* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Metamorphosis of the Aleurone Protein Storage Vacuole
Cytokinesis wikipedia , lookup
Hedgehog signaling pathway wikipedia , lookup
Signal transduction wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Phosphorylation wikipedia , lookup
Endomembrane system wikipedia , lookup
Protein design wikipedia , lookup
Protein folding wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein (nutrient) wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Western blot wikipedia , lookup
Protein purification wikipedia , lookup
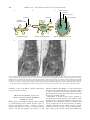
Annals of Botany 82 : 399–412, 1998 Article No. bo980702 REVIEW ARTICLE From Storage Compartment to Lytic Organelle : The Metamorphosis of the Aleurone Protein Storage Vacuole P A U L C. B E T H KE*, S A R A H J. S W A N S O N*, S T E F A N H I L L M E R† and R U S S E L L L. J O N E S*‡ * Department of Plant and Microbial Biology, Uniersity of California, Berkeley, CA 94720 and † Plant Physiology Institute, Uniersity of GoX ttingen, D-37037 GoX ttingen, Germany Received : 4 May 1998 Accepted : 25 May 1998 Protein storage vacuoles are found in a variety of tissues but are especially abundant in the storage organs of fruits and seeds. In this review, we focus on the protein storage vacuoles of cereal aleurone. In the mature grain, these organelles are repositories for reserve nitrogen, carbon and minerals. Following imbibition, protein storage vacuoles of cereal aleurone change from storage compartments to lytic organelles. Changes in protein storage vacuole structure and enzymatic activity during this transition are discussed. It is emphasized that protein storage vacuoles are poised for reserve mobilization, and that gibberellin perception by the aleurone cell initiates a signalling cascade that promotes acidification of the vacuole lumen and activation of enzymes and transporters. # 1998 Annals of Botany Company Key words : Protein storage vacuole, cereal aleurone, gibberellin, abscisic acid, protein body, endosperm reserves. INTRODUCTION Vacuoles are ubiquitous organelles in plant cells. They perform myriad functions and take on a variety of forms (Matile, 1975 ; Boller and Wiemken, 1986). In this review we focus our attention on a specialized class of vacuole, the protein storage vacuole. Protein storage vacuoles are found in a wide variety of tissues, but they are especially abundant in the storage organs of fruits and seeds (Chrispeels, 1985 ; Muntz, 1989 ; Shewry, 1995). This organelle stores proteins, carbohydrates, neutral lipids and minerals that are used to support early growth of the seedling (Bewley and Black, 1994). Protein storage vacuoles are typically 1–5 µm in diameter in mature seeds and grain, and a single plant cell may accumulate hundreds of protein storage vacuoles. Protein storage vacuoles have been intensively investigated in the cotyledons of legumes and in the endosperm of cereals. Despite their functional similarities, differences in protein storage vacuole structure and biogenesis exist between species and within the same tissue of a given species (Chrispeels, 1985 ; Muntz, 1989 ; Okita and Rogers, 1996 ; Robinson and Hinz, 1997). In general, protein storage vacuoles are formed either by budding from the endoplasmic reticulum (ER), or by fragmentation of the central vacuole. The former mechanism is common in the starchy endosperm of cereals and the latter in the cotyledons of dicots, but many exceptions exist. In the starchy endosperm of rice, for example, two types of protein storage vacuole are formed, one containing prolamines, the other glutelins. Rice prolamines are stored in vacuoles formed directly from the ER, ‡ For correspondence. rjones!nature.berkeley.edu Fax 1 510 0305-7364}98}10039914 $30.00}0 642 4995, e-mail but glutelins are stored in Golgi-derived vacuoles (Okita and Rogers, 1996). In the starchy endosperm of wheat yet another mechanism appears to exist for the formation of protein storage vacuoles. Galili et al. (1996) have shown that protein storage vacuoles can arise directly from ER or by a process in which ER-derived vesicles containing storage protein are incorporated into the protein storage vacuole by autophagy. A feature of all protein storage vacuoles is that stored materials accumulate either as large polymers, which is the case for stored carbon and nitrogen, or as complex salts, which is the case for sequestered minerals (Bewley and Black, 1994). Because of the economic and nutritional importance of endosperm and cotyledon storage proteins, much attention has been focused on their synthesis and intracellular deposition within protein storage vacuoles (Shewry, Napier and Tatham, 1995 ; Galili et al., 1996 ; Richard et al., 1996). Much less attention has been focused on the mobilization of these reserves. In cereals, mobilization of protein storage vacuole reserves from dead starchy endosperm cells is accomplished by enzymes secreted into the starchy endosperm by the aleurone or scutellum, and by enzymes released from starchy endosperm cells prior to grain maturation (Fincher, 1989 ; Jones and Jacobsen, 1991), and this topic is not covered here. Mobilization of stored reserves from living cells is unlike the mobilization of starchy endosperm reserves and is preceded by a change in the function of the protein storage vacuole. The protein storage vacuole abandons its role as a storage compartment and becomes an acidic, lytic organelle wherein stored proteins, lipids, carbohydrates and phytin are degraded. In aleurone cells, this change from storage compartment to lytic organelle is hormonally regulated. The dramatic # 1998 Annals of Botany Company 400 Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone changes in structure and enzymatic activity that occur in protein storage vacuoles of barley aleurone during the phase of reserve mobilization are the topics of this review. MATURE ALEURONE PROTEIN STORAGE VACUOLES ARE POISED FOR RESERVE MOBILIZATION The cereal aleurone cell is an excellent model for studying the action of the plant growth regulators gibberellic acid (GA) and abscisic acid (ABA), but more recent investigations have focused on how these hormones regulate gene expression (Fincher, 1989 ; Jacobsen, Gubler and Chandler, 1995 ; Bethke, Schuurink and Jones, 1997). This approach has been eminently successful, and a great deal is known about how GA and ABA affect the transcription of genes for abundantly transcribed enzymes such as α-amylase. GAand ABA-responsive elements have been identified in the promoters of several cereal aleurone genes, and functional trans-acting factors have been found (Jacobsen et al., 1995). Much less is known about other aspects of hormonal regulation in the aleurone cell. GA and ABA receptors have not been identified, and signal transduction pathways that engage cellular targets are only beginning to be described (Bethke et al., 1997). Many key enzymes and transporters in the aleurone cell, however, are not regulated by transcription or translation. These polypeptides are synthesized in the developing grain, but remain inactive. Following imbibition or GA perception, these proteins become active, and the aleurone cell quickly begins the process of synthesizing and secreting hydrolytic enzymes. These enzymes degrade starchy endosperm reserves, making them available for uptake by the scutellum. Since reserves within the barley aleurone cell must be mobilized before the more abundant reserves in the starchy A endosperm can be utilized, protein storage vacuoles must be poised for rapid reserve mobilization. Several lines of experimental evidence support this hypothesis. As described in detail below, all of the transporters that have been identified in the protein storage vacuole tonoplast appear to be present in the dry grain and their amounts are relatively unaffected by GA or ABA. Likewise, some of the enzymes that are needed to mobilize protein storage vacuole reserves are also present in the dry grain. When new protein synthesis is required, it may begin with translation from preexisting mRNAs. Activation of protein storage vacuoles, therefore, may require little more than water and cytosolic signals. A diagram illustrating known components of cereal aleurone protein storage vacuoles is presented in Fig. 1. STRUCTURE OF PROTEIN STORAGE VACUOLES Barley aleurone cells from mature grain contain hundreds of spherical protein storage vacuoles of about 1–5 µm diameter (Fig. 2 A). These vacuoles have also been referred to as protein bodies or aleurone grains. We use the term protein storage vacuole here to emphasize the many features these organelles share with other plant vacuoles. This organelle is easily recognized by light and electron microscopy because of the numerous inclusions in the vacuole lumen and the oleosomes embedded between the inner and outer leaflets of the tonoplast (Figs 2 and 3). Freezefractured (Fernandez and Staehelin, 1985 ; Cornejo et al., 1988) and high-pressure-frozen, freeze-substituted (J. Lonsdale and R. Jones, unpubl. res.) tissue observed by electron microscopy show that protein storage vacuoles are filled with electron-dense material. Histochemical staining of high-pressure-frozen, freeze-substituted tissue shows that the electron-dense material that fills the protein storage B Protein storage vacuole Globoid crystal + 2+ (K , Ca 2+ Mg and P) Protein crystalloid Ca2+ SV channel OA– Organic anion transporter Nuclease Phytase GS-X Protease H2O 7S globulins and other storage proteins (amino acids) + H Lipase α-TIP V-ATPase pH 7.2 PM GSH conjugate transporter H+ pH 5.5 Oleosomes (triglycerides) 2+ + Ca -H antiport H+ Protein storage vacuole V-PPase PM F. 1. Diagram of some of the constituents within cereal aleurone protein storage vacuoles. A, Storage reserves and the enzymes that mobilize these reserves ; B, transporters present in the protein storage vacuole tonoplast. Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone F. 2. Differential interference contrast and fluorescence micrographs of barley aleurone protoplasts. A, Freshly isolated protoplast filled with protein storage vacuoles. Note that each vacuole contains numerous inclusions. B, Barley aleurone protoplast 5 d after treatment with GA. In this cell, the smaller vacuoles have coalesced to form one large protein storage vacuole (PSV). C, Pseudocoloured confocal image of three protoplasts whose vacuoles have been loaded with the pHsensitive, fluorescent probe BCECF. Pseudocolour indicates intensity of fluorescence, where blue is minimum and red is maximum intensity (Reprinted from Swanson and Jones, 1996). D, Fluorescent image of a protoplast whose protein storage vacuoles contain the proteolysis product of the fluorogenic protease substrate ZFR-CMAC. For details see Swanson, Bethke and Jones, 1998. Bars 10 µm. vacuole lumen is likely to be protein. Electron micrographs of storage protein vacuoles in aleurone tissue prepared by chemical fixation which show an organelle only partially filled with protein (e.g. Jones, 1969 a ; Swift and O’Brien, 1972 ; Lott, 1980) are misleading because chemical fixation and solvent dehydration of aleurone tissue extract storage proteins. The lumen of the barley aleurone protein storage vacuole contains 7S globulins, which are the major storage proteins. Yupsanis et al. (1990) characterized the salt-soluble proteins of aleurone protein storage vacuoles from the Himalaya cultivar of barley and found that the major storage proteins included a group of immunochemically related globulins of 50-, 40- and 25-kDa. These peptides had a common Nterminal amino acid sequence which was similar to that in the N-terminus of pea and bean vicilins, as well as cottonseed 7S globulins. Additional peptides of 70 000 kDa also cross-reacted with the anti-oat 7S globulin antibody, but these did not co-purify with the other peptides. This led the authors to suggest that the 70 000 kDa peptides might be part of a separate holoprotein similar to the convicilins in pea and bean. In marked contrast, the predominant storage proteins in the protein storage vacuoles of barley starchy endosperm cells are the alcohol-soluble hordeins, members of the prolamine class of storage proteins (Shewry, 1995). Hordeins have not been found in the aleurone layer. At least two types of inclusions are found in the protein 401 storage vacuoles of barley aleurone cells (Jacobsen, Knox and Pyliotis, 1971 ; Lott, 1980). Histochemistry (Jacobsen et al., 1971) and electron microscopy (Pomeranz, 1973 ; Liu and Pomeranz, 1975 ; Stewart, Nield and Lott, 1988 ; Batten and Lott, 1996) have identified globoid crystals and protein crystalloids in these vacuoles. X-ray microanalysis suggests that the globoid crystal consists of phytin (K, Mg and Ca salt of myo-inositol hexakisphosphate) (Stewart et al., 1988), and electron microscopy shows that the globoid is surrounded by an enveloping membrane of unknown composition (Jones, 1973). The globoid crystal of the aleurone cell is the principal site of K, Mg and P in the mature grain. X-ray microanalysis has shown that 75 % of grain P and K, and 60 % of Mg are in the endosperm. Over 80 % of endosperm P, K and Mg is found in the aleurone layer, largely in the globoid crystals (Liu and Pomeranz, 1975 ; Stewart et al., 1988). The distribution of Ca in Himalaya barley grain differs from that of K, Mg and P in that this element is distributed almost equally between endosperm and embryo (Stewart et al., 1988). Over 80 % of endosperm Ca is found in the protein storage vacuole of the aleurone layer (Stewart et al., 1988). The other type of inclusion in the protein storage vacuole of barley aleurone, the protein crystalloid (Lott, 1980), also referred to as the protein}carbohydrate body (Jacobsen et al., 1971), can be distinguished based on its histochemical staining properties and electron density. Little is known about the composition of this inclusion. In wheat, oat and barley, an inclusion within aleurone protein storage vacuoles is the major site of niacin deposition (Fulcher, O’Brien and Wong, 1981). This inclusion is likely to be the protein crystalloid. Protein storage vacuoles in barley aleurone cells are also a site of neutral lipid storage. Triglycerides are stored in oleosomes that are embedded within the tonoplast (Fig. 3). The presence of oleosomes in the tonoplast provides valuable clues to the origin of the barley aleurone protein storage vacuole. There is widespread agreement that triglycerides are synthesized on ER membranes and deposited in oleosomes as they form between the inner and outer leaflets of smooth ER (Fig. 3 ; Huang, 1992). Oleosomes are therefore unique organelles in that they are surrounded by a half-unit membrane (Huang, 1992). There is disagreement, however, about how oleosomes become associated with protein storage vacuoles. Whereas one model suggests that newly formed oleosomes become detached from the surface of the ER and fuse with the vacuolar membrane (Fernandez and Staehelin, 1985 ; Huang, 1992 ; Napier, Stobart and Shewry, 1996), we propose an alternative model. In this model oleosomes do not become detached from the ER. Rather, the ER membrane that synthesizes the neutral lipid also serves as the site of storage protein accumulation (Fig. 3). This latter model is supported by observations made using electron microscopy which show that oleosomes in mature barley aleurone cells remain attached to the ER, often by long extensions of the outer leaflet of the ER membrane (Fig. 3 D). This model is also consistent with observations regarding lipid mobilization. Empty oleosome ‘ ghosts ’ are not observed when lipids are mobilized from oleosomes, indicating that the oleosome 402 Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone A B C Globoid crystal Oleosome Storage protein Oleosome Oleosome Protein crystalloid Outer ER membrane leaflet ER lumen Storage protein ER lumen Oleosome formation Storage protein deposition Mature protein storage vacuole D F. 3. A model for protein storage vacuole formation in barley aleurone and electron micrographs of a barley aleurone layer. A, Protein storage vacuole formation begins with the production of oleosomes in the endoplasmic reticulum (ER) membrane. B, Storage proteins are deposited within the lumen of the ER and oleosomes are attached to the ER by a stalk whose membrane is contiguous with the outer leaflet of the ER membrane. C, A mature protein storage vacuole containing storage protein, a globoid crystal, and a protein crystalloid, and surrounded by oleosomes. D, Stereo pair of electron micrographs from a barley aleurone layer showing the attachment of oleosomes to the ER by long extensions of the outer leaflet of the ER membrane. Thick sections (gold) were viewed in an intermediate voltage electron microscope (JEOL 4000). membrane reverts to the ER or tonoplast when lipid is removed from the oleosome. PROTEIN STORAGE VACUOLE STRUCTURE DURING RESERVE MOBILIZATION When growth of the seedling is initiated or GA is supplied to de-embryonated grains, isolated aleurone layers, or aleurone protoplasts of barley, a cascade of events is initiated that includes the release of minerals from the aleurone cell and the synthesis and secretion of acid hydrolases (Fincher, 1989 ; Bethke et al., 1997). The protein storage vacuoles, which for months or years have functioned as nutrient repositories, become lytic organelles, rapidly hydrolyzing the stored polymers in their lumen, often with the use of pre-existing enzymes. Mobilization of protein storage vacuole polymers is accompanied by dramatic changes in the structure of this organelle (Jones, 1969 b ; Jones and Price, 1970 ; Ory and Henningsen, 1975). As illustrated in Fig. 2 A and B, protein storage vacuoles increase in size but decrease in number following incubation in GA. When secretory enzyme synthesis has ceased, one large vacuole occupies almost the entire volume of the cell (Fig. 2 B ; Swanson, Bethke and Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone Jones, 1998). The large (approx. 