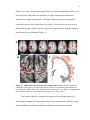
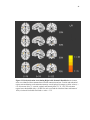
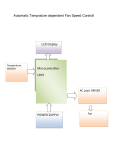
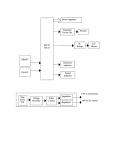
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cortical Involvement During Sustained Lower Limb Contractions
Microneurography wikipedia , lookup
Emotional lateralization wikipedia , lookup
Cognitive flexibility wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Causes of transsexuality wikipedia , lookup
Sex differences in intelligence wikipedia , lookup
Environmental enrichment wikipedia , lookup
Human multitasking wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Electromyography wikipedia , lookup
Premovement neuronal activity wikipedia , lookup
Neuroplasticity wikipedia , lookup
Mind-wandering wikipedia , lookup
Cognitive neuroscience wikipedia , lookup
Neurophilosophy wikipedia , lookup
Sex differences in cognition wikipedia , lookup
Muscle memory wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Embodied language processing wikipedia , lookup
Marquette University e-Publications@Marquette Dissertations (2009 -) Dissertations, Theses, and Professional Projects Cortical Involvement During Sustained Lower Limb Contractions Marnie Lynn Vanden Noven Marquette University Recommended Citation Vanden Noven, Marnie Lynn, "Cortical Involvement During Sustained Lower Limb Contractions" (2014). Dissertations (2009 -). Paper 363. http://epublications.marquette.edu/dissertations_mu/363 CORTICAL INVOLVEMENT DURING SUSTAINED LOWER LIMB CONTRACTIONS By Marnie Lynn Vanden Noven A Dissertation submitted to the Faculty of the Graduate School, Marquette University, in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy Milwaukee, Wisconsin May 2014 ABSTRACT CORTICAL INVOLVEMENT DURING SUSTAINED LOWER LIMB CONTRACTIONS Marnie Lynn Vanden Noven Marquette University, 2014 Despite the critical role of the lower limb during functional tasks such as walking, most studies examining the role of the cortex during muscle contractions have been conducted in upper limb muscles. Modulation of force by the cortex in the lower extremity and the influence of cortical inputs are poorly understood. The purpose of this dissertation was to investigate the role the cortex plays in modulating force control during static contractions with the lower limb and to determine the influence of manipulating cortical inputs. Aim 1 determined the cortical regions involved in force-related changes between low and high forces and those areas that modulate steadiness (force fluctuations) during sustained isometric ankle dorsiflexion contractions in young men and women. This was achieved using functional magnetic imaging (fMRI). Both motor and some typically associated non-motor brain areas were active during lower limb force production and scaled linearly as force increased. Steadiness was associated with both motor and nonmotor brain areas with minimal differences in areas activated between men and women. Aim 2 examined the influence of cognitive demand (null, low-cognitive demand, highcognitive demand) on fatigability and steadiness of low- to moderate-force isometric contractions in young and older men and women. Women demonstrated greater force fluctuations than men during both the low- and moderate-force contractions and their motor output was influenced by changes in cognitive demand. Older adults were less steady than young during low- and moderate-force contractions, had greater age-related reductions in steadiness, and greater variability in fatigability when cognitive demand was increased. This dissertation shows that cortical inputs are very important to lower limb motor control of static voluntary contractions. Cortical motor and non-motor regions that are important for control of force intensity and steadiness of lower limb contractions were identified and are key areas for potential cortical plasticity with impaired or enhanced leg function. Steadiness was altered by increasing cortical inputs (cognitive demand) especially in older adults whose motor performance was impaired and more variable than young. These results have important performance implications for cognitively demanding and low- to moderate-force tasks that are common to daily function in older adults. i ACKNOWLEDGMENTS Marnie Lynn Vanden Noven First and foremost, I would like to thank Dr. Sandra Hunter. I am honored to have had you not only as my mentor, but as my friend. You have taught me so much over the course of this journey, most of it outside of the lab. Thank you for your time, energy, unwavering support, generosity, kindness and the not-so-occasional box of tissues. Thank you also to your family, Jeff and Kennedy Rainwater. Thank you for welcoming me into your home and allowing me to break bread, sleep under your roof, and study at your dining room table. All three of you went above and beyond to help me make this happen. It wouldn’t have happened without you. Thank you. Thank you also to my committee – Dr. Lawrence Pan, Dr. Kristy Nielson, Dr. Alex Ng and Dr. Richard Marklin. I am so thankful that you were willing to work with me throughout this process. I appreciate your wisdom, support and the time you took out of your already busy schedules to make this happen. I enjoyed working with you. Thank you to my friends and colleagues at Marquette University. There are too many of you to name you individually because you are such a fantastic, supportive group! Thank you for all of your smiles, supportive words and enthusiasm. I miss seeing you in the halls and on campus, sharing stories, and your hugs! I am blessed to have had the opportunity to meet you, work with you and call you my friends. Thank you also to all of the Graduate and Undergraduate students that helped with experiments, data collection, data analysis, posters, and the list goes on. I am honored to have worked with ii you! Thank you to Craig Pierce and the staff at the Graduate School. Thank you to Hugo Pereira and Tejin Yoon for your patience with me and your help with data analysis. A special thanks to Dr. Manda Keller-Ross for paving the way and mentoring me through the hardest part! Thank you to everyone at the Medical College of Wisconsin and Belmont University for your support in making this PhD a reality. Thank you also to my PhD sisters Celeste Harvey, Dora Jones, and Felisa Parris. Chance brought us together, but friendship and a common goal kept us together! Thank you for pushing me when I needed it, picking me up when I was down, and reminding me to keep my eye on the prize! You are a blessing! We will all cross that stage! Thank you to NIOSH for grant funding to support study 2 and 3. Thank you also the College of Health Sciences for your support to complete the functional imaging at the Medical College of Wisconsin. Finally, and most important of all, I would like to thank my husband Steve Vanden Noven. Many years ago, I told him about my dream of getting my PhD. He has supported me every step of the way and has been my biggest champion. Thank you for believing in me and knowing that we would get this done together. Thank you for all of the times you left work early, got to work late, and weekends you steered the ship. I am blessed to have you as mine. Thank you to Steven and Sophia Vanden Noven for their patience (most of the time) and understanding how important this PhD is to me. I am proud of you and can’t wait to play! Thank you to my parents, George and Stella Brown, for teaching me the importance of an education and working hard for something you want. I love you all. iii TABLE OF CONTENTS ACKNOWLEDGMENTS…………………………………………………………...........i LIST OF TABLES.............................................................................................................iv LIST OF FIGURES............................................................................................................v LIST OF ABBREVIATIONS...........................................................................................vii CHAPTER I. INTRODUCTION......................................................................................1 II. BRAIN AREAS ASSOCIATED WITH FORCE STEADINESS AND INTENSITY DURING ISOMETRIC ANKLE DORSIFLEXION IN MEN AND WOMEN..........................................................................................27 III. WOMEN ARE LESS STEADY THAN MEN DURING LOW-FORCE ISOMETRIC CONTRACTIONS OF THE LOWER EXTREMITY............................................................................................57 IV. MOTOR VARIABILITY DURING SUSTAINED CONTRACTIONS INCREASES WITH COGNITIVE DEMAND IN OLDER ADULTS....................................................................................................87 V. DISCUSSION..........................................................................................119 BIBLIOGRAPHY............................................................................................................126 iv LIST OF TABLES CHAPTER I. INTRODUCTION Table 1.1. Cortical regions of interest...................................................................10 CHAPTER II. BRAIN ACTIVATION DURING LOWER LIMB CONTRACTIONS Table 2.1. Brain activation areas...........................................................................40 Table 2.2. Brain Areas with Increased Activation Volume...................................45 Table 2.3. Torque fluctuations (SD) and PSC: Correlations................................48 Table 2.4. Torque fluctuations (CV) and PSC: Correlations................................48 CHAPTER III. SEX DIFFERENCES IN LOWER LIMB WITH COGNITIVE DEMAND Table 3.1. Participant Physical Characteristics and Results.................................63 CHAPTER IV. MOTOR VARIABILITY WITH AGING Table 4.1. Participant Physical Characteristics and Results.................................94 v LIST OF FIGURES CHAPTER I. INTRODUCTION Figure 1.1. Somatotopic Representation in the Primary Motor Cortex..................4 Figure 1.2. Standard Deviation and Coefficient of Variation of Force................14 Figure1.3. Characteristics of a Force Task Performed to Failure..........................18 CHAPTER II. BRAIN ACTIVATION DURING LOWER LIMB CONTRACTIONS Figure 2.1. Experimental Protocol.........................................................................33 Figure 2.2. Torque and Steadiness........................................................................38 Figure 2.3. Brain Activation Areas during Right Ankle Isometric Dorsiflexion.41 Figure 2.4. Percent Signal Change in Regions of Interest.....................................44 CHAPTER III. SEX DIFFERENCES IN LOWER LIMB WITH COGNITIVE DEMAND Figure 3.1. Experimental Protocol.........................................................................64 Figure 3.2. State STAI scores................................................................................75 Figure 3.3. Visual Analogue Scale (VAS) Scores for Anxiety and Stress...........76 Figure 3.4. Mean Session Coefficient of Variation (CV) of Torque during the 5% MVC Task..............................................................................................................77 Figure 3.5. Time to Task Failure during the 30% MVC Task..............................79 Figure 3.6. Coefficient of Variation (CV) of Force during the Fatiguing Contraction.............................................................................................................80 CHAPTER IV. MOTOR VARIABILITY WITH AGING Figure 4.1. Visual Analogue Scale for Anxiety (A) and Stress (B).......................97 Figure 4.2. Mean Session Coefficient of Variation of Torque..............................99 Figure 4.3. Individual Times to Task Failure during the Fatiguing Contraction.102 vi LIST OF FIGURES, continued Figure 4.4. Coefficient of Variation of Force during Fatiguing Contraction.......104 Figure 4.5. Mean Arterial Pressure, Heart Rate and Rate Pressure Product.......107 vii LIST OF ABBREVIATIONS AFNI = Analysis of Functional NeuroImages ANOVA = Analysis of Variance BOLD = Blood-Oxygen-Level Dependent C = Coronal CG = Cingulate Gyrus CV = Coefficient of Variation DBP = Diastolic Blood Pressure EMG = Electromyography fMRI = Functional Magnetic Resonance Imaging FWHM = Full Width at Half Maximum GPe = Globus Pallidus external GPi = Globus Pallidus internal High-CD = High-Cognitive Demand I = Inferior L = Left Low-CD = Low-Cognitive Demand M1 = Primary Motor Area MAP = Mean Arterial Pressure MVC = Maximal Voluntary Contraction PAQ = Physical Activity Questionnaire PFC = Prefrontal Cortex viii PSC = Percent Signal Change R = Right RIFG = Right Inferior Frontal Gyrus RMS = Root Mean Square ROI = Region of Interest RPP = Rate Pressure Product RPE = Rating of Perceived Exertion Su = Superior S = Sagittal TE = Echo Time TR = Repetition Time T-T = Talaraich and Tournoux SBP = Systolic Blood Pressure SD = Standard Deviation SEM = Standard Error Measurement SMA = Supplemental Motor Area STAI = State Trait Anxiety Inventory VAS = Visual Analog Scale Z = Axial 1 INTRODUCTION Although steady, controlled movements in the lower extremity are imperative when performing a basic functional activity such as walking, the role of the cortex in producing and controlling movements in the lower extremity is poorly understood. Because most studies investigating the role of the cortex in movement production have been performed in the upper extremity (Dai et al., 2001; eg., Thickbroom et al., 1998; Vaillancourt et al., 2004; van Duinen et al., 2008), the purpose of this dissertation was to determine the influence of cortical input on force control and fatigability during static contractions in the lower extremity. When performing a task under more challenging conditions, such as walking uphill or on an uneven surface, which requires increased force production and greater steadiness to avoid falling, the cortex must modulate force to accommodate the increased force requirements of the task. Lower limb control during static contractions differs from that of the upper limb (eg., Jesunathadas et al., 2012) because lower limb muscles usually have a larger muscle mass and motor unit ratio (Feinstein et al., 1955), and fewer direct corticospinal connections (Brouwer & Ashby, 1990) than upper limb muscles. Thus, aim 1 (study 1) in this dissertation determined the cortical regions involved in forcerelated changes between low and high forces, and modulations of steadiness (force fluctuations) using fMRI during sustained isometric ankle dorsiflexion contractions in young men and women. During a sustained target force contraction, force fluctuations increase as the muscle becomes more fatigued (Hunter, Duchateau, et al., 2004). In an effort to sustain 2 the target force, descending cortical drive will increase (Fuglevand, A. J. et al., 1993). While descending inputs from cortical centers to the motoneurone pool play a critical role in modulation of steadiness and fatigability (Dideriksen et al., 2012; Negro et al., 2009), many of these studies have implicated the role of descending drive with modeling because of the difficulty in studying the intact central nervous system in humans. Recent studies show that in young adults, increased cognitive demand will increase fatigability (decrease time to failure of a sustained contraction) and decrease steadiness (increase force fluctuations) in the arm muscles (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009); the response to increased cognitive demand appears to be exacerbated in older adults (Christou et al., 2004; Marmon, Pascoe, et al., 2011). Aim 2 exposed the role and importance of the cortical centers during lower limb static contractions through two studies. The first study of aim 2, varied the level of cognitive demand and determined the change in motor output (steadiness and fatigability) in young men and women (study 2). In the second study of aim 2, we determined if age-related changes in cortical centers in older men and women would further disrupt motor output during static contractions of the lower extremity muscles (study 3). Thus, aim 2 examined the influence of cognitive demand (null, low-cognitive demand, high-cognitive demand) on fatigability and steadiness of low- to moderate-force isometric contractions in young and older men and women. 3 CHAPTER 1 BACKGROUND Cortical control of voluntary motor tasks with human muscles has been widely studied and yet is still not well understood. This is partly due to the limitations with accessing the central nervous system in awake humans during voluntary movement. In 1968, Evarts published a series of articles showing that the cerebral cortex of macaque monkeys, specifically the pyramidal tract neurons that originate in the motor cortex, were directly involved in wrist flexion and extension force production and direction of displacement. Interestingly, however, despite their role in force production, the pyramidal tract neurons were not responsible for sensing the amount of force needed to perform the movement, indicating that this must occur somewhere else in the brain (Evarts, 1968). Although many of the studies examining motor control have been performed using the upper limb muscles, the exact role of the motor cortex in force/torque production, limb position, velocity and direction of the upper limb still remain unclear (Hatsopoulos, 2005; Sergio et al., 2005). Movement of a limb is very complex and vulnerable to feedback from multiple systems. Voluntary contractions are initiated in cortical centers upstream of motor centers; however, descending drive to the final common pathway (the motor unit) (Sherrington, 1925) is modulated by inputs from other cortical centers, afferent inputs, reflexes, the excitatory and inhibitory spinal circuitry, and the characteristics of the motor unit itself (Enoka & Pearson, 2012). The motoneurone originates in the spinal cord 4 (Squire et al., 2013) such that some inputs from corticospinal connections are direct to the motor unit pools while others are modulated via upper motorneurones and input to interneurons via upper motoneurones. The upper limb has more direct corticospinal connections than the lower limb (Brouwer & Ashby, 1990), therefore the area active in the brain during upper limb activity has a larger representation and is probably influenced more directly by cortical inputs (Figure 1.1). A B C Figure 1.1. Somatotopic representation in the primary motor cortex. Panel (A) illustrates somatotopic order for feet, fist, and lip movements, and (B) is a somatotopic representation for lip, index finger, thumb, elbow, foot, and left fist movement (Lotze et al., 2000). (C) Organization of the primary motor cortex in the homunculus (Squire et al., 2013, p. 607). Fine control of discrete voluntary movements, such as reaching, requires all information descending from supraspinal centers to be integrated at the spinal level with afferent feedback to control and voluntarily modify the task towards successful 5 completion (Drew et al., 2004). Current techniques utilized to record and measure neural activity during discrete motor tasks provide valuable information regarding temporal activity [e.g., electromyography (EMG)] as well as spatial activity (e.g., fMRI) of motoneurones; however, very little in known about cortical contributions to lower extremity movements. Thus, the first study of this dissertation investigates force-related changes in cortical activity across a range of force intensities during sustained isometric ankle dorsiflexion contractions in young men and women. To perform a basic motor task, such as locomotion in the lower limb, the human body employs the use of specific neural networks that are responsible for executing motor programs. These neural networks communicate via groups of interneurons (central pattern generators) with motoneurones to activate the desired movement through activation of agonistic and synergistic muscle groups and inhibition of antagonistic muscle groups (Squire et al., 2013). These specific motor programs are generally located in the spinal cord and brain stem and are capable of producing motor patterns like gait without input from the cortex as exhibited by decerebrate cat preparations (Van de Crommert et al., 1998; Whelan, 1996). Although sensory input from the environment enables modification of motor program patterns (Whelan, 1996), coordination of more complex, goal-directed movements, requires input from other regions of the central nervous system including the sensorimotor cortex and basal ganglia (Burke et al., 2001; Grillner et al., 2008; Heckman et al., 2009). When attempting to sustain a steady contraction, the exerted muscle force tends to fluctuate about the target force (eg., Galganski et al., 1993). The amplitude of force fluctuations (steadiness) can vary between men and women, and the magnitude of the 6 difference will depend on the conditions of the task. For example, women were less steady than men when performing elbow flexion contractions in multiple forearm positions across several force levels for a target-matching task especially at very low forces (Brown et al., 2010); yet, when performing a position task, force task at a fixed 1Newton (N), and precision grip task at a fixed 2N using the first dorsal interosseus, women were more steady than men (Endo & Kawahara, 2011). In a study that compared sex differences in hip extension and hip flexion at very low-to-moderate forces, women were less steady than men at the very low forces during hip extension, but there was no difference during hip flexion (Grunte et al., 2009). Attempting to sustain a task in the presence of increased cognitive demand may influence descending drive from the central nervous system and impair motor output of the upper extremity muscles (Christou et al., 2004; Noteboom, Fleshner, et al., 2001; Yoon et al., 2009). Motor output can also be altered by the age-related changes within the neuromuscular system. As individuals age, there is a loss of motoneurones, which increases the innervation ratio of surviving motor units and the density of muscle fibers innervated by a single motor unit (Drey et al., 2013; Kaya et al., 2013). As a result, steadiness is reduced in the aging adult compared with younger adults when performing sustained isometric contractions at low and moderate force intensities (Galganski et al., 1993). With the loss of motoneurones, older adults also experience a preferential loss of fast-twitch muscle fibers, increasing resistance to muscle fatigability. When compared with young adults, older adults had a longer time to task failure than young adults during static contractions with the ankle dorsiflexor muscles (Griffith et al., 2010). Further, 7 older adults demonstrated a greater increase in force fluctuations than young adults in anticipation of a noxious stressor for a precision grip task (Christou et al., 2004); however the influence of increased arousal (cognitive demand) during performance of a lower limb task is unknown. Section 1: Cortical Contributions to Voluntary Contractions Functional magnetic resonance imaging (fMRI) is a neuroimaging procedure that measures brain activity by detecting changes in magnetization between oxygen-rich and oxygen-poor blood in the brain that is the result of increases or decreases in neural activity as areas of the brain becomes more or less active, respectively. Areas with increased neural activity generate a blood-oxygen-level dependent (BOLD) contrast that can present associated brain activation graphically in the form of a color-coded map that indicates strength of activation across the brain or a particular region of interest (ROI). Changes in BOLD signal contrast are characterized by the intensity percent signal change (PSC, %) and volume of activation (µl) (Huettel et al., 2004). Early fMRI studies were able to confirm the somatotopic organization of the primary motor cortex by having participants perform voluntary movements of different joints; the maps created confirmed that the lower limb is represented medially, the upper limb is represented more laterally, and the hand occupies a large portion of the somatotopic map of the primary motor cortex (M1) due to the precise fine motor movements it performs (Lotze et al., 2000; Rao et al., 1995). fMRI studies have also established the relationship between changes in brain activation and force production. For example, activation in several motor areas of the cortex has been demonstrated 8 during submaximal and fatiguing handgrip tasks (Dai et al., 2001; Liu et al., 2003). Further, during sustained, graded contractions of the hand and first dorsal interosseus abduction, BOLD signal intensity in motor areas, such as the sensorimotor cortex, premotor areas and cerebellum, have also been shown to increase linearly with increased force production (Spraker et al., 2007; van Duinen et al., 2008). Most studies evaluating cortical function during force production use the upper limb; however, the relationship between cortical activation and force intensity in the lower limb has not been determined. Differences exist in cortical activation patterns and intensity between upper and lower limb movement (Luft et al., 2002; Miyai et al., 2001), in part because the lower limb has: 1) a different somatotopic location, 2) reduced fine motor control, 3) larger muscle mass to motor unit ratios (Feinstein et al., 1955), and 4) fewer direct corticospinal connections compared with upper limb muscles (Brouwer & Ashby, 1990). Movement in the lower limb has been shown to correlate with changes in BOLD signal intensity (cortical activation) in the primary motor cortex and sensory cortex (Huda et al., 2008; Orr et al., 2008). Active and passive ankle dorsiflexion and plantarflexion tasks also activated similar cortical regions (Ciccarelli et al., 2006; Dobkin et al., 2004), and graded dorsiflexion movements of the right ankle have produced graded BOLD signal changes in motor areas (MacIntosh et al., 2004); however, the relation between cortical activation and force intensity has not been systematically determined. The intensity, volume and location of cortical activation produced during force production will vary based on many factors, including the amplitude of movement, intensity of force exerted and complexity of the task required. For example, it has been 9 demonstrated that the basal ganglia plays a significant role in the control of force in hand muscles (Kinoshita et al., 2000; Spraker et al., 2007) and BOLD signal activity can vary substantially in the different nuclei of the basal ganglia according to the task selection and prediction, and amplitude and rate of force development (Prodoehl et al., 2009; Spraker et al., 2007). Further, anatomical regions associated with cortical activation during force production and control can be anticipated based on previous evidence. See Table 1.1 for examples of relevant anatomic regions of interest during static contractions in the lower limb across multiple force levels. 