* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Protein functions part 2 File
Nucleic acid analogue wikipedia , lookup
Gene expression wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Magnesium transporter wikipedia , lookup
Bottromycin wikipedia , lookup
Peptide synthesis wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein domain wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Protein folding wikipedia , lookup
Circular dichroism wikipedia , lookup
List of types of proteins wikipedia , lookup
Metalloprotein wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Western blot wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein adsorption wikipedia , lookup
Expanded genetic code wikipedia , lookup
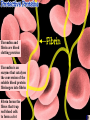
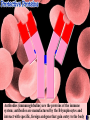
a-keratin is the structural protein of hair, horns and nails Actin and myosin are the contractile proteins of muscle Tubulin is the protein of the microtubules; eukaryotic cilia and mitotic spindles are composed of microtubules Thrombin and fibrin are blood clotting proteins Thrombin is an enzyme that catalyses the conversion of the soluble blood protein fibrinogen into fibrin Fibrin forms the fibres that trap red blood cells to form a clot Antibodies (immunoglobulins) are the proteins of the immune system; antibodies are manufactured by the B-lymphocytes and interact with specific, foreign antigens that gain entry to the body These mussels secrete specialised glue proteins to attach themselves to the rocks Proteins made from monomers called amino acids All amino acid possess amino and carboxylic acid ends There are 20 different naturally occurring amino acids Amino acids differ by in the nature of their R groups Amino acids bond together forming peptide bonds When two amino acids bond during a condensation reaction, the resulting molecule is a dipeptide When many amino acids bond together, the resulting molecule is referred to as a polypeptide Chains of amino acids numbering greater than 100 are generally referred to as proteins Individual amino acids may be neutral, basic or acidic Two amino acids, namely cysteine and methionine, possess sulphur atoms in their R groups The type, number and sequence of amino acids forming the original linear chain of a protein is termed the primary structure of a protein Different proteins have different primary structures The primary structure determines the final shape of the protein molecule The linear chain of amino acids making up the primary structure of the protein bends and folds in various ways to form the secondary structure of the protein Two main types of secondary structure are found in proteins - the beta pleated sheet and the alpha helix The alpha helix forms when the linear chain coils into a right handed helix The beta pleated sheet forms when the linear chain folds back on itself many times Hydrogen bonds play a major part in stabilising the secondary structure of proteins Many proteins bend and fold further to form globular tertiary structures Myoglobin is a globular protein displaying the tertiary level of structure Myoglobin is a protein found in muscle cells Proteins consisting of more than one polypeptide chain display quaternary structure Haemoglobin is a protein consisting of more than one polypeptide chain Haemoglobin consists of four separate polypeptide chains held together by weak van der Waals forces Each polypeptide chain in haemoglobin contains a haem group that binds to molecular oxygen The role of haemoglobin is to transport oxygen molecules from the lungs to the body tissues A variety of different bonds stabilise the secondary and tertiary structures of proteins Hydrogen bonds form between oxygen and hydrogen atoms within the main amino acid chain and between the R groups Disulphide bridges form between sulphur atoms in the R groups of amino acids such as cytseine Ionic bonds form between charged amino groups and charged carboxylic acid groups Hydrophobic interactions occur between R groups that have clustered towards the centre of protein molecules due to their hydrophobic nature The Biuret test is used to detect proteins; a positive result gives a violet/lilac colour