 843 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 165 Mpn213 Mpn035 Mpn035 Mpn035 Mpn035 Mpn035 Mpn622 Mpn561 Mpn561 Mpn343 Mpn097 Mpn014 Mpn078 Mpn688 Mpn688 Mpn656 Mpn250 Mpn250 Mpn678 Mpn623 Mpn596 Mpn596 Mpn684 Mpn438 Mpn388 Mpn388 Mpn609 Mpn609 Mpn609 Mpn281 Mpn663 Mpn363 Mpn277 Mpn474 Mpn123 Mpn518 Mpn518 Mpn518 Mpn518 Mpn125 Mpn125 Mpn599 Mpn309 Mpn337 Mpn091 Mpn091 Mpn305 Mpn525 Mpn179 Mpn179 Mpn204 Mpn622 Mpn518 Mpn179 Mpn616 Mpn520 Mpn035 Mpn518 Mpn179 Mpn615 Mpn091 Mpn688 Mpn289 Mpn014 Mpn520 Mpn416 Mpn671 Mpn609 Mpn305 Mpn337 Mpn578 Mpn036 Mpn114 Mpn671 Mpn525 Mpn195 Mpn238 Mpn358 Mpn250 Mpn599 Mpn573 Mpn341 Mpn114 Mpn518 Mpn266 Mpn178 Mpn035 Mpn561 Mpn474 Mpn486 Mpn650 Mpn281 Mpn578 Mpn623 Mpn615 Mpn097 Mpn678 Mpn388 Mpn035 Mpn561 General principles of cellular organization in Mycoplasma pneumoniae 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 842 166 Mpn114 Mpn114 Mpn204 Mpn635 Mpn486 Mpn289 Mpn036 Mpn036 Mpn616 Mpn520 Mpn520 Mpn520 Mpn195 Mpn650 Mpn549 Mpn187 Mpn515 Mpn345 Mpn020 Mpn606 Mpn352 Mpn443 Mpn061 Mpn155 Mpn434 Mpn600 Mpn327 Mpn208 Mpn081 Mpn616 Mpn315 Mpn046 Mpn445 Mpn316 Mpn636 Mpn208 Mpn115 Mpn042 Mpn042 Mpn042 Mpn615 Mpn118 Mpn307 Mpn155 Mpn383 Mpn063 Mpn480 Mpn674 Mpn189 Mpn025 Mpn684 Mpn277 Mpn213 Mpn578 Mpn125 Mpn078 Mpn596 Mpn520 Mpn035 Mpn035 Mpn688 Mpn036 Mpn388 Mpn125 Mpn345 Mpn515 Mpn187 Mpn549 Mpn352 Mpn443 Mpn020 Mpn606 Mpn600 Mpn616 Mpn208 Mpn061 Mpn081 Mpn434 Mpn327 Mpn155 Mpn042 Mpn042 Mpn042 Mpn636 Mpn316 Mpn115 Mpn208 Mpn315 Mpn046 Mpn445 Mpn081 Mpn025 Mpn063 Mpn189 Mpn674 Mpn307 Mpn520 Mpn383 Mpn155 Mpn118 General principles of cellular organization in Mycoplasma pneumoniae 842 842 842 842 842 842 842 842 842 842 842 842 842 842 841 841 841 841 840 840 840 840 839 839 839 839 839 839 839 839 838 838 838 838 838 838 838 838 838 838 836 836 836 836 836 836 836 836 836 836 167 Mpn004 Mpn081 Mpn520 Mpn072 Mpn573 Mpn023 Mpn106 Mpn683 Mpn324 Mpn430 Mpn516 Mpn487 Mpn446 Mpn177 Mpn674 Mpn526 Mpn479 Mpn615 Mpn278 Mpn155 Mpn078 Mpn176 Mpn542 Mpn563 Mpn042 Mpn650 Mpn020 Mpn106 Mpn394 Mpn520 Mpn178 Mpn218 Mpn350 Mpn480 Mpn153 Mpn618 Mpn516 Mpn171 Mpn166 Mpn571 Mpn354 Mpn021 Mpn306 Mpn020 Mpn378 Mpn140 Mpn619 Mpn618 Mpn483 Mpn430 Mpn072 Mpn615 Mpn480 Mpn004 Mpn106 Mpn324 Mpn573 Mpn430 Mpn023 Mpn683 Mpn177 Mpn446 Mpn487 Mpn516 Mpn526 Mpn674 Mpn078 Mpn176 Mpn542 Mpn042 Mpn479 Mpn615 Mpn278 Mpn650 Mpn155 Mpn563 Mpn394 Mpn520 Mpn020 Mpn106 Mpn480 Mpn350 Mpn218 Mpn178 Mpn618 Mpn153 Mpn166 Mpn571 Mpn516 Mpn171 Mpn021 Mpn354 Mpn483 Mpn430 Mpn619 Mpn618 Mpn378 Mpn140 Mpn306 Mpn020 General principles of cellular organization in Mycoplasma pneumoniae 836 836 836 836 835 835 835 835 835 835 834 834 834 834 834 834 831 831 831 831 831 831 831 831 831 831 830 830 830 830 829 829 829 829 829 829 828 828 828 828 827 827 826 826 826 826 826 826 826 826 168 Mpn516 Mpn668 Mpn671 Mpn580 Mpn329 Mpn492 Mpn636 Mpn173 Mpn126 Mpn283 Mpn556 Mpn289 Mpn219 Mpn219 Mpn342 Mpn520 Mpn230 Mpn117 Mpn029 Mpn561 Mpn221 Mpn005 Mpn321 Mpn515 Mpn678 Mpn480 Mpn420 Mpn065 Mpn521 Mpn165 Mpn191 Mpn239 Mpn073 Mpn394 Mpn658 Mpn520 Mpn573 Mpn573 Mpn340 Mpn490 Mpn430 Mpn050 Mpn105 Mpn116 Mpn516 Mpn155 Mpn656 Mpn346 Mpn623 Mpn197 Mpn173 Mpn219 Mpn342 Mpn126 Mpn636 Mpn283 Mpn329 Mpn516 Mpn580 Mpn492 Mpn520 Mpn219 Mpn668 Mpn289 Mpn671 Mpn556 Mpn520 Mpn658 Mpn321 Mpn480 Mpn678 Mpn521 Mpn029 Mpn165 Mpn221 Mpn561 Mpn394 Mpn073 Mpn005 Mpn515 Mpn239 Mpn191 Mpn065 Mpn420 Mpn117 Mpn230 Mpn050 Mpn105 Mpn116 Mpn430 Mpn490 Mpn573 Mpn573 Mpn340 Mpn165 Mpn635 Mpn623 Mpn124 Mpn656 Mpn393 General principles of cellular organization in Mycoplasma pneumoniae 825 825 825 825 825 825 825 825 825 825 825 825 825 825 825 825 824 824 824 824 824 824 824 824 824 824 824 824 824 824 824 824 824 824 824 824 823 823 823 823 823 823 823 823 822 822 822 822 822 822 169 Mpn182 Mpn393 Mpn165 Mpn239 Mpn309 Mpn124 Mpn635 Mpn169 Mpn221 Mpn515 Mpn340 Mpn434 Mpn167 Mpn508 Mpn426 Mpn571 Mpn283 Mpn658 Mpn551 Mpn480 Mpn379 Mpn257 Mpn207 Mpn307 Mpn340 Mpn653 Mpn544 Mpn551 Mpn590 Mpn254 Mpn686 Mpn361 Mpn497 Mpn280 Mpn360 Mpn360 Mpn243 Mpn278 Mpn515 Mpn636 Mpn546 Mpn280 Mpn165 Mpn331 Mpn006 Mpn294 Mpn171 Mpn515 Mpn123 Mpn115 Mpn239 Mpn197 Mpn516 Mpn182 Mpn169 Mpn346 Mpn155 Mpn309 Mpn340 Mpn167 Mpn221 Mpn283 Mpn515 Mpn426 Mpn508 Mpn658 Mpn434 Mpn571 Mpn480 Mpn551 Mpn207 Mpn497 Mpn379 Mpn360 Mpn254 Mpn551 Mpn361 Mpn653 Mpn360 Mpn340 Mpn280 Mpn544 Mpn257 Mpn686 Mpn307 Mpn590 Mpn515 Mpn280 Mpn243 Mpn546 Mpn636 Mpn278 Mpn331 Mpn165 Mpn123 Mpn115 Mpn515 Mpn171 Mpn006 Mpn294 General principles of cellular organization in Mycoplasma pneumoniae 822 822 822 822 822 822 822 822 821 821 821 821 821 821 821 821 821 821 820 820 819 819 819 819 819 819 819 819 819 819 819 819 819 819 819 819 817 817 817 817 817 817 816 816 815 815 815 815 815 815 170 Mpn315 Mpn023 Mpn061 Mpn357 Mpn393 Mpn662 Mpn222 Mpn324 Mpn357 Mpn142 Mpn022 Mpn656 Mpn388 Mpn674 Mpn397 Mpn220 Mpn061 Mpn515 Mpn167 Mpn228 Mpn179 Mpn032 Mpn567 Mpn567 Mpn416 Mpn668 Mpn328 Mpn246 Mpn246 Mpn246 Mpn192 Mpn192 Mpn559 Mpn479 Mpn479 Mpn001 Mpn266 Mpn351 Mpn278 Mpn185 Mpn595 Mpn347 Mpn387 Mpn387 Mpn533 Mpn592 Mpn282 Mpn033 Mpn606 Mpn606 Mpn357 Mpn061 Mpn023 Mpn315 Mpn662 Mpn393 Mpn324 Mpn222 Mpn220 Mpn397 Mpn388 Mpn674 Mpn022 Mpn656 Mpn142 Mpn357 Mpn032 Mpn179 Mpn228 Mpn167 Mpn515 Mpn061 Mpn192 Mpn300 Mpn559 Mpn389 Mpn024 Mpn185 Mpn311 Mpn342 Mpn567 Mpn110 Mpn416 Mpn533 Mpn606 Mpn367 Mpn282 Mpn387 Mpn562 Mpn246 Mpn545 Mpn232 Mpn351 Mpn060 Mpn479 Mpn232 Mpn266 Mpn593 Mpn479 Mpn389 General principles of cellular organization in Mycoplasma pneumoniae 814 814 814 814 814 814 813 813 812 812 812 812 812 812 812 812 811 811 811 811 811 811 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 171 Mpn367 Mpn423 Mpn311 Mpn232 Mpn232 Mpn490 Mpn320 Mpn389 Mpn389 Mpn593 Mpn300 Mpn300 Mpn545 Mpn024 Mpn644 Mpn645 Mpn562 Mpn332 Mpn342 Mpn060 Mpn110 Mpn110 Mpn401 Mpn434 Mpn383 Mpn656 Mpn678 Mpn352 Mpn292 Mpn629 Mpn595 Mpn269 Mpn515 Mpn606 Mpn166 Mpn316 Mpn178 Mpn677 Mpn515 Mpn034 Mpn191 Mpn556 Mpn616 Mpn252 Mpn229 Mpn003 Mpn434 Mpn034 Mpn599 Mpn628 Mpn001 Mpn332 Mpn246 Mpn347 Mpn592 Mpn645 Mpn644 Mpn668 Mpn606 Mpn033 Mpn567 Mpn110 Mpn595 Mpn328 Mpn320 Mpn490 Mpn278 Mpn423 Mpn246 Mpn387 Mpn192 Mpn300 Mpn352 Mpn629 Mpn656 Mpn383 Mpn292 Mpn401 Mpn678 Mpn434 Mpn269 Mpn595 Mpn166 Mpn316 Mpn515 Mpn606 Mpn515 Mpn034 Mpn178 Mpn677 Mpn616 Mpn252 Mpn191 Mpn556 Mpn034 Mpn434 Mpn003 Mpn229 Mpn628 Mpn599 General principles of cellular organization in Mycoplasma pneumoniae 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 810 809 809 809 809 809 809 809 809 808 808 808 808 808 808 807 807 807 807 807 807 807 807 806 806 806 806 806 806 172 Mpn192 Mpn100 Mpn515 Mpn515 Mpn177 Mpn434 Mpn061 Mpn393 Mpn268 Mpn259 Mpn638 Mpn061 Mpn257 Mpn089 Mpn166 Mpn228 Mpn207 Mpn027 Mpn321 Mpn483 Mpn243 Mpn020 Mpn003 Mpn426 Mpn324 Mpn050 Mpn515 Mpn434 Mpn192 Mpn177 Mpn515 Mpn100 Mpn393 Mpn061 Mpn259 Mpn268 Mpn061 Mpn638 Mpn089 Mpn257 Mpn228 Mpn166 Mpn321 Mpn483 Mpn207 Mpn027 Mpn426 Mpn003 Mpn020 Mpn243 Mpn050 Mpn324 General principles of cellular organization in Mycoplasma pneumoniae 805 805 805 805 805 805 804 804 804 804 803 803 803 803 803 803 802 802 802 802 801 801 801 801 801 801 173 10.3. Table S3: List of protein complexes. This table is a repository of all protein complexes that have been observed in this study. It is divided into i) a set of 116 heteromultimeric complexes and ii) a set of 62 homomultimeric complexes. In part i) the first column gives the assembly ID that specifies the higher order molecular assembly in which a heteromultimeric protein complex clusters. The second column specifies the unique complex ID for each protein complex. Columns 3 and 4 provide complex name and complex function, respectively. In columns 5-8 the systematic name, protein name, UniProt protein name and subunit type of each protein component of a complex are indicated. In part ii) the first column on the left shows the complex ID of the homomultimeric protein complexes. The second column indicates the complex name. Columns 3 and 4 give the annotated systematic and protein name of each homomultimer. Assemb ly ID Comple x ID Complex name Complex function Systema tic name Protein name UniProt protein name Subunit type i) Heteromultimeric complexes XXVIII XXX 40 42 Cohesin like complex Complex 42 Cell division and chromosome partitioning Cell division and chromosome partitioning XXX 43 Complex 43 Cell division and chromosome partitioning III 3 DNA Polymerase III γ complex DNA replication, recombination and repair XX 29 DNA Polymerase III core complex 59 DnaA complex 82 DNA Gyrase complex DNA replication, recombination and repair DNA replication, recombination and repair DNA replication, recombination and repair Mpn300 ScpA Segregation and condensation protein A core Mpn301 ScpB Segregation and condensation protein B core Mpn426 P115 Protein P115 homolog core Mpn423 Mpn423 Uncharacterized protein MG296 homolog core Mpn211 UvrB UvrABC system protein B core Mpn508 Mpn508 Uncharacterized protein MPN508 Mpn332 Lon ATP-dependent protease La core attachm ent attachm ent Mpn573 GroL 60 kDa chaperonin Mpn314 MraZ Protein MraZ core Mpn339 Mpn339 Uncharacterized protein MG243 homolog core Mpn633 Mpn633 Uncharacterized protein Mpn633 core S-adenosyl-L-methionine-dependent methyltransferase attachm ent Mpn315 MraW Mpn314 MraZ Protein MraZ core Mpn315 MraW S-adenosyl-L-methionine-dependent methyltransferase core Mpn007 Mpn007 Uncharacterized protein MG007 homolog core Mpn618 DnaX DNA polymerase III subunit γ/τ core Mpn450 Mpn450 Uncharacterized protein MG315 homolog core Mpn353 DnaG DNA primase core Mpn378 DnaE DNA polymerase III subunit α core Mpn551 Mpn551 Uncharacterized protein MG373 homolog core Mpn118 Mpn118 Uncharacterized protein MG199 homolog core Mannitol-specific phosphotransferase enzyme IIA component Putative ABC transporter ATP-binding protein MG467 homolog attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn563 Mpn563 Uncharacterized GTP-binding protein MG384 homolog Mpn557 GidA tRNA uridine 5-carboxymethylaminomethyl modification enzyme Mpn141 MgpA Adhesin P1 