10 Table 1.1. Regions of interest. ROI for control of lower limb function during static contractions across forces. Cortical Region Function Primary Motor Production and control of voluntary movement; May specify muscle activation levels or Cortex (M1) could be transformed into muscle-specific signals in the spinal cord (Kalaska, 2009) May also adapt to the aging neuromuscular system to assist with production of muscle strength (Plow et al., 2013) Supplemental Contralateral M1 fibers converge with ipsilateral SMA (Luft et al., 2002) Motor Area Helps with assembly of central motor programs, sequences simple movements and (SMA) execution of a motor sequence test (Roland et al., 1980). Basal Ganglia Does not initiate movement as previously thought, but: Contributes to automatic execution of movement sequences, adjust magnitude of globus pallidus internal (GPi) inhibitory output to increase or decrease movement, and permits desired movements and inhibit unwanted competing movements (Squire et al., 2013) Assists with task selection, prediction, and amplitude and rate of force development (Prodoehl et al., 2009) Portions scale in activation intensity with increasing force amplitude with upper limb (Grafton & Tunik, 2011; Spraker et al., 2007; Vaillancourt et al., 2004; Vaillancourt et al., 2007) Prepares motor readiness state in response to danger, transforms emotional responses to behavioral responses (Butler et al., 2007) May play a role in subcortical fight or flight programs (Marchand et al., 2009). Cerebellum Adapts goal-directed arm movements (Tseng et al., 2007), coordinated movement, motor learning, reflex adaptation (Glickstein, 2007), sensorimotor control (Manni & Petrosini, 2004; Stoodley & Schmahmann, 2009; Stoodley & Schmahmann, 2010), and has been shown to scale with force in the hand (Keisker et al., 2009; Kuhtz-Buschbeck et al., 2008) Control of agonist-antagonist activity and inhibiting co-contraction of antagonist muscles (Mari et al., 2014) May influence cognitive and emotional function (O'Reilly et al., 2010; Stoodley & Schmahmann, 2010). Visual Cortex Classifies and interprets environment stimuli enabling appropriate responses to external visual stimuli (Squire et al., 2013) Lingual gyrus may assist target recognition (Gron et al., 2000) Calcarine gyrus (V1) may assist with the central visual field and spatial attention (Martínez et al., 1999) Ipsilateral parietal lobule may assist with oculomotor and attention processes and contribute to appropriate motor output in response to visual sensory input (Clower et al., 2001) Prefrontal Cortex Synthesizes sensory information from internal (affect, memory, reward) and external (PFC) (sensory, cortical and subcortical motor systems) environment to produce goal-directed movement; Assists with planning, decision-making, behavioral control, emotions, moods, and working memory; Communicates with M1 through premotor area and basal ganglia (Squire et al., 2013). Critical in executive function and top-down control of behavior (Miller & Cohen, 2001) Anterior Cingulate Associated with autonomic responses (greater in men than women) (Wong et al., 2007) Cortex (ACC) Conflict and error monitoring and detection, response selection, attention control, as well as emotions, mood, action, anticipation, working memory, visuospatial orientation, and navigation of body in space (Torta & Cauda, 2011) Right Inferior Visual perception (Ishai et al., 1999) Temporal Gyrus Pattern recognition (Herath et al., 2001) Modulation of neural responses that have become motivationally significant (Mesulam, 1998) Left Superior Key component in working memory and involved in coordinating responses to increased Frontal Gyrus executive demand in working memory (Boisgueheneuc et al., 2006) 11 Sex Differences in Cortical Activation during Voluntary Contractions Sex differences in cortical activity have also been shown during performance of cognitive tasks. For example, men demonstrated asymmetric prefrontal activity in response to a difficult mental-math task, whereas women did not demonstrate correlated activity in prefrontal cortex, but instead demonstrated increased activation in limbic regions (Wang et al., 2007). Further, women have demonstrated greater amounts of BOLD signal activity in the ipsilateral frontal and parietal lobes than men during visually guided reaching tasks that required arm and eye movements in the same direction (Gorbet & Sergio, 2007) with greater bilateral distribution (Gorbet et al., 2010); as well as greater frontoparietal activity during a visuospatial navigation task (Gron et al., 2000) than men. However, sex differences in brain activation patterns during lower-extremity motor tasks are unknown. Various indirect measures of motor unit activity (EMG) and assessments of voluntary activation (e.g. techniques of stimulation along the neuraxis to determine neural drive to the muscle and to the motor cortex), indicate no sex difference in activation output from the motor cortex (Hunter et al., 2006; Hunter & Enoka, 2001; Keller et al., 2011). Cortical Contributions to Changes in Motor Output In order to execute a voluntary goal-directed motor task, the cerebral cortex communicates with a desired muscle via the corticospinal tract. The corticospinal neurons originate in the primary motor cortex (M1) and project their axons through the midbrain and pons, decussate in the medulla to the opposite side of the spinal cord. The majority of these neurons terminates in the dorsolateral ventral horn of the spinal cord 12 and communicates with interneurons or motoneurones (decussation of the corticospinal axons will result in activation of the left motor cortex during performance of a right side task and vice versa) (Squire et al., 2013). Motoneurones synapse on multiple muscle fibers via neuromuscular junctions that convert the descending neural input into force output forming a motor unit (a single motoneurone and all of the muscle fibers it innervates (Sherrington, 1925). A single motoneurone will synapse with multiple muscle fibers and a single muscle will be innervated by multiple motor units. Force generation occurs via two strategies: recruitment of motor units (predominantly at lower forces) and rate coding or optimal discharge frequency of the motor unit (predominantly at higher forces) (De Luca et al., 1982a; Monster & Chan, 1977; Van Cutsem et al., 1997). However, for the tibialis anterior muscle, motor unit recruitment is of greater significance across the full range of contraction force intensity because of its large recruitment range (up to ~90% MVC) (Van Cutsem et al., 1997) compared with intrinsic muscles of the hand such as the first dorsal interosseus which has a lower recruitment range to ~50% MVC (De Luca et al., 1982a). Thus, because the motor unit communicates with the motor cortex, factors that influence motor cortex output (descending drive), motor unit recruitment, or motor unit discharge rates will directly influence the motor output of that muscle. Section II: Contributions to Motor Output in the Lower Limb Force Fluctuations during Voluntary Contractions Sustained isometric contractions are important for a wide variety of functional, vocational, and sporting tasks, particularly at submaximal levels (Grabiner & Enoka, 13 1995). In the upper limb, sustained submaximal contractions may be used to grip a pen or hold a glass, or to perform a complex surgical procedure; sustained contractions in the lower extremity may include accelerating or decelerating a vehicle, or walking over various surfaces. Variability of force about an average value can be quantified in absolute terms as the standard deviation (SD) or in relative terms as the coefficient of variation (CV) (Enoka et al., 2003). The SD of force fluctuations characterizes the magnitude of fluctuations in the system output and serves as an index of the average deviation of scores in the distribution from the distribution mean; while the CV indicates the scatter of responses relative to the mean response amplitude and illustrates the way the system output changes over time independent of the magnitude of response (Enoka et al., 2003). Typically, the standard deviation of force increases linearly with increased force production (Figure 1.2) and is usually lowest at the lowest forces (Enoka et al., 2003; Moritz et al., 2005; Taylor, A. M. et al., 2003; Tracy, 2007a). The variation in force (CV) controls for force and is greatest at lower force levels, decreasing as contraction intensity increases (Figure 1.2) (Galganski et al., 1993; Slifkin & Newell, 1999). 14 Figure 1.2. Standard Deviation and Coefficient of Variation of Force. Standard deviation (A) and coefficient of variation (CV; B) for force during constant-force isometric trials performed at 2, 5, 10, and 50% MVC in young and old subjects. Values are means ± SE. *p < 0.05 between age groups. From Tracy and Enoka (2002). At low-to-moderate forces, force fluctuations appear to be the result of primarily two factors that influence the output of motoneurones: synaptic noise and common drive to the motoneurones (De Luca et al., 2009; Dideriksen et al., 2012; Hamilton et al., 2004). In order to sustain a low target force during an isometric contraction, motor units are recruited and the discharge rates modulated in unison through a centrally mediated common drive (De Luca et al., 2009; De Luca et al., 1982b). This modulation of motor 15 unit discharge is characterized as a low-frequency oscillation of 2-3 Hz and is thought to be a major contributor to the force fluctuations during isometric contractions at low to high forces (Mottram et al., 2005; Negro et al., 2009). At very low forces the variability in discharge rate is thought to be the primary contributor to force fluctuations (Jesunathadas et al., 2012; Negro et al., 2009). Relative to muscles in the upper limb (FDI, elbow flexors), the tibialis anterior has fewer synaptic inputs onto motoneurones (Brouwer & Ashby, 1990) which decreases synaptic noise in the system; thus, at low forces, motor unit discharge rate variability probably has its greatest influence on the CV in upper limb muscles and lesser effects in the tibialis anterior (Jesunathadas et al., 2012). Further, at very low force levels (< 5% MVC), descending input from the visual cortex has also been demonstrated to influence the steadiness of sustained voluntary contractions in the lower extremity (Tracy, 2007b). As force increases to moderate and higher levels, the number of recruited motor units decreases (Fuglevand, A. J. et al., 1993) and amplitude of force fluctuations decreases (Moritz et al., 2005). As a result, force fluctuations at higher force levels are related to variability in descending drive from the central nervous system, not synaptic noise or intrinsic motoneurone properties (Dideriksen et al., 2012). Sex Differences in Motor Output The ability to sustain steady low and moderate force contractions can be influenced by a variety of factors: age (Galganski et al., 1993; Laidlaw et al., 2000), fatigue (De Luca et al., 1982b; Singh et al., 2010), arousal (Lorist et al., 2002; Noteboom, Barnholt, et al., 2001), intensity and type of contraction (Cresswell & Loscher, 2000; Griffith et al., 2010; Semmler et al., 2007), and muscle group (Hunter, Yoon, et al., 2008; 16 Jesunathadas et al., 2012; Tracy, Mehoudar, et al., 2007). It is unclear whether sex differences contribute to decreased steadiness because in many studies, sex differences are not assessed. When performing submaximal isometric contractions, women have been reportedly more (Endo & Kawahara, 2011), less (Brown et al., 2010; Brown et al., 2009; Grunte et al., 2009), or similarly (Baweja et al., 2009) steady when compared with men. For example, women demonstrated greater overall force fluctuations than men across multiple force levels (2.5, 5, 10, 25, 50 and 75% of maximal voluntary contraction (MVC) force (Brown et al., 2010). In a target-matching task across similar target forces (2.5, 5, 10, 30, 50 and 80% of MVC force), Tracy (2007a) found differences in force fluctuations between ankle muscle groups (ankle dorsiflexion and plantarflexion), but there were no sex differences between young or older adults. Given the lack of mention in the literature and disparity of results, it remains unclear if women are less steady than men when performing submaximal isometric contractions. Mechanisms related to sex differences in steadiness are equally unclear. Women have been reported to have lower motor unit discharge rates and higher discharge rate variability than men during isometric elbow flexion contractions (Brown et al., 2009). Both of these factors would result in a significant increase in force fluctuations when compared with men (Moritz et al., 2005); however, there is very little additional evidence to support this finding in the literature. Age Differences in Motor Output Greater force fluctuations with advanced age have also been observed across various muscle groups and particularly at the lower intensity contractions (Enoka et al., 2003; Tracy, Dinenno, et al., 2007; Tracy et al., 2005). With advanced age, the 17 motoneurone pool undergoes remodeling that results in decreased motor units numbers and altered relations between discharge rates and recruitment thresholds (Barry et al., 2007); the age difference in force fluctuations therefore appears to be due to age-related changes in the inputs to the motoneurone pool (Barry et al., 2007) with possibly some influence of motor unit discharge rate variability in older adults (Barry et al., 2007; Kornatz et al., 2005; Laidlaw et al., 2000; Tracy et al., 2005). Age-related changes in visual-motor processing may also contribute to altered motoneuronal inputs causing increased force fluctuations during static contractions with age (Fox et al., 2013; Henningsen et al., 1997; Seidler-Dobrin & Stelmach, 1998; Tracy, Dinenno, et al., 2007). Influence of Muscle Fatigue on Motor Output As an isometric contraction is sustained, motor output becomes progressively more variable, the muscle begins to fatigue, and descending cortical drive increases in an effort to sustain the required force (Missenard et al., 2009; Riley et al., 2008). Muscle fatigue is defined as an exercise-induced reduction in muscle force or power caused by impairments within the neuromuscular system that is reversible with rest (Enoka & Duchateau, 2008; Fitts, 1994). It begins at the onset of exercise, progresses during exercise and starts to recover once exercise stops. The magnitude of muscle fatigue caused by exercise, such as a target-matching task, can be quantified as the decline in the maximal force or power measured immediately after the fatiguing contraction (Hunter, Critchlow, Shin, et al., 2004; Taylor, J. L. et al., 1996). If the contraction is sustained it will eventually result in task failure (Gandevia, 2001). As fatigability in a muscle increases, electromyography (EMG) activity will also increase progressively throughout 18 the contraction as the already active fibers lose force and more motor units are recruited (Enoka & Duchateau, 2008; Hunter, Duchateau, et al., 2004) (Figure 1.3). Figure 1.3. Characteristics of a Force Task Performed to Failure. Representative EMG data for a force tasks as performed by one individual using the first dorsal interosseus muscle. The data comprise the EMG for the antagonist (second palmar interosseus), agonist (first dorsal interosseus) muscles, and the abduction force. EMG and force fluctuations increase as fatigability increases (Enoka & Duchateau, 2008). Mechanisms of muscle fatigue are task dependent and will vary based on sex, muscle group, task, and age (Enoka & Duchateau, 2008). For example, when participants performed a sustained isometric contraction of the elbow flexor muscles at 20% and 80% MVC to task failure, men demonstrated a shorter time to task failure than women during the 20% MVC task, but similar times to failure for the 80% MVC task (Yoon et al., 2007). When performing sustained isometric contractions with the elbow flexor muscles and ankle dorsiflexor muscles, participants demonstrated longer times to task failure when performing a force task compared to the position task, and there were no sex differences (Hunter et al., 2002; Hunter, Yoon, et al., 2008). Hunter, et al (2005) found that time to task failure was shorter for a position task compared to a force task for older adults when compared with young adults for elbow flexor muscles (Hunter et al., 2005). However, there was no difference in time to task failure between loads when similar tasks were performed in the lower extremity (Griffith et al., 2010). 19 Similar central mechanisms that contribute to increased force fluctuations will also contribute to muscle fatigue, including suboptimal activation of the motor cortex (Gandevia et al., 1996), decreased neural drive or excitatory input to the motoneurone (Taylor, J. L. & Gandevia, 2008), and decreased responsiveness in motoneurone activation (Herbert & Gandevia, 1999). As a muscle progressively fatigues, descending drive increases, as evidenced by increased EMG and ratings of perceived exertion (Riley et al., 2008), and progressively declines in voluntary activation (level of voluntary drive to the muscle) (Gandevia, 2001). Although various measures of motor unit activity (EMG) and assessments of voluntary activation (e.g. techniques of stimulation along the neuraxis to determine neural drive to the muscle and to the motor cortex), indicate no sex difference in motor cortex output activation (Hunter et al., 2006; Hunter & Enoka, 2001; Keller et al., 2011) women may be more sensitive to changes in common drive than men. This is plausible because women demonstrate differences in motor unit discharge rates and discharge rate variability compared with men at similar intensity contractions (Brown et al., 2009), however, this relationship requires has not been previously investigated. Influence of Cognitive Demand on Motor Output Imposition of increased cognitive demand can increase descending drive, particularly when cognitive demand in increased simultaneously with a sustained isometric contraction, a dual-task. The influence of dual tasks has been shown previously in the literature using multiple combinations of tasks. In young adults, for example, cognitive performance declines and force fluctuations increase when a cognitive task was imposed (reaction time) during sustained isometric tasks with hand muscles (Lorist et al., 2002; Zijdewind et al., 2006); however, force fluctuations were affected more during the 20 isometric fatiguing contractions than during a 5% submaximal non-fatiguing contraction (Lorist et al., 2002). Further, steadiness of the elbow flexor muscles declined (increased force fluctuations) and time to failure of a sustained 20% MVC submaximal task was reduced (increased fatigability) in young adults when simultaneously performing a demanding cognitive task that increased anxiety (counting backwards by 13) (KellerRoss, Pruse, et al., 2014; Yoon et al., 2009). Individuals who were weaker (primarily women) show the largest decrement in time to task failure when the stressful cognitive task was imposed during the fatiguing contraction (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). However, it is not known how changes in cognitive demand in the presence of various force levels would influence steadiness in men and women. Decrements in dual-task performance are also exacerbated with age (Springer et al., 2006). For example, when older and young adults performed an n-back task and a force-tracking task, older and young adults performed each task in isolation similarly; however, during simultaneous performance, older adults demonstrated significantly greater losses in force control than young (Voelcker-Rehage et al., 2006). Older adults are typically weaker than young adults, with older women being weaker than older men for upper and lower limb muscles (Galganski et al., 1993; Laidlaw et al., 2000; Tracy & Enoka, 2002), possibly increasing susceptibility to increased fatigability when a cognitive task is imposed. However, it is not known whether fatigability with increased cognitive demand is exacerbated with advanced age. Furthermore, the effects of increased cognitive demand on lower limb fatigability in young or older adults are not known. Given that fatigue and increased arousal increase descending drive from the central nervous system (Missenard et al., 2009; Noteboom, Fleshner, et al., 2001), potentially 21 further increasing force fluctuations, and that mechanisms that modulate steadiness occur in different areas of the central nervous system, we utilized both fatigue and increased cognitive demand to influence descending drive to determine differences in steadiness between men and women. Therefore, aim 2 of this dissertation examines the influence of cognitive demand on fatigability and steadiness of low- to moderate-force isometric contractions of the lower extremity in young and old men and women. Dual-Tasks Information from multiple areas of the cortex can influence motor output. For example, the primary motor cortex (M1, where production and control of voluntary movements occur) receives information from the cerebellum (which coordinates movement), while the supplemental motor area (responsible for postural stabilization, sequencing of events) will receive input from the basal ganglia (which regulates inhibitory output to regulate movement) (Squire et al., 2013). Further, input from the prefrontal cortex, which receives and synthesizes input from the major sensory systems, basal ganglia and limbic system, provides information to the motor cortex via the premotor cortex to assist with planning, decision-making, and executive function tasks (Squire et al., 2013; Takahara et al., 2012). Executive function (which includes volition, planning, purposive action, and action monitoring), anxiety, and stress are modulated in prefrontal cortical regions and the anterior cingulate cortex (Banich et al., 2009; Miller, 2000; Owen et al., 2005; Schweizer et al., 2013). Changes in performance for older adults in dual-tasks appear to be especially sensitive to cognitive tasks that require executive function (Yogev-Seligmann et al., 2008). Furthermore, increased monoaminergic drive and neuromodulatory inputs potentially increase motor unit 22 discharge rate variability and therefore force fluctuations at very low forces. If a cognitive demand task is perceived to be more of a stressor for the older adults than the young, motor output may become more variable and steadiness may decrease. Thus, there are many different pathways through which the neural connections from areas associated with cognition and anxiety, particularly the cingulate and prefrontal cortices, could directly alter motor function. The sensorimotor cortex provides information to the motor cortex based on stimuli it has interpreted from the environment. Bottom-up processing occurs if the sensory information is perceived to be novel or salient, thus requiring attention or a response. If, however, it is necessary to ignore irrelevant distractions by enhancing or inhibiting neural activity that will ensure successful task completion through premotor cortex modulation , top-down modulation has occurred (Gazzaley & D'Esposito, 2007; Gazzaley & Nobre, 2012). The effort of sustaining attention on a task is affected by fatigue and stress (McDowd, 2007) and can lead to diminished motor and cognitive performance, particularly when the motor and cognitive task are performed simultaneously, or as a dual-task. For example, when performing an auditory choice reaction task and fatiguing submaximal finger abduction simultaneously, increased motor fatigue resulted in a drastic deterioration in the choice reaction task performance and increased force variability compared to when the tasks were performed in isolation (Lorist et al., 2002). Capacity theories of attention assume that there are attentional resource limitations on the ability to perform multiple tasks simultaneously (Hiraga et al., 2009; Kahneman, 1973; McDowd, 2007) and that attempting to perform two tasks simultaneously will result in 23 diminished performance in either one or both of the tasks (Tombu & Jolicoeur, 2003). Thus, attentional limitations during performance of dual-tasks have been demonstrated in both young and older adults and the capacity theory of attention may provide an explanation for decrements in performance found in young and older adults under dualtask conditions. Changes in cognitive demand and stress (Christou et al., 2004; Yoon et al., 2009) have also resulted in increased motor output variability in the upper extremity; however, the influence of changes in cognitive demand and age on motor output to the lower extremity is not known. The central theme of this dissertation is to answer questions regarding the influence of cortical input on force output during isometric contractions of the lower limb. The purpose of aim 1 was to determine the force-related changes in cortical regions across a range of high and low force intensities in the BOLD signal (intensity and volume) during sustained isometric ankle dorsiflexion contractions in young men and women. Thus, study 1, aim 1 in this dissertation determined the cortical regions involved in force-related changes between low and high forces and those areas of the cortex that modulate steadiness (force fluctuations) using fMRI during sustained isometric ankle dorsiflexion contractions in young men and women. Aim 2 was to examine the influence cognitive demand on fatigability and steadiness of low- to moderate-force isometric contractions in young and old men and women. To address aim 2, two different studies were conducted. Study 2, aim 2 exposed the role and importance of the cortical centers during lower limb static contractions, this dissertation used varying levels of cognitive demand and determined the change in motor output (steadiness and fatigue) in young men and women. These experiments were also performed in older men and women 24 (study 3, aim 2) and compared with young adults to determine if age-related changes in cortical centers would further disrupt motor output during the static contraction with the leg muscles. 25 DISSERTATION AIMS AND HYPOTHESES Central Question: What is the influence of cortical input on force output during isometric contractions in the lower extremity? Central Hypothesis: Force output of the lower extremity muscles is controlled by cortical inputs that vary with the intensity of contraction, sex, age and cognitive demand. Specific Aims Aim 1: To determine the force-related changes in cortical regions across a range of high and low force intensities in the BOLD signal (intensity and volume) during sustained isometric ankle dorsiflexion contractions in young men and women. Sub aims: 1. To determine if intensity and volume of activation in the motor areas of the brain demonstrates a linear increase in activity as with increased force intensity. Hypothesis: Motor areas of the brain will scale linearly in intensity and volume with increased force intensity of the ankle dorsiflexor muscles. 2. To identify cortical areas that correlate with the modulation of force fluctuations across a range contraction intensities. Hypothesis: At lower intensities of contraction, larger fluctuations in force will be associated with greater activation of cortical motor areas. 3. To determine whether there were any sex differences that could be detected in brain activity and modulation of the low- and high-force isometric contractions 26 with the lower limb. Hypothesis: Men and women will demonstrate similar changes in brain activity during static contractions of the lower extremity. Aim 2: To examine the influence cognitive demand on fatigability and steadiness of low- to moderate-force isometric contractions in young and old men and women. Sub aims: 1. To compare both fatigability and amplitude of force fluctuations for a low- to moderate-force isometric contractions in the presence and absence of varying levels of cognitive demand in men and women. Hypotheses: Women will show greater fatigability and fluctuations in force during the low-force fatiguing contraction than men in the presence increased cognitive demand (a difficult mental-math task). 2. To compare both the amplitude of force fluctuations and fatigability for low- to moderate-force isometric contractions in the presence and absence of varying levels of cognitive demand in young and old adults. Hypothesis: Old adults will show greater reductions in time to task failure and greater fluctuations in force than young as cognitive demand increased. 3. To compare variability of motor performance (steadiness and time to task failure) between and within young and old adults with increased cognitive demand. Hypothesis: Old adults will exhibit both greater between- and within-subject variability in motor performance as cognitive demand increased. 27 CHAPTER II Brain areas associated with force steadiness and intensity during isometric ankle dorsiflexion in men and women SUMMARY Although maintenance of steady contractions is required for many daily tasks, there is little understanding of brain areas that modulate lower limb force accuracy. Functional magnetic resonance imaging (fMRI) was used to determine brain areas associated with steadiness and force during static (isometric) lower limb target-matching contractions at low and high intensities. Fourteen young adults (6 men, 8 women; 27.1 ± 9.1 years) performed three sets of 16 s isometric contractions with the ankle dorsiflexor muscles at 10, 30, 50 and 70% of maximal voluntary contraction (MVC). Percent signal changes (PSC, %) of the blood oxygenation level dependent (BOLD) response were extracted for each contraction using region of interest (ROI) analysis. Mean PSC increased with contraction intensity in the contralateral primary motor area (M1), supplementary motor area (SMA), putamen, pallidum cingulate cortex, and ipsilateral cerebellum (p<0.05). The amplitude of force fluctuations (SD) increased from 10 to 70% MVC but relative to the mean force (coefficient of variation, CV %) was greatest at 10% MVC. The CV of force was associated with PSC in the ipsilateral parietal lobule (r = 0.28), putamen (r = -0.29), insula (r = -0.33), and contralateral superior frontal gyrus (r = -0.33, p<0.05). There were minimal sex differences in brain activation across the isometric motor tasks indicating men and women were similarly motivated and able to 28 activate cortical motor centers during static tasks. Control of steady lower limb contractions involves cortical and subcortical motor areas in both men and women and provides insight into key areas for potential cortical plasticity with impaired or enhanced leg function. INTRODUCTION Steady postural contractions that stabilize a limb with accuracy are required for successful performance of many daily tasks including carrying objects, driving and walking. Stabilizing contractions across a range of force intensities requires appropriate neural activity in cortical centers. Early work in monkeys established the central role of discharge rates of primary motor cortex (M1) cells during graded voluntary movements of distal upper limb muscles (Evarts, 1968). Subsequent development of imaging techniques in humans including functional magnetic resonance imaging (fMRI) have established positive associations between contraction force intensity of the upper limb and cortical activation indicated by the blood oxygenation level dependent (BOLD) response [e.g. (Keisker et al., 2009; Spraker et al., 2007; Thickbroom et al., 1998; van Duinen et al., 2008)]. However, there remains uncertainty as to the role of intensity and volume of cortical activation in force regulation (Dai et al., 2001) and the motor areas involved with increased activation especially among lower-to-moderate force contractions, which are forces that many daily tasks are performed (Keisker et al., 2009; Spraker et al., 2007; van Duinen et al., 2008). Despite the primary role of lower limb muscles as agonists and stabilizers during functional tasks such as driving and walking, most studies have studied upper limb muscles and little is understood about cortical activation during leg movements. 29 Understanding key cortical areas associated with control of the lower leg in healthy young adults establishes a foundation for identifying the plasticity of these areas with impaired motor function that can occur with aging and neurological conditions as well as enhanced function that is possible with physical exercise in all populations. Some studies have determined areas of activation during foot exercise [e.g. (Ciccarelli et al., 2005; Huda et al., 2008; MacIntosh et al., 2004)] but the relation between activation and force intensity has not been systematically determined. For lower limb muscles however, activation strategies within the cortex (shown as the intensity of the BOLD signal and volume of activation) may differ to that of the more commonly studied hand muscles: the lower limb muscles have a reduced need for fine motor control compared with hand muscles, they have a the larger muscle mass and motor unit ratio (Feinstein et al., 1955), and fewer direct corticospinal connections (Brouwer & Ashby, 1990) compared with upper limb muscles. Further, the tibialis anterior has a greater motor unit recruitment range than for example, the first dorsal interosseus (De Luca et al., 1982a; Moritz et al., 2005; Van Cutsem et al., 1997). Therefore, a purpose of this study was to determine force-related changes across a range of high and low forces in the BOLD signal (intensity and volume) of cortical regions during isometric ankle dorsiflexion contractions in healthy young adults. Because the rate of movement during dynamic or repetitive short isometric contraction mode can affect the BOLD signal (Rao et al., 1996), we used sustained isometric contractions between 10% and 70% of maximal strength. We hypothesized that motor areas of the brain would scale linearly with an increase in force intensity of the ankle dorsiflexor muscles. 