40 µm diameter) vacuole in GA-treated cells is likely to form from the coalescence of smaller protein storage vacuoles (Jones and Price, 1970). Several pieces of evidence from electron microscopy studies support this idea. First, protein storage vacuoles are not separate organelles, but are linked by tonoplast connections (Jones and Price, 1970). Second, the large vacuole formed after GA treatment contains remnants of the phytin globoids found in the smaller protein storage vacuoles. Since there is no evidence that remnants of the phytin globoid leave the protein storage vacuole and enter enlarging vacuoles, we conclude that the latter form from the former. Third, the tonoplast of large vacuoles, like that of smaller protein storage vacuoles, has oleosomes embedded between the inner and outer leaflets of the membrane (Jones and Price, 1970) and contains proteins recognized by anti-αTIP antibodies (Schuurink, Chan and Jones, 1996). The formation of a large vacuole from smaller protein storage vacuoles brings about a substantial reduction in tonoplast surface area and a concomitant reduction in the number of oleosomes. Gluconeogenesis and the synthesis of sucrose occurs in barley aleurone cells in response to GA (Chrispeels, Tenner and Johnson, 1973). GA also brings about an increase in glyoxylate cycle enzymes in barley (Jones, 1972) and germinating wheat (Doig et al., 1975) aleurone cells. These observations suggest that neutral lipids stored in oleosomes are metabolized via the glyoxylate cycle to produce sucrose and other sugars. The ability of the aleurone layer to convert fat to sugar may appear to be an anomaly considering that this tissue lies adjacent to a large store of carbohydrate. However, for starch in the endosperm to be converted to sugars, the aleurone cell must synthesize mRNAs for secretory proteins such as α-amylase. Production of α-amylase and other mRNAs requires a large pool of available ribose, which is likely to come from the pentose phosphate pathway via gluconeogenesis. The ability of the aleurone cell to make sugars at the expense of stored fat would make this tissue independent of other tissues in the grain. As protein storage vacuoles coalesce in response to GA, there is a change in the structure of the phytin globoid and protein crystalloid inclusions. Globoids and crystalloids in aleurone cells from mature grain are electron-dense structures (Jones, 1969 a), but following incubation in GA these inclusions lose their electron opacity, and in the case of the globoid, only the limiting envelope remains in the large protein storage vacuole (Jones and Price, 1970). This loss of electron density coincides with the hydrolysis of phytin. Other materials accumulate in the central vacuole of GAtreated cells, including membrane fragments and lipid droplets of unknown composition (Jones and Price, 1970 ; Jones, 1987). We speculate that these membrane fragments represent the remnants of organelles that are broken down by autophagy in the protein storage vacuole (see below). Aubert et al. (1996) observed the accumulation of phosphoryl choline in the vacuole of sucrose-starved, cultured sycamore cells. They speculated that phosphoryl choline accumulates in these cells as a result of membrane degradation in the vacuole of starved cells undergoing autophagy (Aubert et al., 1996). 403 ENZYMES THAT MOBILIZE PROTEIN STORAGE VACUOLE RESERVES Many hydrolytic enzymes are required for the mobilization of protein storage vacuole reserves (Fig. 1). Storage proteins are degraded by proteases (Bethke, Hillmer and Jones, 1996), and density labelling of newly synthesized proteins with H O") or D O suggests that storage proteins are almost # # completely hydrolyzed to amino acids (Filner and Varner, 1967 ; Chrispeels and Varner, 1973). Amino acids resulting from storage protein breakdown become incorporated into newly synthesized secretory proteins at the ER membrane. Phytate is hydrolyzed by phytase (Gabard and Jones, 1986), and the solubilized minerals are released from the aleurone cell as cations and anions (Jones, 1973). Triglycerides are degraded by lipases, and the resulting free fatty acids are used to produce sugars and ATP. Active nucleases are also present in protein storage vacuoles, but a precise role in DNA or RNA cleavage has not been established. Proteases The mobilization of storage proteins is brought about by proteases, and multiple protease activities have been found in aleurone protein storage vacuoles. In barley, both aspartic and cysteine protease activities have been identified in these organelles (To$ rma$ kangas et al., 1994 ; Bethke et al., 1996). As shown in Fig. 4, these enzymes have pH optima around pH 4 and have no activity at pH 6±5 or greater. Since the pH of protein storage vacuoles in freshly prepared aleurone protoplasts is approx. pH 7 (Swanson and Jones, 1996), these enzymes are unlikely to be active in the dry grain. Ingel assays suggest that as the protein storage vacuole lumen is acidified to pH 5±5 or below (Swanson and Jones, 1996), a sequential activation of proteases may occur (Bethke et al., 1996). In the proteolytic activity gels shown in Fig. 4, for example, no protease activities are detectable at pH 6±5 in extracts of purified protein storage vacuoles from protoplasts treated with GA for 17±5 h (see Bethke et al., 1996 for details of the assay). At pH 5±5, however, two aspartic protease activities (AP1 and AP2) are seen in the lower half of the gel. Further acidification to pH 4±5 results in activation of two slowly migrating cysteine proteases (CP1 and CP2). A third cysteine protease activity (CP3), which is seen between the two aspartic protease activities, is observed at pH 4±5, and this activity is greater at pH 3±5. The consequences of staggered protease activation in barley aleurone are unknown, but in maize starchy endosperm, sequential protease activation promotes efficient hydrolysis of zeins (Bewley and Black, 1994). In barley aleurone protein storage vacuoles isolated 17±5 h after aleurone protoplasts had been treated with GA, two aspartic and three cysteine protease activities were prominent on activity gels (Fig. 4 ; Bethke et al., 1996). One of these activities, the aspartic protease HvAP (Hordeum ulgare aspartic protease) has been well characterized (Sarkkinen et al., 1992 ; Runeberg-Roos et al., 1994 ; To$ rma$ kangas et al., 1994). The identities of the other activities are unknown. HvAP is an aspartic protease similar to mammalian cathepsin D. Purified HvAP exists as 404 P Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone L PSV P pH 6.5 P L L PSV pH 5.5 PSV P L PSV CP1 CP2 AP1 CP3 AP2 pH 4.5 pH 3.5 F. 4. Proteolytic activity gels reveal multiple protease activities in protein storage vacuoles isolated from GA-treated protoplasts. Samples in each of the four gels were : purified protoplasts (P), a lysate depleted of protein storage vacuoles (L) and isolated protein storage vacuoles (PSV). In-gel assays were done essentially as described in Bethke et al., 1996. Denatured haemoglobin was incorporated into each gel to serve as a substrate for proteolysis. Following electrophoresis, gels were incubated for 3 h at 37 °C in solutions buffered to pH 6±5, 5±5, 4±5 or 3±5 and then stained with Coomassie Brilliant Blue. Dark regions are stained haemoglobin, light bands are regions of proteolytic activity. Three cysteine proteases (CP1, CP2 and CP3) and two aspartic proteases (AP1 and AP2) are indicated by arrowheads. Note that proteolytic activity increases with decreasing pH. Bethke and Jones, unpubl. res. two heterodimeric isoforms and it was shown that both isoforms are derived from a single gene by differential processing of the protein precursor (Sarkkinen et al., 1992). The mRNA for HvAP was localized in developing and germinating grain using in situ hybridization (To$ rma$ kangas et al., 1994). HvAP mRNA was not detectable in the starchy endosperm later than 13 d after anthesis, but was detectable in the cells of the mature aleurone layer. In germinating grains, HvAp mRNA was found in the seedling, scutellum and aleurone. Active HvAP has been found in isolated vacuoles from barley leaf protoplasts (Runeberg-Roos et al., 1994), and anti-HvAP antibodies recognize proteins in barley aleurone protein storage vacuoles (Bethke et al., 1996). The location of HvAP protein has been determined for developing and germinating barley grain (To$ rma$ kangas et al., 1994). HvAP protein appeared in the aleurone layer concomitantly with the development of aleurone cells (8–15 d after anthesis). Between 20 and 28 d after anthesis, the amount of immunologically detectable HvAP in the aleurone layer decreased, such that at 28 d after anthesis no signal was present. Before the third day after imbibition, however, HvAP was again seen in aleurone cells. To$ rma$ kangas et al. (1994) suggested that HvAP mRNAs might be stored in the mature grain and are available for translation immediately upon hydration of the grain. Protein blots probed with the anti-HvAP antibody confirmed that HvAP was present in the aleurone layer (Bethke et al., 1996). Aleurone layers imbibed in the absence of hormone and protoplasts incubated for 17±5 h in either ABA or GA contained much greater amounts of HvAP than dry aleurone layers. Since there was little difference in the amount of HvAP protein in protoplasts incubated in ABA or GA, it is likely that the synthesis of HvAP in aleurone layers is stimulated by imbibition not by GA. A role for HvAP in protein processing has been proposed. In itro, HvAP removes 13 of the 15 amino acids in the Cterminal pro sequence of barley lectin (Runeberg-Roos et al., 1994). Since immunoelectron microscopy showed that HvAP and barley lectin were colocalized in barley vacuoles, it was suggested that HvAP participates in the processing of barley lectin (Runeberg-Roos et al., 1994). Additional in io substrates for HvAP are likely to exist but have not been characterized. Aleurone cells synthesize numerous cysteine proteases which are secreted into the starchy endosperm, and some of these effectively proteolyze hordeins (Koehler and Ho, 1988, 1990 a, b). Other cysteine proteases are not secreted, but are found within the protein storage vacuoles of the aleurone (Fig. 4 ; Bethke et al., 1996 ; Swanson et al., 1998). Cysteine protease activities were first identified in barley aleurone protein storage vacuoles using an in-gel activity assay (Bethke et al., 1996). More recently, this localization of cysteine proteases to protein storage vacuoles has been confirmed in io using a fluorogenic protease substrate that is transported into the vacuole and then proteolyzed (Fig. 2 D ; Swanson et al., 1998). When this substrate was conjugated to glutathione, isolated, intact vacuoles were able to take it up in an ATP-dependent manner and the fluorescent proteolysis product accumulated in the vacuole lumen. Accumulation of the proteolyzed product, however, was inhibited by E-64 and leupeptin, two cysteine protease inhibitors. These last two pieces of data suggest that there are active cysteine proteases in the protein storage vacuoles of barley