precursor Mpn059 Gcp Probable O-sialoglycoprotein endopeptidase Mpn653 MtlF Mpn683 Mpn683 Mpn376 Mpn376 Uncharacterized protein Mpn376 core Mpn686 DnaA Chromosomal replication initiator protein dnaA core Mpn003 GyrB DNA gyrase subunit B core Mpn004 GyrA DNA gyrase subunit A core General principles of cellular organization in Mycoplasma pneumoniae 174 XI ParE-GyrA complex DNA replication, recombination and repair Mpn122 ParE DNA topoisomerase 4 subunit B core 102 Mpn004 GyrA DNA gyrase subunit A core DNA Topoisomeras e IV complex DNA replication, recombination and repair Mpn123 ParC DNA topoisomerase 4 subunit A core 17 Mpn122 ParE DNA topoisomerase 4 subunit B core DNA Recombinatio n complex DNA replication, recombination and repair Mpn034 PolC DNA polymerase III PolC-type core Mpn490 RecA Protein RecA core DNA replication, recombination and repair DNA replication, recombination and repair DNA replication, recombination and repair DNA replication, recombination and repair DNA replication, recombination and repair DNA replication, recombination and repair DNA replication, recombination and repair Mpn003 GyrB DNA gyrase subunit B core Mpn486 Mpn486 Uncharacterized protein Mpn486 core Mpn014 Mpn014 Uncharacterized protein MG010 homolog core Mpn377 Mpn377 Uncharacterized protein Mpn377 core Mpn014 Mpn014 Uncharacterized protein MG010 homolog core Mpn235 Ung Uracil-DNA glycosylase core Mpn125 UvrC UvrABC system protein C core Mpn563 Mpn563 Uncharacterized GTP-binding protein MG384 homolog core Mpn023 MetG Methionyl-tRNA synthetase core Mpn619 UvrA UvrABC system protein A core Mpn536 RuvB Holliday junction ATP-dependent DNA helicase RuvB core Mpn663 Mpn663 Uncharacterized protein MG449 homolog core Mpn020 Mpn020 Uncharacterized ATP-dependent helicase MPN020 core Mpn341 UvrD Probable DNA helicase II homolog core 86 XI XXXV 101 GyrB-Mpn486 complex 84 DNA Primase complex 103 DNA Primase complex 90 Complex 90 105 Complex 105 100 Complex 100 104 Helicase complex 15 DNA Recombinatio n complex DNA replication, recombination and repair 114 Complex 114 Transcription 49 RNA polymerase complex Transcription Mpn123 ParC DNA topoisomerase 4 subunit A core Mpn282 Mpn282 Uncharacterized protein Mpn282 core Mpn424 Mpn424 UPF0122 protein Mpn424 core Mpn060 MetK S-adenosylmethionine synthetase core Mpn402 ProS Prolyl-tRNA synthetase core Mpn351 Mpn351 Uncharacterized protein MG248 homolog core Mpn387 Mpn387 Uncharacterized protein MG269 homolog Mpn293 LspA Lipoprotein signal peptidase Mpn490 RecA Protein RecA Mpn122 ParE DNA topoisomerase 4 subunit B core attachm ent attachm ent attachm ent Mpn352 RpoD RNA polymerase σ factor RpoD core Mpn434 DnaK Chaperone protein DnaK core Mpn020 Mpn020 Uncharacterized ATP-dependent helicase Mpn020 core Mpn261 TopA DNA topoisomerase 1 core Mpn352 RpoD RNA polymerase σ factor RpoD σ-A) core Mpn515 RpoC DNA-directed RNA polymerase β' chain core Mpn516 RpoB DNA-directed RNA polymerase β chain core Mpn191 RpoA DNA-directed RNA polymerase α chain core Mpn178 RpsZ 30S ribosomal protein S14 type Z core Mpn154 NusA Transcription elongation protein NusA core Mpn002 Mpn002 DnaJ-like protein MG002 homolog core Mpn539 RplL 50S ribosomal protein L7/L12 core Uncharacterized protein MG010 homolog attachm ent Mpn014 Mpn014 General principles of cellular organization in Mycoplasma pneumoniae 175 V VII VII XXXV 7 10 11 51 Complex 7 AminoacyltRNA synthetase complex PhenylalaninetRNA synthetase complex Complex 51 Translation, ribosomal structure and biogenesis Translation, ribosomal structure and biogenesis Translation, ribosomal structure and biogenesis Translation, ribosomal structure and biogenesis attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn673 Mpn673 Uncharacterized protein MG459 homolog Mpn437 Mpn437 Uncharacterized protein Mpn437 Mpn067 Mpn067 Protein MG054 homolog Mpn265 TrpS Tryptophanyl-tRNA synthetase Mpn678 GltX Glutamyl-tRNA synthetase Mpn379 PolA Probable 5'-3' exonuclease Mpn153 Mpn153 Uncharacterized ATP-dependent helicase MG140 homolog Mpn490 RecA Protein RecA Mpn266 Mpn266 Uncharacterized protein MG127 homolog Mpn009 Mpn009 Uncharacterized deoxyribonuclease MG009 homolog Mpn443 Mpn443 Probable ATP-dependent RNA helicase MG308 homolog Mpn155 InfB Translation initiation factor IF-2 Mpn187 InfA Translation initiation factor IF-1 Mpn252 AsnS Asparaginyl-tRNA synthetase Mpn354 GlyQS Glycyl-tRNA synthetase Mpn219 RplK 50S ribosomal protein L11 Mpn173 RpmC 50S ribosomal protein L29 Mpn016 Mpn016 Uncharacterized protein MG012 homolog core Mpn033 Upp Uracil phosphoribosyltransferase core Mpn133 Mpn133 Uncharacterized lipoprotein MG186 homolog precursor core Mpn635 Mpn635 Uncharacterized protein Mpn635 core Mpn155 InfB Translation initiation factor IF-2 core Mpn346 Mpn346 Uncharacterized protein Mpn346 Mpn064 DeoA Thymidine phosphorylase Mpn156 RbfA Ribosome-binding factor A core attachm ent attachm ent Mpn025 Fba Fructose-bisphosphate aldolase core Mpn105 PheS Phenylalanyl-tRNA synthetase α chain core Mpn669 TyrS Tyrosyl-tRNA synthetase core Mpn061 Ffh Signal recognition particle protein core Mpn023 MetG Methionyl-tRNA synthetase core attachm ent attachm ent attachm ent attachm ent attachm ent Mpn678 GltX Glutamyl-tRNA synthetase Mpn106 PheT Phenylalanyl-tRNA synthetase β chain Mpn322 NrdF Ribonucleoside-diphosphate reductase subunit β Mpn141 MgpA Adhesin P1 precursor Mpn553 ThrS Threonyl-tRNA synthetase Mpn106 PheT Phenylalanyl-tRNA synthetase β chain core Mpn105 PheS Phenylalanyl-tRNA synthetase α chain core Mpn035 Mpn035 Uncharacterized protein Mpn035 core Mpn561 Udk Uridine kinase core Mpn178 RpsZ 30S ribosomal protein S14 type Z core Mpn553 ThrS Threonyl-tRNA synthetase attachm ent General principles of cellular organization in Mycoplasma pneumoniae 176 XXXV XXXV XII XXXV 52 Translation elongation factor complex Translation, ribosomal structure and biogenesis Translation, ribosomal structure and biogenesis Translation, ribosomal structure and biogenesis 53 L29-S12 complex 96 Complex 96 18 Cytidine deaminationribosome complex Translation, ribosomal structure and biogenesis Ribosomecom plex Translation, ribosomal structure and biogenesis 50 Mpn631 Tsf Elongation factor Ts Mpn665 Tuf Elongation factor Tu core core attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn006 Tmk Thymidylate kinase Mpn137 Mpn137 UPF0134 protein Mpn137 Mpn219 RplK 50S ribosomal protein L11 Mpn168 RplB 50S ribosomal protein L2 Mpn173 RpmC 50S ribosomal protein L29 Mpn225 RpsL 30S ribosomal protein S12 Mpn173 RpmC 50S ribosomal protein L29 core Mpn225 RpsL 30S ribosomal protein S12 core Mpn361 PrfA Peptide chain release factor 1 core Mpn556 ArgS Arginyl-tRNA synthetase core Mpn065 Cdd Cytidine deaminase core Mpn643 Mpn643 Uncharacterized lipoprotein MG440 homolog 2 precursor core Mpn539 RplL 50S ribosomal protein L7/L12 core Mpn216 OppC Oligopeptide transport system permease protein OppC attachm ent Mpn616 RpsI 30S ribosomal protein S9 core Mpn622 RpsO 30S ribosomal protein S15 core Mpn115 InfC Translation initiation factor IF-3 core Mpn165 RplC 50S ribosomal protein L3 core Mpn166 RplD 50S ribosomal protein L4 core Mpn167 RplW 50S ribosomal protein L23 core Mpn169 RpsS 30S ribosomal protein S19 core Mpn171 RpsC 30S ribosomal protein S3 core Mpn172 RplP 50S ribosomal protein L16 core Mpn174 RpsQ 30S ribosomal protein S17 core Mpn176 RplX 50S ribosomal protein L24 core Mpn177 RplE 50S ribosomal protein L5 core Mpn179 RpsH 30S ribosomal protein S8 core Mpn182 RpsE 30S ribosomal protein S5 core Mpn189 RpsM 30S ribosomal protein S13 core Mpn192 RplQ 50S ribosomal protein L17 core Mpn208 RpsB 30S ribosomal protein S2 core Mpn220 RplA 50S ribosomal protein L1 core Mpn226 RpsG 30S ribosomal protein S7 core Mpn228 RpsF 30S ribosomal protein S6 core Mpn230 RpsR 30S ribosomal protein S18 core Mpn327 RpmA 50S ribosomal protein L27 core Mpn446 RpsD 30S ribosomal protein S4 core Mpn658 RplS 50S ribosomal protein L19 core Mpn221 Pth Peptidyl-tRNA hydrolase core Mpn660 RpsP 30S ribosomal protein S16 core Mpn685 Putative ABC transporter ATP-binding protein MG468.1 homolog core Mpn685 Mpn518 Mpn518 Uncharacterized protein MG343 homolog core Mpn571 Mpn571 Putative ABC transporter ATP-binding MG390 homolog core Mpn665 Tuf Elongation factor Tu core Mpn178 RpsZ 30S ribosomal protein S14 type Z core General principles of cellular organization in Mycoplasma pneumoniae 177 XXV & XXXI 37 VI & XXXII 9 VI 8 GroEL-GroES complex Dnak-GrpE complex Protein chaperone complex Posttranslatio nal modification, protein turnover, chaperones Posttranslatio nal modification, protein turnover, chaperones Posttranslatio nal modification, protein turnover, chaperones Mpn155 InfB Translation initiation factor IF-2 core Mpn520 IleS Isoleucyl-tRNA synthetase core Mpn091 Mpn091 Uncharacterized protein Mpn091 core Mpn428 Pta Phosphate acetyltransferase core Mpn465 Mpn465 Uncharacterized protein Mpn465 core attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn541 RpsT 30S ribosomal protein S20 Mpn154 NusA Transcription elongation protein NusA Mpn517 Mpn517 Uncharacterized protein MG342 homolog Mpn538 RplJ 50S ribosomal protein L10 Mpn231 RplI 50S ribosomal protein L9 Mpn180 RplF 50S ribosomal protein L6 Mpn117 RplT 50S ribosomal protein L20 Mpn475 EngA GTP-binding protein EngA Mpn636 Frr Ribosome recycling factor Mpn676 Mpn676 Uncharacterized protein Mpn676 Mpn103 Mpn103 Uncharacterized protein Mpn103 Mpn386 Mpn386 Uncharacterized protein MG268 homolog Mpn536 RuvB Holliday junction ATP-dependent DNA helicase RuvB Mpn623 Mpn623 Mpn253 PgsA Mpn524 Mpn524 UPF0134 protein Mpn524 Mpn539 RplL 50S ribosomal protein L7/L12 Mpn553 ThrS Threonyl-tRNA synthetase Mpn072 Mpn072 Uncharacterized protein MG057 homolog Mpn219 RplK 50S ribosomal protein L11 Mpn006 Tmk Thymidylate kinase Mpn137 Mpn137 UPF0134 protein Mpn137 Mpn188 RpmJ 50S ribosomal protein L36 Mpn168 RplB 50S ribosomal protein L2 Mpn317 FtsZ Cell division protein FtsZ Mpn225 RpsL 30S ribosomal protein S12 Mpn573 GroL 60 kDa chaperonin core Mpn574 GroS 10 kDa chaperonin core Mpn120 GrpE Protein GrpE core Mpn434 DnaK Chaperone