30 When sustaining a postural static contraction as is often required during standing, the force exerted to maintain the isometric contraction fluctuates around a mean target force and is often referred to as steadiness (Enoka et al., 2003). The amplitude of the force fluctuations (standard deviation (SD) of the force) increases with force intensity due to activation of more motor units. When the standard deviation is normalized to the mean force (coefficient of variation, CV), the force fluctuations do not increase with intensity and are usually larger at lower intensities of contraction [e.g. (Jesunathadas et al., 2012; Moritz et al., 2005; Taylor, A. M. et al., 2003; Tracy, Mehoudar, et al., 2007). CV of force is mostly mediated by low-frequency oscillations in neural drive (< 2-3 Hz) seen as the oscillations of the trains of motor unit action potentials (Dideriksen et al., 2012; Negro et al., 2009), with a greater influence of synaptic noise and motor unit discharge rate variability at low forces (Jesunathadas et al., 2012). The low-frequency oscillating neural drive reflects an integration of both descending and afferent inputs (Dideriksen et al., 2012; Farina et al., 2012; Negro et al., 2009). A novel aspect of this study was to identify and understand those cortical areas that are related to the force fluctuations across a range of forces in young adults. We hypothesized that at lower intensities of contraction, larger fluctuations in force (CV) would be associated with greater activation of cortical motor areas. Both men and women were tested, so we also determined whether there were any sex differences that could be detected in brain activity and modulation of the low- and high-force isometric contractions with the lower limb. Sex differences have been shown in brain activity during cognitive tasks of equal performance in men and women, such that women have greater brain activation for the same task (Wang et al., 2007) and some 31 differences in motor control during finger tapping (Lissek et al., 2007). Whether there are sex differences in brain activation during motor tasks with the lower limb has not been assessed. Various indirect measures of motor unit activity (electromyography, EMG) and assessments of voluntary activation (e.g. techniques of stimulation along the neuraxis to determine neural drive to the muscle and to the motor cortex), indicate no sex difference in the output of activation from the motor cortex (Hunter et al., 2006; Hunter & Enoka, 2001; Keller et al., 2011). We hypothesized therefore, that men and women would have similar PSC during graded and controlled static contractions. METHODS AND MATERIALS Fourteen healthy young adults (6 men and 8 women; mean ± SD; 27.1 ± 9.1 years, 170.5 ± 9.5 cm in height, 66.5 ± 11.2 kg in body mass) volunteered to participate in the study. All participants were healthy with no known neurological or cardiovascular diseases and were naive to the protocol. Each participant provided informed consent, and the protocol was approved by the institutional review boards at Marquette University and Medical College of Wisconsin. Participants reported to the laboratory once for a familiarization session and then for an experimental session in the MRI scanner. Set Up and Mechanical Recordings. Participants laid supine in a 3.0 Tesla short bore MRI Scanner (General Electric Healthcare, Waukesha, WI) with the hip and knee at 45° of flexion (full extension is 0°). The right foot was assessed with the ankle in a neutral position (0° dorsiflexion). During the static (isometric) voluntary contractions, force of the ankle dorsiflexor muscles was measured with a force transducer (Transducer Techniques, Temecula, CA) mounted at right angles under a footplate that was adjustable for angle and was rigidly secured to the distal end of the scanner bed (polycarbonate 32 platform). The forefoot was secured to the footplate via a strap placed 1-2 cm proximal to the metatarsophalangeal joint of the toes. The forces detected by the transducer were recorded online at 500 samples/s with a Power 1401 A/D converter and Spike2 software (Cambridge Electronics Design, Cambridge, UK). The force signal was displayed via a rear-projection visual display system for participant feedback. During the submaximal contraction the visual display feedback was adjusted for each participant using their maximal strength value. A horizontal cursor representing the baseline was displayed at 12.5% of the height of the screen and a horizontal cursor representing the required target force was displayed at 75% of the height of the screen. The force output was displayed at a refresh rate of 60 Hz and a resolution of 800 × 600 pixels. Each participant was asked to trace the horizontal cursor with the force signal as steadily and accurately as possible. Experimental Protocol. Once the participant was setup in fMRI environment, they performed at least three maximal voluntary contractions (MVC) for 3-5 s duration to obtain maximal strength (Figure 2.1). If peak forces from two of the three trials were not within 5% of each other, additional trials were performed until this was accomplished. The greatest force achieved by the subject was taken as the MVC and used as the reference to calculate the target force. Each participant performed three sets (runs) of isometric contractions at 10, 30, 50 and 70% of MVC in a randomized order. Each contraction was held for 16 s followed by 60 s rest to avoid fatigue. Participants received real-time force feedback during the contraction via a rear projection visual display system and were required to track a horizontal target line on the display screen. 33 Figure 2.1. Experimental protocol. Three maximal voluntary contractions (MVC) of the ankle dorsiflexor muscles were performed to determine the target torque. Each subject performed three sets (runs) of isometric contractions at 10, 30, 50 and 70% of MVC in a randomized order. Each contraction was held for 16 s followed by 60 s rest to avoid fatigue. Participants received realtime torque feedback during the contraction via a rear projection visual display system and were required to track a horizontal target line on the display screen. MRI Acquisition. Magnetic resonance images were collected in General Electric Signa Excite 3.0 Tesla short bore MR Scanner (GE Healthcare, Waukesha, WI). Functional MRI was used to quantify the blood oxygenation level dependent (BOLD) contrast (T2* weighted imaging) overlaid on a T1 weighted anatomical image for each subject. An 8-channel array Radio Frequency receive head coil (GE Healthcare, Waukesha, WI) was used to obtain 36 sagittal plane slices (thickness = 4 mm) across the entire brain volume using an echo-planar imaging sequence (64 × 64 matrix, 240 × 240 mm2 field of view, TE = 25 ms, TR = 2000 ms, and flip angle = 77°). Voxel were 3.75 × 3.75 × 4 mm. Immediately after completion of the protocol, 148 high resolution spoiled GRASS (gradient-recalled at steady state) anatomical images (thickness = 1 mm) were collected (256 × 244 matrix, TE = 3.9 ms, TR = 9.5 ms, and flip angle = 12°). 34 Data Analysis Mechanical Data Analysis: The torque for each MVC and submaximal contractions was calculated as the product of force and the distance between the ankle joint and the point at which the foot attached to the middle of strap of the force transducer. The MVC torque was quantified as the average value over a 0.5s interval that was centered about the peak. The fluctuations in torque for each 16 s contraction were quantified in two ways: (1) the standard deviation (SD) of the torque, and (2) the coefficient of variation (CV = SD/mean × 100%) which normalized the absolute amplitude of the fluctuations to the mean torque exerted during each contraction. fMRI Data Analysis: The public domain software, Analysis of Functional NeuroImages (AFNI, http://afni.nimh.hih.gov/afni/) was used to analyze the fMRI data sets. For each participant, all acquired functional 2-D images from the scanner were converted to AFNI format and were aligned with slice timing correction. The time series of functional image volumes were spatially realigned to correct the effect of head motion. General linear modeling [3dDeconolve, AFNI (Cox, 1996)] was used to regress a model of the contraction and rest blocks with the BOLD data. All contraction blocks (total 12 contractions = 4 intensities × 3 trials) were used for the model to calculate regression coefficient of each voxel, and then used to create task-related activation map. In addition, 3 contraction blocks at each intensity condition were also used for the model to create a task-related activation map at each contraction intensity separately (Figure 2.1). Separate amplitudes were computed for each of the four contraction intensities. These amplitudes were warped to Talairach (Talairach & Tournoux 1988) space and blurred (4 mm FWHM Gaussian), then were used as the percent signal change (PSC, %). The 35 group analysis was performed for contraction versus rest (3dttest). The results were thresholded using AlphaSim (whole brain corrected p = 0.05, cluster size = 168 ul) to create a task-related activation map. This map was used as a mask for regions of interest (ROI) analysis later. The percent signal change was extracted for each contraction intensity for each subject. Repeated measures ANOVAs were performed to test for differences across force levels (10, 30, 50 and 70% of MVC: force effect) in the BOLD PSC of each activated area. To compare the activation volume in each anatomical area separately within the whole brain, we used the CA N27 macro level map in the AFNI toolbox as a mask rather than a task-related activation map. A total of 116 region of interest (ROI) masks were overlapped with PSC amplitude data for the 4 different torque intensities. Data were thresholded with the average value of all PSC calculated from ROI analysis. The volume in each area that showed a higher signal change than the threshold was used as the activation volume. Repeated measures ANOVAs were performed to test the force effect on the activation volume of each 116 regions. One participant was excluded from the study due to severe motion artifact (> 2 mm). Statistical Analysis. Data are reported as means SD within the text, and displayed as means SE in the figures. Three-way ANOVAs (Sex as a fixed factor) with repeated measures on individual run (1, 2, and 3) and force levels (10, 30, 50, and 70% of MVC) were used to test for between- and within-group differences in average torque and torque fluctuations. Two-way ANOVAs (Sex as a fixed factor) with repeated measures on intensity of torque (10, 30, 50, and 70% of MVC) were used to test for between- and within-group differences in percent signal change and activation volume. When the 36 significant main effect of the force level was found, a contrast option was used to compare between each intensity of torque (10, 30, 50, and 70% of MVC) and the trends (linear, quadratic and cubic) were also determined using the tests of within-subjects contrasts table. When a significant sex-related interaction and main effect of sex were found, preplanned t-tests were performed at each intensity to find the sex difference. Pearson product-moment correlations were used to determine the relationships between the force fluctuation and the PSCs of BOLD response in a selected area. We further examined trends in the percent signal change with increased intensity of force (e.g. logarithmic and sigmoidal trends) of using the curve estimation function in SPSS program as others have done (Ashe, 1997; Cheney & Fetz, 1980; Dettmers et al., 1996). A significance level of p < 0.05 was used to identify statistical significance. RESULTS Head Movement Maximal head displacements were 0.54 ± 0.5 mm, 0.27 ± 0.1 mm and 1.37 ± 1.25 mm for anterior-posterior, right-left, inferior-superior directions, respectively. One participant was excluded due to excessive head motion. Torque (Force) and Steadiness Torque: MVC torque of the ankle dorsiflexors was 31.4 ± 6.8 Nm (range 21.0 Nm to 43.9 Nm). For the submaximal target contractions, there was no difference in torque between the three runs [F(2, 26) = 0.49, p = 0.62] so the three runs were averaged for each intensity of torque (Figure 2.2A). Average torque was significantly different between each target torque intensity [main effect of force, F(3, 10) = 81.4, p < 0.001]. Men were 37 stronger than women [men vs. women; 35.7 ± 6.2 vs. 28.2 ± 6.1 Nm, t(12) = 2.29, p = 0.041), but when normalized to the absolute maximal strength (MVC) they performed the contractions at similar intensities. 38 Figure 2.2. Torque and steadiness. (A). Average torque output at 10, 30, 50, and 70% of MVC of the ankle dorsiflexor muscles. Most subjects traced the target line so that the standard errors were relatively small (ranged 0.011 to 0.102 for 10% and 70% conditions respectively) compared with the mean force output. Average torque was significantly different between each target torque level (p < 0.001); (B). Standard deviation (SD) of torque in men (closed circle) and women (open circle). The SD of torque increased as intensity of contraction increased from 10 to 70% of MVC (p < 0.001); (C). Coefficient of variation (CV) of torque in men (closed circle) and women (open circle). CV at the 10% condition was significantly greater than the other conditions (p < 0.001). The CV decreased and was described as a quadratic trend (p < 0.001). There was no difference in torque, SD of torque, and CV of torque between the three runs (p > 0.05) and so all run data were averaged across each torque level. Shown are the means (± SEM). 39 Torque Fluctuations (Steadiness): The SD of torque did not differ between the three runs [F(2, 26) = 1.65, p = 0.21] so SD from the three runs were averaged for each intensity of torque. The SD of torque increased with intensity of contraction from 10 to 70% of MVC [main effect of torque intensity, F(3, 11) = 42.0, p < 0.001] in a linear [F(1, 13) = 90.4, p < 0.001] and quadratic trend [F(1, 13) = 12.6, p = 0.004] (Figure 2.2B). There was no difference SD of torques between men and women [sex effect, F(1, 12) = 0.52, p = 0.48] across contraction intensities [sex × intensity interaction, F(3, 36) = 0.03, p = 0.99]. To determine the CV, the amplitude of torque fluctuations (SD) was normalized to the mean torque exerted for each contraction intensity, for each participant. There was no difference in the CV (%) between the runs [F(2, 12) = 0.83, p = 0.46] so the CV values from the three runs were averaged for each intensity of torque (Figure 2.2C). CV of torque differed between intensities [main effect of torque intensity, F(3, 11) = 32.7, p < 0.001] because the CV at the 10% MVC was greater than the other forces and with difference between 30% and 70% MVC. Thus, the CV decreased with intensity of force and best described as a quadratic trend [F(1, 13) = 83.0, p < 0.001] (Figure 2.2C). There was no difference CV of torques between men and women [sex effect, F(1, 12) = 1.37, p = 0.27] across contraction intensities [sex × intensity interaction, F(3, 10) = 0.36, p = 0.78]. Brain Activation Areas ROI were generated and indicated that both cortical and subcortical regions were significantly activated during right ankle isometric dorsiflexion (See Table 2.1 and Figure 2.3). 40 Table 2.1. Brain activation areas. Areas of brain activation during right ankle isometric dorsiflexion (n = 6 men and 8 women). Coordinates Contraction vs. Cluster (mm) rest Brain Regions Size (µl) x y z t-score z-score L. Putamen 3247 27 4 13 6.52 4.35 L. Lingual Gyrus 1219 14 L. Calcarine Gyrus 1157 4 67 -6 6.15 4.21 67 12 5.9 4.12 R. Cerebellum (III) 1138 -12 35 -22 6.36 4.29 L. Paracentral Lobule (M1) (5mm from L. SMA) 800 4 15 56 6.04 4.17 L. Su. Occipital Gyrus R. I. Parietal Lobule 582 11 85 4 5.92 4.12 542 -33 44 45 6.07 4.18 R. Putamen 484 -30 0 11 5.77 4.06 L. Su. Frontal Gyrus 368 22 13 53 6.07 4.18 R. I. Frontal Gyrus 312 -49 -4 17 5.95 4.14 L. I. Parietal Lobule 312 29 46 49 5.79 4.07 L. Su. Occipital Gyrus 281 17 86 20 5.75 4.05 R. Insula Lobe (Or R.I.F. G=Opercularis) 244 -41 -11 7 6.17 4.22 R. Insula Lobe 236 -39 13 15 6.31 4.27 L. Middle Cingulate Cortex 232 6 4 44 5.99 4.15 L. Pallidum (3mm for putamen; 5 for Thalamus) 205 20 5 1 6.66 4.4 R. I. Temporal Gyrus 195 -39 60 -5 5.8 4.07 R: Right; L: Light; Su: Superior; I: Inferior; Regions are listed accordingly to their cluster size. Coordinates are used in mm in T-T Atlas space. The activation regions were thresholded with p < 0.0001 and an activation cluster minimum of 168.8 µl so that all included voxels had a t-value > 5.32. 41 Figure 2.3. Brain Activation Areas during Right Ankle Isometric Dorsiflexion. Activation areas were identified from contrasts between each contraction and rest. Cortical and subcortical regions activated during each contraction intensity were displayed in orange to yellow colors (t = 6 to 10) on same slice, i.e. on axial, sagittal, and coronal plane (76, 74, 112). The activation regions were thresholded with p < 0.0001 for each voxel and an activation cluster minimum of 168.8 μl so that all included voxels had a t-value > 5.32. 42 ROI Analysis using Activation Map Percent Signal Change (Intensity of BOLD Response) and Torque Left Putamen: There was a main effect of torque intensity for the mean PSC [F(3, 39) = 3.38, p = 0.028]. The PSC increased linearly with greater contraction intensity [F(1, 13) = 5.89, p = 0.030] so that the PSC of the 70% MVC was greater than the 10% MVC (p = 0.036). The PSC also showed logarithmic and sigmoidal increases as the torque increases (logarithmic and sigmoidal; p = 0.041 and 0.048, respectively; Figure 2.4A). Right Cerebellum (III): There was a main effect of torque intensity for the PSC [F(3, 11) = 4.56, p = 0.026]. The PSC increased linearly as contraction intensity increased [F(1, 13) = 12.4, p = 0.004] so that PSC for the 50% and 70% MVC were greater than the 10% MVC (p = 0.032 and p = 0.003, respectively). The PSC also showed logarithmic and sigmoidal increases as the torque increases (logarithmic and sigmoidal; p = 0.001 and p < 0.001, respectively; Figure 2.4B). Left paracentral lobule/SMA: There was a main effect of torque intensity for the mean PSC [F(3, 39) = 6.28, p = 0.001]. PSC increased linearly with the increase in contraction intensity [F(1, 13) = 9.08, p = 0.010] so that the PSC for the 50% and 70% MVC were greater than for the 10% MVC (p = 0.022 and p = 0.011, respectively). The PSC also showed logarithmic and sigmoidal increases as the torque increases (logarithmic and sigmoidal; p = 0.045 and 0.037, respectively; Figure 2.4C). Left middle Cingulate Cortex: There was a main effect of torque intensity for the PSC [F(3, 39) = 4.34, p = 0.010]. PSC increased linearly with increased contraction intensity [F(1, 13) = 6.43, p = 0.025] so that the PSC for the 70% MVC was greater than for the 43 10% and 30% MVC (p = 0.040, 0.013 respectively). The PSC also showed logarithmic and sigmoidal increases as the torque increases (logarithmic and sigmoidal; p = 0.018 and 0.011, respectively; Figure 2.4D). Left Pallidum: There was a main effect of torque intensity for the mean PSC [F(3, 11) = 8.00, p < 0.001]. The PSC increased linearly with increased contraction intensity [F(1, 13) = 34.4, p < 0.001] so that the PSC for the 70% MVC was greater than all the other conditions (p = 0.001 to 0.035). PSC for the 50% MVC was also greater than during the 10% MVC (p = 0.049). The PSC also showed logarithmic and sigmoidal increases as the torque increases (logarithmic and sigmoidal; p = 0.002 and 0.008, respectively; Figure 2.4E). 44 Figure 2.4. Percent signal change in Regions of Interest. Percent signal change of regions of interest (ROI) that scaled with torque intensity. Shown are group mean (± SEM) of percent signal change during 10, 30, 50, and 70% of MVC torque conditions. (A). Left putamen (effect of torque, linearity respectively; p = 0.028, p = 0.03); (B). Right cerebellum III (p = 0.026, p = 0.004); (C). Left paracentral lobule (p = 0.001, p = 0.01); (D). Left middle cingulate cortex (p = 0.01, p = 0.025); (E). Left pallidum (p < 0.001, p < 0.001). 45 Sex Differences in Activation There were no sex differences in intensity of activation (PSC) in the activated areas tested except right inferior temporal gyrus where men showed a greater PSC than women during the 70% MVC [0.65 ± 0.25 vs. 0.32 ± 0.21 respectively; t(12)=2.623, p = 0.022]. Activation Volume (Voxel Number) Several regions showed activation of additional voxels as force increased from low to high intensities of contraction (See Table 2.2). These areas are detailed below. Table 2.2. Brain Areas with Increased Activation Volume. Brain areas with increased activation volume during submaximal contractions with the right ankle isometric dorsiflexor muscles. Areas that had significant linear increases in voxel number with increased force are shown. Regions for CA_N27_ML atlas L. Precentral Gyrus (primary motor cortex, M1) R. Inferior Frontal Gyrus (p. Orbitalis) L. Anterior Cingulate Cortex L. Para Hippocampal Gyrus L. Amygdala R. Amygdala L. Su. Occipital Gyrus R. Su. Occipital Gyrus R. Fusiform Gyrus L. Postcentral Gyrus R. Paracentral Lobule L. Putamen L. Thalamus R. Inferior Temporal Gyrus L. Cerebellum (IV-V) R. Cerebellum (IV-V) R. Cerebellum (VI) Effect of Force Linear Trend p > 0.05 p = 0.02 p = 0.002 p > 0.05 p > 0.05 p > 0.05 p > 0.05 p = 0.037 p > 0.05 p > 0.05 p > 0.05 p = 0.059 p > 0.05 p = 0.053 p = 0.085 p = 0.04 p = 0.059 p = 0.029 p = 0.052 p = 0.033 p = 0.038 p = 0.047 p = 0.026 p = 0.069 p = 0.048 p = 0.044 p = 0.059 p = 0.083 p > 0.05 p = 0.094 p > 0.05 p > 0.05 p = 0.023 p > 0.05 R: Right; L: Light; Su: Superior; I: Inferior; The activation regions were thresholded with the average PSC value of whole activation area. 46 Right inferior frontal gyrus (p. Orbitalis): Activation volumes were 3928 ± 1544, 3077 ± 2010, 4663 ± 1973, 5091 ± 1930 voxels at 10, 30, 50, and 70% MVC intensities respectively. Activation volume increased with a greater torque intensity [main effect of torque intensity, F(3, 11) = 3.67, p = 0.020] in a strong linear trend [F(1, 13) = 4.01, p = 0.052]. Right superior occipital gyrus: Activation volumes were 2070 ± 1538, 1895 ± 1276, 3105 ± 1586, 2949 ± 1771 voxels at 10, 30, 50, and 70% MVC intensities respectively. Activation volume increased with a greater torque intensity [F(3, 11) = 3.12, p = 0.037] in a linear trend [F(1, 13) = 4.781, p = 0.048]. Right cerebellum (IV-V): Activation volumes were 1725 ± 910, 1543 ± 1308, 2157 ± 1045, 2580 ± 1032 voxels at 10, 30, 50, and 70% MVC intensities respectively. Activation volume increased with a greater torque intensity [F(3, 11) = 3.12, p = 0.040] in a linear trend [F(1, 13) = 6.64, p = 0.023]. Left anterior cingulate cortex: Activation volumes were 3363 ± 1432, 1975 ± 1260, 2471 ± 1186, 2120 ± 1099 voxels at 10, 30, 50, and 70% MVC intensities respectively. In contrast to other areas, the left anterior cingulate cortex showed a decrease in activation volume as torque intensity increased [main effect of torque intensity, F(3, 11) = 6.18, p = 0.002]. The decrease was linear [F(1, 13) = 5.69, p = 0.033] so that the activation volume for 30, 50, and 70% MVC were significantly smaller than for the 10% MVC (p = 0.002, p = 0.016, and p = 0.013, respectively). 47 Torque Fluctuations and BOLD PSC To gain insight into which areas of the brain were associated with the torque fluctuations during the submaximal contractions, we tested the association between the brain activity (BOLD signal intensity) and the magnitude of torque fluctuations, first with the SD of torque and then the CV. When all four intensities of contraction were pooled into the one analyses (n = 56), there were significant associations between the absolute torque fluctuations (SD) and mean PSC in several areas including the left putamen [r(56) = 0.36, p =0.007], left calcarine gyrus [r(56) = 0.30, p = 0.027], right cerebellum [r(56) = 0.47, p < .001], M1/SMA (left paracentral lobule) [r(56) = 0.45, p < 0.001], left superior frontal gyrus, [r(56) = 0.29, p = 0.03], right insula [r(56) = 0.28, p = 0.031], left SMA/left middle cingulate cortex [r(56) = 0.43, p = 0.001], and left pallidum [r(56) = 0.42, p = 0.001] (Table 2.3). When the correlation analysis was performed at each contraction intensity (n = 14), significant associations were found at the 10% MVC in the left lingual gyrus [r(14) = -0.607, p = 0.021] , at the 30% MVC in the left occipital gyrus [r(14) = 0.740, p = 0.002] and right insula lobe [r(14) = 0.650, p = 0.012]. Because the SD of force covaries with intensities of contraction, the torque fluctuations were normalized to the mean torque (i.e. CV of torque) and associations with the various brain areas determined. There were significant correlations with the mean PSC in several areas including the right inferior parietal lobule [r(56) = -0.278, p = 0.038], right putamen [r(56) = -0.293, p = 0.028], left superior frontal gyrus [r(56) = 0.331, p = 0.013], and right insula [r(56) = -0.330, p = 0.013] (Table 2.4). When a separate correlation analysis was performed for each contraction intensity, positive correlations were found for the 10% MVC in the right inferior parietal lobule [r(14) = - 48 0.721, p = 0.004]; and for the 30% MVC, in the left superior frontal gyrus [r(14) = 0.548, p = 0.042], left postcentral gyrus [r(14) = -0.793, p = 0.001], left SMA [r(14) = 0.548, p = 0.042], and right middle supramarginal gyrus [r(14) = -0.672, p = 0.008]. Table 2.3. Correlations between torque fluctuations (SD) and PSC (N = 56; All torque conditions pooled) SD of Force SD of Torque L. Putamen L. Calcarine Gyrus L. M1 / SMA R. Cereb L. S. Frontal Gyrus R. Insula L.M. Cing Cortex 1 L. Putamen L. Calcarine Gyrus R. Cerebellum .359** 1 .295* 0.251 1 .472** .613** .550** 1 L. M1/SMA .451** .708** .582** .737** 1 L. Su. Frontal Gyrus .290* .617** .530** .674** .815** 1 R. Insula .277* .428** .735** .492** .700** .696** 1 .433** .545** .680** .668** .693** .546** .654** 1 .418** .426** .421** .601** .553** .479** .384** .561** L.M. Cingulate Cortex L. Pallidum L. Pallidum 1 Note. Cereb: Cerebellum; Cing Cortex: Cingulate Cortex; L: Left; R: Right; Su: Superior, M: Middle; * p < 0.05, **p < 0.01. Table 2.4. Correlations between torque fluctuations (CV) and PSC (N = 56; All torque conditions pooled) CV of Torque CV of Torque R. I. Parietal Lobule R. I. Parietal Lobule R. Putamen L. S. Frontal Gyrus 1 -.278* 1 -.293 * .567** L. Su. Frontal Gyrus -.331 * ** .615** 1 R. Insula -.330* .417** .571** .444** R. Putamen R. Insula .477 1 Note. L: Left; R: Right; Su: Superior; I: Inferior; * p < 0.05, **p < 0.01. 