* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carbonyl Condensation Reactions
Fischer–Tropsch process wikipedia , lookup
Metal carbonyl wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Kinetic resolution wikipedia , lookup
Marcus theory wikipedia , lookup
Elias James Corey wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Stille reaction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Discodermolide wikipedia , lookup
Diels–Alder reaction wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Ene reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Petasis reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Asymmetric induction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
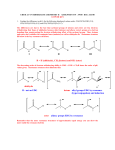
Carbonyl Condensation Reactions These are combination reactions: carbonyl -substitutions + carbonyl nucleophilic additions or substitutions. Usually, a carbonyl molecule is converted to an enolate anion (nucleophile) which attacks a second carbonyl molecule in an addition or substitution reaction. The archetype reaction is the aldol reaction. O + C O C C C H -substitution here nucleophilic addition here 1 H Aldol Condensation — General for aldehydes and ketones with an -hydrogen. This is an equilibrium reaction: product is favored for acetaldehyde and monosubstituted acetaldehyde (R-CH2-CHO) but reactants are favored for disubstituted acetaldehyde (RR'CH-CHO) and most ketones. O H H C + C H H -substitution here NaOH H2O O C H C H H nucleophilic addition here H equilibrium favorable for product: aldol Mechanism — O H O H C + NaOH H2O C H H H C H H O H H C C H + H2O C + O C H C H H 2 H2 O H O C C H H H + OH Other Examples — O H H C + C H O NaOH H2O H C C H H 10% 90% O O 78% NaOH + H H 2O 22% O H3C H C C H H + O NaOH H 2O H3C 5% 95% C H H H C The yield of the product in this case, diacetone alcohol, is quite poor owing to the unfavorable equilibrium constant (Keq = 0.05). Improving the situation by distilling off the product (to sidestep around the unfavorable equilibrium constant problem) is not an option here because the boiling point of the product is much higher than that of acetone (it is twice the molecular weight of acetone and, with its -OH group, can hydrogen bond as donor and acceptor). 3 On the other hand, if somehow the diacetone alcohol, but not the acetone, could be kept away from the base that catalyzes the reaction, the reverse reaction would become very slow. This would lead to a satisfactory yield. So, the idea is to separate some acetone from the diacetone alcohol, expose it to base for a while, then return the acetone - diacetone alcohol mixture to the bulk mixture which is not exposed to base. And do this over and over again until the conversion is complete, or nearly so. But, how? Enter the Soxhlet extractor. It was designed to be used for extracting natural products from their botanical sources. In this reaction, acetone is placed in the round-bottom flask. The filter-paper thimble is charged with barium hydroxide, a base that is not soluble in the compounds involved in the reaction. When the acetone boils its vapors pass up the large exterior tube of the extractor to the condenser, where they are condensed. Warm liquid acetone drips into the chamber that contains barium hydroxide in the paper thimble. A small amount of diacetone alcohol forms. When the chamber fills to the top of the small exterior tube the liquid in the chamber siphons to the round-bottom flask through this tube. Boiling continues, the chamber fills with more acetone (the acetone is more volatile than the diacetone alcohol), some of this is converted to diacetone alcohol, and the chamber empties into Soxhlet Extractor the round-bottom flask. Over and over again — this is an automated process sans computer or moving parts. Meanwhile, in the round-bottom flask very little reaction takes place because there is no catalyst there. Eventually, the acetone is converted to diacetone alcohol in very good yield. 4 If the carbonyl compound does not have an -hydrogen it cannot form an enolate anion and cannot undergo the aldol reaction. However, aldehydes, w/o an -hydrogen can undergo the Cannizzaro reaction in concentrated aqueous base. How about competition between a simple substitution and a condensation? For example, suppose I want to make 2-methyl-3-phenylpropanal by alkylation of propanal with benzyl bromide — base Ph-CH2Br + CH3CH2CHO > Ph-CH2-CH2(CH3)CHO Will the propanal undergo an aldol reaction instead? Well.... This will probably depend on the conditions of the reaction. If the propanal is reacted with LDA and quickly converted (almost) completely to enolate anion, it will not be able to undergo an aldol reaction. The enolate can then be added to benzyl bromide to form 2-methyl-3-phenylpropanal. On the other hand, if a small amount of KOH is added to a mixture of propanal and benzyl bromide, a small equilibrium concentration of propanal enolate would be formed, which could react with either propanal or benzyl bromide, giving both products. [To complicate this situation even further, the OH- could react with the benzyl bromide giving benzyl alcohol.] 