* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download S phase
Tissue engineering wikipedia , lookup
Signal transduction wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Endomembrane system wikipedia , lookup
Kinetochore wikipedia , lookup
Cell nucleus wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Spindle checkpoint wikipedia , lookup
Cell growth wikipedia , lookup
List of types of proteins wikipedia , lookup
Cytokinesis wikipedia , lookup
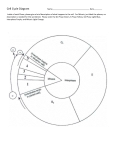
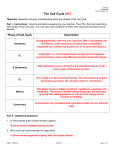
The most important occurrence within the life of a cell are its generation from a progenitor followed by its demise, either through a natural or through a pathological process. Regulation of both processes is critical to ensure that the appropriate number of cells is available to carry out their function within the organism. Safeguard are also needed to protect against uncontrolled growth, which may result in malignancy and the death of organism in which the cell is a participant. The Cell Cycle Dr. Ahmad Salahuddin When an organism requires additional cells, either for growth or to replace those normally lost, new ones must be produced by cell division, or proliferation. Cellular turnover is a normal function. The turnover times for some cells in the adult body are slow or nonexistent (in the endocrine and central nervous system, for example), whereas other cells turnover very rapidly. Somatic cells are generated by the division of existing cells in an orderly sequence of events. For a cell to generate two daughter cells, complete copies of all of the cell’s constituents must be made and shared between the two newly formed daughter cells. This sequence of duplication is known as the cell cycle and is the essential mechanism of eukaryotic reproduction. The cell cycle may be broadly divided into three distinct stages: interphase, mitosis, and cytokinesis. Interphase is the period between successive rounds of nuclear division and is distinguished by cellular growth and new synthesis of DNA. It can be further subdivided into three phases called G1 phase, S phase, and G2 phase. Division of genetic information occurs during the stage of the cell cycle known as mitosis. Mitosis can be divided into five distinct phases called prophase, prometaphase, metaphase, anaphase, and Telophase. Mitosis assures that each daughter cell will have identical complete functional copies of the parent cell’s genetic material. The third stage, cytoplasmic division or cytokinesis, culminates with the separation into two distinct daughter cells that enter interphase. INTERPHASE All cells, whether actively cycling or not, spend the vast majority of their lives in interphase. Interphase is an eventful and important part of the cell cycle and comprises G1, S, and G2 phases. Cellular growth and DNA synthesis occur during interphase, resulting in a duplication of cellular materials so that there are sufficient materials for two complete new daughter cells. G1 and G0 phases G1 phase is generally both a growth phase and a preparation time for DNA synthesis of S phase. During G1 phase: organelles and intracellular structures are duplicated, RNA and protein are synthesized and the cell grows. Those cells in G1 phase that are not committed to DNA synthesis are in a specialized resting state called G0 phase. The restriction point is located within G1 phase and, if passed, will commit a cell to continuing into S phase. The restriction point is critical for cell cycle regulation. The length of G1 phase is the most variable among cell types. Very rapidly dividing cells, such as growing embryonic cells, spend very little time in G1 phase. On the other hand, mature cells that are no longer actively cycling are permanently in G1 phase. S phase Synthesis of nuclear DNA, also known as DNA replication, occurs during S phase. Each of the 46 chromosomes in a human cell is copied to form a sister chromatid. Actively cycling cells spend approximately 6 h in S phase. G2 phase G2 is a safety gap allows the cell to ensure that DNA synthesis is complete before proceeding to nuclear division in mitosis. Also contained with the G2 is a checkpoint where intracellular regulatory molecules assess nuclear integrity. Typically, this phase lasts for approximately 4 h. MITOSIS Mitosis or nuclear division is a continuous process divided into five phases based on progress made to a specific point in the overall nuclear division. Dividing cells spend about 1 h in mitosis. After completion of nuclear division, cytokinesis occurs. Prophase In prophase, the nuclear envelope remains intact and The nucleolus disassembles. Chromosomes of mitotic cells contain