1 49 DISCUSSION This study determined those areas of the brain that were associated with lower limb force and steadiness during isometric contractions over a range of intensities in young men and women. We found that activation in the primary motor and sensory cortices, basal ganglia and cerebellum scaled linearly with increased torque of the ankle dorsiflexor muscles, and similarly in men and women. A unique and important finding of this study was that several motor areas (basal ganglia, cerebellum, M1/SMA, and insula) and some typically non-motor areas (superior frontal gyrus, cingulate cortex), increased in activation as fluctuations in torque increased in higher intensity contractions. Furthermore, in order to account for the increased intensity of contraction, we determined those areas of the brain that were associated with the fluctuations in torque when normalized to the mean target torque (CV). The results indicate that the putamen, insula, contralateral superior frontal gyrus and ipsilateral inferior lobes play an important role in control of steadiness of the lower limb during target matching contractions. The minimal sex differences in brain activation during the steady isometric contractions explain the similar steadiness of men and women during ankle dorsiflexion; these findings also corroborate other work (Hunter et al., 2006) that indicates men and women are similarly motivated and able to activate cortical centers during maximal and submaximal performance of motor tasks. Brain Areas Associated with Increased Contraction Intensity During Isometric Ankle Dorsiflexion 50 This study extended the current literature that has examined cortical activation of foot movements (Ciccarelli et al., 2005; Dobkin et al., 2004; Francis et al., 2009; Huda et al., 2008; MacIntosh et al., 2004; Orr et al., 2008) by identifying those activated areas that control for contraction intensity during an isometric task with the lower limb. The BOLD PSC increased linearly as the level of contraction intensity increased for the lower limb in several cortical and subcortical regions including contralateral primary motor cortex/SMA, the putamen and palladium in the basal ganglia, the cingulate cortex, and ipsilateral cerebellum, with the greatest differences occurring between the 10% and 70% MVC contraction torques. Activation volume did not increase in several of the motor regions (e.g. M1/SMA, basal ganglia) that showed greater PSC of the BOLD as contraction force increased. Typically, the central nervous system uses two strategies to increases force: recruitment of motor units and rate coding of the motor unit (De Luca et al., 1982a; Monster & Chan, 1977; Van Cutsem et al., 1997). For the tibialis anterior muscle recruitment of motor units is adopted to increase force across up to ~90% MVC (Van Cutsem et al., 1997) which is a larger recruitment range than intrinsic muscles of the hand such as the first dorsal interosseus which has a recruitment range to ~50% MVC (De Luca et al., 1982a). Our findings suggest that increased force of the tibialis anterior muscle and the recruitment of motor units are achieved by increased intensity of cortical activation in several key motor areas. These areas are addressed below. BOLD signal intensity in M1/SMA increased linearly with intensity of contraction. This change in BOLD signal intensity for the low and moderate contraction intensities was also observed in several fMRI studies of the hand (Noble et al., 2011; Spraker et al., 2007; van Duinen et al., 2008). Although we did not see a significant 51 difference between 10 and 30% MVC as was observed by Keisker, et al, (2009) for dynamic power grip task, the increase was linear across the forces indicating that brain activation scaled appropriately between low and high forces to achieve greater motor unit recruitment required to increase the contraction intensity. The basal ganglia played a significant role in the control of force of the ankle dorsiflexor muscles similar to what is shown for hand muscles (Kinoshita et al., 2000; Spraker et al., 2007). BOLD signal activity during hand contractions can vary substantially in the different nuclei of the basal ganglia according to the task requirements such as task selection and prediction, and amplitude and rate of the force (Prodoehl et al., 2009). For example, Spraker, et al (Spraker et al., 2007) found that both the globus pallidus external (GPe) and the subthalamic nucleus scaled in activation intensity with increasing force amplitude, however, this was not the case for the globus pallidus internal (GPi), putamen and caudate nucleus. Our results show a linear increase in activation intensity in the putamen with increased force with the lower limb muscles. Although we did not divide the globus pallidus into internal and external portions as others have for the hand exercise (Prodoehl et al., 2009; Spraker et al., 2007; Vaillancourt et al., 2007), we showed that the globus pallidus (pallidum) had a linear increase in BOLD signal with the increase of the force level as others have shown for the hand (Grafton & Tunik, 2011; Spraker et al., 2007; Vaillancourt et al., 2004; Vaillancourt et al., 2007). The cerebellum scaled linearly in intensity of activation as contraction force increased and in contrast to other motor areas, the volume of activation increased in this motor region. Others have shown increased cerebellum activation with increased hand 52 muscle force (Keisker et al., 2009; Kuhtz-Buschbeck et al., 2008). The force-related activation in our study was mainly found in the anterior portion of the cerebellum, which is related to sensorimotor control (Manni & Petrosini, 2004; Stoodley & Schmahmann, 2009; Stoodley & Schmahmann, 2010). We also found force-related activation in the ipsilateral anterior lobe (lobule III and IV) which is thought to be related to sensory function in detecting pain (Iadarola et al., 1998). A growing number of studies support the hypothesis that the cerebellum influences not only the sensorimotor control of movement but also cognitive and emotional function (O'Reilly et al., 2010; Stoodley & Schmahmann, 2010). Thus, the cerebellum along with other motor areas including the primary motor cortex, SMA and basal ganglia play a key role in modulating intensity of force of the lower limb, but the increase in activation and volume also may reflect its role in sensorimotor integration. Appropriate visual cortical centers were activated during the motor task performance because participants were required to utilize visual feedback and trace a horizontal cursor with the target torque presented via a rear-projection visual display system. These visual areas activated however did not scale linearly with intensity of contraction. The visual areas activated during right ankle dorsiflexion during all tasks included the lingual gyrus, calcarine gyrus, and parietal lobule in both parietal and occipital lobes and each of these areas is known to be active in visual processing. The lingual gyrus was likely responsible for assisting with visual recognition of the target (Gron et al., 2000), the calcarine gyrus (V1) for the central visual field and spatial attention (Martínez et al., 1999) and the parietal lobule likely contributed to the ability of 53 participants to respond to visual sensory input with appropriate motor output (Clower et al., 2001). Cortical Areas Associated with Steadiness During an Isometric Contraction A novel finding of this study was the identification of brain areas that control maintenance of a steady contraction during a target matching task with the lower limb of young adults. As expected the amplitude of the torque fluctuations (SD of torque) increased linearly with the level of absolute contraction force (Galganski et al., 1993; Moritz et al., 2005; Tracy, 2007a). Accordingly, those motor areas of the brain that increased activation intensity between low and high forces, the basal ganglia, M1, SMA and cerebellum, were also those associated with the SD of the torque in both men and women. One exception was the positive correlation with the PSC in contralateral calcarine gyrus where the primary visual cortex is located (Rajimehr & Tootell, 2008). Despite the linear correlation found in the visual area when all intensity conditions were pooled, the left lingual gyrus, which is also a visual area, showed a negative relation when correlation analysis was performed separately at each contraction intensity. The reduced activity of some visual areas as force increased may be related to the importance of the visual feedback in maintaining a steady contraction at the lower forces, although in young adults the effect of visual feedback is minimal compared with other populations such as older adults (Tracy, 2007b). Because the intensity of the PSC with increased SD of torque in several motor areas covaried with the contraction intensity, we also examined the CV of torque (amplitude of the fluctuations normalized to the mean torque). The CV of torque was greatest at the lowest intensity of contraction (10% MVC) (see Fig. 2C) for both men and 54 women as found for several upper and lower limb muscles (Burnett et al., 2000; Jesunathadas et al., 2012; Jones et al., 2002; Laidlaw et al., 1999; Taylor, A. M. et al., 2003; Tracy, 2007b). Several areas including two motor areas, the putamen and contralateral superior frontal gyrus, as well as the insula, and ipsilateral inferior lobes were associated with the CV of force when all the contraction intensities were pooled. The putamen, in the basal ganglia, also showed increased PSC as contraction intensity increased (Fig 2.4A). Although not in this current study, the activation in the left superior frontal gyrus was also large during a lower force task of the hand (Kuhtz-Buschbeck et al., 2001), and was associated with force during a power grip task (Kuhtz-Buschbeck et al., 2008). CV of force during isometric contractions is thought to be mostly mediated by low-frequency oscillations in neural drive (< 2-3 Hz) (Dideriksen et al., 2012; Negro et al., 2009) across a range of forces. Thus, our data raises possibility that these motor regions, the putamen and the superior frontal gyrus, are sources or significant conduits of the low-frequency oscillating neural drive that influences the trains of action potentials and ultimately the CV of force. Both the left superior frontal gyrus motor region and the ipsilateral inferior lobes however showed the largest correlations between CV of force and activation for the 10% MVC task, which is the intensity that CV was largest across the intensities (r = -0.55 and -0.72 respectively). The ipsilateral parietal lobule is typically involved in oculomotor and attention processes (Clower et al., 2001). This new finding with the lower extremity control supports the previous observation that visual processes can have a large influence on the motor variability and ability to hold a steady contraction at the lower forces (Aagaard, 2003; Prodoehl & Vaillancourt, 2010; Sosnoff & Newell, 2006b; Tracy, 55 2007b). Further, at low forces up to 10%, synaptic noise and the resultant more variable motor unit discharge rates can contribute to the large CV of force (Dideriksen et al., 2012; Negro et al., 2009). Motor unit discharge rate variability, however probably has its greatest influence on the CV at low forces in upper limb muscles and lesser effects in the tibialis anterior (Jesunathadas et al., 2012). These findings also raise the possibility that along with the superior frontal gyrus (motor region), the ipsilateral parietal lobule, is also an important brain area involved in the increased CV at the lower forces during ankle dorsiflexion. No Sex differences in Brain Activation During Ankle Dorsiflexion Although men were stronger than women, there were no differences in the normalized motor performance tasks indicating that men and women contracted their ankle dorsiflexor muscles at similar relative intensities of force and displayed similar CV and changes in force fluctuations with contraction intensity. Although there are widespread sex differences reported in the brain during cognitive and some motor tasks, we found that activation was similar for young men and women during ankle dorsiflexion for most motor areas. Only in the right inferior temporal gyrus did men display greater activation than the women during the highest contraction intensity (70% MVC). The greater activation of temporal gyrus for men in this study is consistent with that for a finger tapping (Lissek et al., 2007). Our task was relatively simple; sex differences may become more apparent when the task complexity and greater cognitive component are involved (Lissek et al., 2007) or different muscle groups and postures are adopted. Our results also indicate that men and women are similarly motivated and able to activate cortical motor centers during static tasks over a range of forces. 56 Conclusion Activation intensity (PSC) of several cortical and subcortical regions increased with contraction intensity during static ankle dorsiflexion including the primary motor cortex, basal ganglia and cerebellum. In general, activation volume did not increase in motor areas that demonstrated greater activation intensity, and indicating a minimal role of volume of activation in motor unit recruitment to achieve high forces. Activation of a visual (ipsilateral parietal lobule) and motor (contralateral superior frontal gyrus) area were associated with the greater torque fluctuations (CV) at low forces, and therefore may play a role in control of steadiness especially during low intensity contractions in young adults. Activation of the putamen (basal ganglia) was associated with both the CV of force and contraction intensity suggesting this area also plays a central role in the control of steady contractions across the range of forces. Although men were stronger than women, they had similar normalized fluctuations in torque (steadiness) when executed in the supine position and primarily similar areas and intensities of brain activation across the range of low and high intensity contractions. The minimal sex differences in brain activation during the steady isometric contractions indicate that young men and women are equally motivated and able to activate cortical motor centers during static tasks. Therefore, the key cortical and subcortical brain areas that were identified in healthy men and women for control of lower limb steady contractions, could be targeted in future to determine impaired such as occurs with aging and neurologic conditions and enhanced function that can occur with physical training. 57 CHAPTER III Women are less steady than men during low-force isometric contractions of the lower extremity SUMMARY Although steady, controlled movements in the lower extremity are imperative when performing a basic functional activity such as walking, it is unclear if changes in cortical involvement during motor task performance influences lower limb force production and control in men and women differently. To expose sex differences in cortical involvement during motor performance, we compared steadiness (force fluctuations) and fatigability of submaximal isometric contractions with the ankle dorsiflexor muscles in young men and women and with varying levels of cognitive demand imposed. Sixteen young (8 men, 21.5 ± 2 yr., 8 women, 19.3 ± 1.5 yr.) attended three sessions in which they performed a 40 s isometric contraction at 5% maximal voluntary contraction (MVC) force followed by an isometric contraction at 30% MVC until task failure. The cognitive demand required during the submaximal contractions in each session differed as follows: 1) high-cognitive demand session where difficult mental math was imposed (counting backward by 13 from a 4-digit number); 2) low-cognitive demand session which involved simple mental math (counting backward by one); and 3) control session with no mental math. Anxiety was elevated during the high-cognitive demand session compared with other sessions more so for the women than men (p<0.05). Women demonstrated greater force fluctuations than men during the very low (5% MVC) 58 force task (p = 0.005) and the moderate-force (30% MVC) fatiguing contraction (p = 0.002) regardless of cognitive demand compared with men. Women also demonstrated a similar time to task failure as men for the moderate-force task regardless of cognitive demand (6.2 ± 2.4 and 6.7 ± 2.1, respectively; p = 0.060). These findings suggest that both young men and women are more able to successfully achieve a difficult cognitive task and a target matching motor task with the lower limb than with the upper limb. INTRODUCTION Decreased steadiness (increased force fluctuations) during sustained low-tomoderate force isometric contractions can negatively impact achievement of a goaldirected movement (Enoka et al., 2003), and potentially interfere with performance of work-related or functional tasks. For example, decreased hand steadiness diminishes manual task precision (Endo & Kawahara, 2011) and decreased ankle steadiness may increase postural sway in the lower extremity (Kouzaki & Shinohara, 2010), potentially leading to increased fall risk particularly in adults with neurological disorders such as multiple sclerosis (Cameron & Lord, 2010; Findling et al., 2011) and with older adults (Fernie et al., 1982). The ability to sustain steady low and moderate force contractions can be influenced by a variety of factors which can include advanced age (Galganski et al., 1993; Laidlaw et al., 2000), fatigue (De Luca et al., 1982b; Singh et al., 2010), arousal (Lorist et al., 2002; Noteboom, Barnholt, et al., 2001), intensity and type of contraction (Cresswell & Loscher, 2000; Griffith et al., 2010; Semmler et al., 2007), and muscle group (Hunter, Yoon, et al., 2008; Jesunathadas et al., 2012; Tracy, Mehoudar, et al., 2007). The participant’s sex may also contribute to decreased steadiness when 59 contractions are sustained at the same relative intensity; however the influence of sex is still unknown. It is unclear if there is a steadiness sex difference because much of the literature on steadiness neglects to examine if sex differences exist or reports mixed results. For example, when performing submaximal isometric contractions, women are reported to be more (Endo & Kawahara, 2011), less (Brown et al., 2010; Brown et al., 2009; Grunte et al., 2009), or similarly (Baweja et al., 2009) steady compared with men. For example, women demonstrated greater overall force fluctuations during isometric elbow flexion contractions than men across multiple force levels (between 2.5 and 75% of maximal voluntary contraction (MVC) force (Brown et al., 2010). In contrast, Tracy (2007a) found differences in force fluctuations between opposing ankle muscle groups (ankle dorsiflexion and plantarflexion) but no sex differences between young or older adults for forces ranging between 2.5% and 80% MVC force. The first study of this dissertation showed minimal differences in CV of force between the sexes for the ankle dorsiflexors in a stable supine position. Given the disparity of results and the lack of attention to the possibility of sex differences in steadiness, it remains unclear if women are less steady than men when performing submaximal isometric contractions. Mechanism responsible for force fluctuations across the range of forces between low and high intensities isometric contractions is primarily the low frequency oscillations of trains of motor units with some contribution of the motor neuron discharge rate variability and motor unit properties especially at low forces (Barry et al., 2007; Dideriksen et al., 2012; Jesunathadas et al., 2012; Moritz et al., 2005; Negro et al., 2009). At low forces (< 10% MVC) several muscles including those in the lower limb have 60 larger force fluctuations normalized to the target force (CV of force) than moderate to high force fluctuations (Dideriksen et al., 2012). The low frequency oscillations of the trains of motor units appear to originate from descending and afferent inputs (Negro et al., 2009). The first study of this dissertation suggests some of those descending inputs to the motor neuron pool may originate from or are associated with both motor and nonmotor areas of the brain including the ipsilateral parietal lobule, putamen, insula, and contralateral superior frontal gyrus (Chapter 2) (Yoon et al., 2014). How these inputs differ between populations (e.g. men and women, young and older adults) who differ in motor unit properties (Doherty, 2003; Hunter, 2014), and their effects on steadiness is not known. Altering descending inputs to the motoneurone pool occurs during altered states of arousal and fatigue. Women have reduced steadiness with upper limb muscles compared with men when arousal is increased by electric shocks to the back of the hand (Christou et al., 2004), and increased cognitive demand (mental math) (Noteboom, Fleshner, et al., 2001). Further, for the elbow flexor muscles, steadiness is reduced and fatigability increased for both men and women when the cognitive demand is imposed during a low-force (20% MVC) isometric task (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). However, women demonstrated increased in fatigability compared with men and the increased fatigability was related to initial absolute strength (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). Whether such differences in fatigability exist for a lower limb muscle where the number of corticospinal connections are less than for the upper limb (Brouwer & Ashby, 1990) and fatigability is less dependent on initial strength than the elbow flexor muscles (Hunter, 2014) is not known. 61 The first purpose of this study was to determine the influence of increased cognitive demand on the time to task failure for a submaximal contraction of the ankle dorsiflexors in men and women. The second purpose was to examine the influence of varying levels of cognitive demand on the amplitude of force fluctuations during a very low force (5% MVC) and moderate-force (30% MVC isometric contractions in the ankle dorsiflexor muscles in men and women. Increased cognitive demand has been shown to increase force fluctuations in women more so than in men prior to contractions in the elbow flexors; thus, we hypothesize that women will demonstrate greater force fluctuations than men during ankle dorsiflexion and this would increase even further with exposure to high cognitive demand. MATERIALS AND METHODS Sixteen young adults (8 men, 8 women; 18 - 24 years) participated in the study (see Table 3.1 for physical characteristics). All participants were healthy with no known neurological or cardiovascular diseases and were naïve to the protocol. Both men and women had low-to-moderate levels of anxiety (trait) (29.0 ± 8.1; 34.8 ± 7.9; p 0.16) according to the State-Trait Anxiety Inventory (STAI) (Spielberger, 2010) and reported no history of or current mental or psychological pathology, including anxiety or depressive disorders. Participants were right-leg dominant (0.72 ± 0.22 vs.0.77 ± 0.17 for men and women respectively, with a ratio of 1 indicating complete right-leggedness) as estimated using the Edinburgh Handedness Inventory (Oldfield, 1971). The physical activity level for each participant was assessed with a questionnaire that estimated the relative kilocalorie expenditure of energy per week (Kriska & Bennett, 1992). Prior to 62 participation, each subject provided informed consent, and the protocol was approved by the Institutional Review Board at Marquette University. 63 Table 3.1. Participant Physical Characteristics and Results. Participant characteristics and age group means for control, low-cognitive demand (Low-CD) and high-cognitive demand (High-CD) sessions for women and men. Variables reaching significance for main effect of sex are indicated by the asterisk (*). Variable Number of subjects Age (years) Height (cm) Weight (kg) Physical Activity (PAQ) Baseline Trait STAI Scores Baseline State STAI Scores Session Control Low-CD MVC Torque* (Nm) High-CD Total Control MVC Torque Recovery Low-CD (mean % of initial) High-CD Total Control Low-CD Time to Task Failure (min) High-CD Total Control Low-CD 30% MVC CV of Torque* (%) High-CD Total Control Low-CD TA EMG* (% MVC) High-CD Total Control TA EMG Bursting Activity Low-CD (bursts/min) High-CD Total Control Coactivation Ratio – TA:Gastroc Low-CD High-CD Control Coactivation Ratio – TA:Soleus Low-CD High-CD Women Men 8 8 19.3 ± 1.5 21.5 ± 2 170 ± 6.2 167.8 ± 33.9 60.1 ± 6.0 88.8 ± 34.1 73.3 ± 44.7 45.7 ± 26.7 39.1 ± 7.5 33.8 ± 5.7 34.9± 7.9 29 ± 8.1 16.8 ± 2.7 28.1 ± 4.9 17.01 ± 2.4 27.3 ± 5.3 16.9 ± 2.4 28.6 ± 6.5 16.9 ± 2.5 28.0 ± 5.4 95.6 ± 3.1% 96.8 ± 10.3% 94.1 ± 2.3% 96.6 ± 4.5% 94.5 ± 3.9% 95.1 ± 4.1% 96.2 ± 6.6% 94.7 ± 3.1% 6.4 ± 1.7 5.8 ± 2.6 6.1 ± 2.2 6.1 ± 1.9 7.4 ± 2.5 6.8 ± 2.7 6.7 ± 2.1 6.2 ± 2.4 5.4 ± 1.1 3.6 ± 0.5 5.1 ± 0.8 3.8 ± 0.4 5.3 ± 0.7 3.9 ± 0.6 5.26 ± 0.8 3.8 ± 0.5 23.2 ± 3.3 23.7 ± 5.6 23.2 ± 3.1 23.6 ± 6.8 25 ± 2.4 23.3 ± 3.9 23.8 ± 2.9 23.6 ± 5.3 8.1 ± 8.2 12.5 ± 11.1 12.2 ± 10.8 10.7 ± 11 6.8 ± 7.8 12.0 ± 10.9 9.0 ± 8.9 11.7 ± 10.5 1.5 ± 1.1 0.93 ± 0.25 1.2 ± 0.51 0.92 ± 0.39 1.0 ± 0.36 0.87 ± 0.15 0.87 ± 0.49 0.91 ± 0.29 0.77 ± 0.25 0.80 ± 0.21 0.76 ± 0.30 0.71 ± 0.16 P 0.024* 0.861 0.034* 0.156 0.129 0.163 0.0001* 0.0001* 0.0001* 0.0001* 0.757 0.19 0.787 0.476 0.575 0.974 0.627 0.748 0.001* 0.002* 0.001* 0.0001* 0.826 0.885 0.308 0.882 0.381 0.791 0.292 0.473 0.142 0.231 0.254 0.84 0.809 0.672 * Variables reaching statistical significance for main effect of age (p < 0.05). PAQ = Physical Activity Questionnaire, STAI = State-Trait Anxiety Index, MVC = Maximal Voluntary Contraction, CV = Coefficient of Variation, TA = Tibialis Anterior, Gastroc = Gastrocnemius, EMG = Electromyography. 64 Each participant reported to the laboratory on four occasions to perform a protocol that involved a fatiguing contraction with the left ankle dorsiflexor muscles: once for a familiarization session and three experimental sessions (control, low-cognitive demand and high-cognitive demand sessions), with each experimental session being at least 5 days apart. During the low-cognitive demand and high-cognitive demand sessions, each participant performed either a simple mental math task (low-cognitive demand session) or difficult mental math task (high-cognitive demand session) at rest, and also while performing isometric contractions at 5% maximum voluntary contraction (MVC) force (40 s duration) and a 30% MVC for as long as possible until task failure (Figure 3.1). During the control session, each participant performed the motor tasks without performing any mental math. Session order was counterbalanced among participants within each age group. Figure 3.1. Experimental protocol. The top panel illustrates the order of tasks performed by each participant with the ankle dorsiflexor muscles. Maximal voluntary contractions (MVC) (solid bars) were performed at the beginning of the experimental session and during recovery (immediately after the fatiguing contraction and at 1, 2, 5, and 10 min of recovery). The fatiguing contraction (30% MVC), symbolized by the hatched rectangle, was performed until task failure by each participant. The bottom panels indicate with arrows when the State-Trait Anxiety Inventory (STAI; 4 times) was performed and the Visual Analog Scale (VAS) for anxiety and stress were recorded (at 7 time points, T1 – T7). The schematic is not to scale for time or force. 65 Mechanical Recording of Force. Each participant was seated upright in an adjustable chair (Biodex Medical Systems, NY) with the hip and knees at 90° of flexion. The setup is similar to that described elsewhere (Griffith et al., 2010). In brief, the left foot rested on a footplate in a custom made dynamometer to measure forces of the lower leg, with the ankle in a neutral position (0° dorsiflexion). The foot was secured to the footplate via a strap placed over the anterior aspect of the ankle and another strap placed 1–2 cm proximal to the metatarsophalangeal joint. Isometric force of the dorsiflexor muscles was recorded using a force transducer (Transducer Techniques, Temecula, CA) and recorded online at 500 Hz using a Power 1401 analog-to-digital (A/D) converter and Spike2 software (Cambridge Electronics Design (CED), Cambridge, UK). Force displayed on a 19-inch monitor was located at eye level 1.5 m in front of the participant. Each participant was asked to trace a horizontal cursor placed in the middle of the screen with the force signal as it appeared on the screen from the right side of the monitor. Electrical Recordings. Whole muscle EMG signals of the tibialis anterior, medial head of the gastrocnemius, soleus, and rectus femoris were recorded using bipolar surface electrodes (sintered pellet Ag-AgCl, 8mm diameter, with 20 mm between electrodes) taped to the skin over the bellies of each muscle. Reference electrodes were placed on the patella. The recording electrodes on each muscle were placed in line with the muscle fibers and in accordance with locations recommended by the European Recommendations for Surface Electromyography (Hermens et al., 2000). The EMG signals were amplified (1,000 x) and band-pass filtered (13 –1,000 Hz) with Coulbourn bioamplifiers (Coulbourn Instruments, Allentown, Pennsylvania) prior to being recorded directly to a computer using the Power 1401 and Spike2 software (CED). The EMG 66 signals were digitized at 2,000 samples/s and analyzed offline using Spike2 software (CED). Cardiovascular Measurements. Heart rate and blood pressure were monitored during submaximal and fatiguing contractions and periods of rest or mental math with an automated beat-by-beat blood pressure monitor (Finapres 2300, Datex-Ohmeda, Louisville, CO). The blood pressure cuff was placed around the middle finger of the left hand, and the arm was placed on a platform to maintain the hand at heart level. Blood pressure was sampled at 500 samples/s and collected online to PC using Spike2 software (CED). Cognitive Assessment of Anxiety and Stress. Cognitive levels of anxiety and stress were assessed throughout the protocol using a visual analogue scale (VAS) (Yoon et al., 2009) and the state portion of the STAI questionnaire (Spielberger, 2010). Each VAS (one for anxiety and another for stress) had a 10-cm line anchored at the far left by “none” and at the far right by “as bad as it could be.” The right anchor corresponded to the most stressful or most anxious moment in the life of the participant. Anxiety was defined as the participant’s negative feelings regarding the immediate future, whereas stress represented the physical changes (e.g., increase in heart rate and perspiration) occurring during the test perceived by the participant that were above and beyond the expectation for their level of exertion (Christou et al., 2004). VAS for anxiety and stress were recorded at seven time points (T1 - T7) during the protocol: one baseline assessment before intended arousal (T1); during the rest period after each 2 × 2-min bout of mental math (low-cognitive demand or high-cognitive demand session) or quiet rest (control session) (T2, T3); immediately after the 5% MVC submaximal contraction (T4); 67 immediately after the fatiguing contraction/MVC (T5); and 5 and 10 min after the fatiguing contraction (T6, T7) (Figure 3.1). The state portion of the STAI- questionnaire consisted of 20 statements that required a response on a four-point Likert-type scale. Baseline trait and state assessments were conducted during the familiarization session. There was no significant difference between young and older adults in baseline trait STAI scores (p = 0.54) or baseline state STAI scores taken during the familiarization session (p = 0.66) (Table 3.1). State STAI assessments were also conducted at four different time points during the experimental protocol: baseline assessment before arousal; after 2 × 2-min bouts of quiet sitting (control session) or mental math (low-cognitive demand and high-cognitive demand sessions); immediately after the fatiguing contraction/MVC; and 10 min after completion of the fatiguing contraction (Figure 3.1). Cognitive Demand Conditions. Difficult mental math is an established psychosocial technique used to induce cognitive demand (Kajantie & Phillips, 2006) and was used for the high-cognitive demand task (Noteboom, Fleshner, et al., 2001). Each participant performed serial subtraction from a four-digit number by 13 with one response required every 3 s (Noteboom, Fleshner, et al., 2001). If the participant made an error in serial subtraction or was unable to provide the correct answer within 3 s, they were asked to restart the mental math from the first number in the series. After three errors, the investigator asked the participant to begin with a new four-digit number. The simple mental math task, performed during the low-cognitive demand session, was designed to increase cognitive demand above control without elevating arousal. Participants serially counted backward by 1’s from 50 to 0 at a slow, even pace. If the 68 participant made an error in counting, they were asked to restart counting from 50. During the control session participants were instructed to rest quietly during the 2 × 2min bouts, 5% MVC submaximal contraction (40 s) and 30% MVC fatiguing contraction. During the low-cognitive demand and high cognitive demand sessions, participants performed the mental math task while at rest (2 × 2-min bouts), and then continuously during the 5% MVC submaximal contraction and 30% MVC fatiguing contraction until task failure. Experimental protocol. The protocol for each experimental session (control, lowcognitive demand and high-cognitive demand sessions) involved procedures in the following order: 1) MVCs of the ankle dorsiflexor, ankle plantarflexor and knee extensor muscles; 2) assessment of cognitive and physiological arousal before and after 2 × 2-min bout of either quiet sitting (control session), simple mental math (low-cognitive demand session), or difficult mental math (high-cognitive demand session); performance of: 3) one submaximal isometric contraction at 5% MVC force sustained for 40 s with assessment of cognitive and physiological arousal immediately following the contraction; 4) a submaximal fatiguing isometric contraction at 30% MVC force sustained until task failure; and 5) recovery MVCs immediately following the fatiguing contraction, and at 1, 2, 5, and 10 min recovery with assessment of anxiety and stress levels (Figure 3.1). Participants performed two MVCs of the knee extensor and plantar flexor muscles each at the beginning of each experimental session in order to obtain peak EMG for the gastrocnemius, soleus and rectus femoris muscles. Participants rested for 60 s between each trial. For both muscle groups, MVCs were performed with the participant seated in a position the same as for testing the ankle dorsiflexors muscles (described above). The aim 69 was to obtain peak EMG values for each muscle group: forces were not recorded during these contractions for knee extension and plantar flexion. Each participant was asked to push as hard as possible against an immovable restraint for 3–4 s to activate either the knee extensor or ankle plantar flexor muscles. For the knee extensor muscles, manual resistance was applied to the distal leg (just above the lateral malleolus) so that the lower leg was restrained at 90° of flexion while the participant performed maximal knee extension. For the ankle plantar flexor muscles, the foot of each participant was placed on the footplate, and vertical movement was minimized during each MVC by a block that eliminated movement of the footplate. The MVC trial with the greatest amount of EMG activity was used to normalize the EMG recordings during the fatiguing contractions of the rectus femoris, medial head of the gastrocnemius, and soleus muscles. Participants performed three to four MVC trials with the ankle dorsiflexors while their foot was attached to the footplate. Each participant was asked to dorsiflex as hard as possible for 3–4 s. Participants were given visual feedback on a display monitor and strong verbal encouragement to achieve and maintain maximal force. Participants rested for 60 s between each trial. If the peak force achieved for two of the first three trials was not within 5% of each other, additional trials were performed until this criterion was met. The greatest MVC force achieved with the ankle dorsiflexor muscles was used as the reference to calculate the target level for both the submaximal contractions at 5% MVC and the fatiguing contraction at 30% MVC. The MVC with the greatest amount of EMG activity was used to normalize the EMG recordings during the fatiguing contractions of the tibialis anterior muscle. MVCs of the ankle dorsiflexor muscles were also performed during recovery (Figure 3.1). 