protein DnaK core Mpn538 RplJ 50S ribosomal protein L10 core Mpn434 DnaK Chaperone protein DnaK core Mpn021 DnaJ Chaperone protein DnaJ core Mpn142 Mpn142 Mgp-operon protein 3 precursor core SpoT Probable guanosine-3',5'-bis(diphosphate) 3'pyrophosphohydrolase core Mpn397 Probable ATP-dependent RNA helicase MG425 homolog CDP-diacylglycerol-glycerol-3-phosphate 3phosphatidyltransferase General principles of cellular organization in Mycoplasma pneumoniae 178 Mpn441 VIII XXXIII 12 46 92 XXXII 45 Glycolytic enzyme complex 1 Complex 46 Complex 92 Glycolytic enzyme complex 2 Carbohydrate transport and metabolism Carbohydrate transport and metabolism Carbohydrate transport and metabolism Carbohydrate transport and metabolism 38 Peptidase complex Amino acid transport and metabolism Mpn297 Uncharacterized protein MG211 homolog Mpn002 Mpn002 DnaJ-like protein MG002 homolog Mpn531 ClpB Chaperone ClpB Mpn124 HrcA Heat-inducible transcription repressor HrcA Mpn247 Mpn247 Putative protein phosphatase Mpn354 GlyQS Glycyl-tRNA synthetase Mpn394 Nox Probable NADH oxidase Mpn429 Pgk Phosphoglycerate kinase Mpn120 GrpE Protein GrpE Mpn547 Mpn547 Uncharacterized protein MG369 homolog 39 Complex 39 Metabolism core attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn025 Fba Fructose-bisphosphate aldolase core Mpn430 GapA Glyceraldehyde-3-phosphate dehydrogenase core Mpn303 Pyk Pyruvate kinase core Mpn606 Eno Enolase core Mpn629 TpiA Triosephosphate isomerase core Mpn569 Mpn569 Putative metalloprotease Mpn569 core Mpn316 Mpn316 Uncharacterized protein MG223 homolog core Mpn258 Mpn258 Putative carbohydrate transport ATP-binding protein Mpn258 core Mpn259 Mpn259 Uncharacterized protein MG120 homolog core Mpn430 GapA Glyceraldehyde-3-phosphate dehydrogenase core Mpn429 Pgk Phosphoglycerate kinase core core Mpn606 Eno Enolase Mpn434 DnaK Chaperone protein DnaK core attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn120 GrpE Protein GrpE Mpn002 Mpn002 DnaJ-like protein MG002 homolog Mpn531 ClpB Chaperone ClpB Mpn124 HrcA Heat-inducible transcription repressor Mpn247 Mpn247 Putative protein phosphatase Mpn354 GlyQS Glycyl-tRNA synthetase Mpn394 Nox Probable NADH oxidase GatB Aspartyl/glutamyl-tRNA(Asn/Gln) amidotransferase subunit B core Mpn250 Pgi Glucose-6-phosphate isomerase core Mpn358 Mpn358 Uncharacterized protein MG255 homolog core Mpn572 PepA Probable cytosol aminopeptidase core Mpn609 PstB Phosphate import ATP-binding protein PstB core Mpn611 Mpn611 Uncharacterized lipoprotein MG412 homolog precursor core Mpn197 PepF Oligoendopeptidase F homolog core Mpn218 OppF Oligopeptide transport ATP-binding protein OppF core Mpn372 Mpn372 ADP-ribosylating toxin CARDS core Mpn671 FtsH Cell division protease FtsH homolog core GpmI 2,3-bisphosphoglycerate-independent phosphoglycerate mutase attachm ent Mpn628 XXVII Uncharacterized protein Mpn441 Mpn297 Mpn238 XXVI Mpn441 Mpn203 Mpn203 Putative adhesin P1-like protein Mpn203 core Mpn668 Mpn668 Organic hydroperoxide resistance protein-like core Mpn391 PdhC Dihydrolipoyllysine-residue acetyltransferase core General principles of cellular organization in Mycoplasma pneumoniae 179 94 XXXI 44 54 93 XXXIV XIX XXIV XXIV 47 28 34 35 95 109 Ribonucleosid e-diphosphate reductase complex Pyruvate dehydrogenas e complex Phosphotransf er system complex ScpA-ThyA complex ATP synthase complex Restriction enzyme complex Restriction enzyme complex Restriction enzyme complex Mpn420 Mpn420 Uncharacterized protein MG293 homolog attachm ent Mpn322 NrdF Ribonucleoside-diphosphate reductase subunit β core Mpn324 NrdE Ribonucleoside-diphosphate reductase α subunit core Mpn392 PdhB Pyruvate dehydrogenase E1 component subunit β core Mpn393 PdhA Pyruvate dehydrogenase E1 component subunit α core Mpn573 GroL 60 kDa chaperonin core Mpn390 PdhD Dihydrolipoyl dehydrogenase Mpn574 GroS 10 kDa chaperonin Mpn006 Tmk Thymidylate kinase Mpn306 ArcB Ornithine carbamoyltransferase, catabolic Mpn023 MetG Methionyl-tRNA synthetase Mpn008 TrmE Probable tRNA modification GTPase TrmE Mpn009 Mpn009 Uncharacterized deoxyribonuclease MG009 homolog Mpn321 FolA Dihydrofolate reductase Mpn426 P115 Protein P115 homolog Metabolism Metabolism Metabolism Metabolism Energy production and conversion Defense mechanisms Defense mechanisms Defense mechanisms Restriction enzyme complex Defense mechanisms Restriction enzyme complex Defense mechanisms core attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn553 ThrS Threonyl-tRNA synthetase Mpn207 PtsG PTS system glucose-specific EIICBA component core Mpn268 Mpn268 Putative phosphotransferase enzyme IIB component core Mpn300 ScpA Segregation and condensation protein A core Mpn320 ThyA Thymidylate synthase core Mpn598 AtpD ATP synthase subunit β core Mpn600 AtpA ATP synthase subunit α core Mpn602 AtpF ATP synthase B chain precursor core Mpn153 Mpn153 Uncharacterized ATP-dependent helicase MG140 homolog Mpn688 Mpn688 ParA family protein Mpn688 attachm ent attachm ent Mpn347 Mpn347 Mpn638 Mpn638 Putative type I restriction enzyme MpnORFDP R protein part 1 Putative type I restriction enzyme specificity protein Mpn638 (S protein) core core Mpn185 Adk Adenylate kinase core Mpn438 Mpn438 Uncharacterized protein Mpn438 core Mpn342 Mpn342 Putative type I restriction enzyme MpnORFDP M protein core Mpn663 Mpn663 Uncharacterized protein MG449 homolog core Cell division protease FtsH homolog core Mpn671 FtsH Mpn342 Mpn342 Mpn507 Mpn507 Mpn089 Mpn089 Mpn345 Mpn345 Putative type-1 restriction enzyme MpnORFDP R protein part 2 core Mpn365 Mpn365 Putative type I restriction enzyme specificity protein Mpn365 (S protein) core Mpn089 Mpn089 Putative type I restriction enzyme specificity protein Mpn089 (S protein) core Mpn497 Mpn497 Uncharacterized protein Mpn497 core Putative type I restriction enzyme MpnORFDP M protein Putative type I restriction enzyme specificity protein Mpn507 (S protein) Putative type I restriction enzyme specificity protein Mpn089 (S protein) General principles of cellular organization in Mycoplasma pneumoniae core core core 180 113 115 XIX II 27 2 81 IV IV IV IX X XIII 4 5 6 13 14 19 Restriction enzyme complex Defense mechanisms Restriction enzyme complex Defense mechanisms Restriction enzyme complex Complex 2 Complex 81 Complex 4 Complex 5 Complex 6 Complex 13 Complex 14 Complex 19 Defense mechanisms Cell envelope biogenesis, outer membrane Cell envelope biogenesis, outer membrane Function unknown Function unknown Function unknown Function unknown Function unknown Function Mpn365 Mpn365 Putative type I restriction enzyme specificity protein Mpn365 (S protein) core Mpn343 Mpn343 Putative type I restriction enzyme specificity protein Mpn343 (S protein) core Mpn365 Mpn365 Putative type I restriction enzyme specificity protein Mpn365 (S protein) core Mpn615 Mpn615 Putative type I restriction enzyme specificity protein Mpn615 (S protein) core Mpn116 RpmI 50S ribosomal protein L35 core Mpn254 Mpn254 Protein MG115 homolog core Mpn232 DnaB Replicative DNA helicase core Mpn526 Mpn526 Uncharacterized protein MG350 homolog core Mpn347 Mpn347 Putative type I restriction enzyme MpnORFDP R protein part 1 core Mpn257 GalE UDP-glucose 4-epimerase core Mpn592 Mpn592 Uncharacterized lipoprotein Mpn592 precursor core Mpn638 Mpn638 Putative type I restriction enzyme specificity protein Mpn638 (S protein) Mpn656 Mpn656 Uncharacterized protein MG442 homolog attachm ent attachm ent Mpn367 Mpn367 Putative mgpC-like protein Mpn367 core Mpn514 Mpn514 Uncharacterized protein Mpn514 core Mpn200 Mpn200 Uncharacterized lipoprotein Mpn200 precursor core Mpn499 Mpn499 Uncharacterized protein Mpn499 core Mpn645 Mpn645 Uncharacterized lipoprotein MG439 homolog 2 precursor core Mpn100 Mpn100 UPF0134 protein Mpn100 core Mpn650 Mpn650 Uncharacterized lipoprotein Mpn650 precursor core Mpn140 Mpn140 Mgp-operon protein 1 core Mpn148 Mpn148 Uncharacterized protein Mpn148 core Mpn450 Mpn450 Uncharacterized protein MG315 homolog core Mpn311 Mpn311 Uncharacterized protein MG218.1 homolog core Mpn246 Def Peptide deformylase core Mpn011 Mpn011 Uncharacterized lipoprotein Mpn011 precursor core Mpn466 Mpn466 Uncharacterized protein Mpn466 core attachm ent attachm ent attachm ent attachm ent Mpn204 Mpn204 UPF0134 protein Mpn204 Mpn245 Gmk Guanylate kinase Mpn419 AlaS Alanyl-tRNA synthetase Mpn095 Mpn095 Uncharacterized protein Mpn095 Mpn246 Def Peptide deformylase core Mpn245 Gmk Guanylate kinase core Mpn095 Mpn095 Uncharacterized protein Mpn095 core Mpn311 Mpn311 Uncharacterized protein MG218.1 homolog core Mpn041 Mpn041 Uncharacterized protein Mpn041 core Mpn400 Mpn400 Uncharacterized protein MG281 homolog core Mpn118 Mpn118 Uncharacterized protein MG199 homolog core Mpn298 AcpS Holo-[acyl carrier protein] synthase Mpn563 Mpn563 Uncharacterized GTP-binding protein MG384 homolog Mpn557 GidA tRNA uridine 5-carboxymethylaminomethyl modification enzyme attachm ent attachm ent core Mpn066 ManB Phosphomannomutase core Mpn564 Adh Probable NADP-dependent alcohol dehydrogenase core Mpn048 Mpn048 Uncharacterized protein Mpn048 core Mpn320 ThyA Thymidylate synthase core General principles of cellular organization in Mycoplasma pneumoniae 181 unknown XIV 20 Complex 20 Function unknown Mpn076 Mpn076 Uncharacterized protein MG061 homolog 1 core Mpn284 Mpn284 Uncharacterized lipoprotein Mpn284 precursor core Mpn353 DnaG DNA primase core Mpn083 Mpn083 Uncharacterized lipoprotein Mpn083 precursor core Mpn142 Mpn142 Mgp-operon protein 3 precursor core Mpn582 Uncharacterized lipoprotein Mpn582 precursor attachm ent Mpn088 Mpn088 Uncharacterized protein Mpn088 core Mpn145 Mpn145 UPF0134 protein Mpn145 core Mpn620 Mpn620 Uncharacterized protein MG422 homolog core Mpn612 Mpn612 Uncharacterized protein MG414 homolog core Mpn221 Pth Peptidyl-tRNA hydrolase core Mpn292 Mpn292 Uncharacterized RNA pseudouridine synthase MG209 homolog core Mpn094 Mpn094 UPF0134 protein Mpn094 core Mpn305 ArcA Putative arginine deiminase Mpn582 XV XVI XVI XVII XVIII XVIII XXI 21 22 23 24 25 26 31 Complex 21 Complex 22 Complex 23 Complex 24 Complex 25 Complex 26 Complex 31 Function