aleurone protoplasts. Unlike the aspartic protease activities in barley aleurone protein storage vacuoles, at least some of the cysteine protease activities are strongly upregulated by GA treatment of barley aleurone protoplasts. Protein storage vacuoles isolated from freshly prepared protoplasts show only one band of cysteine protease activity in the activity gel assay (Bethke et al., 1996). Vacuoles from cells incubated in GA for 17±5 h, however, showed greatly increased activity in two slowly migrating bands of cysteine protease activity. Whether this increase in activity is the result of new protein synthesis, as it is for HvAP, is not known. An equally likely hypothesis is that cysteine protease zymogens are processed to their active form in a GA-dependent manner. Processing and activation of zymogens could be coupled to the acidification of the protein storage vacuole lumen. The amount of the cysteine protease aleurain is also upregulated by GA. Aleurain, however, is not localized to Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone the protein storage vacuoles, but is found in a smaller organelle termed the aleurain-containing vacuole (Holwerda et al., 1990). The function of the aleurain-containing vacuole is unknown, as is its relationship to the protein storage vacuole. Aleurain has been cloned and the sequence encodes a cysteine protease of 361 amino acids. Aleurain is synthesized as a 42 kDa propeptide which is subsequently cleaved in a post-Golgi compartment to the mature 32 kDa form (Holwerda et al., 1990 ; Holwerda and Rogers, 1992). The increase in aleurain activity that occurs following GAstimulation results from increased transcription of the aleurain mRNA and subsequent translation and posttranslational processing (Holwerda et al., 1990 ; Holwerda and Rogers, 1992). Because purified aleurain functions as an aminopeptidase, it has been suggested that aleurain functions as a processing enzyme. Phytases The mobilization of phytin is brought about by phytase (Cosgrove, 1980). Two phytase activities have been recognized by the IUPAC-UB : 3-phytase (EC 3.1.3.8) from microbes and 6-phytase (EC 3.1.3.26) from plants. 3-Phytase dephosphorylates phytic acid (myo-inositol-1,2,3,4,5,6hexakisphosphate ; IP ) beginning at the D-3 position, and ' 6-phytase dephosphorylates phytic acid beginning at the L6 position (Cosgrove, 1980). 6-Phytase has been isolated from a variety of plant sources, but it is especially abundant in seeds and pollen. Phytase has been carefully studied in wheat bran (mostly the aleurone and testa pericarp of the wheat endosperm), and two classes of phytase, F1 and F2, have been identified (Cosgrove, 1980). F1 phytase is a 6phytase producing myo-inositol 1,2,3,4,5 pentaphosphate followed by removal of phosphate from carbons 5, 4, 3}1 and 1}3 yielding myo-inositol 2-phosphate. F2 phytase is a 2-phytase that dephosphorylates IP to inositol 1-phosphate. ' Inositol monophosphate is hydrolyzed to free inositol and phosphate by a separate enzyme, inositol monophosphatase (Cosgrove, 1980). Phytase has recently been purified to homogeneity from several higher plant tissues, most notably from the roots and shoots of Zea mays (Laboure, Gagnon and Lescure, 1993 ; Hubel and Beck, 1996). Corn 6-phytase consists of a dimer of two identical 38-kDa subunits (Laboure et al., 1993 ; Hubel and Beck, 1996). The pH optimum of the corn enzyme is around 5, confirming earlier reports that 6phytase from a variety of plant sources, including seeds, has an acidic pH optimum (Cosgrove, 1980 ; Hubel and Beck, 1996). Northern blot analysis of 6-phytase RNA in germinating corn kernels showed low transcript abundance in embryos from dry kernels and in embryos from kernels soaked in aerated water for 12 h. Phytase mRNA accumulated after 24 h of imbibition and before radicle protrusion. Transcript amount was maximal 2 d after the beginning of imbibition, the time when the radicle protruded (Maugenset, Martinez and Lescure, 1997). Maugenset et al. (1997) concluded that 6-phytase from corn was likely to be involved in the mobilization of phosphate from phytic acid. Although 6-phytase activity has been studied intensively in wheat grain (Cosgrove, 1980), this gene has not been 405 cloned from the small grain cereals. We showed that phytin globoids isolated from barley aleurone layers have associated phosphatases with high phytase activity (Gabard and Jones, 1986). As determined by activity gel assays of density gradient fractions, these candidate phytases were present in the aleurone of dry grain. Their activity did not change with imbibition in water but declined upon incubation in GA (Gabard and Jones, 1986). Since these data are not consistent with the observations of Maugenset et al. (1997), further experimentation is required. Because of the role that inositol 1,4,5-triphosphate (InsP ) $ plays in cellular signalling in eukaryotes, there is interest in knowing the nature of the products that result from phytin hydrolysis. There is substantial evidence that plant cells contain InsP (Drobak, 1992 ; Crain, 1993), and recent $ reports confirm that InsP can arise from phosphatidyl$ inositol 4,5-bisphosphate (PiP ) by the action of phospho# lipase C (Brearley, Parmar and Hanke, 1997). There is no convincing evidence, however, that InsP arises from the $ hydrolysis of phytic acid in seeds or grains (Cosgrove, 1980). A detailed study of inositol phosphates in barley aleurone layers failed to detect the presence of InsP , but $ both inositol 1,2,3- and inositol 1,2,6-trisphosphate were identified (Brearley and Hanke, 1996). The spectrum of inositol phosphates in barley aleurone cells found by Brearley and Hanke (1996) were very similar to the in itro products of phytic acid hydrolysis by wheat bran phytase(s), indicating that the inositol phosphates in aleurone extracts were the products of phytic acid degradation. Although attention has been focused on InsP as a signalling molecule $ in eukaryotic cells, evidence is accumulating that other inositol phosphates including IP can serve as signalling ' molecules in microorganisms and mammals (Huisamen and Lochner, 1996 ; Van Haastert and Van Dijken, 1997). As yet a role in signal transduction for inositol phosphates other than IP has not been identified in plant cells. $ Lipases Triglycerides in oleosomes are hydrolyzed to free fatty acids by lipases. The localization of lipase in barley aleurone was examined using sucrose density gradient centrifugation of homogenates from aleurone layers imbibed in buffer or treated with ABA and GA (Fernandez and Staehelin, 1987). In freshly prepared aleurone layers, no lipase activity was associated with purified oleosomes. Instead, lipase activity was found in a 10 000 g pellet. GA treatment of aleurone layers caused a shift in lipase activity from the 10 000 g pellet to the oleosome-containing fraction. This shift in enzymatic activity began 1 h after incubation in GA and was complete 1 h later, when approx. 75 % of all lipase activity was associated with the oleosome-containing fraction. Total lipase activity increased only slightly following GA treatment. Aleurone layers treated with ABA did not show this shift in lipase activity, and the shift in activity was reduced when layers were treated with both GA and ABA. Because protein storage vacuole membranes could be identified in the 10 000 g pellet, and because membrane connections between oleosomes and protein storage vacuoles were observed by freeze-fracture electron microscopy (Fernan- 406 Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone dez and Staehelin, 1987), the authors suggested that lipase was transferred from protein storage vacuoles to oleosomes following perception of a GA signal. This transfer might occur along the continuous phospholipid layer that links oleosomes and protein storage vacuoles. A similar situation was found in wheat aleurone, where acid lipase activity in freshly isolated aleurone layers was found in density gradient fractions containing protein storage vacuoles but not in oleosome containing fractions (Jelsema et al., 1977). Nucleases Nucleases are also contained within aleurone protein storage vacuoles (Holstein et al., 1991 ; A. Fath and R. Jones, unpubl. res.). A type I nuclease was purified from barley aleurone (Brown and Ho, 1987 ; Brown, Mecham and Ho, 1988), and immunoelectron microscopy localized this enzyme to the protein storage vacuole (Holstein et al., 1991). Protein blotting of fractions enriched in intact protein storage vacuoles has confirmed this observation (J-Z. Huang and R. Jones, unpubl. res.). Nuclease I is a 32 kDa endonuclease that hydrolyzes both DNA and RNA. Based on immunoelectron microscopy it was inferred that barley Nuclease I was delivered to the protein storage vacuoles via the Golgi apparatus since both ER and Golgi labelled with anti-Nuclease I antibodies. The existence of Nuclease I within protein storage vacuoles raises interesting questions about its function. One hypothesis was that it might hydrolyze the small amount of RNA present in these organelles (Holstein et al., 1991). An extension of this hypothesis is that following GA perception, unwanted DNA or RNA fragments are transported into protein storage vacuoles for destruction. Rapid hydrolysis of nuclear DNA occurs in living aleurone cells as they approach death (A. Fath, P. Bethke and R. Jones, unpubl. res.), and much of the nuclease activity seen in these cells at that time is in the protein storage vacuoles. No data exist to suggest how nucleic acids might be transported through the tonoplast and into the vacuole. Autophagic organelles or vesicles, however, may fulfil the role of delivering unwanted cytosolic macromolecules or multi-molecular structures to the vacuole for destruction or inactivation. TRANSPORT THROUGH THE TONOPLAST Transport through the tonoplast is an essential feature of aleurone protein storage vacuoles. Most of the reserves stored there must pass through the vacuolar membrane before they benefit either the aleurone cell or the embryo. At the same time , harmful or surplus compounds may be transported into protein storage vacuoles for storage, detoxification or destruction. Although much remains to be learned about the transporters in the protein storage vacuole tonoplast, it is clear that an extensive, carefully regulated system exists. By using biochemical, immunological and biophysical approaches, researchers have identified several transporters. These include the aquaporin αTIP, the slow vacuolar (SV) channel, a Ca#+}nH+ antiporter, the H+ATPase and H+-PPase, as well as at least two ATP-binding cassette (ABC) transport activities (Fig. 1 B). Significantly, the transporters that are used for the export of nutrient reserves from the vacuole to the cytosol have not been characterized. Tonoplast intrinsic protein αTIP (α-tonoplast intrinsic protein) is an integral tonoplast protein having six transmembrane helices that was first purified from bean cotyledons, where it constitutes approx. 2 % of total extractable protein (Johnson, Herman and Chrispeels, 1989). αTIP is highly expressed in the tonoplast of seed storage tissues but is absent or present in very low amounts in other membranes and tissues (Maurel, 1997). αTIP is a member of the MIP family of transporters, and can function as a water channel when expressed in Xenopus oocytes. The water channel activity of αTIP in oocytes was dependent on phosphorylation (Maurel et al., 1995). Oocytes injected with αTIP cRNA had a water permeability that was four to eight-fold greater than that of control oocytes. Treatment of injected oocytes with compounds that increased the activity of endogenous protein kinase A increased water permeability by an additional 80–100 %. When putative phosphorylation sites in the αTIP cRNA were disrupted by site-directed mutagenesis, injection of mutant cRNA caused less of an increase in permeability of the oocyte membrane than injection of wild type cRNA. Since αTIP is phosphorylated in plant vacuoles, it was suggested that phosphorylation of αTIP might be a means for regulating tonoplast osmotic permeability (Maurel et al., 1995). αTIP is present in the dry barley aleurone (Bethke et al., 1996), as well as in ABA- or GA-treated aleurone layers or protoplasts (Schuurink et al., 1996). On protein blots, αTIP appears as a doublet of approx. 25 kDa, and the relative amount of the upper band with respect to the lower band varies with hormone treatment and duration. Although αTIP is an abundant protein, its function is unclear. A role in water transport is presumed (Schaffner, 1998), but it is not known if this is to facilitate grain desiccation or rehydration. What is clear is that protein storage vacuoles that are rapidly hydrolyzing stored proteins and solubilizing stored minerals may have a relatively large requirement for water if they are to remain iso-osmotic with the cytosol. αTIP may also function to buffer the osmolality of the cytosol from changes in water potential in the free space of the wall (Maurel et al., 1993). In this way, TIPs in the tonoplast would ensure that water entry into the protein storage vacuole was at least as rapid as water entry into the cytosol. This might minimize the osmotic shock experienced by other organelles following imbibition. The slow acuolar channel Another abundant tonoplast transporter found in aleurone cells is the slow vacuolar (SV) channel. This class of channel is ubiquitous in the tonoplast of plants and was found in the tonoplast of both ABA- and GA-treated barley aleurone protoplasts (Bethke and Jones, 1994). The SV channel is a voltage-regulated, cation channel that is selective for Ca#+ but also readily transports K+. Like αTIP, the role Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone of the SV channel in the aleurone is unknown. A role in facilitating calcium-induced calcium release has been proposed for the SV channel in guard cells (Ward and Schroeder, 1994), but this remains controversial. Under one set of experimental conditions, calcium release through the SV channel was found to be thermodynamically unfavourable (Pottosin et al., 1997). Although the function of the aleurone SV channel remains unknown, its regulation has been studied in detail. The SV channel in aleurone is voltage regulated, requiring a positive (cytosol-vacuole) membrane potential of approx. 20–40 mV for opening (Bethke and Jones, 1994). The conditions under which this occurs are not known. Channel opening is also promoted by calcium and calmodulin (CaM) (Bethke and Jones, 1994). Cytosolic calcium concentrations greater than about 1 µ are required for opening, and in the absence of added CaM the probability of the channel being in the open state increases with cytosolic free calcium up to at least 100 µ. Plant CaM, e.g. Chenopodium rubrum (Weiser, Blum and Bentrup, 1991) or spinach (Bethke and Jones, 1994), but not bovine brain CaM, also stimulated channel opening and increased the sensitivity of the channel toward calcium. It seems likely, therefore, that the effects of calcium on channel opening are at least partly the effect of Ca#+ interacting with membrane-bound CaM. Immunolocalization of CaM in barley aleurone protoplasts has shown strong binding of an anti-CaM antibody to an antigen in the barley tonoplast. Activation of the SV channel by increases in Ca and CaM suggests that the transport activity of this channel may be upregulated by GA. Protein storage vacuoles isolated from barley aleurone protoplasts treated with GA had whole vacuolar SV currents that were 240 % of currents in protein storage vacuoles isolated from ABA-treated protoplasts (Bethke and Jones, 1994). One explanation for this difference is that protein storage vacuoles isolated from GA-treated cells have more CaM associated with them than those isolated from ABA-treated cells. GA treatment of barley aleurone layers increased the amount of CaM mRNA and protein (Schuurink et al., 1996). In barley and wheat, GA treatment resulted in increased cytosolic calcium concentrations (Bush, 1995). SV channel opening, however, may be inhibited by GAinduced acidification of the vacuole lumen. This could provide another link to GA signalling. It is not known, however, if SV channel activity in aleurone is decreased by vacuolar acidification as it was in Vicia faba and Beta ulgaris (Schultz-Lesdorf and Hedrich, 1995). The barley aleurone SV channel is also regulated by protein phosphorylation (Bethke and Jones, 1997). When protein storage vacuoles isolated from ABA-treated barley aleurone protoplasts were treated with the protein phosphatase inhibitor okadaic acid, the activity of the SV channel decreased. This decrease could be overcome by addition of an okadaic-acid-insensitive protein phosphatase. SV channel activity could also be inhibited by the addition of 200 µ ATP, and this inhibition was prevented by the protein kinase inhibitor H-7. These experiments suggest that phosphorylation can decrease the activity of the channel, and that the protein phosphatase and protein 407 kinase that regulate this activity are localized to the protein storage vacuole tonoplast. The effect of ATP addition, however, was dependent on its concentration. At 2 m, ATP increased the activity of the SV channel. Two millimolar ATP is close to the presumed cytosolic concentration of ATP in most plant cells. Addition of recombinant CDPK in the presence of either 200 µ or 2 m ATP strongly stimulated activity of the channel. These data were interpreted to mean that there are at least two phosphorylation sites that regulate the activity of the SV channel. Increased phosphorylation of the first site relative to the second led to an increase in channel activity (Bethke and Jones, 1997). The regulation of the SV channel is complex, and it is worth noting that with the exception of Ca#+ most of the known regulatory components for this channel are not cytosolic but rather are located on the tonoplast. It will be interesting to learn if other tonoplast transporters in protein storage vacuoles are accompanied by a similar suite of regulatory elements. Ca#+-transporters At least three calcium-transport activities have been identified in microsomal vesicles isolated from GA-treated wheat aleurone layers (Bush and Wang, 1995). After separation on an isopycnic sucrose density gradient, one of these activities, referred to as Type II, was found in a fraction enriched in tonoplast marker enzymes. It is likely, therefore, that the Type II activity was localized to the protein storage vacuole membrane. Type II calcium transport activity was stimulated by calmodulin and inhibited by the calmodulin antagonist W7. Nitrate and compounds such as FCCP that prevent the formation of a proton gradient inhibited Type II calcium transport by over 60 %. Kinetic analysis revealed that this transport activity had both a low affinity (Km ¯ 12 µ) and a high affinity (Km ¯ 0±33 µ) for Ca#+. This led the authors to suggest that Type II activity had characteristics of both a primary Ca#+ transporter and a secondary Ca#+}nH+ antiport. Bush and Wang (1995) were not able to determine if these two Ca#+ transport mechanisms exist in the same transporter or in two separate transporters, but suggested that Type II calcium transporters are likely to be important in the regulation of cytosolic calcium concentrations. Tonoplast proton pumps In the cells of most higher plants the vacuole is an acidic compartment (Boller and Wiemken, 1986 ; Kurkdjian and Guern, 1989). The protein storage vacuole of the cereal aleurone cell is one of a few interesting exceptions (Davies et al., 1996 ; Swanson and Jones, 1996). The pH of the protein storage vacuoles in mature aleurone cells from barley (Swanson and Jones, 1996) and wild oat (Davies et al., 1996) is near neutrality. Incubation of aleurone protoplasts in the absence of hormone results in a gradual decline in the pH of the PSV lumen (Davies et al., 1996), and in barley this acidification is accelerated by incubation in GA (Swanson and Jones, 1996). Proton pumps contribute to the regulation of vacuolar 408 Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone A B 440 nm 0 hr 4 hr 8 hr +ABA 6.8 6.4 pH ABA GA3 +GA3 6.0 5.6 0 pH 5.0 6.0 7.0 2 4 6 Time (hr) 8 10 F. 5. Pseudocolour images of barley aleurone protoplasts loaded with the pH-sensitive probe BCECF, and quantification of vacuolar pH. A, Protein storage vacuoles in living barley aleurone protoplasts were loaded with BCECF then treated with either ABA or GA . The fluorescence $ emission at 440 nm is presented in the left panels. The pseudocoloured fluorescent signal from protoplasts 0, 4 and 8 h after hormone addition is presented in the three right-hand panels and corresponds to the pH scale bar below. B, Quantification of the data in A. Note that GA treatment resulted in acidification of protein storage vacuoles but ABA treatment did not. Reprinted from Swanson and Jones, 1996. pH (pHV) in plants (Kurkdjian and Guern, 1989), and two types of proton pumps, the vacuolar H+ ATPase (VATPase ; EC 3.6.1.3) and the vacuolar pyrophosphatase (VPPase ; EC 3.6.1.1) (Nelson and Taiz, 1989 ; Rea and Poole, 1993), are found in the plant tonoplast. Both V-ATPase and V-PPase have been identified in the tonoplast of aleurone protein storage vacuoles (Swanson and Jones, 1996). Immunofluorescence microscopy of aleurone protoplasts and protein blotting of purified protein storage vacuoles established that V-ATPase and V-PPase were present in the tonoplast of barley aleurone protoplasts. These enzymes were found in the tonoplast of freshly isolated protoplasts, and the amount of these pumps was not affected by incubation of protoplasts in GA or ABA (Swanson and Jones, 1996). Using the ratioable, pH-sensitive dye BCECF we have shown that pHV of the aleurone protein storage vacuole is regulated by GA (Swanson and Jones, 1996). BCECF was used to monitor pHV in living aleurone cells since this dye could be loaded into protoplasts non-invasively using the membrane-permeant AM (acetoxy methyl ester) form. BCECF specifically accumulated in the lumen of the protein storage vacuole (Fig. 2 C) and did not affect the ability of the aleurone cells to respond to GA or ABA (Swanson and Jones, 1996). In freshly isolated aleurone protoplasts pHV was around 6±7, but following exposure to GA for 8 h, pHV dropped to around pH 5±8 (Fig. 5 ; Swanson and Jones, 1996). Acidification of the protein storage vacuole in GAtreated protoplasts began within 2 h of adding GA and reached a maximum about 8 h after hormone addition (Swanson and Jones, 1996). From this we can conclude that 2 h is the maximum time required for a GA signal transduction cascade to reach the protein storage vacuoles. ABA slowed the acidification of protein storage vacuoles, and protoplasts incubated in ABA for 21 h had a pHV that was slightly higher than the pHV of protoplasts incubated in the absence of hormones. We have shown that pHV in ABAtreated protoplasts remains higher than that of GA-treated protoplasts after incubation for 4 or more days (Swanson et al., 1998). Ratio imaging of BCECF has also shown that the VATPase and V-PPase can acidify the lumen of isolated barley aleurone protein storage vacuoles (Swanson and Jones, 1996). When either ATP or PPi were added to intact isolated protein storage vacuoles, pHV fell rapidly to a new level in less than 5 min. Acidification of the vacuole lumen occurred regardless of whether vacuoles were isolated from ABA-, GA- or non-hormone-treated protoplasts. Acidification following ATP addition was inhibited by NO−, and $ acidification following PPi addition was prevented by Ca#+ suggesting that both V-ATPase and V-PPase can act to lower pHV (Swanson and Jones, 1996). Although these experiments demonstrate that V-ATPase and V-PPase can lower pHV in itro, we have not established the contribution of these proton pumps to regulation in pHV in io. Indeed, the in io regulation of pHV in barley aleurone cells is likely to be complex since vacuolar acidification is much faster in GA-treated cells than in ABA-treated cells despite the presence of comparable amounts of V-ATPase and V-PPase in both (Swanson and Jones, 1996). Since acidification in itro occurred in minutes and acidification in io required hours, acidification cannot result simply from the activation of the proton pumps. There is only limited information to suggest how VATPase and V-PPase activities might be regulated in protein storage vacuoles (Davies, 1997). Transcriptional regulation of the V-ATPase has been reported (Low et al., 1996 ; Tsiantis, Batholomew and Smith, 1996), but our experiments showing that GA or ABA had no effect on the amount of either V-ATPase or V-PPase in aleurone protoplasts suggest that in barley aleurone protein storage vacuoles, regulation of H+-pump activities is likely to be post-translational. Ca#+ and protein phosphorylation are attractive candidates for signals that control pHV in the aleurone cell. Ca#+ at low micromolar concentrations is a potent inhibitor of the VPPase (Rea et al., 1992). Because the SV channel may Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone 409 T 1. Subcellular distribution of fluorescent probes in barley aleurone protoplasts Probe purpose and name ABA-treated protoplasts GA-treated protoplasts F. 6. Fluorescence from protein storage vacuoles in ABA- or GAtreated (A and B, respectively) barley aleurone protoplasts. Protoplasts were incubated with non-fluorescent monochlorobimane which becomes fluorescent after conjugation to cytosolic glutathione. Transport into the vacuole by a glutathione conjugate transporter results in fluorescence from the protein storage vacuoles. Note that the amount of vacuolar fluorescence in ABA- and GA-treated cells is approximately the same. This suggests that rates of uptake into these organelles are comparable. See Swanson, Bethke and Jones, 1998 for details. transport Ca#+ from the vacuole to the cytosol (Ward and Schroeder, 1994), the local concentration of Ca#+ at the tonoplast of the aleurone protein storage vacuole may be high and this would effectively inhibit the activity of this pump. Protein phosphorylation may also play a role in the regulation of the V-ATPase. Martiny-Baron et al. (1991) showed that the V-ATPase of zucchini hypocotyls is likely to be regulated by a lysophospholipid-activated protein kinase. Phosphorylation of V-ATPase and H+ pumping in zucchini membranes was stimulated by phospholipid, leading Martiny-Baron et al. (1991) to conclude that proton pumping by the V-ATPase was regulated by phosphorylation. By analogy with the role that protein phosphorylation plays in the regulation of the SV channel of the aleurone protein storage vacuole, we suggest that the activity of the V-ATPase may be regulated by protein phosphorylation. ABC transporters Proteins that can transport a variety of potentially harmful toxins and naturally occurring molecules into the vacuole are located in the tonoplast (Kreuz, Tommasini and Martinoia, 1996 ; Coleman, Blake-Kalff and Davies, 1997 ; Rea et al., 1998). These transporters belong to the ABC family of transport proteins. Two subclasses of these proteins have been identified in plants, the MDRs (multidrug resistance proteins) and MRPs (multidrug resistance associated proteins) (Rea et al., 1998). We have demonstrated the presence of two ABC transport activities on the tonoplast of barley aleurone protein storage vacuoles, a glutathione (GS) conjugates transport activity and an organic anion transport activity (Swanson et al., 1998). These transporters were present in freshly isolated protoplasts and, unlike the H+ pumps on the tonoplast, their activities were not markedly affected by incubation in GA or ABA (Fig. 6). Thus, accumulation of fluorescent probes that are transported Glutathione}sulfhydryls Monochlorobimane Monobromobimane Cell tracker green pH BCECF-AM Lysosensor yellow}blue Calcium Indo-1 ff Indo-1 Fluo-3 Protease substrate ZFR-CMAC Other fluorophores Lucifer yellow Oregon green Fluorescein Location of fluorescence Cytoplasm then vacuole Cytoplasm then vacuole Cytoplasm then vacuole Cytoplasm then vacuole Vacuole Cytoplasm Cytoplasm Cytoplasm Vacuole No uptake Cytoplasm then vacuole Cytoplasm then vacuole Reprinted from Swanson, Bethke and Jones, 1998. into vacuoles by one of these transporters was not altered by incubation of protoplasts in the presence or absence of hormones. The ability of protein storage vacuoles in freshly isolated protoplasts to accumulate fluorescent probes is a very useful attribute. It allows non-hormone-treated cells to be loaded with probes that can measure subsequent responses to hormones, as was done for vacuole acidification (Fig. 5). Our interest in characterizing the ABC transporters on the tonoplast of protein storage vacuoles was sparked by the observation that these organelles accumulate a wide variety of fluorescent probes (Table 1). With the exception of the Ca#+-sensitive dyes, including Indo and Fura, all other cell permeant fluorescent dyes that we have examined are taken up and sequestered in the vacuole of aleurone cells (Table 1, Figs 2, 5 and 6 ; Swanson et al., 1998). By monitoring the uptake and accumulation of dyes such as monobromobimane (MBB) and monochlorobimane (MCB) that fluoresce only after binding to sulphydryls such as glutathione (Coleman et al., 1997), we surmised that at least one of the ABC transport activities was a transporter of GS conjugates (Fig. 6). Using isolated protein storage vacuoles, we confirmed that compounds such as MCB and MBB are taken up by ATP-dependent GS conjugates transporters, but that other dyes, including the pH-sensitive dye BCECF, are transported into protein storage vacuoles by ATPdependent organic anion transporters (Swanson et al., 1998). Whereas vanadate was a potent inhibitor of both ABC transport activities in the protein storage vacuole membrane, probenicid inhibited only the transport activity that facilitates BCECFs accumulation. Conversely, GSconjugate transport, but not organic anion transport activity was competitively inhibited by other GS-conjugated substrates (Swanson et al., 1998). The experiments showing that accumulation of fluorescent dyes into aleurone vacuoles is driven by at least two types of ATP-dependent transport activities allow for the rational 410 Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone design of compounds that can be targeted to the plant vacuole. For example, cell permeable electrophiles that readily form glutathione conjugates are likely to be taken up into the vacuole in io. In itro conjugation of these same compounds to glutathione provides a means for their introduction into isolated vacuoles. The ability of probenecid to prevent the uptake of dyes into vacuoles in io provides a way to alter the partitioning of dyes between vacuole and cytoplasm. Further, an understanding of the chemistry of compounds such as indo-1ff that accumulate in the cytosol but not in the vacuole of aleurone cells provides a way of targeting probes to this compartment. CONCLUSIONS Much has been learned about the composition and function of the aleurone protein storage vacuole. Figure 1 illustrates constituents of the barley aleurone protein storage vacuole, and Fig. 7 illustrates changes that occur in these organelles following imbibition of whole grain or GA treatment of isolated aleurone layers or protoplasts. Although these diagrams are only cartoon depictions of this organelle, until recently even these rudimentary aspects of protein storage vacuoles were unknown. Yet for each new thing that has been learned about protein storage vacuoles, multiple unknowns exist. Globulin storage proteins have been identified in barley aleurone protein storage vacuoles, but the amount and identity of other storage proteins remain unknown. Proteases exist in protein storage vacuoles, but in io substrates have not been determined, and a more precise A characterization of protein storage vacuole proteases is needed. Nuclease I has been localized to the protein storage vacuole, but the number of other nucleases and their role in aleurone physiology are unclear. Lipase activities are associated with protein storage vacuoles, but how this activity is regulated needs further investigation. Although it is known that stores of protein and minerals are deposited into protein storage vacuoles, influx and efflux transporters for mineral reserves and efflux transporters for amino acids or small peptides have not been identified. αTIP and the SV channel are abundantly present in the tonoplast, yet the function of these transporters remains speculative. And although it is clear that the activities of the vacuole are coordinated with those of the cytosol, there is only fragmentary evidence to suggest how this might be done. As we learn more, we are likely to unearth new layers of complexity and discover new levels of interaction. A complete picture of the aleurone protein storage vacuole will not be ours for many years, yet what we can see already suggests that it will be a fascinating picture indeed. LITERATURE CITED Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. 1996. Ultrastructural and biochemical characterization of autophagy in higher plant cells subject to carbon deprivation : Control by the supply of mitochondria with respiratory substrates. Journal of Cell Biology 133 : 1251–1263. Batten GD, Lott JNA. 1996. The influence of phosphorus nutrition on the appearance and composition of globoid crystals in wheat aleurone cells. Cereal Chemistry 63 : 14–18. B Phytin globoid PSV Protein storage vacuole Inactive protease Active protease Active nuclease pH 5 + H Storage proteins Inactive phytase Nucleotides pH 7 Amino nitrogen Active phytase Protein crytalloid Inactive lipase Inactive nuclease Ca2+ CaM Active lipase – 2+ Ca CaM Oleosome ABA H2PO4 K+ PM Mature grain or ABA-treated GA Cytosolic signals PM Imbibed grain or GA-treated F. 7. A model for changes that occur in barley aleurone protein storage vacuoles following imbibition of whole grain or GA treatment of isolated aleurone layers or protoplasts. Note that GA is perceived at the plasma membrane (PM) and cytosolic signals, including Ca#+ and calmodulin (CaM), act to promote coalescence of protein storage vacuoles (PSVs), acidification of the vacuole lumen, activation of enzymes, and the hydrolysis of stored reserves. Although labels for the enzymes present in protein storage vacuoles of mature grain are placed on separate vacuoles, it is likely that each protein storage vacuole contains phytase, protease, nuclease and lipase. Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone Bethke PC, Jones RL. 1994. Ca#+-calmodulin modulates ion channel activity in storage protein vacuoles of barley aleurone cells. The Plant Cell 6 : 277–285. Bethke PC, Jones RL. 1997. Reversible protein phosphorylation regulates the activity of the slow-vacuolar ion channel. The Plant Journal 11 : 1227–1235. Bethke PC, Hillmer S, Jones RL. 1996. Isolation of intact protein storage vacuoles from barley aleurone. Plant Physiology 110 : 521–529. Bethke PC, Schuurink RC, Jones RL. 1997. Hormonal signaling in cereal aleurone. Journal of Experimental Botany 48 : 1337–1356. Bewley JD, Black M. 1994. Seeds : Germination, structure and composition. New York : Plenum. Boller T, Wiemken A. 1986. Dynamics of vacuolar compartmentation. Annual Reiew of Plant Physiology 37 : 137–164. Brearley CA, Hanke DE. 1996. Inositol phosphates in barley (Hordeum ulgare L.) aleurone tissue are stereochemically similar to the products of breakdown of InsP in itro by wheat germ phytase. ' Biochemistry Journal 318 : 279–286. Brearley CA, Parmar PN, Hanke DE. 1997. Metabolic evidence of PtdTns(4,5)P-2 directed phospholipase C in permeabilized plant protoplasts. Biochemistry Journal 324 : 123–131. Brown PH, Ho T-HD. 1987. Biochemical properties and hormonal regulation of barley nuclease. European Journal of Biochemistry 52 : 1–8. Brown PH, Mecham RP, Ho T-HD. 1988. Hormonal regulation of barley nuclease : investigations using a monoclonal antibody. Plant, Cell and Enironment 11 : 747–753. Bush DS. 1995. Calcium regulation in plant cells and its role in signaling. Annual Reiew of Plant Physiology and Plant Molecular Biology 46 : 95–122. Bush DS, Wang T. 1995. Diversity of calcium-efflux transporters in wheat aleurone cells. Planta 197 : 19–30. Chrispeels MJ. 1985. The role of the Golgi apparatus in the transport and post-translational modifications of vacuolar (protein body) proteins. Oxford Surey of Plant Molecular Biology 2 : 21–53. Chrispeels MJ, Varner JE. 1973. A test for the de noo synthesis of enzymes in germinating seeds : density labeling with D O. In : # Chrispeels MJ, ed. Molecular techniques and approaches in deelopmental biology. New york : John Wiley, 79–92. Chrispeels MJ, Tenner AJ, Johnson KD. 1973. Synthesis and release of sucrose by the aleurone layer of barley : regulation by gibberellic acid. Planta 113 : 35–46. Coleman JOD, Blake-Kalff MMA, Davies TGE. 1997. Detoxification of xenobiotics by plants : chemical modification and vacuolar compartmentation. Trends in Plant Science 2 : 144–151. Cornejo MJ, Platt-Aloia KA, Thomson WW, Jones RL. 1988. Effects of GA and Ca#+ on barley aleurone protoplasts : a freeze-fracture $ study. Protoplasma 146 : 157–165. Cosgrove DJ, 1980. Inositol phosphates. Their chemistry, biochemistry and physiology. Amsterdam : Elsevier. Crain R. 1993. Biochemistry of phosphoinositides. Annual Reiew of Plant Physiology and Plant Molecular Biology 44 : 333–356. Davies JM. 1997. Vacuolar energization : pumps, shunts and stress. Journal of Experimental Botany 48 : 633–641. Davies TGE, Steele SH, Walker DJ, Leigh RA. 1996. An analysis of vacuole development in oat aleurone protoplasts. Planta 198 : 356–364. Doig RI, Colborne AJ, Morris G, Laidman DL. 1975. The induction of glyoxysomal enzyme activities in the aleurone cells of germinating wheat. Journal of Experimental Botany 26 : 387–398. Drobak BK. 1992. The plant phosphoinositide system. Biochemical Journal 288 : 697–712. Fernandez DE, Staehelin LA. 1985. Structural organization of ultrarapidly frozen barley aleurone cells actively involved in protein secretion. 165 : 455–468. Fernandez DE, Staehelin LA. 1987. Does gibberellic acid induce the transfer of lipase from protein bodies to lipid bodies in barley aleurone cells ? Plant Physiology 85 : 487–496. Filner P, Varner JE. 1967. A test of de noo synthesis of enzymes : density labelling with H O") of barley α-amylase induced by # gibberellic acid. Proceedings of the National Academy of Sciences USA 58 : 1520–1526. 411 Fincher GB. 1989. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annual Reiew of Plant Physiology and Plant Molecular Biology 40 : 305–346. Fulcher RG, O’Brien TP, Wong SI. 1981. Microchemical detection of niacin, aromatic amine, and phytin reserves in cereal bran. Cereal Chemistry 58 : 130–135. Gabard KA, Jones RL. 1986. Localization of phytase and acid phosphatase isoenzymes in aleurone layers of barley. Plant Physiology 67 : 182–192. Galili G, Shimoni Y, Giorini-Silfen S, Levanony H, Altschul Y, Shani N. 1996. Wheat storage proteins : Assembly, transport and deposition in protein bodies. Plant Physiology and Biochemistry 34 : 245–252. Holstein SEH, Kobert B, Hillmer S, Brown PH, Ho T-HD, Robinson DG. 1991. Subcellular localization of nuclease in barley aleurone. Physiologia Plantarum 83 : 255–264. Holwerda, BC, Rogers JC. 1992. Purification and characterization of aleurain. Plant Physiology 99 : 848–855. Holwerda BC, Galvin NJ, Baranski TJ, Rogers JC. 1990. In itro processing of aleurain, a barley vacuolar thiol protease. Plant Cell 2 : 1091–1106. Huang AHC. 1992. Oil bodies and oleosins in seeds. Annual Reiew of Plant Physiology and Plant Molecular Biology 43 : 177–200. Hubel F, Beck E. 1996. Maize root phytase. Plant Physiology 112 : 1429–1436. Huisamen B, Lochner A. 1996. Inositolpolyphosphates and their binding proteins—a short review. Molecular and Cellular Biochemistry 157 : 229–232. Jacobsen JV, Gubler F, Chandler PM. 1995. Gibberellin action in germinating cereal grains. In : Davies PJ, ed. Plant hormones : physiology, biochemistry and molecular biology. Dordrecht, Netherlands : Kluwer. Jacobsen JV, Knox RB, Pyliotis NA. 1971. The structure and composition of aleurone grains in the barley aleurone layer. Planta 101 : 189–209. Jelsema CL, Morre DJ, Ruddat M, Turner C. 1977. Isolation and characterization of the lipid reserve bodies, spherosomes, from aleurone layers of wheat. Botanical Gazette 138 : 139–149. Johnson KD, Herman EM, Chrispeels MJ. 1989. An abundant, highly conserved tonoplast protein in seeds. Plant Physiology 91 : 1006–1013. Jones RL. 1969 a. The fine structure of barley aleurone cells. Planta 85 : 359–374. Jones RL. 1969 b. Gibberellic acid and the fine structure of barley aleurone cells. I. Changes during the lag-phase of α-amylase synthesis. Planta 87 : 119–133. Jones RL. 1972. Fractionation of the enzymes of the barley aleurone layer : evidence for a soluble mode of enzyme release. Planta 103 : 95–109. Jones RL. 1973. Gibberellic acid and ion release from barley aleurone tissue. Plant Physiology 52 : 303–308. Jones RL. 1987. Localization of ATPase in the endoplasmic reticulum and Golgi apparatus of barley aleurone. Protoplasma 138 : 73–88. Jones RL, Jacobsen JV. 1991. Regulation of synthesis and transport of secreted proteins in cereal aleurone. International Reiew of Cytology 126 : 49–88. Jones RL, Price JM. 1970. Gibberellic acid and the fine structure of barley aleurone cells. III. Vacuolation of the aleurone cell during the phase of ribonuclease release. Planta 94 : 191–202. Koehler S, Ho T-HD. 1988. Purification and characterization of gibberellic acid-induced cysteine endoprotease in barley aleurone layers. Plant Physiology 87 : 95–103. Koehler S, Ho T-HD. 1990 a. A major gibberellic acid-induced barley aleurone cysteine protease which digests hordein. Plant Physiology 94 : 251–258. Koehler SM, Ho T-HD. 1990 b. Hormonal regulation, processing, and secretion of cysteine proteinase in barley aleurone layers. The Plant Cell 2 : 769–783. Kreuz K, Tommasini R, Martinoia E. 1996. Old enzymes for a new job. Plant Physiology 111 : 349–353. Kurkdjian A, Guern J. 1989. Intracellular pH : Measurement and importance in cell activity. Annual Reiew of Plant Physiology and Plant Molecular Biology 40 : 271–303. 412 Bethke et al.—Protein Storage Vacuoles in Cereal Aleurone Laboure AM, Gagnon J, Lescure AM. 1993. Purification and characterization of phytase (myo-inositol-hexakisphosphate) accumulated in maize (Zea mays) seedlings during germination. Biochemical Journal 295 : 413–419. Liu DJ, Pomeranz Y. 1975. Distribution of minerals in barley at the cellular level using x-ray microanalysis. Cereal Chemistry 52 : 620–629. Lott JNA. 1980. Protein bodies. In : Tolbert NE, ed. The biochemistry of plants. San Diego : Academic Press, 589–623. Lower R, Rockel B, Kirsch M, Ratajczak R, Hortensteiner S, Martinoia E, Luttge U, Rausch T. 1996. Early salt stress effects on the differential expression of vacuolar H+-ATPase genes in roots and leaves of Mesembryanthemum crystallinum. Plant Physiology 110 : 259–265. Martiny-Baron G, Manolson MF, Poole RJ, Hecker D, Scherer GFE. 1991. Proton transport and phosphorylation of tonoplast polypeptides from zucchini are stimulated by the phospholipid platelet activating factor. Plant Physiology 99 : 1635–1641. Matile P, 1975. The lytic compartment of plant cells. Vienna, New York : Springer-Verlag. Maugenest S, Martinez I, Lescure AM. 1997. Cloning and characterization of a cDNA encoding a maize seedling phytase. Biochemical Journal 322 : 511–517. Maurel C. 1997. Aquaporins and water permeability of plant membranes. Annual Reiew of Plant Physiology and Plant Molecular Biology. 48 : 399–429. Maurel C, Kado RT, Guern J, Chrispeels MJ. 1995. Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-TIP. The EMBO Journal 14 : 3028–3035. Maurel C, Reizer J, Schroeder JI, Chrispeels MJ. 1993. The vacuolar membrane protein γ-TIP creates water channels in Xenopus oocytes. EMBO Journal 12 : 2241–2247. Mu$ ntz K. 1989. Intracellular protein sorting and the formation of protein reserves in storage tissue cells of plant seeds. Biochemie und Physiologie der Pflanzen 185 : 315–335. Napier JA, Stobart AK, Shewry PR. 1996. The structure and biogenesis of plant oil bodies : The role of the ER membrane and the oleosin class of proteins. Plant Molecular Biology 31 : 945–956. Nelson N, Taiz L. 1989. The evolution of H+-ATPases. Trends in Biochemical Science 14 : 113–116. Okita TW, Rogers JC. 1996. Compartmentation of proteins in the endomembrane system of plant cells. Annual Reiew of Plant Physiology and Plant Molecular Biology 47 : 327–350. Ory RL, Henningsen KW. 1975. Changes in protein bodies during germination of barley seeds. Bios 6 : 71–76. Pomeranz Y. 1973. Structure and mineral composition of cereal aleurone cells as shown by scanning electron microscopy. Cereal Chemistry 50 : 504–511. Pottosin II, Tikhonova LI, Hedrich R, Schoenknecht G. 1997. Slowly activating vacuolar channels can not mediate Ca#+-induced Ca#+ release. The Plant Journal 12 : 1387–1398. Rea PA, Li Z-S, Lu Y-P, Drozdowicz Y, Martinoia E. 1998. From vacuolar GS-X pumps to multispecific ABC transporters. Annual Reiew of Plant Physiology and Plant Molecular Biology 49 : 727–760. Rea PA, Britten CJ, Jennings IR, Calvert CM, Skiera LA, Leigh RA, Sanders D. 1992. Regulation of vacuolar H+-pyrophosphatase by free calcium. Plant Physiology 100 : 1706–1715. Rea PA, Poole RJ. 1993. Vacuolar H+-translocating pyrophosphatase. Annual Reiew of Plant Physiology and Plant Molecular Biology 44 : 157–180. Richard G, Mark PF, Napier JA, Shewry PR. 1996. Transport and deposition of cereal prolamins. Plant Physiology and Biochemistry 34 : 237–243. Robinson DG, Hinz G. 1997. Vacuole biogenesis and protein transport to the vacuole : a comparison with the yeast vacuole and mammalian lysosome. Protoplasma 197 : 1–25. Runeberg-Roos P, Kervinen J, Kovaleva V, Raikhel NV, Gal S. 1994. The aspartic protease of barley is a vacuolar enzyme that processes probarley lectin. Plant Physiology 105 : 321–329. Sarkkinen P, Kalkkinen P, Tilgmann C, Siuro J, Kervinen J, Mikola L. 1992. Aspartic protease from barley grains is related to mammalian lysosomal cathepsin D. Planta 186 : 317–323. Schaffner AR. 1998. Aquaporin function, structure and expression : are there more surprises to surface in water relations ? Planta 204 : 131–139. Schultz-Lesdorf B, Hedrich R. 1995. Protons and calcium modulate SVtype channels in the vacuolar-lysosomal compartment-channel interaction with calmodulin inhibitors. Planta 197 : 655–671. Schuurink RC, Chan PV, Jones RL. 1996. Modulation of calmodulin mRNA and protein levels in barley aleurone. Plant Physiology 111 : 371–380. Shewry P. 1995. Plant storage proteins. Biological Reiew 70 : 375–426. Shewry PR, Napier JA, Tatham AS. 1995. Seed storage proteins : Structures and biosynthesis. The Plant Cell 7 : 945–956. Stewart A, Nield H, Lott JNA. 1988. An investigation of the mineral content of barley grains and seedlings. Plant Physiology 86 : 93–97. Swanson SJ, Jones RL. 1996. Gibberellic acid induces vacuolar acidification in barley aleurone. The Plant Cell 8 : 2211–2221. Swanson S, Bethke PC, Jones RL. 1998. Barley aleurone cells contain two types of vacuoles : Characterization of lytic compartments by use of fluorescent probes. The Plant Cell 10 : 685–698. Swift JG, O’Brien TP. 1972. The fine structure of wheat scutellum during germination. Australian Journal of Biological Science 25 : 468–486. To$ rma$ kangas K, Kervinen J, O> stman A, Teeri T. 1994. Tissue-specific localization of aspartic proteinase in developing and germinating barley grains. Planta 195 : 116–125. Tsiantis MS, Batholomew DM, Smith JAC. 1996. Salt regulation of transcript levels for the c subunit of a leaf vacuolar H+-ATPase in the halophyte Mesembryanthemum crystallinum. The Plant Journal 9 : 729–736. Van Haastert PJM, Van Dijken P. 1997. Biochemistry and genetics of inositol phosphate metabolism in Dictyostelium. FEBS Letters 410 : 39–43. Ward J, Schroeder JI. 1994. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. The Plant Cell 6 : 669–683. Weiser T, Blum W, Bentrup FW. 1991. Calmodulin regulates the Ca#+ dependent slow-vacuolar ion channel in the tonoplast of Chenopodium rubrum suspension cells. Planta 185 : 440–442. Yupsanis T, Burgess SR, Jackson PJ, Shewry PR. 1990. Characterization of the major protein component from aleurone cells of barley (Hordeum ulgare L.). Journal of Experimental Botany 41 : 385–392.