70 A fatiguing contraction was performed with the ankle dorsiflexor muscles at 30% MVC during each experimental session. Each participant was asked to trace a horizontal cursor with the force signal as it appeared on the screen from the left side of the monitor in order to match the vertical target force as displayed on the monitor. Participants were encouraged to sustain the force for as long as possible. The fatiguing contraction was terminated when the force declined by 10% of the target force. To minimize the influence of transient fluctuations in motor output on the criteria for task failure, the task was terminated only after force fell below the predetermined threshold for 2.5 s of a 5 s interval. Participants were not informed of their time to task failure. Rating of perceived effort (RPE) was assessed using the modified Borg 10-point scale (Borg, 1982). Each participant was instructed to focus their assessment of effort on the ankle muscles performing the fatiguing task. The scale was anchored so that 0 represented the resting state and 10 corresponded to the strongest contraction that the ankle muscles could perform. The RPE was recorded at the beginning of the fatiguing contraction and every minute thereafter until task failure. In order to obtain RPE while the participant was performing mental math during the low-cognitive demand and highcognitive demand sessions, the participant was interrupted and asked to report their RPE. After reporting their RPE, participants resumed the mental math task of serial counting from 50 to 0 during the low cognitive demand session or serial subtraction using a new 4digit number during the high cognitive demand session. Data Analysis All data collected during the experiments were recorded online using a Power 1401 A/D converter and analyzed using Spike2 (CED). The MVC torque was quantified 71 as the average value over a 0.5-s interval that was centered about the peak. The torque for the MVCs, submaximal and fatiguing contractions was calculated as the product of force and the distance between the ankle joint and the point at which the ankle was attached to the force transducer. The maximal EMG for each muscle was determined as the root mean square (RMS) value over a 0.5-s interval about the same peak interval of the MVC torque measurement. The maximal EMG value of the involved muscles was then used to normalize the RMS EMG values recorded during the fatiguing contraction. The RMS value of the 5% MVC dorsiflexion task was averaged for each muscle over the middle 30 s of the 40 s contraction for the tibialis anterior, medial gastrocnemius, soleus and rectus femoris. During the 30% MVC fatiguing contraction, the RMS EMG signal for each muscle was quantified at the following time intervals: the first 30 s; 15 s on both sides of 25, 50, and 75% of time to task failure; and the last 30 s of the task duration. The EMG activity of each muscle was normalized to the RMS EMG value obtained during the MVC for each respective muscle. The level of coactivation was quantified by calculating the ratio between the RMS EMG (% peak) of the agonist muscle (tibialis anterior) and antagonist muscle (medial gastrocnemius or soleus) (Coactivation = antagonist/agonist x 100) [e.g. (Griffith et al., 2010)]. To quantify the bursts of EMG activity of the tibialis anterior during the 30% MVC fatiguing contraction, the EMG signal was first rectified, smoothed (averages of 1-s duration, 500 data points), and then differentiated over 0.25-s averages. The differentiated signal represents the rate of change and was used to identify rapid changes in the rectified and smoothed EMG signal. The threshold for establishing if a burst of EMG had occurred was determined by first finding the minimum SD of the differentiated 72 EMG during the fatiguing contraction using a 30-s moving window; the threshold was then defined as the mean + 3 SD of the minimum differentiated signal. The minimal burst duration was 0.1 s. The EMG bursting activity (bursts/min) was quantified for five continuous intervals of 20% of the time to task failure. The amplitude of the force fluctuations was quantified as the coefficient of variation (SD/mean × 100) for the 5% MVC task and 30% MVC fatiguing contraction. The fluctuations in force during the 5% MVC task were quantified over the middle 30 s of the 40 s contraction and for the fatiguing contraction at 30% MVC for five continuous intervals of 20% of the time to task failure. Mean arterial pressure (MAP) and heart rate (HR) were evaluated only on participants not currently taking blood pressure medications with normal blood pressure. MAP and HR recorded during the 30% MVC fatiguing contraction were analyzed by comparing ~15 s averages at 25% intervals throughout the fatiguing contraction; during the 5% MVC submaximal contraction they were quantified over the middle 30s of the 40s contraction. For each interval, the blood pressure signal was analyzed for the mean peaks [systolic blood pressure (SBP)], mean troughs [diastolic blood pressure (DBP)], and number of pulses per second (multiplied by 60 to determine heart rate). MAP was calculated for each epoch with the following equation: MAP = DBP + ⅓ (SBP - DBP). Rate pressure product (RPP) was calculated as the product of heart rate and MAP for the equivalent time periods as stated above. Statistical Analysis Data were reported as means ± SD within the text and displayed as means ± SE in the figures. Repeated measures ANOVAs, with sex (men and women) as a between- 73 subject factor, were used to compare the various dependent variables. Repeated measures factors during different tests included session (control, low-cognitive demand and highcognitive demand) and either fatigue (pre-, post-fatiguing contraction), recovery (immediately after fatiguing task failure, 1 min, 2 min, 5 min, and 10 min after fatiguing task failure) or time (state STAI scores were taken at baseline, after 2 × 2-min of quiet sitting or dual task, fatiguing contraction and 10 min recovery; VAS was taken at baseline, after each 2 min of quiet sitting or dual task, 5% submaximal contraction, immediately after task failure, and at 5 min and 10 min of recovery (T1 – T7; Figure 3.3). Post hoc analyses (Tukey) were used to test for differences among pairs when appropriate. Independent t-tests (one-tailed) were used to compare subject physical characteristics, percent decline in MVC force across the fatiguing tasks, and rates of increase in various dependent variables as a function of absolute time. A significance level of p < 0.05 was used to identify statistical significance. Time to task failure was compared across sessions using repeated measures ANOVA with sex as a between group factor. The contribution of several variables to time to task failure was analyzed using multiple linear regressions. These variables included the rate of change in RMS EMG activity of each muscle, EMG bursting activity of the tibialis anterior, MAP, heart rate, RPE, fluctuations in force, and MVC force (SPSS version 22). RESULTS MVC Torque At baseline, men were stronger than women (28.0 ± 5.4 Nm vs. 16.9 ± 2.5 Nm; sex effect, p = 0.0001) on all three days of testing (Table 3.1). The relative reduction (%) 74 in MVC torque after the fatiguing contraction was similar across sessions (session effect, p = 0.539), and similar for men and women (sex effect, p = 0.0476). During recovery, MVC torque increased to near baseline levels within 10 minutes of completing the fatiguing contraction similarly for men and women across all sessions (session × sex, p = 0.407; Table 3.1). Furthermore, at the end of the recovery period, relative strength was similar men and women (p > 0.05). Arousal State STAI Scores. Men and women demonstrated similar baseline state STAI scores prior to each experimental session (p = 0.163; Table 3.1). State STAI scores taken immediately after the 2×2-min bout were significantly elevated after the high-cognitive demand session compared with the control and low-cognitive demand sessions (p < 0.0001), but there was no interaction (session × sex, p = 0.064), or difference between men and women (sex effect, p = 0.140). State STAI scores taken immediately after the fatiguing were significantly elevated for women compared to men across sessions (session × sex, p = 0.003; Figure 3.2), but there was no main effect of sex (p = 0.318); scores taken during recovery were similar across sessions for men and women (session, p = 0.349; sex effect, p = 0.995; session × sex, p = 0.334). State STAI scores were higher for women than men after the fatiguing contraction during the high-cognitive demand session (women, 56.6 ± 11.9; men, 42.6 ± 11.1; p = 0.029). 75 State STAI Scores Men Women 44 State STAI Score 42 40 38 36 34 32 30 28 26 24 Control Low-CD High-CD Session Figure 3.2. State STAI scores. State STAI scores for men (closed symbols) and women (open symbols) during the control, low-cognitive demand (low-CD) and high-cognitive demand (highCD) sessions. The values shown are mean ± SE. State STAI scores taken immediately after the fatiguing were significantly elevated for women compared to men across sessions (session × sex, p = 0.003). VAS for Stress and Anxiety. There was no difference between men and women for baseline stress and anxiety VAS taken at the beginning of each session (p > 0.05). Stress and Anxiety VAS were greater during the high-cognitive demand session than control and low-cognitive demand sessions (session, p = 0.0001), and over time (time, p = 0.001, p = 0.002), but there were no sex differences (sex effect, p = 0.744, p = 0.899) and no interactions (p > 0.05). VAS for stress and anxiety were elevated after the 2×2 bout, and 5% MVC contraction during the high-cognitive demand session compared with the control and low-cognitive demand sessions (session, p < 0.05), but there were no sex differences (sex effect, p > 0.05) and no interactions (session × sex, p > 0.05; Figure 3.3). Both stress and anxiety scores were significantly elevated after the fatiguing contraction 76 across all three sessions (session, p = 0.0001), and although there were no interactions for stress (session × sex, p > 0.05), anxiety was significantly higher for women across sessions than men (session × sex, p = 0.040). A Men Control Women Control Men Low-CD Women Low-CD Men High-CD Women High-CD 60 Anxiety VAS (cm) 50 40 30 20 10 0 -10 T1 T2 T3 B T4 T5 T6 T7 T5 T6 T7 Time 60 Stress VAS (cm) 50 40 30 20 10 0 -10 T1 T2 T3 T4 Time Figure 3.3. Visual Analogue Scale (VAS) scores for anxiety (A) and stress (B). Mean (±SE) VAS scores for young adults (closed symbols) and older adults (open symbols) are shown for anxiety (A) and stress (B) throughout the experimental protocol during the control session (circles), low-cognitive demand session (Low-CD, triangles), and high-cognitive demand session (High-CD, squares). Time intervals were as follows: baseline (T1), after the first bout of 2 min of quiet rest/mental math (T2), after the second bout of 2 min of quiet rest/mental math (T3), after the 5% submaximal contraction (T4), during recovery immediately after task failure (T5), and then at 5 min (T6) and 10 min of recovery (T7). 77 Low-Intensity Sustained Contraction (5% MVC) Fluctuations in Torque. Women had greater fluctuations in torque (CV) than men across all three sessions (sex effect, p = 0.005). Women demonstrated greater fluctuations in torque than men because the interaction did not reach significance (linear trend interaction, session × sex, p = 0.060; Figure 3.4). Fluctuations in force were negatively correlated with strength during the control session (r2 = -0.863, n = 15, p = 0.0001), and the low-cognitive demand session (r2 = -0.719, n = 15, p = 0.002), but not during the high-cognitive demand session (r2 = -0.437, n = 15, p = 0.104). 15 Men Women 14 CV (%) 13 12 11 10 9 8 7 Control Low-CD High-CD Session Figure 3.4. Mean Session Coefficient of Variation (CV) of Torque during the 5% MVC Task. Mean Session Coefficient of Variation (CV) of Torque during the 5% MVC Task for men (closed symbols) and women (open symbols) during the control, low-cognitive demand (Low-CD), and high-cognitive demand (High-CD) sessions. Women had greater fluctuations in torque (CV) than men across all three sessions (sex effect, p = 0.005) during the 5% MVC task. 78 Mean arterial pressure and heart rate. Mean arterial pressure during the 5% MVC contraction was significantly higher during the high-cognitive demand session (99.4 ± 14.0 mmHg) than the low-cognitive demand session (93.9 ± 8.9 mmHg) and the control session (86.4 ± 10.1 mmHg; session effect, p = 0.003), but there was no influence of sex on mean arterial pressure (sex main effect, p = 0.258). Heart rate was similar across sessions (p = 0.073) for men and women (p = 0.73). Rate pressure product was significantly higher during the high-cognitive demand session (session, p = 0.002), but there was no difference between men and women (p = 0.925). EMG Activity. Gastrocnemius, rectus femoris, soleus, and tibialis anterior RMS EMG (% MVC) were similar across sessions (p = 0.077, p = 0.446, p = 0.159, p = 0.670, respectively) for men and women (p = 0.855, p = 0.373, p = 0.534, p = 0.492, respectively). Fatiguing Contraction (30%MVC) Time to Task Failure. There was no difference in time to task failure across sessions (p = 0.060), interactions (sex × sessions, p = 0.748), or between men and women (sex effect, p = 0.675; Table 3.1 and Figure 3.5). There was no correlation between strength and time to task failure for any of the sessions. 79 Time to Failure (min) 10 Men Women 8 6 4 Control Low-CD High-CD Session Figure 3.5. Time to task failure during the 30% MVC Task. Time to task failure during the 30% MVC task for men (closed symbols) and women (open symbols) are shown during the control, low-cognitive demand (Low-CD), and high-cognitive demand (High-CD) sessions. There was no difference in time to task failure across sessions (p = 0.060) or between men and women (sex effect, p = 0.675). Fluctuations in Torque. Fluctuations in torque (CV) increased over time across sessions (session × time, p = 0.0001) and women had significantly higher fluctuations in torque than men (sex effect, p = 0.002; Figure 3.6). CV of torque was significantly higher during the high-cognitive demand session over time (session × time, p = 0.030) particularly for women (sex effect, p = 0.0001; Figure 3.6). Women demonstrated greater fluctuations in torque than men throughout the fatiguing contraction. CV of torque was negatively correlated with strength during the low-cognitive demand session (r2 = -0.642, n = 16, p = 0.007) and the high-cognitive demand (r2 = -0.556, n = 16, p = 0.025) sessions, but not during the control session (r2 = -0.431, n = 16, p = 0.095). 80 Men Control Women Control Men Low-CD Women Low-CD Men High-CD Women High-CD 8 CV of Torque (%) 7 6 5 4 3 2 1 0 20 40 60 80 100 Time (%) Figure 3.6. Coefficient of variation (CV) of force during the fatiguing contraction. Coefficient of variation (CV) of force during the fatiguing contraction (30% MVC) across time for men (closed symbols) and women (open symbols) during the control session (circle), low-cognitive demand session (Low-CD, triangle), and high-cognitive demand session (High-CD, square). The CV of torque is shown for the control (circle), low cognitive demand (triangle), and high cognitive demand (square) sessions for men (closed symbols) and women (open symbols) during five continuous intervals of 20% during the fatiguing contraction. The x-axis intervals indicate the last time point of data averaged in that interval (20% on the axis for example, is equivalent to the 0-20% of the contraction, and the 100% represents 80-100% of the time to failure). Fluctuations in torque (CV) increased over time across sessions (session × time, p = 0.0001) and women had significantly higher fluctuations in torque than men (sex effect, p = 0.002). EMG Activity of Agonist Muscles. The amplitude of the RMS EMG (% MVC) for the tibialis anterior (TA, ankle dorsiflexor muscle), increased during the fatiguing contraction (time, p < 0.001) similarly across sessions (session × time, p = 0.836) and between men and women (sex effect, p = 0.882). Bursting activity (bursts per minute) increased over time during the fatiguing contraction across sessions (time, p = 0.005), similarly for men and women (sex effect, p = 0.471), with no interactions (p > 0.05). There was no difference in bursting activity between men and women within sessions (p > 0.05). EMG Activity of Antagonist and Accessory Muscles. The RMS EMG amplitude (% EMG) for the gastrocnemius, soleus, and rectus femoris increased over time during 81 the fatiguing contractions (time effect, p < 0.001 for all muscles) but was similar across sessions (session effect, p > 0.05 for all muscles) and sex (sex effect, p > 0.05) with no interactions (session × sex, p > 0.05). Coactivation ratios for gastrocnemius relative to the tibialis anterior were similar across sessions (session, p = 0.164), over time (time, p = 0.161), and for men and women (p = 0.129), with no interactions (p > 0.05). Coactivation ratios for the soleus relative to the tibialis anterior were also similar across sessions (session, p = 0.125), over time (time, p = 0.245), for men and women (sex effect, p = 0.977), and there were no interactions (p > 0.05). Physiological Response during Fatiguing Contraction. Mean arterial pressure (MAP) increased over time during the fatiguing contraction (time, p = 0.001), was higher during the high-cognitive demand session for men than women (session × sex, p = 0.031), but there was no main effect of sex (sex effect, p = 0.170), and there were no interactions (p > 0.05). Heart rate was significantly higher during the high-cognitive demand session (session, p = 0.004), over time (time, p = 0.0001), but there was no difference between men and women (sex effect, p = 0.458) and no interactions (p > 0.05). Rate-pressure product (RPP) was higher during the high-cognitive demand session (session, p = 0.014) over time (time effect, p = 0.0001), but there was no difference between men and women (sex effect, p = 0.856) and no interactions (p > 0.05). Ratings of Perceived Exertion (RPE) increased during the fatiguing contraction (time effect, p = 0.0001) similarly across sessions (session effect, p = 0.709). RPE was similar for men and women (sex effect, p = 0.514), with no interactions (p > 0.05). 82 DISCUSSION This study compared the force fluctuations during very low and moderate intensity contractions and the time to task failure for a moderate-force fatiguing contractions of the ankle dorsiflexor muscles in the presence and absence of increased cognitive demand for young men and women. The novel findings of this study were that women demonstrated: 1) greater force fluctuations than men during the very low (5% MVC) force task and the moderate-force (30% MVC) fatiguing contraction regardless of cognitive demand, and 2) a similar time to task failure as men for the moderate-force task regardless of cognitive demand. In contrast to the elbow flexor muscles (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009), the fatigability of the dorsiflexor muscles in young men and women was not greater with superimposition of either a high cognitive demand task, despite elevated levels of reported stress and anxiety. Force fluctuations in the upright posture however, were greater for women than men for the low and moderate force contractions. Force fluctuations at low-to-moderate forces appear to be the result of multiple factors that influence the output of motoneurones including synaptic noise and the common input or drive to the motoneurones (Dideriksen et al., 2012; Hamilton et al., 2004; Jesunathadas et al., 2012; Negro et al., 2009). Synaptic noise in the motoneurone pool will increase motor unit discharge rates and likely influence the force fluctuations at the very low forces (Jesunathadas et al., 2012; Moritz et al., 2005). The primary mechanism for force fluctuations however across the range of low to high forces is the thought to be due to centrally mediated common drive that oscillates at < 2-3 and influence the discharge rates of the trains of motoneurones in unison (De Luca et al., 83 2009; De Luca et al., 1982b; Negro et al., 2009). Fluctuations in force at lower force levels (5% MVC) may also be exacerbated by the use of visual feedback during a sustained contractions (Tracy, 2007b). Although there is some agreement on the mechanisms that contribute to force fluctuations during sustained contractions, the majority of participants in theses previous studies were men providing very little insight regarding the cause of greater force fluctuations found in women. Women demonstrated greater force fluctuations during both the low-force (5% MVC) and moderate-force (30% MVC) contractions with the ankle dorsiflexion. Some but not all studies have shown these sex differences in the elbow flexor muscles and hip extensors. For example, Brown, et al (2010) demonstrated that women had overall decreased steadiness during elbow flexion tasks; women were also less steady than men while performing a target-matching task of the hip extensors, this was only seen at low target values (Grunte et al., 2009). Several factors may have influenced the decreased steadiness found in women in this study. Women have been reported to have lower motor unit discharge rates and higher discharge rate variability than men during isometric elbow flexion contractions (Brown et al., 2009). Both of these factors would result in a significant increase in force fluctuations when compared with men (Moritz et al., 2005); however, there is very little additional evidence to support this finding in the literature. Other possible mechanisms for the sex difference in force fluctuations include muscle strength and simultaneous activation of the antagonist muscles. While others indicate that coactivation has also been reported as a potential contributor to decreased steadiness during low-force tasks (Kouzaki et al., 2004), we found were no differences in coactivation between men and women at either low- or moderate-intensity contractions in 84 this study. Thus coactivation did not appear to explain the sex difference in force fluctuations. We found a negative correlation between steadiness and strength during the control session for the 5% MVC task indicating that stronger adults (men) had a lower CV. However, there was no relationship between steadiness and strength during the 30% MVC task. Brown, et al (2010) also reported a strong relationship (r = -0.49) between increased force fluctuations and decreased strength demonstrated by women compared with men at all submaximal force levels across three positions of the forearm (neutral, pronated and supinated). Thus, although women were consistently weaker and less steady than men during the ankle dorsiflexion task, it is unlikely that strength influenced steadiness during the moderate-intensity contraction (30% MVC), but it may have been a factor during the low-intensity contraction (5% MVC). This is consistent with reports in the literature that stronger joints generate less variable torques and that stronger muscles produce less force noise due to having higher motor unit numbers and firing rates than weaker muscles (Hamilton et al., 2004), further potentially causing women with less strength to perform with greater force fluctuations than men. In contrast to the elbow flexor muscles and small muscle of the hand, cognitive demand and increased stress did not increase the force fluctuations during the isometric contractions (Christou et al., 2004; Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). Unexpectedly, women appeared to have improved their steadiness during the high cognitive demand compared with the control and low cognitive demand, although this did not reach statistical significance but the men did not. For the control and low-cognitive demand sessions, fluctuations in force were also negatively correlated with strength, but 85 not during the high-cognitive demand session. As a response to increased acute anxiety, the sympathetic nervous system may have released neuromodulators that evoked a response from the motor system or spinal cord that altered recruitment or rate coding of motor units enabling the participant to perform the motor task with greater steadiness (Marmon & Enoka, 2010). However, why women appeared to improve steadiness and men did not, is unclear. Time to failure of the 30% MVC task did not alter with cognitive demand for both men and women. These results for the ankle dorsiflexor muscles are in contrast to that for the elbow flexor muscles (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). The increased fatigability with high cognitive demand was associated with initial strength for the elbow flexor muscles such that weaker subjects had greater reduction in time to failure when the cognitive high-cognitive demand was imposed (Keller et al 2014). For the ankle dorsiflexors, there was no correlation between strength and time to task failure [as has been seen before (Avin et al., 2010)], nor were there indications of significant differences in cardiovascular responses between men and women as has been observed for the elbow flexor muscles. For the elbow flexor muscles, one explanation for the reduction in time to failure was a strength-related perfusion mechanisms such that for weaker subjects the perfusion advantage at the start of a contraction (due to less intramuscular pressure) was altered with increased cognitive demand and arousal (Yoon et al 2009; Keller et al 2014). For the dorsiflexor muscles, the reduced association between strength and time to failure (Avin et al., 2010) and the slightly higher intensity contraction, may have ameliorated any perfusion related mechanism for influencing the time to task failure. 