two chromatids connected to each other at a centromere. Specialized protein complexes, called kinetochores, form and associate with each chromatid. Mitotic spindle microtubules will attach to each kinetochore. The microtubules of the cytoplasm disassemble and then reorganize on the surface of the nucleus to form the mitotic spindle. Two centriole pairs push away from each other by growing bundles of microtubules forming the mitotic spindle. Prometaphase The disassembly of the nuclear envelope marks the beginning of pro-metaphase. Spindle microtubules bind to kinetochores and chromosomes are pulled by the microtubules of the spindle. Metaphase Metaphase is characterized by chromatids aligned at the equator of the spindle, halfway between the two poles. The aligned chromatids form the metaphase plate. Anaphase The mitotic poles are pushed further apart as a result of polar microtubules elongating. Each centromere splits in two and paired kinetochores also separate. Sister chromatids migrate toward the opposite poles of the spindle. Telophase Telophase is characterized by kinetochore microtubule disassembly and mitotic spindle dissociation. Nuclear envelopes form around each of the two nuclei containing the chromatids. The chromatids decondense into dispersed chromatin and nucleoli reform in the daughter nuclei. CYTOKINESIS - COMPLETION OF A CELL CYCLE In order to create two distinct, separate daughter cells, cytoplasmic division follows nuclear division. An actin microfilament ring forms. Contraction of this actin-based structure results in the formation of a cleavage furrow that is seen beginning in anaphase. The furrow deepens until opposing edges meet. Plasma membranes fuse on each side of the deep cleavage furrow and the result is the formation of two separate daughter cells, each identical to the other and to the original parent cell. CELL CYCLE REGULATORS Cell cycle regulators control cell cycle progression. Cell cycle mediators are categorized as cyclins or as cyclindependent kinases (CDKs). Complexes of cyclins with CDKs (cyclin-CDKs complexes) possess enzymatic activity. Cyclin-dependent kinase inhibitors (CKI) can inhibit cyclin-CDKs complexes. Cyclins The cyclins, categorized as cyclins D, E, A, or B, are a family of cell cycle regulatory proteins that regulate specific phases of the cell cycle. Cyclins D are G1 regulators critical for progression through the restriction point. S phase cyclins include cyclins E and A. Mitotic cyclins include cyclins B and A. Cyclin-dependent kinases Certain cyclins form complexes with certain CDKs to stimulate the kinase activity of the CDKs. Cyclin Kinase Function D CDK4 CDK6 Progression past the restriction point at the G1/S boundary. E A CDK2 B CDK1 Transition from G1 to S. Initiation of DNA synthesis in early S phase. Transition from G2 to M. Initiation of mitosis. CHECKPOINT REGULATION Checkpoints placed at critical points in the cell cycle monitor the completion of critical events and, if necessary, delay the progression to the next stage of the cell cycle. One such checkpoint is the restriction point in G1 and G2. Tumor suppressors and checkpoints Tumor suppressor proteins normally function to halt the cell cycle progression within G1 when the cell should not continue past the restriction point. Mutated tumor suppressor genes may encode proteins that permit cell cycle progression at inappropriate times. Cancer cells often show mutations of tumor suppressor genes. G1 checkpoint: It is important that nuclear synthesis of DNA not begin until all the appropriate cellular growth has occurred during G1. Therefore, there are key regulators that ensure that G1 is completed prior to the start of S phase (eg: RB and p53). Retinoblastoma (RB) protein: This tumor suppressor functions to halt a cell in the resting or G1 phase of the cell cycle. The RB gene is mutated in an inherited eye malignancy known as hereditary RB. p53: Nuclear DNA damage results in activation of p53. Activated p53 stimulates CKI transcription to produce a protein named p21, to halt cell cycle progression to allow for DNA repair. If the damage is irreparable, p53 triggers apoptosis. Unregulated cell cycle progression can occur in the presence of mutated p53 because it is incapable of arrest the cell cycle. Over 50% of all human cancers show p53 mutations. G2 checkpoint It is important for the integrity of the genome and of the cell that nuclear division (mitosis) does not begin before DNA is completely duplicated during S phase. Therefore, the G2 checkpoint, which occurs after S and before the initiation of mitosis, is also a critical regulatory point within the cell cycle. CDK1 controls the transition from G2 to M.