* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lec 16: Nitrogen (ammonia) assimilation
Peptide synthesis wikipedia , lookup
Western blot wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemical cascade wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Plant nutrition wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Point mutation wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Genetic code wikipedia , lookup
Proteolysis wikipedia , lookup
Catalytic triad wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Metalloprotein wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
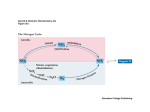
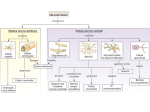
Lec 16: Nitrogen (ammonia) assimilation Reference material Biochemistry 4th edition, Mathews, Van Holde, Appling, Anthony‐Cahill. Pearson ISBN:978‐0‐13‐800464‐4 Lehninger Principles of Biochemistry 4th edition, David L. Nelson, Michael M. Cox. W. H. Freeman ISBN:978‐0716743392 Breakdown of Topics that we will talk about: • How NH3 is assimilated into metabolism • How to extract energy from proteins and (therefore) amino acids? • Protein degradation • Urea cycle • Metabolic pathways for amino acid degradation (catabolism) • How to synthesize amino acids and their derivative? • Metabolic pathways for amino acid degradation (anabolism) • Biosynthesis of other molecules (e.g. neural transmitters, hemes, etc) from amino acids • Metabolism of nucleotides 國立交通大學生物科技學系蘭宜錚老師 1 How ammonia is incorporated into metabolism Reactions in assimilation of ammonia and major fates of the fixed nitrogen: 1.Glutamate dehydrogenase 2.Glutamine synthetase 3.Asparagine synthetase 4.Carbamoyl phosphate synthetase 5.Glutamate synthase. Glutamate dehydrogenase – reductive amination of α‐KG • The most important/major entrance of NH3 into organic Nitrogen is through glutamate dehydrogenase. • Glutamate dehydrogenase catalyzes the following reaction: Note the hydrogen here indicating reduction of that carbon • While this enzyme is reversible (even with NH3 having high Km of 1 mM), it functions primarily towards glutamate for bacteria and plants. • On the other hand, this reaction occurs in the αKG formation direction for animals because intracellular concentration of NH3 is expected to be low… as NH3 is toxic to animal. (Animals do not utilize NH3 directly as nitrogen source!) • In animals, this enzyme is located in the inner mitochondrial membrane. It is also inhibited by GTP and ATP. These characteristics are representative of catabolic enzyme (Energy generation). 國立交通大學生物科技學系蘭宜錚老師 2 Glutamate Synthase – taking NH3 from glutamine • glutamate synthase occurs only in microorganisms, plants, and lower eukaryotes. • It catalyzes a reaction comparable to that catalyzed by glutamate dehydrogenase but functions primarily in glutamate biosynthesis: Glutamine synthetase – formation of amide • To further incorporate NH3 into metabolism. NH3 can be added to Glutamate at the carboxylate, forming an amide. • This reaction is ATP dependent. Therefore this is called a “Synthetase” and not “synthase” • Mn2+ is used a a cofactor for this enzyme. • Glutamine is subsequently used to synthesize variety of different metabolites, including nucleotides and other amino acids. • Because it is the precursor to many downstream products, its production is extensively regulated… 國立交通大學生物科技學系蘭宜錚老師 3 Glutamine synthetase – formation of amide • 2 modes of regulation for glutamine synthetase (E. coli): 1. Allosteric cumulative feedback inhibition: 8 inhibitors are products downstream of glutamine (i.e.tryptophan, histidine, glucosamine‐6P, carbamoyl‐P, CTP, AMP, and Indicators of the general status of amino acid metabolism (ala, gly)) Each of the inhibitors provide partial inhibition. If all inhibitors are present, then it becomes completely inhibited. Why is this a good idea? 2. Covalent modification by adenylylation which inhibits glutamine synthetase. Around the active site of the enzyme, there is key tyrosine group. This tyrosine group can be adenylylated. Once the adenylylation occurs, active site is inactived. This enzyme is 12‐mer (i.e. 12 GlnA polypeptide forms the function enzyme). Similar to cumulative feedback inhibition above, partial adenylylation inhibits the enzyme partially. How come? Structure of Salmonella typhimurium glutamine synthetase: Mn2+ at active site Tyrosine to be adenylylated 國立交通大學生物科技學系蘭宜錚老師 4 Regulation cascade of Glutamine synthetase adenylylation Complicated? I think so too… Regulation cascade of Glutamine synthetase adenylylation Active GS protein – catalyzes glutamate to glutamine Inactive GS protein – inactivated by Adenylyltransferase (AT) Adenylyltransferase (AT) – can activate or deactivate GS, depending on PII that binds to it Small regulatory protein, also affecting the transcription of many nitrogen regulated genes. Whe NOT uridylylated, it binds to AT and turns OFF GS Whe uridylylated, it binds to AT and turns ON GS Bifunctional enzyme that can uridylylate or deuridylylate PII 國立交通大學生物科技學系蘭宜錚老師 5 Regulation cascade of Glutamine synthetase adenylylation Regulation cascade of Glutamine synthetase adenylylation When there are a lot of αKG… Glutamine production is allowed 國立交通大學生物科技學系蘭宜錚老師 6 Regulation cascade of Glutamine synthetase adenylylation When too much glutamine is made… Glutamine synthetase is shutdown. Depending on the situation… different enzymes are used • If NH4+ levels are high, the glutamate dehydrogenase/glutamine synthetase pathway operates: • When NH4+ levels are low, the glutamate synthase/glutamine synthetase pathway predominates: NOTE the ATP requirement is different 國立交通大學生物科技學系蘭宜錚老師 7 Asparagine synthetase – formation of amide.. But AMP forming • This reaction is similar to glutamine synthetase with two key differences: 1. It forms AMP from ATP… (instead of forming ADP as in glutamine synthetase) 2. “Ammonia” can be free ammonia OR the amide of glutamine It turns out… using glutamine is preferred over using ammonia in this case. Therefore, this enzyme is not a major enzyme for ammonia assimilation. Carbamoyl Phosphate synthetase • carbamoyl phosphate synthetase makes carbamoyl phosphate, which participates in biosynthesis of arginine, urea, and pyrimidine nucleotides • Either free ammonia or glutamine can serve as the nitrogen donor. In the 2 ATP dependent reaction, carbamoyl phosphate is produced as follow: • Bacterial carbamoyl phosphate synthetase prefers using glutamine instead of NH3. Similarly eukaryotic cytosolic (also called form II) carbamoyl phosphate synthetase also preferably uses glutamine. • On the other hand, eukaryotic mitochondrial (also called form I) carbamoyl phosphate synthetase prefers NH3 as substrate. It is used in Urea cycle, which is for detoxification of NH3. 國立交通大學生物科技學系蘭宜錚老師 8