86 Alternatively, the lack of increase in the force fluctuations and no change in time to failure with increased cognitive demand for both young men and women may also have been a consequence of the reduced number of corticospinal inputs to the motoneurone pool in the lower limb relative to the upper limb resulting in less modulation at of the motor unit output. Taken together with previous studies (Christou et al., 2004; Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009), our findings suggest that because both young men and women demonstrate similar times to task failure and force fluctuations during the 30% MVC fatiguing task regardless of cognitive demand as well and similar force fluctuations during a 5% MVC task in the presence of high-cognitive demand, they are more able to successfully achieve a difficult cognitive task and a target matching motor task with the lower limb than with the upper limb. 87 CHAPTER IV Motor variability during sustained contractions increases with cognitive demand in older adults SUMMARY To expose cortical involvement in age-related changes in motor performance, we compared steadiness (force fluctuations) and fatigability of submaximal isometric contractions with the ankle dorsiflexor muscles in older and young adults and with varying levels of cognitive demand imposed. Sixteen young (20.4 ± 2.1 yr: 8 men, 8 women) and 17 older adults (68.8 ± 4.4: 9 men, 8 women) attended three sessions and performed a 40 s isometric contraction at 5% maximal voluntary contraction (MVC) force followed by an isometric contraction at 30% MVC until task failure. The cognitive demand required during the submaximal contractions in each session differed as follows: 1) high-cognitive demand session where difficult mental math was imposed (counting backward by 13 from a 4-digit number); 2) low-cognitive demand session which involved simple mental math (counting backward by one); and 3) control session with no mental math. Anxiety was elevated during the high-cognitive demand session compared with other sessions for both age groups but more so for the older adults than young adults (p<0.05). Older adults had larger force fluctuations than young adults during: 1) the 5% MVC task as cognitive demand increased (p=0.007), and 2) the fatiguing contraction for all sessions (p=0.002). Time to task failure did not differ between sessions or age groups (p>0.05), but the variability between sessions (standard deviation [SD] of 3 sessions) was 88 greater for older adults than young (2.02 ± 1.05 min vs. 1.25±0.51 min, P<0.05). Thus, variability in lower limb motor performance for low- and moderate-force isometric tasks increased with age and was exacerbated when cognitive demand was imposed during a very low-force contraction indicating that age-related variability of sustained contractions can originate from central sources. These data have significant performance implications for cognitively demanding low-force motor tasks that are relevant to functional and ergonomic in an aging workforce. INTRODUCTION Aging results in marked declines in both motor performance and cognitive function. For example, older adults are weaker and less steady (i.e., they exhibit greater fluctuations in force around a target force) than young adults (Enoka et al., 2003; Hunter, Todd, et al., 2008). Decreased steadiness with age is greatest during low-intensity isometric contractions in lower and upper extremity muscles (Enoka et al., 2003; Marmon, Pascoe, et al., 2011; Tracy, 2007a) probably due to age-related changes in inputs to the motoneurone pool (Barry et al., 2007). Cognitive impairment can also be marked but subtle and often subclinical in early stages of cognitive dysfunction (Aine et al., 2011; Chen et al., 2001; Morris et al., 2001); it is often observed as degradation in short-term memory and executive function resulting in a decreased ability to perform daily tasks that are dependent upon memory-related abilities (Artero et al., 2001; Farias et al., 2012; Morris et al., 2001), including planning and decision-making in lower limb activities such as gait (Yogev-Seligmann et al., 2008). Age-related declines in motor and cognitive function are usually studied separately but are often performed simultaneously in daily tasks. The current study assessed motor function with a focus on steadiness and 89 muscle fatigability in young and older adults while they were presented with low and high levels of cognitive demand. In young adults, cognitive performance declines and force fluctuations increase when a cognitive task is imposed (reaction time) during sustained isometric tasks with hand muscles (Lorist et al., 2002; Zijdewind et al., 2006); however, force fluctuations were affected more during the isometric fatiguing contractions than during a 5% maximal voluntary contraction (MVC) submaximal non-fatiguing contraction (Lorist et al., 2002). We also previously found that steadiness of the elbow flexor muscles declined (increased force fluctuations) and time to failure of a sustained 20% MVC submaximal task was reduced (increased fatigability) in young adults when simultaneously performing a demanding cognitive task (‘high cognitive demand’) that increased anxiety (counting backwards by 13). Accordingly heart rate and blood pressure, which are indices of increased sympathetic activity with arousal (Kajantie & Phillips, 2006), were elevated during the ‘high-cognitive demand’ session compared with control (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). Individuals who were weaker (primarily the women) showed the largest decrement in time to task failure when the stressful cognitive task was imposed during the fatiguing contraction (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). Older adults are typically weaker than young adults, and older women being weaker than older men for upper and lower limb muscles (Galganski et al., 1993; Laidlaw et al., 2000; Tracy & Enoka, 2002), possibly increasing susceptibility to increased fatigability when a cognitive task is imposed. However, it is not known whether fatigability with increased cognitive demand is exacerbated with advanced age. 90 Furthermore, the effects of increased cognitive demand on lower limb fatigability and steadiness in young or older adults are not known. Initial evidence would suggest that age-related decrements in motor function of the upper limb (e.g., reduced steadiness) become larger with greater cortical involvement of non-motor centers (Fraser et al., 2010; Voelcker-Rehage & Alberts, 2007; VoelckerRehage et al., 2006) and may increase between- and within-participant variability particularly in older adults (Enoka et al., 2003; Sosnoff & Newell, 2006a). Older adults display greater between- and within-participant variability than young in activation of supraspinal centers during maximal contractions with the upper limb (Hunter, Todd, et al., 2008) indicating that variability within cortical motor areas is larger with advanced age. Increased cognitive involvement, particularly with tasks that tax attentional resources and that require the use of short-term memory or executive function, may contribute further to age-related variability in motor performance for older adults during functional tasks (Sommervoll et al., 2011; Voelcker-Rehage et al., 2006; YogevSeligmann et al., 2008). Increased cognitive involvement however, can increase anxiety and stress levels (e.g., Noteboom, Fleshner, et al., 2001) which may further decrease steadiness of upper limb muscles in older adults compared with young. Force fluctuations, for example, increased for older adults more than young when exposed to a noxious stressor (unpredictable electrical stimulation to the hand) prior to performing the pinch grip task (Christou et al., 2004). The increased force fluctuations therefore, could be due to agerelated changes in monoaminergic drive modulating inputs to the motoneurone pool (Barry et al., 2007). A novel aspect of this current study is that we varied the level of 91 cognitive demand administered simultaneously during motor task of the lower limbs to determine its influence on lower limb fatigability and steadiness and any accompanying changes in anxiety and stress. The purpose of this study was to compare both the amplitude of force fluctuations (steadiness) and time to task failure (fatigability) for low-to-moderate-force isometric contractions in the presence and absence of varying levels of cognitive demand in young and older adults. Participants were exposed to two different levels of cognitive load, lowand high-cognitive demand while performing a motor task with the ankle dorsiflexor muscles, which are muscles that play a functional role controlling the position of the foot during walking and while maintaining balance. We hypothesized that older adults would show greater reductions in time to task failure and greater force fluctuations than young as cognitive demand increased. We also compared variability of fatigability between and within young and older adults with increased cognitive demand. We hypothesized that as cognitive demand increased, older adults would exhibit both greater between- and withinparticipant variability in fatigability. To understand the perceived and physiological arousal responses with the varying levels of cognitive demand in young and older adults, during each session we quantified perceived levels of stress and anxiety, as well as heart rate, blood pressure and perceived effort of contraction. MATERIALS AND METHODS Sixteen young adults (8 men, 8 women; 18 - 24 years) and 17 older adults (9 men, 8 women; 62 - 79 years) participated in the study (see Table 3.1 for physical characteristics). All participants were healthy with no known neurological or cardiovascular diseases, had controlled blood pressure and were naïve to the protocol. 92 Six of the older adults were on medication to control blood pressure. Both young and older adults had low-to-moderate levels of anxiety (trait) according to the State-Trait Anxiety Inventory (STAI) (Spielberger, 2010) and reported no history of current mental or psychological pathology, including anxiety or depressive disorders. Participants were right-leg dominant (0.81 ± 0.40 vs. 0.80 ± 0.54 for young and older adults, respectively, with a ratio of 1 indicating complete dominance of the right-leg) (Oldfield, 1971). The physical activity level for each participant was assessed with a questionnaire that estimated the relative kilocalorie expenditure of energy per week (Kriska & Bennett, 1992). Prior to participation, each participant provided informed consent, and the protocol was approved by the Institutional Review Board at Marquette University. The experimental Materials and Methods for Chapter IV are identical to Chapter III. To avoid redundancy, all experimental Materials and Methods information has been removed from Chapter IV. Please refer to Chapter III for experimental procedures, protocol, data acquisition, analysis information, etc. Statistical Analysis Data were reported as means ± SD within the text, and displayed as means ± SE in the figures. Analyses of variances (ANOVA) models were used to compare the various dependent variables. Specifically, separate ANOVAs with repeated measures on session (control, low-cognitive demand and high-cognitive demand), and with age (young and old) and sex (men and women) as fixed factors, were used to compare MVC torque and STAI (state) and VAS for anxiety and stress at baseline, MAP, heart rate, CV of torque and EMG activity during the 5% MVC task, error rates (only low- and high-cognitive demand sessions included for error rate analysis) during the fatiguing contraction and the 93 time to task failure of the fatiguing contraction. This ANOVA model was also used to compare the SD of the time to failure from the three sessions, and the SD of the mean CV of torque during the fatiguing contraction obtained from the three sessions. ANOVAs with repeated measures on session and time, and with age and sex as fixed factors, were used to compare VAS for stress and anxiety, STAI (state) and MVC torques throughout the sessions i.e. before and after the fatiguing contraction (see Figure 3.1 for time points). ANOVAs with repeated measures on session and time (five time points for each: see data analysis section), and with age and sex as fixed factors, were used to compare the following variables during the fatiguing contraction: CV of torque, RMS EMG, EMG bursting activity, heart rate, MAP, rate pressure product and rating of perceived exertion. Post hoc analyses (Tukey) were used to test for differences among pairs when appropriate. Univariate ANOVAs were used to compare young and old men and women for the following variables: physical characteristics (age, height and weight), physical activity level, handedness and STAI (trait). The strength of an association is reported as the Pearson product-moment correlation coefficient (r). The statistical significance adopted was 5% (p <0.05) and all analysis were performed in IBM Statistical Package for Social Sciences (SPSS) version 19. 94 Table 4.1. Participant Physical Characteristics and Results. Participant physical characteristics and age group means (± SD) for control, low-cognitive demand (Low-CD) and high-cognitive demand (HighCD) sessions. Variable Session Young Older Number of Participants Age (years) Height (cm) Women Age (years) Men Age (years) Weight (kg) Physical Activity (PAQ) Baseline Trait STAI Scores Baseline State STAI Scores MVC Torque* (Nm) MVC Torque Recovery (% of initial) Time to Task Failure (min) 30% MVC CV of Torque* (%) TA EMG* (% MVC) TA EMG Bursting Activity (bursts/min) Soleus EMG (% MVC) Gastrocnemius EMG (% MVC) Rectus Femoris EMG (% MVC) Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total Control Low-CD High-CD Total 16 20.4 ± 2.1 168.9 ± 23.6 19.3 ± 1.5 21.5 ± 2.0 74.4 ± 27.9 59.5 ± 38.3 36.4 ± 7 26.0 ± 6.7 22.4 ± 6.9 22.2 ± 6.6 22.8 ± 7.7 22.5 ± 7.0 96.3 ± 7.4% 95.4 ± 3.7% 94.8 ± 3.9% 96.2 ± 7.4% 6.1 ± 2.2 6.1 ± 2.0 7.1 ± 2.5 6.4 ± 2.2 4.5 ± 1.2 4.5 ± 0.8 4.6 ± 2.7 4.5 ± 1.0 23.5 ± 5.6 23.5 ± 6.2 24.1 ± 4.8 23.7 ± 4.3 10.3 ± 9.7 11.5 ± 10.6 9.4 ± 9.6 10.4 ± 9.8 23.8 ± 38.9 63.0 ± 98.9 65.5 ± 102.3 49.3 ± 80.8 26.7 ± 15.8 26.3 ± 9.9 21.1 ± 6.1 24.2 ± 11.3 22.8 ± 42.2 72.0 ± 124.6 73.3 ± 126.9 40.6 ± 82.1 17 68.8 ± 4.4* 167.5 ± 11.3 68.4 ± 3.6 69.2 ± 4.9 77.8 ± 15.2 22.0 ± 21.8* 34.8 ± 7.3 26.2 ± 5.3 19.1 ± 6.6 18.3 ± 5.8 18.7 ± 6.3 18.7 ± 6.1* 97.9 ± 6.8% 93.2 ± 8.5% 94.7 ± 5.5% 97.6 ± 6.5% 8.0 ± 3.2 7.6 ± 3.1 8.7 ± 3.8 8.1 ± 3.4 6.0 ± 3.0 7.2 ± 4.1 6.8 ± 2.3 6.7 ± 3.2* 29.1 ± 6.0 27.2 ± 4.7 28.7 ± 8.4 28.3 ± 5.9* 5.7 ± 5.7 9.0 ± 9.2 7.7 ± 7.6 7.4 ± 7.6 35.6 ± 61.5 40.1 ± 57.8 56.2 ± 63.5 46.6 ± 58.9 32.9 ± 13.8 30.6 ± 11.8 29.9 ± 13.8 31.1 ± 12.9 30.3 ± 47.9 51.7 ± 83.8 52.0 ± 85.9 46.0 ± 73.8 * Variables reaching statistical significance for main effect of age (p < 0.05). PAQ = Physical Activity Questionnaire, STAI = State-Trait Anxiety Index, MVC = Maximal Voluntary Contraction, CV = Coefficient of Variation, TA = Tibialis Anterior, Gastroc = Gastrocnemius, EMG = Electromyography. 95 RESULTS MVC Torque At baseline, young adults were stronger than the older adults (22.5 ± 7 Nm vs. 18.7 ± 6.1 Nm respectively: age effect, p = 0.005) and men were stronger than women (sex effect, p = 0.0001) on all three days of testing, with no interaction between sex and age (age × sex, p = 0.69; Table 4.1). The relative reduction (%) in MVC torque after the fatiguing contraction was similar across sessions (session effect, p = 0.98), and similar for young and older adults (session × age, p = 0.26). During recovery, MVC torque increased to near baseline levels within 10 minutes of completing the fatiguing contraction similarly for young and older adults across all sessions (session × age, p = 0.57; Table 4.1). Furthermore, at the end of the recovery period, the MVC (% of baseline) was similar for young and older men and women (p > 0.05). Anxiety and Stress Levels State STAI Scores. Baseline state STAI scores taken at the beginning of each experimental session were similar for young and older men and women (age effect, p = 0.88; sex effect, p = 0.57; Table 4.1). State STAI scores taken after exposure to the 2 × 2 min of difficult mental math were higher during the high-cognitive demand session compared with the control and low-cognitive demand sessions (control, 25.1 ± 6.6; lowcognitive demand, 30.4 ± 10.0; high-cognitive demand, 42.8 ± 13.1; session effect, p = 0.0001) and immediately after the fatiguing contraction (control, 36.2 ± 9.2; lowcognitive demand, 38 ± 9; high-cognitive demand , 50.4 ± 13.8; session effect, p = 0.0001). There was no difference between young and older adults across all three session 96 after completing the fatiguing contraction (age effect, p = 0.72). Older adults however, demonstrated higher state STAI scores than young adults after the fatiguing contraction when exposed to the high-cognitive demand (session × time × age, p = 0.02). VAS for Stress and Anxiety. VAS scores for stress and anxiety were similar at baseline for young and older adults, and men and women (p > 0.05; Figure 4.1). Anxiety VAS was significantly higher during the high-cognitive demand session compared to the control and low-cognitive demand sessions (session × time, p = 0.0001), and increased more for older adults than young over time (time × age, p = 0.01; Figure 4.1A). There were no other interactions. Stress VAS scores were significantly higher for older adults than young during the high-cognitive demand session compared with the control and lowcognitive demand sessions (session × age, p = 0.02; Figure 4.1B) and for older adults than young over time (time × age, p = 0.001). There were no main effects of sex and no other interactions. 97 A Young Control Old Control Young Low-CD Old Low-CD Young High-CD Old High-CD Anxiety VAS (cm) 6 4 2 0 Baseline Cognitive Demand T1 T3 T2 T4 Recovery T5 T6 T7 Time B Stress VAS (cm) 6 4 2 0 Baseline Cognitive Demand T1 T2 T3 Recovery T4 T5 Time T6 T7 Figure 4.1. Visual Analogue Scale (VAS) scores for anxiety (A) and stress (B). Mean (±SE) VAS scores for young adults (closed symbols) and older adults (open symbols) are shown for anxiety (A) and stress (B) throughout the experimental protocol during the control session (circles), lowcognitive demand session (Low-CD, triangles), and high-cognitive demand session (High-CD, squares). Time intervals were as follows: baseline (T1), after the first bout of 2 min of quiet rest/mental math (T2), after the second bout of 2 min of quiet rest/mental math (T3), after the 5% submaximal contraction (T4), during recovery immediately after task failure (T5), and then at 5 min (T6) and 10 min of recovery (T7). 98 Low-Intensity Sustained Contraction (5% MVC) Fluctuations in Torque. Older adults had greater fluctuations in torque (coefficient of variation of force: CV) compared with young across all three sessions (age effect, p = 0.007; Figure 4.2) and women had higher fluctuations in torque than men (sex effect, p = 0.001). On average, the older adults had a linear increase in fluctuations in torque as cognitive demand increased while young adults showed no change (linear interaction for session × age, p = 0.04; Figure 4.2A). Women demonstrated higher force fluctuations than men (p = 0.001), but there were no other interactions (p > 0.05). 99 A 20 Young Old CV of Torque (%) 18 16 14 12 10 8 6 Control Low-CD High-CD Session Figure 4.2. Mean Session Coefficient of Variation (CV) of torque during the 5% MVC task for young (closed symbols) and older (open symbols) adults during the control, lowcognitive demand (Low-CD), and high-cognitive demand (High-CD) sessions (A), and representative force tracings of a young and older adult (B). Older adults had significantly higher CV of torque than young adults (age effect, p = 0.007). 100 MAP and Heart Rate. Cardiovascular measures were analyzed for only those older participants who were not currently taking blood pressure medications at the time of the experiment (young, n = 16; older, n = 11). During the 5% MVC task, MAP was higher during the high-cognitive demand session (105.1 ± 18.8 mmHg) than the lowcognitive demand session (87.2 ± 28.3 mmHg) and the control session (88.8 ± 23 mmHg; session effect, p = 0.001). There was no influence of age or sex on MAP (p > 0.05). Similarly, heart rate was greater during the high-cognitive demand session (79.6 ± 15 beats.min-1) compared with the low-cognitive demand (71.6 ± 28 beats.min-1) and control sessions (68.2 ± 19.4 beats.min-1, session effect, p = 0.04) regardless of age of sex (p > 0.05, i.e. no interactions). Consequently, the rate pressure product was higher during the high-cognitive demand session than the control and low-cognitive demand sessions (session effect, p = 0.0001), but there was no difference between young and older adults (age effect, p = 0.52) or men and women (sex effect, p = 0.70). EMG Activity. During the 5% MVC task, older adults had higher soleus RMS EMG (% MVC) (antagonist muscle) than young adults during the high-cognitive demand session (session × age, p = 0.04), but there were no other interactions, or main effects of age or sex or session for soleus or any other muscles (tibialis anterior, gastrocnemius, and rectus femoris). Fatiguing Contraction (30%MVC) Time to Task Failure. There was no difference in time to task failure across sessions or with age (p > 0.05; Table 4.1 and Figure 4.3). There was no difference in time to task failure between men and women (sex effect, p = 0.96) and no interactions for age, sex and session (p > 0.05). Variability between the three sessions in the time to task 101 failure (comparison of standard deviation [SD] generated from the three sessions for each participant) however, was greater for older adults than young adults (2.02 ± 1.05 min vs. 1.25 ± 0.51 min respectively, p = 0.02), but similar for men and women (sex effect, p = 0.53; sex × age, p = 0.57; Figure 4.3). Furthermore, we compared the SD for time to task failure between sessions (SD for control and low-cognitive demand session versus SD for control and high-cognitive demand session) to determine if variability increased with difficulty of the mental math. While the age effect remained (age effect, p = 0.03), there were no effects of session (session effect, p = 0.38) or sex (sex effect, p = 0.69), and no interactions. Thus, although the older adults were more variable than young between the three sessions due to addition of a cognitive task, the variability between the older adults did not increase with to the difficulty of the cognitive task. 102 Young Time to Failure (min) A 25 Men Women (n = 8) (n = 8) Control Low-CD High-CD 20 15 10 5 0 Mean Subject Old Time to Failure (min) B 25 Men Women (n = 9) (n = 8) 20 15 10 5 0 Subject Mean Figure 4.3. Time to task failure during the fatiguing contraction for individual young (A) and older adults (B) during the control (circle), low-cognitive demand (Low-CD, triangle), and high-cognitive demand (High-CD, square) sessions. Time to task failure is shown for each young (A) and older (B) man and woman for each session (separated by dashed line). The aggregate mean (±SE) is shown for each session after the solid vertical line. The range and variability of time to task failure among the older adults was greater than for the young adults for each session. Older adults were more variable than young between the three sessions due to addition of a cognitive task (age effect, p = 0.03), but variability did not increase with task difficulty (session effect, p = 0.38). 103 Fluctuations in Torque. Fluctuations in torque (CV) increased over time during the sustained contraction (time, p < 0.001) similarly across sessions for both age groups (session x time, p = 0.56; Figure 4.4C). Older adults however, had larger fluctuations in torque than young adults (age effect, p = 0.002) and this difference was similar across sessions (session × age, p = 0.11). Women also had larger fluctuations in torque than men (sex effect, p = 0.005) and this sex difference was similar across sessions (session × sex, p = 0.69). There were no other interactions (p > 0.05). Because older and young adults can demonstrate more variability in motor performance than young adults, we evaluated variability of the fluctuations in torque (CV of torque) during the 30% MVC task (comparison of standard deviation [SD] generated from the three sessions for each participant) between young and older adults. Variability was greater for older adults than young adults (p = 0.01), and greater for women than men (sex effect, p = 0.02), but there were no interactions (sex × age, p = 0.94; Figure 4.4A-B). 104 Young CV of Torque (%) A Old Men Women (n = 8) (n = 8) 20 B 20 15 15 10 10 5 5 Subject C Mean Men Women (n = 9) (n = 8) Subject Control Low-CD High-CD Mean CV of Torque (%) 10 Young Control Old Control Young Low-CD Old Low-CD Young High-CD Old High-CD 8 6 4 2 0 0 0 0 0 0 0-2 20-4 40-6 60-8 0-10 8 Time (%) Figure 4.4. Coefficient of Variation of Force during Fatiguing Contraction. Coefficient of variation (CV) of force during the fatiguing contraction (30% MVC) for individual young (A) and older adults (B), and across time for young and older adults (C) during the control session (circle), low-cognitive demand session (Low-CD, triangle), and high-cognitive demand session (High-CD, square). CV of force is shown for each young (A) and older (B) man and woman for each session. The aggregate mean is shown for each session after the solid vertical line. In panel (C), the CV of torque is shown for the control (circle), lowcognitive demand (triangle), and high-cognitive demand (square) sessions for young (closed symbols) and older (open symbols) adults during five continuous intervals of 20% during the fatiguing contraction. The x-axis intervals for panel (C) indicate the last time point of data average along in that interval (20% on the axis for example, is equivalent to the 0-20% of the contraction, and the 100% represents 80-100% of the time to failure). Older adults had significantly higher CV of torque than young adults (age effect, p = 0.002). 105 EMG Activity of Agonist Muscles. The amplitude of the RMS EMG (% MVC) for the tibialis anterior (ankle dorsiflexors), increased during the fatiguing contraction (time effect, p < 0.001) and similarly across sessions (session × time, p = 0.38). Furthermore, tibialis anterior EMG activity was greater for the older adults than young during all three sessions (age effect, p = 0.002; Table 4.1), but there was no difference between men and women (sex effect, p = 0.80), and no interactions. EMG bursting activity (bursts per minute) increased over time during the fatiguing contraction (30% MVC) for all three sessions (time effect, p = 0.002); however, there was no difference across the sessions (session effect, p = 0.50). Neither age nor sex influenced the bursting activity during the fatiguing contraction (p > 0.05; Table 4.1). EMG Activity of Antagonist and Accessory Muscles. The RMS EMG amplitude (% MVC) for the soleus, gastrocnemius and rectus femoris increased over time during the fatiguing contractions (time effect, p < 0.001 for all muscles) but was similar across sessions (session effect, p > 0.05 for all muscles). Older adults however, had higher RMS EMG for the soleus (age effect, p = 0.04), gastrocnemius (p = 0.01) and rectus femoris (p = 0.03) than young adults. There was no effect of sex for gastrocnemius and soleus (sex effect, p > 0.05) and there were no interactions (p > 0.05). For the rectus femoris, however, women had higher RMS EMG amplitudes than men (sex effect, p = 0.04), but there were no interactions (p > 0.05). There was a correlation between rectus femoris RMS EMG and CV of torque for the high-cognitive demand session only, indicating that participants who had greater torque fluctuations during the high-cognitive demand session also had greater rectus femoris EMG (r33 = 0.31, p = 0.04). 106 MAP and Heart Rate. Cardiovascular measures were included only for older participants who were not currently taking blood pressure medications at the time of the experiment (young, n = 16; older, n = 11). MAP, heart rate, and rate pressure product increased over time (time, p < 0.05; Figure 4.5). MAP, heart rate and rate pressure product were higher during the high-cognitive demand session than the control or lowcognitive demand sessions (session effect, p < 0.05). MAP was higher for older adults during the fatiguing contraction in the high-cognitive demand session over time than other sessions (session × time × age, p = 0.02). Heart rate and rate pressure product, however, were similar for older adults over time across sessions (session × time × age, p > 0.05), with no main effects of age or sex (p > 0.05). 107 A 140 Young Control Old Control Young Low-CD Old High-CD Young High-CD Old High-CD MAP (mmHg) 130 120 110 100 90 80 0 B 25 50 75 Time (%) 100 130 Heart Rate (b.min-1) 120 110 100 90 80 70 60 50 0 Rate Pressure Product (x10 3) C 25 50 75 100 Time to Failure (%) 13 12 11 10 9 8 7 6 0 25 50 75 100 Time (%) Figure 4.5. Mean Arterial Pressure (MAP), Heart Rate and Rate Pressure Product. Mean arterial pressure, heart rate and rate pressure product during the fatiguing contraction for young and older adults across sessions. The values are mean ± SE at 25% increments of time to task failure for young (closed symbols) and older (open symbols) adults during the control (circles), low-cognitive demand (Low-CD, triangles) and high-cognitive demand (High-CD, squares) sessions for mean arterial pressure (A), heart rate (B), and rate pressure product (C) during the fatiguing contraction. Averages of 15-s intervals were used for the MAP and heart rate. Rate-pressure product was the product of heart rate and MAP for the equivalent time periods in panels (A) and (B). 108 Rating of Perceived Exertion (RPE). Perceived exertion increased during the fatiguing contraction (time effect, p = 0.0001) similarly across sessions (session effect, p = 0.59). RPE was similar for young and older adults (age effect, p = 0.82) and men and women (sex effect, p = 0.62), with no interactions (p > 0.05). Mean RPE across all three sessions was 4.2 ± 1.5 and 4.2 ± 1.8 at the beginning of the fatiguing contraction for young and older adults respectively and increased to 8.7 ± 2.3 vs. 9.1 ± 1.4 by the end of the fatiguing contraction. Error rate. The mental math error rate during the fatiguing contraction (errors/min) was significantly higher during the high-cognitive demand session (2.9 ± 1.3 errors/min) compared with the low-cognitive demand session (0.4 ± 0.4 errors/min, session effect, p < 0.001). There was no main effect of age or sex (p > 0.05). There was also no correlation between error rate and CV of torque during the fatiguing contraction for the low-cognitive demand (r33 = -0.17, p = 0.35) or high-cognitive demand (r33 = 0.26, p = 0.14) sessions; nor were there significant associations between error rate during the fatiguing contraction and the time to task failure for the low-cognitive demand (r33 = 0.12, p = 0.52) or high-cognitive demand (r33 = -0.06, p = 0.74) sessions. DISCUSSION This study imposed several levels of cognitive demand during sustained low- and moderate-force isometric contractions with the ankle dorsiflexor muscles to determine the influence of increased cortical involvement on motor function and fatigability in young and older adults. The novel findings of this study were that as cognitive demand increased, steadiness decreased (i.e. CV of torque increased) during the very low-force contraction (5% MVC) for older adults but did not change for the young adults. While 109 fatigability (time to failure) of a moderate intensity contraction (30% MVC) was not essentially different with imposed cognitive demand for young or older adults, variability in the time to failure and in the torque fluctuations across sessions was greater for the older adults than the young adults. These results provide evidence that increased cortical involvement of motor and non-motor cortical areas can disrupt motor performance of low-to-moderate intensity isometric contractions of the lower limb more so in older adults than young adults. Steadiness was Reduced with Age in the Lower Limb Torque fluctuations (CV) were greater (steadiness reduced) during the lowintensity contraction (5% MVC) than at the start of the 30% MVC task (prior to fatigue), and also greater for older adults than young across all sessions. Larger torque fluctuations with advanced age have also been observed under control conditions across various muscle groups and particularly at the lower intensity contractions for both young and older men and women (Enoka et al., 2003; Tracy, Dinenno, et al., 2007; Tracy et al., 2005). Typically, the CV (%) will decrease as contraction intensity increases (Enoka et al., 2003; Moritz et al., 2005; Taylor, A. M. et al., 2003; Tracy, 2007a) and as we observed. Because we showed increased torque fluctuations during ankle dorsiflexion for the older adults during both 5% MVC and 30% MVC tasks compared with young, the age-related mechanism for reduced steadiness under control conditions influences both the low- and moderate-force tasks in the ankle dorsiflexor muscles. CV of torque across a range of low-to-high forces appear to be primarily modulated by low-frequency oscillations (< 2 -3 Hz) in neural drive found in motor unit action potentials trains (Dideriksen et al., 2012; Negro et al., 2009) with some contribution from increased motor 110 unit variability at very low forces (Dideriksen et al., 2012; Jesunathadas et al., 2012). This low-frequency oscillating neural drive likely reflects an integration of both descending and afferent inputs onto the motoneurone pool (Dideriksen et al., 2012; Farina et al., 2012; Negro et al., 2009). With advanced age, the motoneurone pool undergoes remodeling that results in decreased motor units numbers and altered relations between discharge rates and recruitment thresholds (Barry et al., 2007); the age difference in torque fluctuations, therefore, appears to be due to age-related changes in the inputs to the motoneurone pool (Barry et al., 2007) with possibly some influence of greater motor unit discharge rate variability in older adults (Barry et al., 2007; Kornatz et al., 2005; Laidlaw et al., 2000; Tracy et al., 2005). Age-related changes in visual-motor processing may also contribute to altered motoneuronal inputs causing increased torque fluctuations during static contractions with age (Fox et al., 2013; Henningsen et al., 1997; Seidler-Dobrin & Stelmach, 1998; Tracy, Dinenno, et al., 2007). Cardiovascular Responses and Anxiety were Elevated with High Cognitive Demand While mental math was used to manipulate different levels of cognitive demand in this study, it can also increase anxiety and stress (Kajantie & Phillips, 2006) as it did for the young but more so in the older adults during the high-cognitive demand task (see Figure 4.1). Accordingly, MAP and heart rate were elevated when the difficult mental math was performed during both the 5% MVC and 30% MVC tasks, although similarly for the young and older adults. Older adults have reduced maximal heart rates compared with young, in less than for young, explaining the similar age-related increase in heart rate despite older adults reporting they felt more anxious and stressed. Because both MAP and heart rate were elevated, rate-pressure product was elevated for both young and 111 older adults indicating increased cardiac work and myocardial oxygen consumption (Gobel et al., 1978; Wasmund et al., 2002) during the 5% MVC task and 30% MVC fatiguing contraction when cognitive demand was high. Chronicity of high blood pressure has been associated with an increased risk of stroke, cognitive decline and dementia, especially in older adults with untreated high blood pressure (Tzourio, Christophe 2007; Tzourio, Christophe et al., 1999). In the short term, increased stress and anxiety can increase monoaminergic drive; neuromodulatory inputs alter excitability of the motoneurone pool (Heckman et al., 2009) and potentially alter motor neuron output especially at low forces. Steadiness Decreased with Cognitive Demand in Older Adults A novel finding was that the age difference in CV of torque grew linearly (i.e. steadiness decreased) with the increased levels of cognitive demand during a lowintensity contraction (5% MVC) of the lower limb. Although steadiness during the 30% MVC task was similar across sessions, older adults had greater variability in the CV of torque between sessions than the young. Increased torque fluctuations during the 5% MVC task between the low- and high-cognitive demand sessions indicate that the decline in steadiness was not solely due to the added distraction or challenge of talking because the low-cognitive demand task controlled for those factors. Because motor unit discharge rate variability can contribute to the force fluctuations at very low forces (Jesunathadas et al., 2012; Tracy et al., 2005), increased variability of the motor unit pool in the older adults may have been altered when high-cognitive demand was imposed. Our findings indicate that increased antagonist muscle activation may also have contributed to the larger force fluctuations in the older adults when cognitive demand was 112 high during the 5% MVC task. The older adults had greater soleus muscle activation during the high-cognitive demand session relative to the other sessions and compared with the young adults: this suggests less inhibition of the antagonist muscle from descending cortical sources. Increased antagonist muscle activity is a strategy adopted by older adults to stiffen joints and reduce movement variability with age (Hortobagyi & DeVita, 2006). For the higher force task (30% MVC) the torque fluctuations were larger with age, but there was no increase in the CV of torque with cognitive demand for young or older adults. Agonist (tibialis anterior), antagonist (gastrocnemius, soleus) and synergist (rectus femoris) activations were greater in the older adults than the young across all sessions, possibly contributing to the larger torque fluctuations with age. Because both the agonist (tibialis anterior) and antagonist activation were greater in the older adults compared with the young, activation differences had minimal effect on the greater CV of torque in the older adults. There are numerous age-related changes along the neuraxis that can alter inputs to the motoneurone pool and perhaps make it more susceptible to altering motor output when cognitive demand is imposed. Cortical size (Raz et al., 2007) and processing are diminished with age, along with reduced corticospinal fibers numbers (Eisen et al., 1996), and changes in spinal reflex pathways (Kido et al., 2004) which result in decreased cortical inhibition of both cognitive and motor processes with age (Hunter, Todd, et al., 2008; Peinemann et al., 2001; Sale & Semmler, 2005). Age-related changes along the neuraxis can also result in increased activation of antagonist muscles (Hortobagyi & DeVita, 2006; Macaluso et al., 2002) and may have been responsible for the greater activation of antagonist muscles with age in the current study. 113 Several theories of motor control assert that the division of attentional resources during a dual-task paradigm has limits and these limitations increase with advanced aging due to diminished cortical processing (Woollacott & Shumway-Cook, 2002). Consequently some studies show older adults have less ability to simultaneously perform a cognitive task and motor task as well as they can be performed individually (Fraser et al., 2010; Johnson & Shinohara, 2012; Voelcker-Rehage & Alberts, 2007). Changes in performance for older adults in dual-tasks appear to be especially sensitive to cognitive tasks that require executive function (Yogev-Seligmann et al., 2008). Executive function (which included working memory, which was varied in this study), anxiety, and stress are modulated in prefrontal cortical regions and the anterior cingulate cortex (Banich et al., 2009; Miller, 2000; Owen et al., 2005; Schweizer et al., 2013). Prefrontal connections to motor areas (Takahara et al., 2012) along with input to neural connections between these and other cortical centers associated with cognition, anxiety and motor function could directly alter motor output, as was observed in this study. Capacity theories of attention that assume attentional resource limitations on the ability to perform multiple tasks simultaneously (Hiraga et al., 2009; Kahneman, 1973; McDowd, 2007) would predict even greater decrements in steadiness for older adults when descending drive from the motor cortex increased during the 30% MVC task and as the fatiguing contraction progressed. Interestingly, error rates in mental math (executive function task) during the fatiguing contraction did not differ across the age groups for the low-cognitive demand sessions and high-cognitive demand, indicating that mental math performance was not diminished in the older adults compared with the young. Although the variability in the CV of torque across the sessions was greater for the older adults than 114 the young, the mean values in CV of torque were similar across the three sessions during the 30% MVC task and increased at similar rates to the young adults during the fatiguing contraction. Time to failure was also similar across sessions for each age group, and the increase in EMG activity, EMG bursting activity and perceived effort during the fatiguing contraction progressed at similar rates across sessions. Increases in EMG and RPE during a fatiguing contraction are the result of increased descending drive to recruit more motor units in an effort to maintain the required force as the working muscle becomes progressively fatigued (Riley et al., 2008). Thus, while some older adults are clearly more affected than others by the increased cognitive demand during the sustained contractions (Figure 4.4B), capacity limitations in cortical regions of older adults cannot alone explain the loss of steadiness, especially at the very low forces when descending drive was not large. Another explanation for the reduced steadiness in older adults as cognitive demand increased is that descending and afferent inputs to the motoneurone pool differed for the older and young adults. One input that likely differed between the young and older adults was monoaminergic drive (Christou et al., 2004). Increased monoaminergic drive to the spinal cord from the brainstem enables motoneurone activation and is essential for exercise (Heckman, 2003), but monoaminergic drive is attenuated in older adults (Meltzer et al., 1998; Reynolds & Meltzer, 1999; Seals & Esler, 2000), potentially leading to decreased motor output and altered responses to increased anxiety and stress compared with young. 115 Sex Differences in Steadiness Both young and old women demonstrated heightened levels of stress and anxiety, and greater torque fluctuations during the very low-intensity and fatiguing contractions for all three sessions compared with men, regardless of the magnitude of cognitive demand. Similar sex differences in stress and anxiety (Christou et al., 2004) and in torque fluctuations have been shown previously in the upper limb (Brown et al., 2010; KellerRoss, Pruse, et al., 2014; Yoon et al., 2009); however, this is the first study to demonstrate increased torque fluctuations in women when performing submaximal contractions of the lower limb with varying levels of cognitive demand. Greater torque fluctuations in women when performing upper extremity tasks have been attributed to strength difference between men and women (Brown et al., 2010), although the mechanism is not known. When exposed to a stressful noxious stimulus prior to task performance, increased torque fluctuations have been attributed to greater activation of central neural mechanisms in response to increased stress and anxiety (Christou et al., 2004); however, in the current study, women demonstrated greater torque fluctuations and reported higher stress and anxiety than men regardless of the magnitude of cognitive demand (i.e. in both low- and high-cognitive demand tasks compared with control). Because both aging and decreased levels of estrogen in postmenopausal women possibly contribute to changes in monoaminergic drive (Meltzer et al., 1998), women may be even more vulnerable than men to diminished motor output with aging (Figure 4.4B); however, it remains unclear if greater torque fluctuations in women are related to activation of alternate neural pathways, or a strength-related mechanism. 116 Increased Variability in Fatigability with Cognitive Demand and Aging Fatigability (time to task failure) of the ankle dorsiflexor muscles was similar across age groups and sessions. Hence, there was no systematic decrease in fatigability when cognitive demand was imposed during the sustained contraction with the ankle dorsiflexor muscles. In contrast, the elbow flexor muscles were more fatigable when high-cognitive demand was imposed in young men and women (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). Similarly, handgrip muscles were more fatigable in both young men and women when high-cognitive demand was imposed for high intensity contractions (Bray et al., 2012; Bray et al., 2008) but not for relatively strong men during a low-intensity sustained contraction (Keller-Ross, Schlinder-Delap, et al., 2014). The largest increases in fatigability were related to the initial muscle strength such that weaker participants experienced the greatest increases in fatigability (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). Perfusion associated changes within the muscle in response to a mental-math task (which was used to induce high-cognitive demand) was implicated but only partially explains these findings (Keller-Ross, Pruse, et al., 2014; Yoon et al., 2009). In contrast to the elbow flexor muscles (Hunter, Critchlow, & Enoka, 2004), the ankle dorsiflexor muscles exhibit lesser differences between sub-populations including men and women (Avin et al., 2010), and young and older adults (Griffith et al., 2010; Kent-Braun et al., 2002) and we show here the fatigability of this muscle group is also less responsive to cognitive demand. Christie and Kamen (2009) attribute the lack of difference in fatigability of the dorsiflexor muscles between young and older adults to a lack of difference in motor unit discharge rates, suggesting young and older adults adopted similar neural adaptations during the fatiguing contractions. We found that the 117 increase in EMG activity of the tibialis anterior muscle during the fatiguing contraction was similar across sessions for young and older adults, although older adults had greater EMG relative to the young. Another possible explanation for the different responses in fatigability with and without cognitive demand is a decreased number of corticospinal connections and larger motor unit ratio (motoneurone to fibers) in large lower limb muscles compared with the upper limb (Feinstein et al., 1955). A reduced number of corticomotor inputs to the dorsiflexor muscles relative to the upper limb muscles may minimize the modulating inputs from higher centers imposed with high-cognitive demand and lessen the responsiveness during the sustained fatiguing contractions at the moderate intensity. While there was no systematic reduction in time to failure of the ankle dorsiflexor muscles as we have observed with the upper limb, older adults, particularly older women, demonstrated significantly more variability in their time to task failure between sessions than young adults (Figure 4.3). Variability between trials of a motor task can be exacerbated with increased cognitive demand in young people (Lorist et al., 2002) but is often greater with age as shown here. The greater variability in performance with advanced age can be due greater variability in cortical and motor nerve activation during motor tasks (Hunter, Todd, et al., 2008; Yoon et al., 2008). This greater age-related variability in a motor task when a cognitive task was imposed further demonstrates the important role of cognitive control in determining reliability of performance of motor tasks such as those in the work force. 118 Conclusion This study demonstrated that older adults exhibit more variability than young adults in fatigability and less steadiness while performing low-force and moderate isometric with the ankle dorsiflexor muscles. For very low-force contractions, steadiness decreased further as greater cognitive demand increased. The reduced steadiness in older adults compared with the young, may be related to modulation of synergist and antagonist muscles and an altered neural strategy with age. Older adults also exhibited greater variability in steadiness between sessions and in fatigability as cognitive demand was imposed. Increased variability in lower extremity tasks may negatively impact activities of daily living and work tasks that require high-cognitive demand in an aging population. These data also expose differences within an older adult but also between older adults. Our results provide evidence that increased involvement of non-motor cortical areas can disrupt motor performance of low-to-moderate intensity isometric contractions of the lower limb more so in older adults than young adults. These findings have significant implications related to successful aging and performance of activities of daily living with advanced age especially those activities that require simultaneous execution of a cognitive task that involves working memory and maintenance of a static motor task. 119 CHAPTER V Discussion This dissertation highlights the importance of cortical involvement in lower limb motor function, in particular during low-to-moderate force static contractions. Despite the critical role of force production in the lower limb to perform functional tasks, such as walking, studies investigating the role of the cortex in force production have been predominantly performed on the upper extremity resulting in a lack of information regarding the role of the cortex in the lower limb. This dissertation first investigated the cortical activation during static contractions of the lower limb muscles in young men and women using fMRI (study 1, aim 1). This study was then followed by determining the influence of cortical inputs on force control in young and old men and women, particularly force fluctuations and fatigability (time to task failure), in the lower limb during static contractions by manipulating factors that potentially alter cortical inputs from including force intensity and cognitive demand (study 2 and study 3, aim 2). One of the first novel findings of this dissertation was that several motor and sensory areas of the cortex scaled linearly with increased torque (primary motor and sensory cortices, basal ganglia and cerebellum) (study 1, aim 1). Although lower limb muscle control during static contractions is different from the upper limb (eg., Jesunathadas et al., 2012) and lower limb muscles have fewer direct corticospinal connections (Brouwer & Ashby, 1990) than upper limb muscles, the active areas in the cortex associated with lower limb muscles were not unexpected because previous studies 120 have demonstrated similar areas of activation during active ankle movements (Ciccarelli et al., 2005; Trinastic et al., 2014). Previous studies have also demonstrated scaled cortical activity with increased force production (in volume, intensity or both) in the upper limb (Spraker et al., 2007; Thickbroom et al., 1998; Vaillancourt et al., 2004; Vaillancourt et al., 2007), however this is the first study to demonstrate similar scaling with force in the lower limb. Furthermore, cortical areas that are important to modulation of steadiness were identified. Study 1 determined that there are several motor (basal ganglia, cerebellum, M1/SMA, and insula) and typically non-motor (superior frontal gyrus, cingulate cortex) areas that increased activation with increased force fluctuations during high intensity contractions of the ankle dorsiflexors. The amplitude of the force fluctuations (SD of torque) increased with force intensity due to activation of more motor units (Laidlaw et al., 2000) and corresponded with motor areas that increased activation intensity between low and high forces. More importantly, the results also indicated that other areas of the cortex (putamen, insula, contralateral superior frontal gyrus and ipsilateral inferior lobes) were correlated with control of steadiness of the lower limb during target matching contractions. CV of force (amplitude of the fluctuations normalized to the mean torque) does not increase with force intensity and is usually largest at lower intensities of contraction [e.g. (Jesunathadas et al., 2012; Moritz et al., 2005; Taylor, A. M. et al., 2003; Tracy, Mehoudar, et al., 2007)]. During the 10% MVC task the CV was largest and both the left superior frontal gyrus motor region and the ipsilateral inferior lobes showed the largest correlations between CV of force and cortical activation. The CV of force is thought to be mostly mediated by low-frequency oscillations in neural drive (< 2 -3 Hz) 121 during isometric contractions (Dideriksen et al., 2012; Negro et al., 2009) across a range of forces. This data raises possibility that these motor regions, particularly the putamen and the superior frontal gyrus, both of which are associated with limb movement regulation (Ciccarelli et al., 2005) and force production control (Kuhtz-Buschbeck et al., 2008) and showed the largest correlations between CV of force and activation for the 10% MVC task, which is the intensity that CV was largest across the intensities, may be the sources of the low-frequency oscillating neural drive that influences action potentials trains and ultimately the CV of force. Cognitive demand however exacerbated the age-related increases in force fluctuations during the light load contractions. Contrary to the young adults, older adults demonstrated decreased steadiness while performing a low-force (5% MVC) ankle dorsiflexion contraction with increased cognitive demand (study 3, aim 2). Increased force fluctuations during the 5% MVC contraction in the presence of increased cognitive demand in older adults appears to be the result of two age-related consequences that occur with normal aging in the neuromuscular system and cognitive processes. The extensive alterations in the neuromuscular system with aging (Doherty, 2003; Plow et al., 2013; Semmler et al., 2006) includes altered relations between discharge rates and recruitment thresholds (Barry et al., 2007), as well as increased in motor unit discharge rate variability (Barry et al., 2007; Kornatz et al., 2005; Laidlaw et al., 2000; Tracy et al., 2005), resulting in increased force fluctuations. Similar age-related changes appear to occur in the prefrontal cortex (Hedden & Gabrieli, 2004, 2005), making cognitive tasks that demand executive function (Miller & Cohen, 2001; West, 1996) and neural connectivity from the prefrontal cortex to other essential cortical regions during executive 122 function tasks (Madden et al., 2010) particularly vulnerable with age. Attentional resource limitations alter the older adult’s ability to perform multiple tasks (motor and cognitive tasks) simultaneously (Hiraga et al., 2009; Kahneman, 1973; McDowd, 2007), subsequently resulting in diminished performance in either one or both of the tasks (Tombu & Jolicoeur, 2003; Yogev-Seligmann et al., 2008). This could be particularly disruptive when trying to perform a low-force functional task like accelerating or decelerating a car while talking or answering a question. Unlike the elbow flexor muscles (Yoon et al 2009, Keller-Ross 2014) fatigability was not increased for the ankle dorsiflexor muscles for young or older adults. Muscle fatigue is specific not only to the demands of the task (Hunter, 2014; Hunter, Duchateau, et al., 2004) but is specific to the muscle group involved. Some muscle groups such as the ankle dorsiflexors are less susceptible to sex and age differences (Avin & Law, 2011) but as shown in this dissertation, they may also be less susceptible to cortical inputs manipulated with cognitive demand. Although fatigability was not altered with imposed cognitive demand in young or older adults, variability in the time to failure across sessions was greater for the older adults than the young adults. The greater variability in performance of maximal contractions with advanced age can be due greater variability in cortical and motor nerve activation during motor tasks (Hunter, Todd, et al., 2008; Yoon et al., 2008). The results in this dissertation show that this age-related variability is particularly large during submaximal task of the lower limb and that cognitive demand will exacerbate this variability for a participant and between old adults. These results indicate cortical involvement in the greater variability possibly due to reduced inhibition onto the 123 motoneurone pool. For example, older adults had greater soleus muscle activation during the high-cognitive demand session relative to the other sessions and compared with the young adults (study 3, aim 2): this suggests less inhibition of the antagonist muscle from descending cortical sources (Baudry et al., 2010). Age-related changes along the neuraxis can also result in increased antagonist muscle activation (Hortobagyi & DeVita, 2006; Macaluso et al., 2002), which may be a response to decreased intracortical inhibition that appears to only occur in older adults as a consequence of the increased demands place on the remodeled neuromuscular system (Plow et al., 2013). Future studies that involve assessment of cortical inhibition [which can be done with paired pulse cortical stimulation (eg., McGinley et al., 2010)] with and without cognitive demand during a motor task may provide insight into the role of age-related cortical inhibition and its contribution to the increased in motor variability among older men and women. The results of this dissertation contribute important information to the current body of literature regarding the role of the cortex in force production and control in the lower limb. Understanding the key cortical areas associated with force control in the lower leg in healthy young adults, and the influence of factors that increased descending drive from the cortex, such as increased cognitive demand or fatigue, establishes a foundation for identifying the plasticity of these areas with impaired motor function that can occur with neurological conditions and aging as well as enhanced function that is possible with physical exercise in all populations. These results also provide evidence that increased involvement of motor and nonmotor cortical areas can disrupt motor performance of low-to-moderate intensity isometric contractions in the lower limb more so in older adults than young adults. These 124 results have important performance implications for cognitively demanding and low-tomoderate-force tasks that are common to daily function in older adults. Key cortical and subcortical brain areas associated with control of lower limb steady contractions were also identified in healthy men and women and could be targeted in future studies to better understand the influence of cortical control on neuromuscular impairments such as those that occurs with neurologic conditions as well as normal and pathologic aging. Moderate, regular exercise (Granacher et al., 2010; Krebs et al., 1998) and practice (Marmon, Gould, et al., 2011; Silsupadol et al., 2009; Voelcker-Rehage & Alberts, 2007; Yogev-Seligmann et al., 2008) may reduce variability in motor performance and encourage efficient adaptive allocation of cognitive resources (Schaefer & Schumacher, 2011) to better ensure successful task performance. In a clinical setting, it may be a valuable use of time to evaluate the cost of dual tasking through the use of clinical tests (such as Stops walking while talking, and walking while performing mental math or verbal fluency tasks)(Yogev-Seligmann et al., 2008) and utilize practice to ameliorate the effects of dual tasking particularly in older adults because they have been shown to improve performance with practice (Yogev-Seligmann et al., 2008). The information provided in this dissertation can be helpful in understanding cortical contributions to force control in the lower extremity, although many questions still remain. The adult neuromuscular system undergoes significant changes with advance age, and acquiring a better understanding of the associated neural processes and cortical control can help with design of recovery programs from conditions that impact both cognitive and motor function such as stroke. Examples of age-related changes in cortical activity include decreased lateralization in M1 activity during motor task performance 125 (Langan et al., 2010) and the prefrontal cortex during cognitive performance (Cabeza, 2002) found in older adults compared with young adults. These changes can have implications regarding the compensatory strategies older adults utilize in order to perform the same tasks as young adults. Further, because changes in cognitive demand have been demonstrated to influence motor performance in the older adult, understanding the cortical regions associated with dual-task performance in the lower limb of young and old men and women may provide valuable information that can be utilized to improve functional task performance. 126 BIBLIOGRAPHY Aagaard, P. (2003). Training-Induced Changes in Neural Function. Exerc Sport Sci Rev, 31(2), 61-67. Aine, C.J., Sanfratello, L., Adair, J.C., Knoefel, J.E., Caprihan, A., & Stephen, J.M. (2011). Development and Decline of Memory Functions in Normal, Pathological and Healthy Successful Aging. Brain Topogr, 24(3-4), 323-339. doi: 10.1007/s10548-011-0178-x Artero, S., Touchon, J., & Ritchie, K. (2001). Disability and Mild Cognitive Impairment: A Longitudinal Population‐Based Study. International Journal of Geriatric Psychiatry, 16(11), 1092-1097. doi: 10.1002/gps.477 Ashe, J. (1997). Force and the Motor Cortex. Behav Brain Res, 87(2), 255-269. Avin, K.G., & Law, L.A. (2011). Age-Related Differences in Muscle Fatigue Vary by Contraction Type: A Meta-Analysis. Phys Ther, 91(8), 1153-1165. doi: ptj.20100333 [pii] 10.2522/ptj.20100333 Avin, K.G., Naughton, M.R., Ford, B.W., Moore, H.E., Monitto-Webber, M.N., Stark, A.M., . . . Law, L.A. (2010). Sex Differences in Fatigue Resistance Are Muscle Group Dependent. Med Sci Sports Exerc, 42(10), 1943-1950. doi: 10.1249/MSS.0b013e3181d8f8fa Banich, M.T., Mackiewicz, K.L., Depue, B.E., Whitmer, A.J., Miller, G.A., & Heller, W. (2009). Cognitive Control Mechanisms, Emotion and Memory: A Neural Perspective with Implications for Psychopathology. Neuroscience & Biobehavioral Reviews, 33(5), 613-630. doi: http://dx.doi.org/10.1016/j.neubiorev.2008.09.010 Barry, B.K., Pascoe, M.A., Jesunathadas, M., & Enoka, R., M. (2007). Rate Coding Is Compressed but Variability Is Unaltered for Motor Units in a Hand Muscle of Old Adults. Journal of Neurophysiology, 97, 3206-3218. doi: 10.1152/jn.01280.2006 Baudry, S., Maerz, A.H., & Enoka, R.M. (2010). Presynaptic Modulation of Ia Afferents in Young and Old Adults When Performing Force and Position Control. J Neurophysiol, 103, 623-631. doi: 10.1152/jn.00839.2009 Baweja, H.S., Patel, B.K., Martinkewiz, J.D., Vu, J., & Christou, E.A. (2009). Removal of Visual Feedback Alters Muscle Activity and Reduces Force Variability During Constant Isometric Contractions. Exp Brain Res, 197(1), 35-47. doi: 10.1007/s00221-009-1883-5 127 Boisgueheneuc, F.d., Levy, R., Volle, E., Seassau, M., Duffau, H., Kinkingnehun, S., . . . Dubois, B. (2006). Functions of the Left Superior Frontal Gyrus in Humans: A Lesion Study. Brain, 129(12), 3315-3328. doi: 10.1093/brain/awl244 Borg, G.A. (1982). Psychophysical Bases of Perceived Exertion. Med Sci Sports Exerc, 14(5), 377-381. Bray, S.R., Graham, J.D., Martin Ginis, K.A., & Hicks, A.L. (2012). Cognitive Task Performance Causes Impaired Maximum Force Production in Human Hand Flexor Muscles. Biol Psychol, 89(1), 195-200. Bray, S.R., Martin Ginis, K.A., Hicks, A.L., & Woodgate, J. (2008). Effects of SelfRegulatory Strength Depletion on Muscular Performance and Emg Activation. Psychophysiology, 45(2), 337-343. Brouwer, B., & Ashby, P. (1990). Corticospinal Projections to Upper and Lower Limb Spinal Motoneurons in Man. Electroencephalogr Clin Neurophysiol, 76(6), 509519. Brown, R.E., Edwards, D., L. , & Jakobi, J., M. . (2010). Sex Differences in Force Steadiness in Three Positions of the Forearm. European Journal of Applied Physiology, 110, 1251-1257. doi: 10.1007/s00421-010-1600-x Brown, R.E., Edwards, D.L., Kenno, K.A., & Jakobi, J.M. (2009). Effect of Tendon Vibration on Elbow Flexor Force Steadiness and Motor Unit. Medicine and Science in Sports and Exercise, 45(S5 ), S21. Burke, R.E., Degtyarenko, A.M., & Simon, E.S. (2001). Patterns of Locomotor Drive to Motoneurons and Last-Order Interneurons: Clues to the Structure of the Cpg. Journal of Neurophysiology, 86(1), 447-462. Burnett, R.A., Laidlaw, D.H., & Enoka, R.M. (2000). Coactivation of the Antagonist Muscle Does Not Covary with Steadiness in Old Adults. Journal of Applied Physiology, 89(1), 61-71. Butler, T., Pan, H., Tuescher, O., Engelien, A., Goldstein, M., Epstein, J., . . . Silbersweig, D.A. (2007). Human Fear-Related Motor Neurocircuitry. Neuroscience, 150(1), 1-7. doi: http://dx.doi.org/10.1016/j.neuroscience.2007.09.048 Cabeza, R. (2002). Hemispheric Asymmetry Reduction in Older Adults: The Harold Model. Psychol Aging, 17(1), 85-100. Cameron, M.H., & Lord, S. (2010). Postural Control in Multiple Sclerosis: Implications for Fall Prevention. Current Neurology and Neuroscience Reports, 10, 407-412. doi: 10.1007/s11910-010-0128-0 128 Chen, P., Ratcliff, G., Belle, S.H., Cauley, J.A., DeKosky, S.T., & Ganguli, M. (2001). Patterns of Cognitive Decline in Presymptomatic Alzheimer Disease: A Prospective Community Study. Arch Gen Psychiatry, 58, 853-858. Cheney, P.D., & Fetz, E.E. (1980). Functional Classes of Primate Corticomotoneuronal Cells and Their Relation to Active Force. J Neurophysiol, 44(4), 773-791. Christie, A., & Kamen, G. (2009). Motor Unit Firing Behavior During Prolonged 50% Mvc Dorsiflexion Contractions in Young and Older Adults. J Electromyogr Kinesiol, 19(4), 543-552. doi: 10.1016/j.jelekin.2008.03.005 Christou, E.A., Jakobi, J.M., Critchlow, A., Fleshner, M., & Enoka, R.M. (2004). The 1to 2-Hz Oscillations in Muscle Force Are Exacerbated by Stress, Especially in Older Adults. J Appl Physiol, 97(1), 225-235. doi: 10.1152/japplphysiol.00066.2004 97/1/225 [pii] Ciccarelli, O., Toosy, A.T., Marsden, J.F., Wheeler-Kingshott, C.M., Miller, D.H., Matthews, P.M., & Thompson, A.J. (2006). Functional Response to Active and Passive Ankle Movements with Clinical Correlations in Patients with Primary Progressive Multiple Sclerosis. J Neurol, 253(7), 882-891. Ciccarelli, O., Toosy, A.T., Marsden, J.F., Wheeler-Kingshott, C.M., Sahyoun, C., Matthews, P.M., . . . Thompson, A.J. (2005). Identifying Brain Regions for Integrative Sensorimotor Processing with Ankle Movements. Exp Brain Res, 166(1), 31-42. Clower, D.M., West, R.A., Lynch, J.C., & Strick, P.L. (2001). The Inferior Parietal Lobule Is the Target of Output from the Superior Colliculus, Hippocampus, and Cerebellum. J Neurosci, 21(16), 6283-6291. doi: 21/16/6283 [pii] Cresswell, A.G., & Loscher, W.N. (2000). Significance of Peripheral Afferent Input to the Alpha-Motoneurone Pool for Enhancement of Tremor During an Isometric Fatiguing Contraction. Eur J Appl Physiol, 82(1-2), 129-136. Dai, T., Liu, J., Sahgal, V., Brown, R., & Yue, G. (2001). Relationship between Muscle Output and Functional Mri-Measured Brain Activation. Experimental Brain Research, 140(3), 290-300. doi: 10.1007/s002210100815 De Luca, C.J., Gonzalez-Cueto, J.A., Bonato, P., & Adam, A. (2009). Motor Unit Recruitment and Proprioceptive Feedback Decrease the Common Drive. Journal of Neurophysiology, 101, 1620-1628. doi: 10.1152/jn.90245.2008 De Luca, C.J., LeFever, R.S., McCue, M.P., & Xenakis, A.P. (1982a). Behaviour of Human Motor Units in Different Muscles During Linearly Varying Contractions. J Physiol, 329, 113-128. 129 De Luca, C.J., LeFever, R.S., McCue, M.P., & Xenakis, A.P. (1982b). Control Scheme Governing Concurrently Active Human Motor Units During Voluntary Contractions. J Physiol, 329, 129-142. Dettmers, C., Connelly, A., Stephan, K.M., Turner, R., Friston, K.J., Frackowiak, R.S., & Gadian, D.G. (1996). Quantitative Comparison of Functional Magnetic Resonance Imaging with Positron Emission Tomography Using a Force-Related Paradigm. Neuroimage, 4(3 Pt 1), 201-209. Dideriksen, J.L., Negro, F., Enoka, R.M., & Farina, D. (2012). Motor Unit Recruitment Strategies and Muscle Properties Determine the Influence of Synaptic Noise on Force Steadiness. J Neurophysiol, 107(12), 3357-3369. doi: 10.1152/jn.00938.2011 Dobkin, B.H., Firestine, A., West, M., Saremi, K., & Woods, R. (2004). Ankle Dorsiflexion as an Fmri Paradigm to Assay Motor Control for Walking During Rehabilitation. Neuroimage, 23(1), 370-381. doi: S1053811904003179 [pii] 10.1016/j.neuroimage.2004.06.008 Doherty, T.J. (2003). Invited Review: Aging and Sarcopenia. J Appl Physiol, 95(4), 1717-1727. Drew, T., Prentice, S., & Schepens, B. (2004). Cortical and Brainstem Control of Locomotion. In D. G. S. Shigemi Mori & W. Mario (Eds.), Progress in Brain Research (Vol. Volume 143, pp. 251-261): Elsevier. Drey, M., Grösch, C., Neuwirth, C., Bauer, J.M., & Sieber, C.C. (2013). The Motor Unit Number Index (Munix) in Sarcopenic Patients. Experimental Gerontology, 48(4), 381-384. doi: http://dx.doi.org/10.1016/j.exger.2013.01.011 Eisen, A., Entezari-Taher, M., & Stewart, H. (1996). Cortical Projections to Spinal Motoneurons: Changes with Aging and Amyotrophic Lateral Sclerosis. Neurology, 46(5), 1396-1404. Endo, H., & Kawahara, K. (2011). Gender Differences in Hand Stability of Normal Young People Assessed at Low Force Levels. Ergonomics, 54(3), 273-281. doi: dx.doi.org/10.1080/00140139.2010.547607 Enoka, R.M., Christou, E.A., Hunter, S.K., Kornatz, K.W., Semmler, J.G., Taylor, A.M., & Tracy, B.L. (2003). Mechanisms That Contribute to Differences in Motor Performance between Young and Old Adults. Journal of Electromyography and Kinesiology, 13(1), 1-12. doi: 10.1016/s1050-3411(02)00084-6 Enoka, R.M., & Duchateau, J. (2008). Muscle Fatigue: What, Why and How It Influences Muscle Function. J Physiol, 586(1), 11-23. doi: jphysiol.2007.139477 [pii] 10.1113/jphysiol.2007.139477 130 Enoka, R.M., & Pearson, K.G. (2012). The Motor Unit and Muscle Action Principles of Neural Science (5th ed., pp. 1760): McGraw-Hill. Evarts, E.V. (1968). Relation of Pyramidal Tract Activity to Force Exerted During Voluntary Movement. J Neurophysiol, 31(1), 14-27. Farias, S.T., Mungas, D., Reed, B.R., Harvey, D., Cahn-Weiner, D., & DeCarli, C. (2012). Mci Is Associated with Deficits in Everyday Functioning : Alzheimer Disease & Associated Disorders. Alzheimer Dis Assoc Disord, 20(4), 217 - 223. Farina, D., Negro, F., Gizzi, L., & Falla, D. (2012). Low-Frequency Oscillations of the Neural Drive to the Muscle Are Increased with Experimental Muscle Pain. J Neurophysiol, 107(3), 958-965. doi: 10.1152/jn.00304.2011 Feinstein, B., Lindegard, B., Nyman, E., & Wohlfart, G. (1955). Morphologic Studies of Motor Units in Normal Human Muscles. Acta Anat (Basel), 23(2), 127-142. Fernie, G.R., Gryfe, C.I., Holliday, P.J., & Llewellyn, A. (1982). The Relationship of Postural Sway in Standing to the Incidence of Falls in Geriatric Subjects Age and Ageing, 11(1), 11-16. doi: 10.1093/ageing/11.1.11 Findling, O., Sellner, J., Meier, N., Allum, J.H.J., Vibert, D., Lienert, C., & Mattle, H.P. (2011). Trunk Sway in Mildly Disabled Multiple Sclerosis Patients with and without Balance Impairment. Experimental Brain Research, 213(4), 363-370. doi: 10.1007/s00221-011-2795-8 Fitts, R.H. (1994). Cellular Mechanisms of Muscle Fatigue. Physiol Rev, 74(1), 49-94. Fox, E.J., Baweja, H.S., Kim, C., Kennedy, D.M., Vaillancourt, D.E., & Christou, E.A. (2013). Modulation of Force Below 1 Hz: Age-Associated Differences and the Effect of Magnified Visual Feedback. PLoS One, 8(2), e55970. doi: 10.1371/journal.pone.0055970 Francis, S., Lin, X., Aboushoushah, S., White, T.P., Phillips, M., Bowtell, R., & Constantinescu, C.S. (2009). Fmri Analysis of Active, Passive and Electrically Stimulated Ankle Dorsiflexion. Neuroimage, 44(2), 469-479. Fraser, S.A., Li, K.Z., & Penhune, V.B. (2010). Dual-Task Performance Reveals Increased Involvement of Executive Control in Fine Motor Sequencing in Healthy Aging. J Gerontol B Psychol Sci Soc Sci, 65(5), 526-535. doi: 10.1093/geronb/gbq036 Fuglevand, A.J., Winter, D.A., & Patla, A.E. (1993). Models of Recruitment and Rate Coding Organization in Motor-Unit Pools. Journal of Neurophysiology, 70, 24702488. 131 Fuglevand, A.J., Zackowski, K.M., Huey, K.A., & Enoka, R.M. (1993). Impairment of Neuromuscular Propagation During Human Fatiguing Contractions at Submaximal Forces. J Physiol, 460, 549-572. Galganski, M.E., Fuglevand, A.J., & Enoka, R.M. (1993). Reduced Control of Motor Output in a Human Hand Muscle of Elderly Subjects During Submaximal Contractions. J Neurophysiol, 69(6), 2108-2115. Gandevia, S.C. (2001). Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol Rev, 81(4), 1725-1789. Gandevia, S.C., Allen, G.M., Butler, J.E., & Taylor, J.L. (1996). Supraspinal Factors in Human Muscle Fatigue: Evidence for Suboptimal Output from the Motor Cortex. Gazzaley, A., & D'Esposito, M. (2007). Top-Down Modulation and Normal Aging. Ann N Y Acad Sci, 1097, 67-83. Gazzaley, A., & Nobre, A.C. (2012). Top-Down Modulation: Bridging Selective Attention and Working Memory. Trends in Cognitive Sciences, 16(2), 129-135. doi: http://dx.doi.org/10.1016/j.tics.2011.11.014 Glickstein, M. (2007). What Does the Cerebellum Really Do? Curr Biol, 17(19), R824827. doi: 10.1016/j.cub.2007.08.009 Gobel, F.L., Norstrom, L.A., Nelson, R.R., Jorgensen, C.R., & Wang, Y. (1978). The Rate-Pressure Product as an Index of Myocardial Oxygen Consumption During Exercise in Patients with Angina Pectoris. Circulation, 57(3), 549-556. Gorbet, D.J., Mader, L.B., & Staines, W.R. (2010). Sex-Related Differences in the Hemispheric Laterality of Slow Cortical Potentials During the Preparation of Visually Guided Movements. Exp Brain Res, 202(3), 633-646. doi: 10.1007/s00221-010-2170-1 Gorbet, D.J., & Sergio, L.E. (2007). Preliminary Sex Differences in Human Cortical Bold Fmri Activity During the Preparation of Increasingly Complex Visually Guided Movements. Eur J Neurosci, 25(4), 1228-1239. Grabiner, M.D., & Enoka, R.M. (1995). Changes in Movement Capabilities with Aging. Exerc Sport Sci Rev, 23, 65-104. Grafton, S.T., & Tunik, E. (2011). Human Basal Ganglia and the Dynamic Control of Force During on-Line Corrections. J Neurosci, 31(5), 1600-1605. doi: 31/5/1600 [pii] 10.1523/JNEUROSCI.3301-10.2011 Granacher, U., Muehlbauer, T., Bridenbaugh, S., Bleiker, E., Wehrle, A., & Kressig, R.W. (2010). Balance Training and Multi-Task Performance in Seniors. Int J Sports Med, 31(5), 353-358. doi: 10.1055/s-0030-1248322 132 Griffith, E.E., Yoon, T., & Hunter, S.K. (2010). Age and Load Compliance Alter Time to Task Failure for a Submaximal Fatiguing Contraction with the Lower Leg. J Appl Physiol (1985), 108(6), 1510-1519. doi: 10.1152/japplphysiol.01396.2009 Grillner, S., Wallén, P., Saitoh, K., Kozlov, A., & Robertson, B. (2008). Neural Bases of Goal-Directed Locomotion in Vertebrates-an Overview. Brain Research Reviews, 57(1), 2-12. doi: doi:10.1016/j.brainresrev.2007.06.027 Gron, G., Wunderlich, A.P., Spitzer, M., Tomczak, R., & Riepe, M.W. (2000). Brain Activation During Human Navigation: Gender-Different Neural Networks as Substrate of Performance. Nat Neurosci, 3(4), 404-408. Grunte, I., Hunter, G.R., McCurry, B.D., Bolding, M.S., Roy, J.L., & McCarthy, J.P. (2009). Age and Gender Differences in Hip Extension and Flexion Torque Steadiness. Gerontology, 56(6), 533-541. doi: 10.1159/000311935 Hamilton, A.F., Jones, K.E., & Wolpert, D.M. (2004). The Scaling of Motor Noise with Muscle Strength and Motor Unit Number in Humans. Exp Brain Res, 157(4), 417430. doi: 10.1007/s00221-004-1856-7 Hatsopoulos, N.G. (2005). Encoding in the Motor Cortex: Was Evarts Right after All? Focus on "Motor Cortex Neural Correlates of Output Kinematics and Kinetics During Isometric-Force and Arm-Reaching Tasks". J Neurophysiol, 94(4), 22612262. Heckman, C.J. (2003). Active Conductances in Motoneuron Dendrites Enhance Movement Capabilities.[Article]. Exercise & Sport Sciences Reviews, 31(2), 96101. Heckman, C.J., Mottram, C.J., Quinlan, K., Theiss, R., & Schuster, J. (2009). Motoneuron Excitability: The Importance of Neuromodulatory Inputs. Clinical Neurophysiology, 120, 2040–2054. doi: 10.1016/j.clinph.2009.08.009 Hedden, T., & Gabrieli, J.D. (2004). Insights into the Ageing Mind: A View from Cognitive Neuroscience. Nat Rev Neurosci, 5(2), 87-96. Hedden, T., & Gabrieli, J.D. (2005). Healthy and Pathological Processes in Adult Development: New Evidence from Neuroimaging of the Aging Brain. Curr Opin Neurol, 18(6), 740-747. Henningsen, H., Knecht, S., & Ende-Henningsen, B. (1997). Influence of Afferent Feedback on Isometric Fine Force Resolution in Humans. Exp Brain Res, 113(2), 207-213. Herath, P., Kinomura, S., & Roland, P.E. (2001). Visual Recognition: Evidence for Two Distinctive Mechanisms from a Pet Study. Human Brain Mapping, 12(2), 110119. doi: 10.1002/1097-0193(200102)12:2<110::AID-HBM1008>3.0.CO;2-0 133 Herbert, R.D., & Gandevia, S.C. (1999). Twitch Interpolation in Human Muscles: Mechanisms and Implications for Measurement of Voluntary Activation. J Neurophysiol, 82(5), 2271-2283. Hermens, H.J., Freriks, B., Disselhorst-Klug, C., & Rau, G. (2000). Development of Recommendations for Semg Sensors and Sensor Placement Procedures. J Electromyogr Kinesiol, 10(5), 361-374. doi: S1050-6411(00)00027-4 [pii] Hiraga, C.Y., Garry, M.I., Carson, R.G., & Summers, J.J. (2009). Dual-Task Interference: Attentional and Neurophysiological Influences. Behav Brain Res, 205(1), 10-18. doi: 10.1016/j.bbr.2009.07.019 Hortobagyi, T., & DeVita, P. (2006). Mechanisms Responsible for the Age-Associated Increase in Coactivation of Antagonist Muscles. Exercise & Sport Sciences Reviews, 34(1), 29-35. Huda, S., Rodriguez, R., Lastra, L., Warren, M., Lacourse, M.G., Cohen, M.J., & Cramer, S.C. (2008). Cortical Activation During Foot Movements: Ii Effect of Movement Rate and Side. Neuroreport, 19(16), 1573-1577. doi: 10.1097/WNR.0b013e328311ca1c 00001756-200810290-00004 [pii] Huettel, S.A., Song, A.W., & McCarthy, G. (2004). Functional Magnetic Resonance Imaging. Sunderland, Mass.: Sinauer Associates. Hunter, S.K. (2014). Sex Differences in Human Fatigability: Mechanisms and Insight to Physiological Responses. Acta Physiologica, n/a-n/a. doi: 10.1111/apha.12234 Hunter, S.K., Butler, J.E., Todd, G., Gandevia, S.C., & Taylor, J.L. (2006). Supraspinal Fatigue Does Not Explain the Sex Difference in Muscle Fatigue of Maximal Contractions. J Appl Physiol (1985), 101(4), 1036-1044. doi: 10.1152/japplphysiol.00103.2006 Hunter, S.K., Critchlow, A., & Enoka, R.M. (2004). Influence of Aging on Sex Differences in Muscle Fatigability. J Appl Physiol, 97, 1723-1732. Hunter, S.K., Critchlow, A., Shin, I.S., & Enoka, R.M. (2004). Fatigability of the Elbow Flexor Muscles for a Sustained Submaximal Contraction Is Similar in Men and Women Matched for Strength. J Appl Physiol, 96(1), 195-202. doi: 10.1152/japplphysiol.00893.2003 00893.2003 [pii] Hunter, S.K., Duchateau, J., & Enoka, R.M. (2004). Muscle Fatigue and the Mechanisms of Task Failure. Exerc Sport Sci Rev, 32(2), 44-49. Hunter, S.K., & Enoka, R.M. (2001). Sex Differences in the Fatigability of Arm Muscles Depends on Absolute Force During Isometric Contractions. J Appl Physiol, 91(6), 2686-2694. 134 Hunter, S.K., Rochette, L., Critchlow, A., & Enoka, R.M. (2005). Time to Task Failure Differs with Load Type When Old Adults Perform a Submaximal Fatiguing Contraction. Muscle Nerve, 31(6), 730-740. doi: 10.1002/mus.20325 Hunter, S.K., Ryan, D.L., Ortega, J.D., & Enoka, R.M. (2002). Task Differences with the Same Load Torque Alter the Endurance Time of Submaximal Fatiguing Contractions in Humans. J Neurophysiol, 88(6), 3087-3096. doi: 10.1152/jn.00232.2002 Hunter, S.K., Todd, G., Butler, J.E., Gandevia, S.C., & Taylor, J.L. (2008). Recovery from Supraspinal Fatigue Is Slowed in Old Adults after Fatiguing Maximal Isometric Contractions. J Appl Physiol, 105(4), 1199-1209. doi: 10.1152/japplphysiol.01246.2007 Hunter, S.K., Yoon, T., Farinella, J., Griffith, E.E., & Ng, A.V. (2008). Time to Task Failure and Muscle Activation Vary with Load Type for a Submaximal Fatiguing Contraction with the Lower Leg. J Appl Physiol, 105(2), 463-472. Iadarola, M.J., Berman, K.F., Zeffiro, T.A., Byas-Smith, M.G., Gracely, R.H., Max, M.B., & Bennett, G.J. (1998). Neural Activation During Acute Capsaicin-Evoked Pain and Allodynia Assessed with Pet. Brain, 121(5), 931-947. doi: 10.1093/brain/121.5.931 Ishai, A., Ungerleider, L.G., Martin, A., Schouten, J.L., & Haxby, J.V. (1999). Distributed Representation of Objects in the Human Ventral Visual Pathway. Proceedings of the National Academy of Sciences, 96(16), 9379-9384. doi: 10.1073/pnas.96.16.9379 Jesunathadas, M., Klass, M., Duchateau, J., & Enoka, R.M. (2012). Discharge Properties of Motor Units During Steady Isometric Contractions Performed with the Dorsiflexor Muscles. J Appl Physiol, 112(11), 1897-1905. doi: 10.1152/japplphysiol.01372.2011 Johnson, A.N., & Shinohara, M. (2012). Corticomuscular Coherence with and without Additional Task in the Elderly. J Appl Physiol, 112(6), 970-981. doi: 10.1152/japplphysiol.01079.2011 Jones, K.E., Hamilton, A.F., & Wolpert, D.M. (2002). Sources of Signal-Dependent Noise During Isometric Force Production. J Neurophysiol, 88(3), 1533-1544. Kahneman, D. (1973). Attention and Effort. Englewood Cliffs, NJ: Prentice Hall. Kajantie, E., & Phillips, D.I. (2006). The Effects of Sex and Hormonal Status on the Physiological Response to Acute Psychosocial Stress. Psychoneuroendocrinology, 31(2), 151-178. doi: 10.1016/j.psyneuen.2005.07.002 135 Kalaska, J.F. (2009). From Intention to Action: Motor Cortex and the Control of Reaching Movements. . Advances in Experimental Medicine & Biology, 629, 139178. doi: http://dx.doi.org/10.1007/978-0-387-77064-2_8 Kaya, R.D., Nakazawa, M., Hoffman, R.L., & Clark, B.C. (2013). Interrelationship between Muscle Strength, Motor Units, and Aging. Experimental Gerontology, 48(9), 920-925. doi: http://dx.doi.org/10.1016/j.exger.2013.06.008 Keisker, B., Hepp-Reymond, M.C., Blickenstorfer, A., Meyer, M., & Kollias, S.S. (2009). Differential Force Scaling of Fine-Graded Power Grip Force in the Sensorimotor Network. Hum Brain Mapp, 30(8), 2453-2465. doi: 10.1002/hbm.20676 Keller-Ross, M.L., Pruse, J., Yoon, T., Harkins, A., & Hunter, S. (2014). StressorInduced Increase Muscle Fatigability of Young Men and Women Is Predicted by Strength, but Not Voluntary Activation. Keller-Ross, M.L., Schlinder-Delap, B., Doyel, R., Larson, G., & Hunter, S.K. (2014). Muscle Fatigability and Control of Force in Men with Posttraumatic Stress Disorder. Med Sci Sports Exerc. doi: 10.1249/MSS.0000000000000244 Keller, M.L., Pruse, J., Yoon, T., Schlinder-Delap, B., Harkins, A., & Hunter, S.K. (2011). Supraspinal Fatigue Is Similar in Men and Women for a Low-Force Fatiguing Contraction. Med Sci Sports Exerc, 43(10), 1873-1883. doi: 10.1249/MSS.0b013e318216ebd4 Kent-Braun, J.A., Ng, A.V., Doyle, J.W., & Towse, T.F. (2002). Human Skeletal Muscle Responses Vary with Age and Gender During Fatigue Due to Incremental Isometric Exercise. J Appl Physiol, 93(5), 1813-1823. Kido, A., Tanaka, N., & Stein, R.B. (2004). Spinal Excitation and Inhibition Decrease as Humans Age. Canadian Journal of Physiology and Pharmacology, 82(4), 238248. doi: 10.1139/y04-017 Kinoshita, H., Oku, N., Hashikawa, K., & Nishimura, T. (2000). Functional Brain Areas Used for the Lifting of Objects Using a Precision Grip: A Pet Study. Brain Res, 857(1-2), 119-130. doi: S0006-8993(99)02416-6 [pii] Kornatz, K.W., Christou, E.A., & Enoka, R.M. (2005). Practice Reduces Motor Unit Discharge Variability in a Hand Muscle and Improves Manual Dexterity in Old Adults. J Appl Physiol, 98(6), 2072-2080. doi: 01149.2004 [pii] 10.1152/japplphysiol.01149.2004 Kouzaki, M., & Shinohara, M. (2010). Steadiness in Plantar Flexor Muscles and Its Relation to Postural Sway in Young and Elderly Adults. Muscle Nerve, 42(1), 7887. doi: 10.1002/mus.21599 136 Kouzaki, M., Shinohara, M., Masani, K., & Fukunaga, T. (2004). Force Fluctuations Are Modulated by Alternate Muscle Activity of Knee Extensor Synergists During Low-Level Sustained Contraction. J Appl Physiol, 97(6), 2121-2131. Krebs, D.E., Jette, A.M., & Assmann, S.F. (1998). Moderate Exercise Improves Gait Stability in Disabled Elders. Arch Phys Med Rehabil, 79(12), 1489-1495. Kriska, A., & Bennett, P. (1992). An Epidemiological Perspective of the Relationship between Physical Activity and Niddm: From Activity Assessment to Intervention. Diabetes Metab Rev, 8, 355-372. Kuhtz-Buschbeck, J.P., Ehrsson, H.H., & Forssberg, H. (2001). Human Brain Activity in the Control of Fine Static Precision Grip Forces: An Fmri Study. European Journal of Neuroscience, 14(2), 382-390. Kuhtz-Buschbeck, J.P., Gilster, R., Wolff, S., Ulmer, S., Siebner, H., & Jansen, O. (2008). Brain Activity Is Similar During Precision and Power Gripping with Light Force: An Fmri Study. Neuroimage, 40(4), 1469-1481. doi: 10.1016/j.neuroimage.2008.01.037 Laidlaw, D.H., Bilodeau, M., & Enoka, R.M. (2000). Steadiness Is Reduced and Motor Unit Discharge Is More Variable in Old Adults. Muscle Nerve, 23(4), 600-612. doi: 10.1002/(SICI)1097-4598(200004)23:4<600::AID-MUS20>3.0.CO;2-D [pii] Laidlaw, D.H., Kornatz, K.W., Keen, D.A., Suzuki, S., & Enoka, R.M. (1999). Strength Training Improves the Steadiness of Slow Lengthening Contractions Performed by Old Adults. Journal of Applied Physiology, 87(5), 1786-1795. Langan, J., Peltier, S.J., Bo, J., Fling, B.W., Welsh, R.C., & Seidler, R.D. (2010). Functional Implications of Age Differences in Motor System Connectivity. Front Syst Neurosci, 4, 17. doi: 10.3389/fnsys.2010.00017 Lissek, S., Hausmann, M., Knossalla, F., Peters, S., Nicolas, V., Gunturkun, O., & Tegenthoff, M. (2007). Sex Differences in Cortical and Subcortical Recruitment During Simple and Complex Motor Control: An Fmri Study. Neuroimage, 37(3), 912-926. Liu, J.Z., Shan, Z.Y., Zhang, L.D., Sahgal, V., Brown, R.W., & Yue, G.H. (2003). Human Brain Activation During Sustained and Intermittent Submaximal Fatigue Muscle Contractions: An Fmri Study. J Neurophysiol, 90, 300-312. doi: 10.1152/jn.00821.2002 Lorist, M.M., Kernell, D., Meijman, T.F., & Zijdewind, I. (2002). Motor Fatigue and Cognitive Task Performance in Humans. J Physiol, 545(Pt 1), 313-319. Lotze, M., Erb, M., Flor, H., Huelsmann, E., Godde, B., & Grodd, W. (2000). Fmri Evaluation of Somatotopic Representation in Human Primary Motor Cortex. Neuroimage, 11(5 Pt 1), 473-481. 137 Luft, A.R., Smith, G.V., Forrester, L., Whitall, J., Macko, R.F., Hauser, T.K., . . . Hanley, D.F. (2002). Comparing Brain Activation Associated with Isolated Upper and Lower Limb Movement across Corresponding Joints. Hum Brain Mapp, 17(2), 131-140. Macaluso, A., Nimmo, M.A., Foster, J.E., Cockburn, M., McMillan, N.C., & De Vito, G. (2002). Contractile Muscle Volume and Agonist-€ •Antagonist Coactivation Account for Differences in Torque between Young and Older Women. Muscle & Nerve, 25(6), 858-863. doi: 10.1002/mus.10113 MacIntosh, B.J., Mraz, R., Baker, N., Tam, F., Staines, W.R., & Graham, S.J. (2004). Optimizing the Experimental Design for Ankle Dorsiflexion Fmri. Neuroimage, 22(4), 1619-1627. Madden, D.J., Costello, M.C., Dennis, N.A., Davis, S.W., Shepler, A.M., Spaniol, J., . . . Cabeza, R. (2010). Adult Age Differences in Functional Connectivity During Executive Control. NeuroImage, 52(2), 643-657. doi: http://dx.doi.org/10.1016/j.neuroimage.2010.04.249 Manni, E., & Petrosini, L. (2004). A Century of Cerebellar Somatotopy: A Debated Representation. Nat Rev Neurosci, 5(3), 241-249. Marchand, W.R., Lee, J.N., Healy, L., Thatcher, J.W., Rashkin, E., Starr, J., & Hsu, E. (2009). An Fmri Motor Activation Paradigm Demonstrates Abnormalities of Putamen Activation in Females with Panic Disorder. Journal of Affective Disorders, 116(1–2), 121-125. doi: http://dx.doi.org/10.1016/j.jad.2008.10.026 Mari, S., Serrao, M., Casali, C., Conte, C., Giovanni, M., Ranavolo, A., . . . Pierelli, F. (2014). Lower Limb Antagonist Muscle Co-Activation and Its Relationship with Gait Parameters in Cerebellar Ataxia. The Cerebellum, 13(2), 226-236. doi: 10.1007/s12311-013-0533-4 Marmon, A.R., & Enoka, R.M. (2010). Comparison of the Influence of Two Stressors on Steadiness During Index Finger Abduction. Physiol Behav, 99(4), 515-520. doi: 10.1016/j.physbeh.2010.01.002 Marmon, A.R., Gould, J.R., & Enoka, R.M. (2011). Practicing a Functional Task Improves Steadiness with Hand Muscles in Older Adults. Med Sci Sports Exerc, 43(8), 1531-1537. doi: 10.1249/MSS.0b013e3182100439 Marmon, A.R., Pascoe, M.A., Schwartz, R.S., & Enoka, R.M. (2011). Associations among Strength, Steadiness, and Hand Function across the Adult Life Span. Med Sci Sports Exerc, 43(4), 560-567. doi: 10.1249/MSS.0b013e3181f3f3ab Martínez, A., Anllo-Vento, L., Sereno, M.I., Frank, L.R., Buxton, R.B., Dubowitz, D.J., . . . Hillyard, S.A. (1999). Involvement of Striate and Extrastriate Visual Cortical Areas in Spatial Attention. Nat Neurosci, 2(4), 364-369. doi: 10.1038/7274 138 McDowd, J.M. (2007). An Overview of Attention: Behavior and Brain. J Neurol Phys Ther, 31(3), 98-103. doi: 10.1097/NPT.0b013e31814d7874 McGinley, M., Hoffman, R.L., Russ, D.W., Thomas, J.S., & Clark, B.C. (2010). Older Adults Exhibit More Intracortical Inhibition and Less Intracortical Facilitation Than Young Adults. Experimental Gerontology, 45(9), 671-678. doi: http://dx.doi.org/10.1016/j.exger.2010.04.005 Meltzer, C.C., Smith, G., DeKosky, S.T., Pollock, B., G. , Mathis, C.A., Moore, R.Y., . . . Reynolds, C.F. (1998). Serotonin in Aging, Late-Life Depression, and Alzheimer's Disease: The Emerging Role of Functional Imaging. Neuropsychopharmacology, 18, 407-430. doi: doi:10.1016/S0893133X(97)00194-2 Mesulam, M.M. (1998). From Sensation to Cognition. Brain, 121(6), 1013-1052. doi: 10.1093/brain/121.6.1013 Miller, E.K. (2000). The Prefontral Cortex and Cognitive Control. Nat Rev Neurosci, 1(1), 59-65. Miller, E.K., & Cohen, J.D. (2001). An Integrative Theory of Prefrontal Cortex Function Annual Review of Neuroscience, 24(1), 167-202. doi: doi:10.1146/annurev.neuro.24.1.167 Missenard, O., Mottet, D., & Perrey, S. (2009). Factors Responsible for Force Steadiness Impairment with Fatigue. Muscle & Nerve, 40(6), 1019-1032. doi: 10.1002/mus.21331 Miyai, I., Tanabe, H.C., Sase, I., Eda, H., Oda, I., Konishi, I., . . . Kubota, K. (2001). Cortical Mapping of Gait in Humans: A near-Infrared Spectroscopic Topography Study. NeuroImage, 14(5), 1186-1192. doi: http://dx.doi.org/10.1006/nimg.2001.0905 Monster, A.W., & Chan, H. (1977). Isometric Force Production by Motor Units of Extensor Digitorum Communis Muscle in Man. J Neurophysiol, 40(6), 14321443. Moritz, C.T., Barry, B.K., Pascoe, M.A., & Enoka, R.M. (2005). Discharge Rate Variability Influences the Variation in Force Fluctuations across the Working Range of a Hand Muscle. J Neurophysiol, 93(5), 2449-2459. doi: 10.1152/jn.01122.2004 Morris, J.C., Storandt, M., Miller, J.P., McKeel, D.W., Price, J.L., Rubin, E.H., & Berg, L. (2001). Mild Cognitive Impairment Represents Early-Stage Alzheimer Disease. Archives of Neurology, 58(3), 397-405. doi: 10-1001/pubs.Arch Neurol.ISSN-0003-9942-58-3-noc00280 139 Mottram, C.J., