* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cell Apoptosis
Biochemical switches in the cell cycle wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell growth wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell membrane wikipedia , lookup
Cytokinesis wikipedia , lookup
Signal transduction wikipedia , lookup
Endomembrane system wikipedia , lookup
Programmed cell death wikipedia , lookup
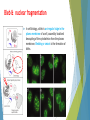
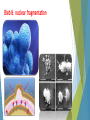
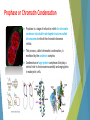
Cell Apoptosis Puria Rafsanjani Arvin Honari Pardis Taheri Apoptosis is the process of programmed cell death (PCD) that may occur in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentation. Between 50 and 70 billion cells die each day due to apoptosis in the average human adult. For an average child between the ages of 8 and 14, approximately 20 billion to 30 billion cells die a day. Excessive apoptosis causes atrophy, whereas an insufficient amount results in uncontrolled cell proliferation, such as cancer. Bleb & nuclear fragmentation In cell biology, a bleb is an irregular bulge in the plasma membrane of a cell, caused by localized decoupling of the cytoskeleton from the plasma membrane. Blebbing or zeiosis is the formation of blebs. Bleb & nuclear fragmentation Prophase or Chromatin Condensation Prophase is a stage of mitosis in which the chromatin condenses into double rod-shaped structures called chromosomes in which the chromatin becomes visible. This process, called chromatin condensation, is mediated by the condensin complex. Condensins are large protein complexes that play a central role in chromosome assembly and segregation in eukaryotic cells. Unlike necrosis, apoptosis produces cell fragments called apoptotic bodies that phagocytic cells are able to engulf and quickly remove before the contents of the cell can spill out onto surrounding cells and cause damage. What may lead to apoptosis ? Extracellular signals may include toxins, hormones, growth factors, nitric oxide or cytokines, that must either cross the plasma membrane or transduce to effect a response. A cell initiates intracellular apoptotic signaling in response to a stress, which may bring about cell suicide. The binding of nuclear receptors by glucocorticoids, heat, radiation, nutrient deprivation, viral infection, hypoxia and increased intracellular calcium concentration, for example, by damage to the membrane, can all trigger the release of intracellular apoptotic signals by a damaged cell. Glucocorticoids (GC) are a class of steroid hormones that bind to the glucocorticoid receptor (GR), which is present in almost every vertebrate animal cell. A number of cellular components, such as poly ADP ribose polymerase, may also help regulate apoptosis. Two main methods of regulating apoptosis Targeting mitochondria functionality or directly transducing the signal via adaptor proteins to the apoptotic mechanisms. Adaptor proteins contain a variety of protein-binding modules that link protein-binding partners together and facilitate the creation of larger signaling complexes. These proteins tend to lack any intrinsic enzymatic activity themselves but instead mediate specific protein–protein interactions that drive the formation of protein complexes. Another pathway for initiation identified in several toxin studies is an increase in calcium concentration within a cell caused by drug activity, which also can cause apoptosis via a calcium binding protease calpain. There is also a growing body of evidence indicating that nitric oxide is able to induce apoptosis by helping to dissipate the membrane potential of mitochondria and therefore make it more permeable. Cytochrome c Cytochrome c is also released from mitochondria due to formation of a channel and serves a regulatory function as it precedes morphological change associated with apoptosis. Once cytochrome c is released it binds with Apoptotic protease activating factor - 1 (Apaf-1) and ATP, which then bind to pro-caspase-9 to create a protein complex known as an apoptosome. The apoptosome cleaves the pro-caspase to its active form of caspase9, which in turn activates the effector caspase-3. Caspase-3 has been found to be necessary for normal brain development as well as its typical role in apoptosis, where it is responsible for chromatin condensation and DNA fragmentation. MAC, also called "Mitochondrial Outer Membrane Permeabilization Pore" is regulated by various proteins. Bcl-2 proteins are able to promote or inhibit apoptosis by direct action on MAC/MOMPP. Bax and/or Bak form the pore, while Bcl-2, Bcl-xL or Mcl-1 inhibit its formation. Execution ! Many pathways and signals lead to apoptosis, but there is only one mechanism that actually causes the death of a cell. After a cell receives stimulus, it undergoes organized degradation of cellular organelles by activated proteolytic caspases. Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. This generally occurs by the hydrolysis of the peptide bond, and is most commonly achieved by cellular enzymes called proteases. characteristic morphology of a cell undergoing apoptosis Cell shrinkage and rounding are shown because of the breakdown of the proteinaceous cytoskeleton by caspases. The cytoplasm appears dense, and the organelles appear tightly packed. Chromatin undergoes condensation into compact patches against the nuclear envelope in a process known as pyknosis, a hallmark of apoptosis. The nuclear envelope becomes discontinuous and the DNA inside it is fragmented in a process referred to as karyorrhexis. The nucleus breaks into several discrete chromatin bodies or nucleosomal units due to the degradation of DNA. The cell membrane shows irregular buds known as blebs. The cell breaks apart into several vesicles called apoptotic bodies, which are then phagocytosed.