5 Dehydration of Aldols — Aldols are alcohols and alcohols can undergo dehydration under acidic conditions, eg warming t-butyl alcohol, (CH3)3COH, with acid gives isobutylene, CH2=C(CH3)2. Aldols are enolized in acid and easily dehydrate to give the conjugated enone — H O C C H H + C H C H C H O H3O H H O H H C H + H3O H H H O + C H C H 6 H H Ordinary alcohols are not dehydrated under basic conditions (E2) owing to OH- being a poor leaving group. However, aldols do undergo dehydration under basic conditions via an enolate anion — O H C C H H H OH O - H C C H H O H C C H + H Consequently an aldol may spontaneously dehydrate to a conjugated enone as it is formed, especially if reaction conditions are pushed, eg high temperature. Mixed or "crossed" Aldol Reactions — If two different carbonyl compounds are allowed to react in an aldol reaction four products usually result; each carbonyl compound forms an enolate and each enolate forms two aldols, one with the carbonyl compound from which it was formed and one with the other carbonyl compound. 7 However — if one component does not have any -hydrogens (eg formaldehyde, benzaldehyde), and has an electrophilic carbonyl group, a good yield of one cross-product may be obtained. The carbonyl compound having the hydrogen(s) is slowly added to a basic solution of the one which does not. O O O H H3C H H H When the aldol product can form an enone in which the C-C double bond is further conjugated, dehydration is usually spontaneous. O NaOEt EtOH H3C NaOEt EtOH + OH H3 C + NaOH Chem 221 Experiment, Preparation of Dibenzalacetone— O C + H OH C H O NaOH H2O + C H OH C C H H 8 C H if one component is especially acidic it will "completely" convert to enolate and this enolate will make a nucleophilic attack on the other compontent. O EtO C O O CH2 C OEt NaOEt EtOH EtO C O CH C OEt Na O EtO C O CH C OEt O O NaOEt EtOH + EtO C O C C OEt Na Unsaturated aldehydes and ketones can be reduced to give saturated aldehydes and ketones with Pt/H2. They can also be reduced to saturated alcohols with LAH or unsaturated alcohols with NaBH4. 9 Reactions Related to the Aldol Condensation These two examples are mixed or crossed reactions of the aldol type in which one component does not have αhydrogens. Perkin Condensation — RCH2CO Na Ar C O + (RCH2C)2O H O Ar CH CH C O C OH R O RCH2CO Na O CH2R Ar CH C C O C O Ar CH C C O C R O O R O CH2R H2O CH2R O Ar CH C C OH + HO C R O O CH2R O Knoevenagel Reaction — This is a reaction between aldehydes or ketones not containing an a-hydrogen and a compound of the type Z-CH2-Z’ or Z-CHR-Z’, where Z and Z’ are electron withdrawing. Ar C O + H2C(COOC2H5)2 2o amine Ar C H H 10 C(COOC2H5)2 R C L + R CH2 Z :B R O C CHZ + :L + HB O R C Z= CN NO2 CF3 O Claisen Condensation: synthesis of -ketoesters — 2 RCH2 C OEt + NaOEt EtOH RCH2 C Na C C OEt + 2 C2H5OH O R O O 11 Mechanism — RCH2 C OEt + NaOEt Na CH C OEt + HOEt EtOH O R Na C OEt + CH C OEt EtOH RCH2 O EtOH RCH2 R C O RCH2 O Na C CH C OEt OEt R CH C OEt + NaOEt O R O O EtOH O Na C C OEt + C2H5OH It is only in this last step --RCH2 C the formation of the -ketoester O R O salt --- that the equilibrium is favorable. This step pulls the overall reaction. Consequently esters which do not have 2 protons do not undergo this reaction. Dieckmann Condensation — This is just an intramolecular Claisen condensation. O EtO C (CH2)4 O C OEt NaOEt H3 O + O C OEt O This reaction is good for making compounds of 5, 6, or 7 membered rings and rings of more than 12 carbons. 12 Mixed or Crossed Claisen Condensations — Generally useful only when one of the reactants has no hydrogens (cf. mixed aldol condensations). O O EtO EtO C CH2 C + OEt O O C CH C OEt OEt NaOEt This is phenylmalonic ester. It cannot be made by reacting sodiomalonic ester with an aryl halide + HOEt 13 The Michael Addition Reaction — nucleophilic addition of (typically stable) enolate anions to -unsaturated carbonyl compounds. CH CH C OEt + NaOEt EtOH O CH CH C OEt + O CH CH C OEt CH CH C OEt O O CH CH2 C OEt + + EtOH EtO O Another example — H H CH3 C C H COOEt + NaOEt EtOH CH3 H C C H COOEt 14