unknown Function unknown Function unknown Function unknown Function unknown Function unknown Function unknown Mpn547 Mpn547 Mpn247 Mpn247 Putative protein phosphatase Mpn292 Mpn292 Uncharacterized RNA pseudouridine synthase MG209 homolog core Mpn547 Mpn547 Uncharacterized protein MG369 homolog core Mpn525 Mpn525 Uncharacterized protein MG349 homolog core Mpn567 P200 Protein P200 core Mpn110 Mpn110 Uncharacterized protein Mpn110 32 Complex 32 Function unknown XXIII 33 Complex 33 Function unknown XXV 36 Complex 36 Function unknown core attachm ent attachm ent Mpn295 Mpn295 Uncharacterized protein MG210.1 homolog Mpn095 Mpn095 Uncharacterized protein Mpn095 Mpn258 Mpn258 Putative carbohydrate transport ATP-binding protein core Mpn341 UvrD Probable DNA helicase II homolog core Mpn114 Mpn114 Putative acetyltransferase core Mpn241 Mpn241 Uncharacterized protein MG103 homolog core Mpn262 Mpn262 Uncharacterized protein MG123 homolog core Mpn269 Mpn269 UPF0144 protein MG130 homolog core attachm ent attachm ent Mpn223 HprK HPr kinase/phosphorylase Mpn415 P37 High affinity transport system protein p37 precursor Mpn341 UvrD Probable DNA helicase II homolog core Mpn223 HprK HPr kinase/phosphorylase core Mpn121 Mpn121 Uncharacterized protein MG202 homolog core Mpn135 Mpn135 Probable ABC transporter permease protein MG188 homolog core Mpn366 Mpn366 Putative mgpC-like protein Mpn366 core Uncharacterized protein MG328 homolog attachm ent Mpn474 XXII Uncharacterized protein MG369 homolog core attachm ent attachm ent Mpn474 Mpn158 RibF Putative riboflavin biosynthesis protein core Mpn209 PacL Probable cation-transporting P-type ATPase core Mpn331 Tig Trigger factor core Mpn172 RplP 50S ribosomal protein L16 core Mpn418 Mpn418 Putative Holliday junction resolvase core Mpn642 Uncharacterized lipoprotein MG439 homolog 4 precursor core Mpn211 UvrB UvrABC system protein B core Mpn573 GroL 60 kDa chaperonin core Mpn194 CbiO2 Cobalt import ATP-binding protein CbiO 2 core Mpn264 Mpn264 Uncharacterized protein MG125 homolog core Mpn642 General principles of cellular organization in Mycoplasma pneumoniae 182 XI XXIX XXXIV I XX 16 41 48 1 Complex 16 Complex 41 Complex 48 Complex 1 Function unknown Function unknown Function unknown Function unknown attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent attachm ent Mpn574 GroS 10 kDa chaperonin Mpn267 PpnK Probable inorganic polyphosphate/ATP-NAD kinase Mpn008 TrmE Probable tRNA modification GTPase TrmE Mpn009 Mpn009 Uncharacterized deoxyribonuclease MG009 homolog Mpn321 FolA Dihydrofolate reductase Mpn426 P115 Protein P115 homolog Mpn553 ThrS Threonyl-tRNA synthetase Mpn387 Mpn387 Uncharacterized protein MG269 homolog core Mpn293 LspA Lipoprotein signal peptidase core Mpn289 Mpn289 Putative type I restriction enzyme specificity protein Mpn289 (S protein) core Mpn479 AzoR FMN-dependent NADH-azoreductase core Mpn389 LplA Probable lipoate-protein ligase A core Uncharacterized protein Mpn040 attachm ent Mpn040 Mpn040 Mpn602 AtpF ATP synthase B chain precursor core Mpn153 Mpn153 Uncharacterized ATP-dependent helicase MG140 homolog core Mpn195 Mpn195 Uncharacterized protein MG181 homolog core Mpn395 Apt Adenine phosphoribosyltransferase core Mpn367 Mpn367 Putative mgpC-like protein Mpn367 core Mpn001 DnaN DNA polymerase III subunit β core Mpn388 Mpn388 Uncharacterized protein MG269.1 homolog core Mpn551 Mpn551 Uncharacterized protein MG373 homolog core MtlF Mannitol-specific phosphotransferase enzyme IIA component core 30 Complex 30 Function unknown 55 Complex 55 Function unknown Mpn444 Mpn444 Putative esterase/lipase 1 core Mpn578 Mpn578 Uncharacterized protein Mpn578 core 56 Complex 56 Function unknown Mpn071 Mpn071 UPF0011 protein MG056 homolog core Mpn273 Mpn273 Uncharacterized 16.1 kDa HIT-like protein core Function unknown Mpn462 Mpn462 Uncharacterized protein Mpn462 core Mpn471 RpmG1 50S ribosomal protein L33 1 core Mpn281 Mpn281 Uncharacterized lipoprotein Mpn281 precursor core Mpn509 Mpn509 Uncharacterized protein Mpn509 core Mpn294 Mpn294 Uncharacterized protein Mpn294 core Mpn445 Uncharacterized lipoprotein MG309 homolog precursor core 57 Complex 57 Mpn653 58 Complex 58 Function unknown 60 Complex 60 Function unknown 61 Complex 61 Function unknown Mpn328 Nfo Probable endonuclease 4 core Mpn330 Mpn330 Uncharacterized protein MG237 homolog core Function unknown Mpn062 DeoD Purine nucleoside phosphorylase DeoD-type core Mpn079 FruK Putative 1-phosphofructokinase core NrdI Protein NrdI core Mpn407 Mpn407 Uncharacterized protein Mpn407 core 62 Complex 62 Mpn445 63 Complex 63 Function unknown Mpn323 64 Complex 64 Function unknown Mpn029 Efp Elongation factor P core Mpn586 Mpn586 Uncharacterized protein Mpn586 core 65 Complex 65 Function unknown Mpn248 Mpn248 Putative serine/threonine-protein kinase core Mpn504 Mpn504 UPF0134 protein Mpn504 core core 66 67 Complex 66 Complex 67 Function unknown Mpn493 UlaD Probable 3-keto-L-gulonate-6-phosphate decarboxylase Mpn596 Mpn596 Uncharacterized protein MG397 homolog core Function unknown Mpn350 Mpn350 UPF0078 membrane protein Mpn350 core Mpn401 GreA Transcription elongation factor GreA core General principles of cellular organization in Mycoplasma pneumoniae 183 Mpn329 Mpn329 Uncharacterized protein MG236 homolog core Mpn440 Mpn440 Uncharacterized protein Mpn440 core Mpn302 PfkA 6-phosphofructokinase core Mpn644 Mpn644 Uncharacterized lipoprotein MG439 homolog 3 precursor core Function unknown Mpn097 Mpn097 Uncharacterized lipoprotein Mpn097 precursor core Mpn562 NadE Probable NH3-dependent NAD synthetase core Mpn337 Uncharacterized protein MG241 homolog core 68 Complex 68 Function unknown 69 Complex 69 Function unknown 70 Complex 70 + 71 Complex 71 Function unknown Mpn337 Mpn416 P29 Probable ABC transporter ATP-binding protein p29 core 72 Complex 72 Function unknown Mpn047 Mpn047 Uncharacterized protein MG037 homolog core Mpn063 DeoC Deoxyribose-phosphate aldolase core 73 Complex 73 Function unknown 74 Complex 74 75 76 77 Complex 75 Complex 76 Complex 77 Mpn529 Mpn529 Uncharacterized protein MG353 homolog core Mpn646 Mpn646 Uncharacterized lipoprotein MG440 homolog 1 precursor core Function unknown Mpn051 Mpn051 Uncharacterized protein MG039 homolog core Mpn627 PtsI Phosphoenolpyruvate-protein phosphotransferase core Function unknown Mpn461 Mpn461 Uncharacterized protein MG323 homolog core Mpn476 Cmk Cytidylate kinase core Mpn436 Uncharacterized lipoprotein MG307 homolog precursor core Function unknown Mpn436 Mpn492 UlaE Probable L-ribulose-5-phosphate 3-epimerase core Function unknown Mpn126 Mpn126 Putative metallophosphoesterase MG207 homolog core Mpn580 Mpn580 Uncharacterized protein Mpn580 core Mpn537 Uncharacterized protein MG360 homolog core Peptide methionine sulfoxide reductase core 78 Complex 78 Function unknown Mpn537 Mpn662 MsrB 79 Complex 79 Function unknown Mpn307 Mpn307 Carbamate kinase-like protein core Mpn590 Mpn590 Uncharacterized lipoprotein Mpn590 precursor core core 80 83 Complex 80 Complex 83 Function unknown Mpn081 Mpn081 Putative ABC transporter ATP-binding protein MG065 homolog Mpn505 Mpn505 Uncharacterized protein Mpn505 core Function unknown Mpn005 SerS Seryl-tRNA synthetase core Mpn340 Mpn340 Probable DNA helicase core Mpn027 Mpn027 Uncharacterized protein Mpn027. core Mpn628 GpmI 2,3-bisphosphoglycerate-independent phosphoglycerate mutase core 85 Complex 85 Function unknown 87 Complex 87 Function unknown Mpn036 Mpn036 Uncharacterized protein Mpn036 core Mpn676 Mpn676 Uncharacterized protein Mpn676 core Function unknown Mpn073 Prs Ribose-phosphate pyrophosphokinase core Mpn404 Mpn404 Uncharacterized protein MG285 homolog core Mpn103 Uncharacterized protein Mpn103 core 88 Complex 88 89 Complex 89 Function unknown Mpn103 Mpn280 Mpn280 UPF0036 protein MG139 homolog core 91 Complex 91 Function unknown Mpn210 SecA Preprotein translocase subunit SecA core Mpn379 PolA Probable 5'-3' exonuclease core Complex 97 Function unknown Mpn386 Mpn386 Uncharacterized protein MG268 homolog core Mpn688 Mpn688 ParA family protein Mpn688 core Mpn413 Uncharacterized protein Mpn413 core Mpn475 EngA GTP-binding protein EngA core 97 98 Complex 98 Function unknown Mpn413 99 Complex 99 Function unknown Mpn419 AlaS Alanyl-tRNA synthetase core Mpn582 Mpn582 Uncharacterized lipoprotein Mpn582 precursor core 106 Complex 106 Function unknown Mpn025 Fba Fructose-bisphosphate aldolase core Mpn573 GroL 60 kDa chaperonin core Function unknown Mpn060 MetK S-adenosylmethionine synthetase core Mpn048 Mpn048 Uncharacterized protein Mpn048 core 107 Complex 107 General principles of cellular organization in Mycoplasma pneumoniae 184 108 Complex 108 Function unknown Mpn073 Prs Ribose-phosphate pyrophosphokinase core Mpn556 ArgS Arginyl-tRNA synthetase core 110 Complex 110 Function unknown Mpn189 RpsM 30S ribosomal protein S13 core Mpn629 TpiA Triosephosphate isomerase core Mpn623 Probable ATP-dependent RNA helicase MG425 homolog core 111 Function unknown 112 Complex 112 Function unknown 116 Complex 116 Function unknown Complex ID 117 118 119 120 121 122 123 124 125 126 127 128 129 130 131 132 133 134 135 136 137 138 139 140 141 142 143 144 145 146 147 148 149 150 151 Complex 111 Mpn623 Mpn222 TilS tRNA(Ile)-lysidine synthase core Mpn340 Mpn340 Probable DNA helicase Mpn340 core Mpn521 Mpn521 Uncharacterized tRNA/rRNA methyltransferase MG346 homolog core Mpn573 GroL 60 kDa chaperonin core Mpn665 Tuf Elongation factor Tu core Systematicname Protein name ii) Homomultimeric complexes DNA polymerase III subunit β DNA gyrase subunit B Thymidylate kinase Uncharacterized protein MG007 homolog Mpn001 Mpn003 Mpn006 Mpn007 DnaN GyrB Tmk Mpn007 Uncharacterized ATP-dependent helicase Mpn020 Putative proline iminopeptidase Methionyl-tRNA synthetase Fructose-biphosphate aldolase DNA polymerase III PolC-type Glycerol kinase Mpn020 Mpn022 Mpn023 Mpn025 Mpn034 Mpn050 Mpn020 Pip MetG Fba PolC GlpK Uncharacterized protein MG039 homolog Deoxyribose-phosphate aldolase Phosphomannomutase Putative 1-phosphofructokinase Phenylalanyl-tRNA synthetase α chain Phenylalanyl-tRNA synthetase β chain Mpn051 Mpn063 Mpn066 Mpn079 Mpn105 Mpn106 Mpn051 DeoC ManB FruK PheS PheT Uncharacterized protein MG202 homolog Putative Metallophosphoesterase DNA-directed RNA polymerase subunit α HPr kinase/phosphorylase Single stranded DNA binding protein Replicative DNA helicase Uncharacterized protein MG127 homolog Probable inorganic polyphosphate/ATP-NAD kinase 2',3'-cyclic-nucleotide 2'-phosphodiesterase Mpn121 Mpn126 Mpn191 Mpn223 Mpn229 Mpn232 Mpn266 Mpn267 Mpn269 Mpn121 Mpn126 RpoA HprK Ssb DnaB Mpn266 PpnK Mpn269 Uncharacterized protein MG134 homolog 6-phosphofructokinase /Phosphohexokinase Pyruvate kinase Protein MraZ Cell division protein Thymidylate synthase Ribonucleoside-diphosphate reductase subunit β Ribonucleoside-diphosphate reductase subunit α Probable endonuclease 4 Mpn275 Mpn302 Mpn303 Mpn314 Mpn317 Mpn320 Mpn322 Mpn324 Mpn328 Mpn275 PfkA Pyk MraZ FtsZ ThyA NrdF NrdE Nfo Uncharacterized protein MG236 homolog Mpn329 Mpn329 Complex name General principles of cellular organization in Mycoplasma pneumoniae 185 152 153 154 155 156 157 158 159 160 161 162 163 164 165 166 167 168 169 170 171 172 173 174 175 176 177 178 Uncharacterized protein MG246 homolog Uncharacterized protein MG259 homolog Uncharacterized protein MG263 homolog Dihydrolipoyl lysine-residue acetyltransferase Pyruvate dehydrogenase E1 component subunit β Pyruvate dehydrogenase E1 component subunit α Alanyl-tRNA synthetase Mpn349 Mpn362 Mpn381 Mpn391 Mpn392 Mpn393 Mpn419 Mpn349 Mpn362 Mpn381 PdhC PdhB PdhA AlaS Uncharacterized protein MG293 homolog Glyceraldehyde-3-phosphate-dehydrogenase Putative Xaa-Pro aminopeptidase FMN-dependent NADH-azoreductase Recombinase A Probable L-ribulose-5-phosphate 3-epimerase UlaE Mpn420 Mpn430 Mpn470 Mpn479 Mpn490 Mpn492 Mpn420 GapA PepP AzoR RecA UlaE Uncharacterized protein MG350 homolog Chaperone protein ClpB Acetate kinase Probable thiamine biosynthesis protein Mpn526 Mpn531 Mpn533 Mpn550 Mpn526 ClpB AckA ThiL Uncharacterized protein MG377 homolog Ribosomal RNA small subunit methyltransferase G Arginine deiminase-like protein Probable NH3-dependent NAD+ synthetase 60 kDa chaperonin Enolase Putative type I restriction enzyme specificity protein Elongation factor Tu L-lactate dehydrogenase Mpn555 Mpn558 Mpn560 Mpn562 Mpn573 Mpn606 Mpn638 Mpn665 Mpn674 Mpn555 RsmG Mpn560 NadE GroL Eno Mpn638 Tuf Ldh Uncharacterized protein MG461 homolog Mpn677 Mpn677 General principles of cellular organization in Mycoplasma pneumoniae 186 10.4. Table S4: List of literature curated protein complexes. This table is a repository of literature evidence for i) 31 heteromultimeric and ii) 78 homomultimeric protein complexes that may exist in M. pneumoniae based on observed homologs in other species. In i) the first column states complex name. In columns 2-4 the table describes the protein components present in each complex, with their annotated systematic name, protein name and subunit/function, if known. In column 5, the PubMed ID or database evidence is highlighted. Column 6 specifies the organism for which the complex is observed. In ii) the first column provides the complex name. Columns 2 and 3 give the systematic and protein name of a homomultimer. Column 4 states the multimerization state of each complex. In column 5, the PubMed ID or database evidence is highlighted. Column 6 specifies the organism for which the complex is observed. Components Complex name Systematic name Protein name Mpn003 GyrB B-subunit Mpn004 GyrA A-subunit Mpn105 SyfA a-subunit Mpn106 SyfB b-subunit Mpn024 RpoE Probable δ-factor Mpn191 RpoA a-subunit Mpn352 RpoD σ-factor Mpn516 RpoB b-subunit PubMed ID or source Genus/ species Expasy M. pneumoniae Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity Expasy by homology to Bacillus subtilis Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity Expasy M. pneumoniae Expasy M. pneumoniae by similarity Expasy M. pneumoniae Subunit/function i) Heteromultimeric complexes DNA Gyrase complex Phenylalanine-tRNA synthetase complex RNA polymerase complex Transcription antitermination factor complex Pyruvate dehydrogenase complex Mpn515 RpoC b'-subunit Mpn067 NusG Transcription antitermination Mpn024 RpoE Probable δ-factor Mpn191 RpoA a-subunit Mpn352 RpoD σ-factor Mpn516 RpoB b-subunit Mpn515 RpoC b'-subunit Mpn390 PdhD E3 component Dihydrolipoyl dehydrogenase Mpn391 PdhC E2 component Dihydrolipoamide acetyltransferase Mpn392 PdhB E1 component b-subunit Mpn393 PdhA E1 component a-subunit Ribonucleosidediphosphate reductase complex Mpn322 NrdF a-subunit Mpn324 NrdE b-subunit DNA Topoisomerase IV complex Mpn122 ParE b-subunit Mpn123 ParC a-subunit Mpn001 DnaN b-subunit Mpn007 Mpn007 d-subunit Mpn034 PolC Mpn378 DnaE a-subunit Mpn450 Mpn450 d'-subunit Mpn618 DnaX g/t-subunit Mpn631 Tsf DNA polymerase III complex Translation elongation General principles of cellular organization in Mycoplasma pneumoniae 187 factor complex Mpn665 GroEL-GroES complex Mpn573 GroL Mpn574 GroS Mpn089 Mpn089 S protein Mpn201 Mpn201 S protein Mpn285 Mpn285 S protein Mpn289 Mpn289 S protein Mpn290 Mpn290 S protein Mpn342 Mpn342 M protein Mpn343 Mpn343 S protein Mpn345 Mpn345 R protein Mpn347 Mpn347 R protein Mpn365 Mpn365 S protein Mpn507 Mpn507 S protein Restriction enzyme complexes Ribosome complex Tuf Mpn615 Mpn615 S protein Mpn638 Mpn638 S protein Mpn069 RpmG2 50S ribosomal protein L33 2 Mpn116 RpmI 50S ribosomal protein L35 Mpn117 RplT 50S ribosomal protein L20 Mpn164 RpsJ 30S ribosomal protein S10 Mpn165 RplC 50S ribosomal protein L3 Mpn166 RplD 50S ribosomal protein L4 Mpn167 RplW 50S ribosomal protein L23 Mpn168 RplB 50S ribosomal protein L2 Mpn169 RpsS 30S ribosomal protein S19 Mpn170 RplV 50S ribosomal protein L22 Mpn171 RpsC 30S ribosomal protein S3 Mpn172 RplP 50S ribosomal protein L16 Mpn173 RpmC 50S ribosomal protein L29 Mpn174 RpsQ 30S ribosomal protein S17 Mpn175 RplN 50S ribosomal protein L14 Mpn176 RplX 50S ribosomal protein L24 Mpn177 RplE 50S ribosomal protein L5 Mpn178 RpsZ/RpsN 30S ribosomal protein S14 type Z Mpn179 RpsH 30S ribosomal protein S8 Mpn180 RplF 50S ribosomal protein L6 Mpn181 RplR 50S ribosomal protein L18 Mpn182 RpsE 30S ribosomal protein S5 Mpn183 RplO 50S ribosomal protein L15 Mpn188 RpmJ 50S ribosomal protein L36 Mpn189 RpsM 30S ribosomal protein S13 Mpn190 RpsK 30S ribosomal protein S11 Mpn192 RplQ 50S ribosomal protein L17 Mpn208 RpsB 30S ribosomal protein S2 Mpn219 RplK 50S ribosomal protein L11 Mpn220 RplA 50S ribosomal protein L1 Mpn225 RpsL 30S ribosomal protein S12 Mpn226 RpsG 30S ribosomal protein S7 General principles of cellular organization in Mycoplasma pneumoniae Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity Expasy M. pneumoniae 188 Translation initiation factor 1-Ribosome complex Translation initiation factor 2-Ribosome complex Cohesin-like complex RbfA-30S-ribosomal subunit complex Glycolytic enzyme complex 1 Dnak-GrpE-DnaJ complex Glycolytic enzyme complex 2 Era-30S-ribosomal Mpn228 RpsF 30S ribosomal protein S6 Mpn230 RpsR 30S ribosomal protein S18 Mpn231 RplI 50S ribosomal protein L9 Mpn296 RpsU 30S ribosomal protein S21 Mpn325 RplU 50S ribosomal protein L21 Mpn327 RpmA 50S ribosomal protein L27 Mpn360 RpmE 50S ribosomal protein L31 Mpn446 RpsD 30S ribosomal protein S4 Mpn471 RpmG1 50S ribosomal protein L33 1 Mpn538 RplJ 50S ribosomal protein L10 Mpn539 RplL 50S ribosomal protein L7/L12 Mpn540 RpmF 50S ribosomal protein L32 Mpn541 RpsT 30S ribosomal protein S20 Mpn616 RpsI 30S ribosomal protein S9 Mpn617 RplM 50S ribosomal protein L13 Mpn622 RpsO 30S ribosomal protein S15 Mpn624 RpmB 50S ribosomal protein L28 Mpn658 RplS 50S ribosomal protein L19 Mpn660 RpsP 30S ribosomal protein S16 Mpn682 RpmH 50S ribosomal protein L34 Mpn187 InfA Translation initiation factor IF-1 Ribosome Ribosome Mpn155 InfB Ribosome Ribosome Mpn300 ScpA Mpn301 ScpB Mpn426 Smc Mpn156 RbfA 30S ribosomal subunit 30S ribosomal subunit Mpn430 GapA Glyceraldehyde-3-phosphate dehydrogenase Mpn025 Fba Fructose-bisphosphate aldolase Mpn674 Ldh L-lactate dehydrogenase Translation initiation factor IF-2 17329809, 11959450 by homology to Mycobacterium tuberculosis Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity 15866174 by homology to Thermus thermophilus 15701694 by homology to Mus musculus 15632130 By homology to Escherichia coli 8613464 by homology to MCF-7 cells (eukaryotes) 15866174 by homology to Ribosome-binding factor A Mpn303 Pyk Pyruvate kinase Mpn302 PfkA 6-phosphofructokinase Mpn120 GrpE Protein GrpE Mpn434 DnaK Chaperone protein DnaK Mpn021 DnaJ Chaperone protein dnaJ Mpn430 GapA Glyceraldehyde-3-phosphate dehydrogenase Mpn429 Fba Phosphoglycerate kinase Mpn606 Ldh Enolase Mpn303 Pyk Pyruvate kinase Mpn568 Era General principles of cellular organization in Mycoplasma pneumoniae 189 subunit complex Thermus thermophilus 30S ribosomal subunit 30S ribosomal subunit Mpn401 GreA Transcription elongation factor Mpn024 RpoE probable δ-factor Mpn191 RpoA a-subunit Mpn352 RpoD σ-factor Mpn516 RpoB b-subunit Mpn515 RpoC b'-subunit Dihydrofolate reductase-thymidylate synthase complex Mpn321 FolA Mpn320 ThyA Holliday junction ATPdependent helicase complex Mpn536 RuvB Mpn535 RuvA Aspartyl-glutamyltRNA(Asn/Gln) amidotransferase complex Mpn238 GatB Mpn237 GatA Mpn236 GatC Mpn125 UvrC Mpn619 UvrA GreA-RNA polymerase complex UvrABC complex Phosphotransfer system maltose complex DnaB-DnaG complex Phosphotransfer system glucose complex Phosphotransfer system fructose complex Phosphotransfer system maltose complex tRNA modification complex Mpn211 UvrB Mpn053 PtsH Mpn651 MtlA Mpn653 MtlF Mpn232 DnaB Mpn353 DnaG Mpn053 PtsH Mpn207 PtsG Mpn053 PtsH Mpn078 FruA Mpn053 PtsH Mpn494 UlaC Mpn495 UlaB Mpn496 UlaA Mpn557 MnmG Mpn008 TrmE Protein function 17207814 M. pneumoniae by similarity Expasy other Expasy M. pneumoniae Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity 17803963 by homology to Escherichia coli 17947583 by homology to Escherichia coli Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity Expasy M. pneumoniae by similarity 17062623 by homology to Escherichia coli Subunits PubMed per ID or complex source ii) Homomultimeric complexes Systematic Protein name name Cell division MraZ Mpn314 MraZ 8 Expasy Pyruvate kinase Mpn303 Pyk 4 Expasy Thymidylate synthase Mpn320 ThyA 2 Expasy L-lactate dehydrogenase Mpn674 Ldh 4 Expasy General principles of cellular organization in Mycoplasma pneumoniae Genus/ species M. pneumoniae M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity 190 Fructose-biphosphate aldolase Fba 2 Expasy M. pneumoniae by similarity Mpn317 FtsZ multiple/ aggregates to form a ring-like structure 17977836 M. pneumoniae by similarity Mpn223 HprK 6 Expasy M. pneumoniae Mpn302 PfkA multiple Mpn479 AzoR 2 Mpn430 GapA 4 Acetate kinase Mpn533 AckA 2/4 Alanyl-tRNA synthetase Mpn419 AlaS 2 Cell division FtsZ HPr kinase/ phosphorylase 6-phosphofructokinase /Phosphohexokinase FMN-dependent NADHazoreductase Glyceraldehyde-3phosphate-dehydrogenase Mpn025 by homology to Escherichia coli by homology to 16684776 Escherichia coli M. pneumoniae by Expasy similarity 216674, by homology to 29037, Veillonella 230181 alcalescens by homology to 9207019 Thermus thermophilus 16289704 Probable inorganic polyphosphate/ATP-NAD kinase Mpn267 PpnK 2 11006082 by homology to Micrococcus flavus & Mycobacterium tuberculosis Recombinase A Mpn490 RecA 6 17955055 by homology to Escherichia coli Chaperone protein ClpB Mpn531 ClpB 6 Expasy M. pneumoniae by similarity Replicative DNA helicase Mpn232 DnaB 6 17947583 by homology to Escherichia coli Glycerol kinase Mpn050 GlpK 2/4 9930671 by homology to Escherichia coli Putative proline iminopeptidase Mpn022 Pip multiple 8885412 by homology Xanthomonas campestris pv. citri Methionyl-tRNA synthetase Mpn023 MetG Enolase Mpn606 Eno 2 Expasy Putative Metallophosphoesterase Mpn126 Mpn126 4 17586769 Single stranded DNA binding protein Mpn229 Ssb 4 16041080 by homology to Mycobacterium smegmatis Arginine deiminase-like protein Mpn560 Mpn560 2/3 17223359, 11911465 by homology to Lactococcus lactis DNA polymerase III subunit β Mpn001 DnaN 2 Expasy by homology to Streptococcus pneumoniae 2126467 General principles of cellular organization in Mycoplasma pneumoniae by homology to Escherichia coli M. pneumoniae by similarity by homology to Escherichia coli 191 by homology to Thermus thermophilus by homology to Homo 11071809 sapiens by homology to 11545747 Pyrococcus furiosus M. pneumoniae by Expasy similarity DNA gyrase subunit B Mpn003 GyrB 2 Thymidylate kinase Mpn006 Tmk 2 Mpn007 Mpn007 6 Mpn034 PolC 2 Deoxyribose-phosphate aldolase Mpn063 DeoC 1/2 Phosphomannomutase Mpn066 ManB 2 Putative 1phosphofructokinase Mpn079 FruK 8 Mpn105 PheS 2 Mpn106 PheT 2 Mpn191 RpoA 2 Mpn322 NrdF 2 Mpn324 NrdE 2 Mpn328 Nfo 2 Mpn391 PdhC 60 Mpn392 PdhB 2 Mpn393 PdhA 2 Putative Xaa-Pro aminopeptidase Mpn470 PepP 2 12377124 by homology to Pyrococcus horikoshii Probable L-ribulose-5phosphate 3-epimerase UlaE Mpn492 UlaE 4 11732895 by homology to Pseudomonas cichorii Probable thiamine biosynthesis protein Mpn550 ThiL 2 16343540 by homology to Bacillus anthracis Ribosomal RNA small subunit methyltransferase G Mpn558 RsmG 2 18667428 by homology to Geobacter sulfurreducens Probable NH(3)-dependent NAD(+) synthetase Mpn562 NadE 2 15645437 60 kDa chaperonin Mpn573 GroL 2x7 Expasy Putative type I restriction enzyme specificity protein Mpn638 Mpn638 2 16038930 Uncharacterized protein MG007 homolog DNA polymerase III polCtype Phenylalanyl-tRNA synthetase α chain Phenylalanyl-tRNA synthetase β chain DNA-directed RNA polymerase subunit α Ribonucleosidediphosphate reductase subunit β Ribonucleosidediphosphate reductase subunit α Probable endonuclease 4 Dihydrolipoyllysine-residue acetyltransferase Pyruvate dehydrogenase E1 component subunit β Pyruvate dehydrogenase E1 component subunit α General principles of cellular organization in Mycoplasma pneumoniae 11850422 by homology to Escherichia coli by homology to 16540464 Sulfolobus Tokodaii by homology to Homo 2527305 sapiens by homology to 7664121 Thermus thermophilus by homology to 7664121 Thermus thermophilus M. pneumoniae by Expasy similarity by homology to 16301799 Salmonella typhimurium by homology to 8052308 Salmonella typhimurium by homology to 10458614 Escherichia coli M. pneumoniae by 9990008 similarity M. pneumoniae by Expasy similarity M. pneumoniae by Expasy similarity 7675789 by homology to Bacillus subtilis M. pneumoniae by similarity by homology to Methanocaldococcus jannaschii 192 by homology to Escherichia coli M. pneumoniae by similarity Elongation factor Tu Mpn665 Tuf 2 16257965 Dihydrolipoyl dehydrogenase Mpn390 PdhD 2 Expasy 10 kDa chaperonin Mpn574 GroS 7 Expasy DNA topoisomerase 4 subunit B Mpn122 ParE 2 Expasy Mpn332 Lon 4 Expasy Mpn008 TrmE 2 Expasy Mpn278 Glf 2 10089346 Mpn632 PyrH 6 17297917 Mpn595 RpiB 4 15162497 Mpn186 Map 2 12297034 FAD synthetase Mpn158 RibF 2 15468322 GMP kinase Mpn245 Def 2 17012781 Mpn298 AcpS 2 16788183 M. pneumoniae Mpn395 Apt 2 10393170, 11535055 Threonyl-tRNA synthetase Mpn553 ThrS 2 Expasy Thymidine phosphorylase Mpn064 DeoA multiple Expasy Ribose-phosphate pyrophosphokinase Mpn073 Prs multiple 2169413 Protein GrpE Mpn120 GrpE 2 Expasy UDP-glucose 4-epimerase Mpn257 GalE 2 Expasy Mpn265 TrpS 2 Expasy Mpn686 DnaA multiple 16829961 Mpn629 TpiA 2 Expasy Mpn576 GlyA 4 Expasy Mpn627 PtsL 2 Expasy by homology to Leishmania donovani M. pneumoniae by similarity M. pneumoniae by similarity by homology to Bacillus subtilis M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity by homology to Aquifex aeolicus M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity ATP-dependent protease Lon Probable tRNA modification GTPase UDP-galactopyranose mutase Uridylate Kinase Mpn632 Ribose-5-phosphateisomerase-B Methionine aminopeptidase, Peptidase M Acyl carrier protein (ACP) synthase Adenine phosphoribosyltransferase Tryptophanyl-tRNA synthetase Replication initiator protein DnaA Triosephosphate isomerase Serine hydroxymethyltransferase Phosphoenolpyruvateprotein transferase General principles of cellular organization in Mycoplasma pneumoniae M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity by homology to Thermotoga maritima by homology to Escherichia coli by homology to Sulfolobus solfataricus by homology to Thermotoga maritima by homology to Bacillus stearothermophilus by homology to Thermotoga maritima by homology to Staphylococcus aureus 193 Glycyl-tRNA synthetase Mpn354 GlyqS 2 Expasy Seryl-tRNA synthetase Mpn005 SerS 2 Expasy Bifunctional protein FolD Mpn017 FolD 2 Expasy Lysyl-tRNA synthase Mpn277 LysS 2 Expasy Histidyl-tRNA synthase Mpn045 HisS 2 Expasy Thioredoxin reductase Mpn240 TrxB 2 Expasy Chaperone protein dnaJ Mpn021 DnaJ 2 Expasy General principles of cellular organization in Mycoplasma pneumoniae M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity M. pneumoniae by similarity 194 10.5. Table S5: List of modeled protein complexes. This table is a list of binary interactions that can be modeled for all protein complexes in this study. It is divided into i) a set of 29 heteromultimeric complexes and ii) a set of 57 homomultimeric complexes. In part i) the first column gives the unique complex ID for each protein complex modeled. Column 2 provides the complex name. In columns 3 and 4 give the systematic name and protein name of the first interactor modeled, columns 5 and 6 give the systematic name and protein name of the second interactor modeled. Columns 7 and 8 give the PDB ID and the species this information was obtained from. In part ii) the first column on the left shows the complex ID of the homomultimeric protein complexes modeled. The second column indicates the complex name. Columns 3 and 4 give the annotated systematic and protein name of each homomultimer. Columns 5 and 6 give the PDB ID and the species this information was obtained from. (* novel homomultimers supported by structural models) Interactor 1 Interactor 2 ComComplex PDB PDB species plex Systematic Protein Systematic Protein name ID ID name name name name i) Heteromultimeric complexes Mpn007 Mpn007 Mpn450 Mpn450 1sxj S. cerevisiae DNA Polymerase 3 Mpn007 Mpn007 Mpn618 DnaX 1njf E. coli III γ complex Mpn450 Mpn450 Mpn618 DnaX 1iqp P. furiosus Mpn002 Mpn002 Mpn434 DnaK 2qwq B. taurus Mpn021 DnaJ Mpn002 Mpn002 2och C. elegans Protein 8 chaperone Mpn021 DnaJ Mpn434 DnaK 2qwq B. taurus complex Mpn120 GrpE Mpn434 DnaK 1dkg E. coli Mpn120 GrpE Mpn531 ClpB 2tma O. cuniculus 9 Dnak-GrpE complex Mpn120 GrpE Mpn434 DnaK 1dkg E. coli 10 Mpn023 Aminoacyl-tRNA Mpn105 synthetase Mpn105 complex Mpn106 MetG PheS PheS PheT Mpn678 Mpn106 Mpn553 Mpn553 GltX PheT ThrS ThrS 1g59 2rhs 2zcd 2e3c T. thermophilus S. haemolyticus M. mazei M. mazei 11 PhenylalaninetRNA synthetase Mpn105 complex PheS Mpn106 PheT 2rhs S. haemolyticus 15 DNA Recombination complex Mpn122 ParE Mpn123 ParC 1bjt S. cerevisiae 17 DNA Topoisomerase IV complex Mpn122 ParE Mpn123 ParC 1bjt S. cerevisiae 19 25 Complex 19 Complex 25 Mpn076 Mpn223 Mpn076 Mpn284 HprK Mpn258 Mpn284 2r6g E. coli Mpn258 1kkm L. casei Adk FtsH 34 Restriction Mpn185 enzyme complex Mpn671 General principles of cellular organization in Mycoplasma pneumoniae 2rhm C. aurantiacus 195 35 Restriction Mpn089 enzyme complex 36 Mpn089 Mpn507 Mpn507 1yf2 Complex 36 Mpn008 Mpn194 Mpn574 TrmE CbiO2 GroS Mpn426 Mpn426 Mpn573 P115 P115 GroL 2ocy S. cerevisiae 1mv5 L. lactis 1gru E. coli 37 GroEL-GroES complex Mpn574 GroS Mpn573 GroL 1gru E. coli 38 Peptidase complex Mpn218 OppF Mpn609 PstB 2ouk G. stearothermophilus 40 Cohesin like complex Mpn426 P115 Mpn508 Mpn508 3bat Mpn008 TrmE Mpn426 P115 44 Pyruvate dehydrogenase complex Mpn392 PdhB Mpn393 PdhA GroS Mpn002 GrpE GrpE Mpn573 Mpn434 Mpn434 Mpn531 GroL DnaK DnaK ClpB S. cerevisiae G. 1w85 stearothermophilus 1gru E. coli 2qwq B. taurus 1dkg E. coli 2tma O. cuniculus AtpD Mpn600 AtpA 2jj1 B. taurus Mpn020 Mpn020 Mpn153 Mpn153 RpoA RpoA RpoA AsnS TopA TopA RpoD RpoD RpoC Tmk Tmk Mpn137 Mpn137 Mpn137 InfB InfB InfB RplC RplW RpsS RplE RpsZ RpsH RpsH Mpn153 Mpn443 Mpn443 Mpn490 Mpn266 Mpn515 Mpn516 Mpn354 Mpn515 Mpn516 Mpn515 Mpn516 Mpn516 Mpn386 Mpn536 Mpn155 Mpn475 Mpn524 Mpn475 Mpn524 Mpn665 Mpn658 Mpn174 Mpn189 Mpn446 Mpn171 Mpn182 Mpn208 Mpn153 Mpn443 Mpn443 RecA Mpn266 RpoC RpoB GlyQS RpoC RpoB RpoC RpoB RpoB Mpn386 RuvB InfB EngA Mpn524 EngA Mpn524 Tuf RplS RpsQ RpsM RpsD RpsC RpsE RpsB 2qeq 8ohm 2qeq 2gbl 1z3e 2a69 1ynj 3bju 2nvq 2r7z 2a69 1smy 1ynn 2ccg 2ar7 2ocy 2ocy 2ba2 2zej 2eqb 2qmu 1voy 1ml5 2vqe 1ml5 2i2p 2avy 1s1h Kunjin virus Hepatitis C virus Kunjin virus S. elongatus B. subtilis T. thermophilus T. aquaticus H. sapiens S. cerevisiae S. cerevisiae T. thermophilus T. thermophilus T. aquaticus S. aureus H. sapiens S. cerevisiae S. cerevisiae M. pneumoniae H. sapiens S. cerevisiae S. solfataricus E. coli E. coli T. thermophilus E. coli E. coli E. coli S. cerevisiae 45 47 49 50 Mpn574 Mpn002 Glycolytic enzyme complex Mpn120 2 Mpn120 ATP synthase complex Mpn598 Mpn020 Mpn020 Mpn153 Mpn153 Mpn191 Mpn191 RNA polymerase Mpn191 complex Mpn252 Mpn261 Mpn261 Mpn352 Mpn352 Mpn515 Ribosome Mpn006 complex Mpn006 Mpn137 Mpn137 Mpn137 Mpn155 Mpn155 Mpn155 Mpn165 Mpn167 Mpn169 Mpn177 Mpn178 Mpn179 Mpn179 General principles of cellular organization in Mycoplasma pneumoniae M. jannaschii A. irradians 2ocy 196 Mpn182 Mpn230 Mpn386 Mpn475 Mpn475 Mpn536 RpsE RpsR Mpn386 EngA EngA RuvB Mpn446 Mpn228 Mpn536 Mpn524 Mpn665 Mpn571 RpsD RpsF RuvB Mpn524 Tuf Mpn571 1fjg 2vho 1sxj 2eqb 2zej 2chg Mpn571 Mpn571 Mpn685 Mpn685 2olk T. thermophilus E. coli S. cerevisiae S. cerevisiae H. sapiens A. fulgidus G. stearothermophilus 52 Translation elongation factor Mpn631 complex Tsf Mpn665 Tuf 54 Phosphotransfer Mpn207 system complex PtsG Mpn268 Mpn268 1o2f E. coli Mpn004 GyrA S. cerevisiae 1xb2 B. taurus 82 DNA Gyrase complex Mpn003 GyrB 92 Complex 92 Mpn258 Mpn258 Mpn259 Mpn259 2onk A. fulgidus 93 ScpA-ThyA complex Mpn300 ScpA Mpn320 ThyA 2oip C. hominis 94 Ribonucleosidediphosphate reductase complex Mpn322 NrdF Mpn324 NrdE 2bq1 S. typhimurium 102 ParE-GyrA complex Mpn004 GyrA Mpn122 ParE 1bjt S. cerevisiae 1bjt 113 Restriction Mpn343 enzyme complex Mpn343 Mpn365 Mpn365 1yf2 M. jannaschii 115 Restriction Mpn365 enzyme complex Mpn365 Mpn615 Mpn615 1yf2 M. jannaschii Com plex ID Complex name Systematic name Protein name PDB ID PDB species DnaN GyrB Tmk Mpn007 Mpn020 Pip Fba PolC GlpK Mpn051 DeoC ManB FruK PheS PheT 1mmi 1kij 2uvq 1njf 2qeq 1xkt 1rv8 2p1j 2dpn 1ryi 1jcj 2f7l 1gqt 1eqr 3bu2 E. coli T. thermophilus H. sapiens E. coli Kunjin virus H. sapiens T. aquaticus T. maritima T. thermophilus B. subtilis E. coli S. tokodaii E. coli E. coli S. saprophyticus ii) Homomultimeric complexes 117 118 119 120 121* 122 124 125 126 127* 128 129 130 131 132 DNA polymerase III subunit β DNA gyrase subunit B Thymidylate kinase Uncharacterized protein MG007 homolog Uncharacterized ATP-dependent helicase Mpn020 Putative proline iminopeptidase Fructose-biphosphate aldolase DNA polymerase III PolC-type Glycerol kinase Uncharacterized protein MG039 homolog Deoxyribose-phosphate aldolase Phosphomannomutase Putative 1-phosphofructokinase Phenylalanyl-tRNA synthetase α chain Phenylalanyl-tRNA synthetase β chain Mpn001 Mpn003 Mpn006 Mpn007 Mpn020 Mpn022 Mpn025 Mpn034 Mpn050 Mpn051 Mpn063 Mpn066 Mpn079 Mpn105 Mpn106 General principles of cellular organization in Mycoplasma pneumoniae 197 134 135 136 137 139* 140 141* 142* 143 Putative Metallophosphoesterase DNA-directed RNA polymerase subunit α HPr kinase/phosphorylase Single stranded DNA binding protein Uncharacterized protein MG127 homolog Probable inorganic polyphosphate/ATP-NAD kinase 2',3'-cyclic-nucleotide 2'-phosphodiesterase Uncharacterized protein MG134 homolog 6-phosphofructokinase /Phosphohexokinase Mpn126 Mpn191 Mpn223 Mpn229 Mpn266 Mpn267 Mpn269 Mpn275 Mpn302 Mpn126 RpoA HprK Ssb Mpn266 PpnK Mpn269 Mpn275 PfkA 1z2w 2cw0 1knx 1s3o 1r7h 1y3h 2pau 1j8b 1pfk 144 146 147 148 149 Pyruvate kinase Cell division protein Thymidylate synthase Ribonucleoside-diphosphate reductase subunit β Ribonucleoside-diphosphate reductase subunit α Mpn303 Mpn317 Mpn320 Mpn322 Mpn324 Pyk FtsZ ThyA NrdF NrdE 2e28 1w58 1hw4 2bq1 2bq1 150 151* 152* 153* 154* 155 Probable endonuclease 4 Uncharacterized protein MG236 homolog Uncharacterized protein MG246 homolog Uncharacterized protein MG259 homolog Uncharacterized protein MG263 homolog Dihydrolipoyl lysine-residue acetyltransferase Mpn328 Mpn329 Mpn349 Mpn362 Mpn381 Mpn391 Nfo Mpn329 Mpn349 Mpn362 Mpn381 PdhC 1did 2fe3 1t71 2ipx 2p9j 1c4t 156 157 158 159* Pyruvate dehydrogenase E1 component subunit β Pyruvate dehydrogenase E1 component subunit α Alanyl-tRNA synthetase Uncharacterized protein MG293 homolog Mpn392 Mpn393 Mpn419 Mpn420 PdhB PdhA AlaS Mpn420 1w85 2r8p 1vhx 2pz0 160 161 162 163 164 166 167 168 169* 170 171 172 173 174 175 176 177 Glyceraldehyde-3-phosphate-dehydrogenase Putative Xaa-Pro aminopeptidase FMN-dependent NADH-azoreductase Recombinase A Probable L-ribulose-5-phosphate 3-epimerase UlaE Chaperone protein ClpB Acetate kinase Probable thiamine biosynthesis protein Uncharacterized protein MG377 homolog Ribosomal RNA small subunit methyltransferase G Arginine deiminase-like protein Probable NH3-dependent NAD+ synthetase 60 kDa chaperonin Enolase Putative type I restriction enzyme specificity protein Elongation factor Tu L-lactate dehydrogenase Mpn430 Mpn470 Mpn479 Mpn490 Mpn492 Mpn531 Mpn533 Mpn550 Mpn555 Mpn558 Mpn560 Mpn562 Mpn573 Mpn606 Mpn638 Mpn665 Mpn674 GapA PepP AzoR RecA UlaE ClpB AckA ThiI Mpn555 GidB Mpn560 NadE GroL Eno Mpn638 Tuf Ldh 1nqa 1wn1 2z9c 1oft 1k77 2tma 1g99 1vbk 1zxj 1ne2 2ci5 1wxe 2nwc 1pdz 1yf2 2bvn 1uxg M. musculus T. thermophilus M. pneumoniae H. sapiens C. ammoniagenes M. tuberculosis E. coli H. Influenzae E. coli G. stearothermophilus M. jannaschii H. sapiens S. typhimurium S. typhimurium Arthrobacter sp. nrrl B. subtilis M. pneumoniae H. sapiens A. aeolicus E. coli G. stearothermophilus E. coli B. subtilis T. tengcongensis G. stearothermophilus P. horikoshii E. coli P. aeruginosa E. coli O. cuniculus M. thermophila P. horikoshii M. pneumoniae T. acidophilum B. taurus E. coli E. coli H. gammarus M. jannaschii E. coli C. aurantiacus Mpn677 Mpn677 2q14 B. thetaiotaomicron 178* Uncharacterized protein MG461 homolog General principles of cellular organization in Mycoplasma pneumoniae 198 10.6. Table S6: List of multifunctional proteins. This table gives a list of all multifunctional proteins. The first column indicates the annotated systematic name. The second column provides the protein name.The third column gives all complexes a multifunctional protein clusters to. Systematic name Protein name Complexes Mpn003 GyrB 35 83 58 59 112 99 34 46 56 101 96 29 Mpn004 GyrA 104 67 102 26 17 106 105 100 82 6 75 83 36 3 94 111 47 Mpn006 Tmk 90 17 15 Mpn007 Mpn007 28 3 10 105 Mpn011 Mpn011 6 4 99 3 5 Mpn014 Mpn014 84 103 106 97 10 12 Mpn020 Mpn020 104 11 26 17 48 93 106 3 97 61 94 108 98 101 43 10 86 Mpn025 Fba 28 106 97 9 105 Mpn027 Mpn027 109 85 79 Mpn034 PolC 112 90 21 83 86 Mpn042 Mpn042 74 60 1 24 14 Mpn048 Mpn048 14 107 62 54 10 105 82 105 110 Mpn060 MetK 16 17 15 107 Mpn061 Ffh 28 97 105 47 Mpn062 DeoD 92 11 56 97 10 Mpn063 DeoC 67 72 79 Mpn067 Mpn067 Mpn073 Prs 108 96 55 12 22 20 33 60 106 65 88 18 Mpn078 FruA 71 23 41 Mpn081 Mpn081 87 80 115 Mpn089 Mpn089 35 109 Mpn094 Mpn094 22 75 Mpn097 Mpn097 69 70 Mpn100 Mpn100 81 53 95 Mpn103 Mpn103 33 89 51 Mpn106 PheT Mpn108 Mpn108 Mpn115 InfC 11 28 94 97 112 71 83 73 60 51 55 110 Mpn118 Mpn118 99 106 13 105 29 Mpn122 ParE 104 95 102 17 82 Mpn124 HrcA 73 13 Mpn141 MgpA 59 11 Mpn142 Mpn142 62 20 Mpn153 Mpn153 38 4 97 48 59 112 37 101 73 20 3 Mpn155 InfB 7 13 5 110 Mpn165 RplC 52 98 32 116 23 Mpn166 RplD 52 98 33 116 110 Mpn169 RpsS 51 110 55 Mpn177 RplE 111 79 Mpn189 RpsM 51 23 110 Mpn191 RpoA 67 53 51 18 106 Mpn192 RplQ 24 68 110 10 General principles of cellular organization in Mycoplasma pneumoniae 199 Mpn207 PtsG 3 64 68 12 47 79 48 98 18 93 86 54 Mpn210 SecA 74 67 33 32 90 91 98 103 56 24 43 86 Mpn211 UvrB 33 93 Mpn218 OppF 38 67 Mpn221 Pth 112 22 83 Mpn222 TilS 103 94 23 111 51 110 105 100 85 Mpn228 RpsF 33 53 57 Mpn230 RpsR 59 51 105 Mpn246 Def 6 4 34 3 100 Mpn257 GalE 112 27 109 28 83 Mpn258 Mpn258 25 92 3 68 86 Mpn264 Mpn264 36 75 Mpn269 Mpn269 25 77 Mpn277 LysS 25 53 76 Mpn280 Mpn280 53 64 89 Mpn284 Mpn284 19 86 Mpn294 Mpn294 60 57 Mpn300 ScpA 69 93 Mpn307 Mpn307 85 79 Mpn311 Mpn311 6 Mpn314 MraZ 42 Mpn315 MraW 43 105 Mpn316 Mpn316 46 68 Mpn320 ThyA 69 Mpn321 FolA Mpn322 Mpn324 40 24 4 3 68 5 71 66 68 43 104 93 94 19 67 64 105 NrdF 11 63 94 NrdE 94 41 Mpn328 Nfo 61 24 Mpn329 Mpn329 11 67 Mpn330 Mpn330 60 61 Mpn331 Tig 32 90 13 65 Mpn340 Mpn340 112 27 28 83 68 5 47 48 102 82 79 101 Mpn341 UvrD 25 104 102 26 82 Mpn342 Mpn342 109 35 34 30 100 5 Mpn345 Mpn345 81 95 100 17 Mpn346 Mpn346 27 77 73 7 13 31 79 Mpn347 Mpn347 112 27 67 28 83 10 105 Mpn350 Mpn350 67 85 61 51 Mpn352 RpoD 84 103 67 37 Mpn354 GlyQS 60 28 97 105 Mpn361 PrfA 35 74 99 56 108 96 Mpn365 Mpn365 95 105 115 79 113 14 Mpn366 Mpn366 28 56 31 Mpn378 DnaE 105 20 Mpn379 PolA 116 91 Mpn387 Mpn387 76 16 17 15 107 Mpn388 Mpn388 1 24 105 Mpn390 PdhD 75 44 Mpn392 PdhB 106 36 71 66 37 Mpn393 PdhA 64 71 97 10 105 97 17 General principles of cellular organization in Mycoplasma pneumoniae 200 Mpn394 Nox 97 41 14 Mpn401 GreA 67 28 10 68 105 79 Mpn402 ProS 86 17 Mpn416 P29 71 100 Mpn419 AlaS 99 96 Mpn426 P115 93 86 Mpn430 GapA 12 33 85 53 56 106 67 33 32 7 80 26 17 99 88 100 110 28 75 40 95 108 112 69 49 10 104 11 53 78 107 93 106 46 23 96 65 50 3 51 111 9 114 58 12 15 8 52 81 34 45 86 19 5 10 105 110 36 108 112 34 37 10 19 86 5 Mpn434 DnaK 56 37 101 Mpn436 Mpn436 76 17 15 3 Mpn440 Mpn440 6 4 Mpn461 Mpn461 75 105 68 Mpn476 Cmk 75 58 Mpn480 ValS 18 30 51 Mpn486 Mpn486 90 101 82 Mpn487 Csd 98 76 111 Mpn497 Mpn497 109 85 Mpn509 Mpn509 81 58 Mpn514 Mpn514 1 39 21 2 Mpn515 RpoC 67 53 57 61 111 48 18 106 110 Mpn516 RpoB 53 57 61 111 51 17 47 107 106 87 106 43 44 96 29 20 44 Mpn520 IleS Mpn526 Mpn526 11 97 108 51 112 27 28 21 Mpn531 ClpB 79 110 Mpn536 RuvB 95 71 100 Mpn547 Mpn547 81 25 23 Mpn550 ThiI 65 55 Mpn551 Mpn551 35 18 40 30 Mpn553 ThrS 11 97 51 12 38 106 37 Mpn556 ArgS 4 87 3 68 108 41 96 Mpn562 NadE 59 70 89 Mpn563 Mpn563 81 39 90 Mpn567 P200 6 24 Mpn568 Era 87 23 100 88 93 106 105 100 48 38 4 106 105 Mpn573 GroL 11 90 80 Mpn574 GroS 60 37 9 Mpn582 Mpn582 99 20 13 26 Mpn592 Mpn592 27 75 Mpn596 Mpn596 87 66 108 55 Mpn598 AtpD 3 97 12 47 Mpn599 AtpG 67 85 58 Mpn606 Eno 46 41 110 Mpn609 PstB 38 68 Mpn612 Mpn612 21 46 110 Mpn615 Mpn615 87 80 115 Mpn619 UvrA 75 105 Mpn622 RpsO 60 51 Mpn623 Mpn623 71 111 Mpn629 TpiA 3 46 113 110 General principles of cellular organization in Mycoplasma pneumoniae 201 Mpn635 Mpn635 7 55 Mpn644 Mpn644 69 93 Mpn650 Mpn650 81 90 Mpn656 Mpn656 109 71 100 Mpn658 RplS 33 23 26 100 Mpn662 MsrB 78 44 90 53 100 54 Mpn665 Tuf Mpn668 Mpn668 104 33 32 39 90 41 Mpn669 TyrS 106 97 79 Mpn671 FtsH Mpn673 Mpn673 38 92 34 67 61 Mpn676 Mpn676 87 80 Mpn677 Mpn677 21 26 Mpn678 GltX 22 103 23 Mpn685 Mpn685 56 37 51 110 Mpn686 DnaA 70 102 20 59 48 116 18 101 29 82 106 23 96 110 50 36 51 111 47 52 49 37 54 115 89 General principles of cellular organization in Mycoplasma pneumoniae 202 10.7. Table S7: List of literature curated multifunctional proteins. This table is a repository of literature evidence for multifunctional proteins that may exist in M. pneumoniae based on observed homologs in other species. In total 50 multifunctional proteins are reported in this list. The first column on the left gives the annotated systematic name of each multifunctional protein. The second and third column provide the protein name and protein functions. The last column gives the PubMed ID or database, in which this evidence is reported. PMID or Systematic Protein description Functions, enzymatic activities other name source: Mpn002 DnaJ-like protein 17919282 Mpn008 tRNA modification GTPase mnmE DnaJ has a function as co-chaperone of dnaK, but is also reported to be involved in DNA replication in Escherichia coli DNA gyrase catalyzes the interconversion of various topological isomers of double-stranded DNA rings, including catenanes and knotted rings DNA gyrase catalyzes the interconversion of various topological isomers of double-stranded DNA rings, including catenanes and knotted rings Thymidylate kinase can use purine and pyrimidine nucleosides as substrates Has both GTPase and tRNA modification activities Mpn003 DNA gyrase subunit B Mpn004 DNA gyrase subunit A Mpn006 Thymidylate kinase Mpn025 Fructose-bisphosphate Glycolytic enzyme. aldolase In eukaryotes associates with vacuolar H+ATPase; contribute to its assembly, activity. Aldolase interacts with the endocytic proteins sorting nexin 9 (SNX9) Role in actin cytoskeleton assembly; bind Factin. Also binds g-tubuline, heparin, phospholipase Dand the glucose transporter Glut4. Histidyl-tRNA Is a class-II aminoacyl-tRNA synthetase and is synthetase reported to interact with translation elongation factors in mammals Aspartyl-tRNA Is a class-II aminoacyl-tRNA synthetase and is synthetase reported to interact with translation elongation factors in mammals Signal recognition Respond to acid stress particle protein Protein export and membrane biogenesis 18453690 15299020 Mpn045 Mpn046 Mpn061 Mpn105 Phenylalanyl-tRNA synthetase α-chain Mpn106 Phenylalanyl-tRNA synthetase β-chain Mpn155 Translation initiation factor IF-2. Expasy Expasy 18477629 12730230 12362348 12362348 11274114 Is a class-II aminoacyl-tRNA synthetase and is 12362348 reported to interact with translation elongation factors in mammals Is a aminoacyl-tRNA synthetase and is reported 12362348 to interact with translation elongation factors in mammals Is involved in the hydrolysis of GTP during the Expasy formation of the 70S ribosomal complex Protects formylmethionyl-tRNA from spontaneous hydrolysis and promotes its binding to the 30S ribosomal General principles of cellular organization in Mycoplasma pneumoniae 203 Mpn164 Mpn166 30S ribosomal protein S10 50S ribosomal protein L4 Mpn171 30S ribosomal protein S3 Mpn172 50S ribosomal protein L16 Mpn177 50S ribosomal protein L5 Mpn178 50S ribosomal protein S14 type Z Mpn179 30S ribosomal protein S8 Mpn189 30S ribosomal protein S13 Mpn211 UvrABC system protein B 50S ribosomal protein L1 Mpn220 Involved in translation and NusB mediated antitermination of transcription Suppresses translation of S10 operon mRNA also inhibits transcription of the s10 operon, binds to L4 mRNA and inhibits translation Is involved in translation and has an endonuclease activity in Homo sapiens & Escherichia coli and a lyase function in Drosophila melanogaster Is involved in translation; It is also seems to make contacts with the A and possibly P site tRNAs Is involved in translation and L5-5S rRNA complex stimulates aminoacyl-tRNA synthetases Is involved in translation, binds to S14 gene and inhibits transcription 8871397 Is involved in translation and inhibits translation by binding to mRNAs in specific ribosomal cistrons Is involved in translation and it associates with the ribosomal protein S19 and RNA fragments 16S rRNA recognition though its C-terminal domain Helicase, nuclease and hydrolase activities 8871397 8871397 8871397 Expasy 8871397 8871397 8193163 GO Is involved in translation and inhibits translation by binding to mRNAs in specific ribosomal cistrons Peptidyl-tRNA Releases tRNA from peptidyl-tRNAs by hydrolase cleavage of ester bond Pth is also capable of hydrolysing an amide bond between the peptide and the 3'-amino group of the modified ribose at the end of the tRNA. It also associates with the 30S ribosomal subunit 30S ribosomal protein Is involved in translation and inhibits translation S7 by binding to mRNAs in specific ribosomal cistrons Uracil-DNA Glyceraldehyde-3-phosphate dehydrogenase glycosylase and transcriptional activation Thioredoxin Thioredoxin activity; synthesis of deoxyribonucleotides. Also binds to the Escherichia coli the T7 DNA polymerase Segregation and Chromosome segregation and condensation condensation protein A DNA repair and transcription regulation 8871397 Mpn303 Pyruvate Kinase 15877277 Mpn320 Mpn332 Thymidylate synthase ATP-dependent protease La Mpn221 Mpn226 Mpn235 Mpn263 Mpn300 Final step of glycolysis—the conversion of phosphoenolpyruvate to pyruvate. In mammalian muscle it binds thyroid hormone. It regulates the transcriptional responses of the thyroid-hormone receptor. In bacteria, it can synthesize nucleotide triphosphate (NTP) under anaerobic conditions Pyrimidine metabolism. Translation inhibitor Chaperone General principles of cellular organization in Mycoplasma pneumoniae 16849786 8871397 1924305 768986 15009890 1924359 9149530 204 Mpn345 Mpn347 Mpn356 Putative type-1 restriction enzyme MpnORFDP R protein part 2 Putative type I restriction enzyme MpnORFDP R protein part 1 Cysteinyl-tRNA synthetase Mpn379 DNA polymerase (PolA) Mpn384 Leucyl-tRNA synthetase Mpn430 Glyceraldehyde-3phosphate dehydrogenase Chaperone protein dnaK Mpn434 Mpn446 30S ribosomal protein S4 Mpn480 Valyl-tRNA synthetase Mpn520 Isoleucyl-tRNA synthetase Mpn538 50S ribosomal protein L10 Mpn539 50S ribosomal protein L7/L12 Mpn541 30S ribosomal protein S20 Enolase Mpn606 Mpn616 30S ribosomal protein S9 Mpn622 30S ribosomal protein S15 It has an ATP dependent nuclease as well as DNA translocation function 16126220 It has an ATP dependent nuclease as well as DNA translocation function 16126220 Is a class-I aminoacyl-tRNA synthetase and is reported to interact with translation elongation factors 5'-3' exonuclease, but also reverse transcriptase activity in Streptomyces; part of the Streptomyces telomere complex Is a class-I aminoacyl-tRNA synthetase and is reported to interact with translation elongation factors in mammals Converts glyceraldehyde-3-phosphate to 1,3diphosphoglycerate, but also binds RNA, RNA polymerase and HPr in Bacillus subtilis Besides its role as protein chaperone dnaK was also shown to be involved in DNA replication Escherichia coli Is involved in translation, nucleats assembly of the body of 30S ribosome by binding directly to the 16S rRNA and inhibits translation by binding to mRNAs in specific ribosomal cistrons in Homo sapiens Is a class-I aminoacyl-tRNA synthetase and has two distinct active sites: one for aminoacylation and one for editing.Additionally it is reported to interact with translation elongation factors in mammals Is a class-I aminoacyl-tRNA synthetase and has two distinct active sites: one for aminoacylation and one for editing. Additionally it is reported to specifically interact with HSP90 in mammals Is involved in translation and inhibits translation by binding to mRNAs in specific ribosomal cistrons in Escherichia coli Inhibits the translation of specific mRNAs including its own in Homo sapiens 12362348 Is involved in translation and polyamine biosynthesis in Saccharomyces cerevisiae Catalyzes the conversion of 2-phosphoglycerate to phosphoenolpyruvate in glycolysis, but further it is reported to function also as heat-shock protein, to bind cytoskeleton and chromatin structures Is involved in translation and participates in the error prone SOS repair process in Escherichia coli Is involved in translation and seems to selectively stabilize the temperature sensitive cap-binding subunit of eIF-4E in Saccharomyces cerevisiae General principles of cellular organization in Mycoplasma pneumoniae 15353591 12362348 17142398 15877277 17919282 2470510 Uniprot 12362348 12362348 2470510 8871397 2470510 7539334 15877277 8871397 8871397 205 Mpn623 ATP-dependent RNA helicase Mpn665 Elongation factor Tu Mpn671 Cell division protease ftsH homolog Mpn678 Glutamyl-tRNA synthetase Some members of the same family are involved in RNA transcription, mRNA transport, translation initiation and cell cycle regulation in Homo sapiens Elongation factor Tu promotes the GTPdependent binding of aminoacyl-tRNA to the Asite of ribosomes during protein biosynthesis, in addition it has a direct function in host cell adherence in M. pneumoniae Metalloprotease, but has also chaperone function in Escherichia coli Is a class-I aminoacyl-tRNA synthetase and is reported to specifically interact with HSP90 in mammals General principles of cellular organization in Mycoplasma pneumoniae 17979704 17709513 9149530 12362348 206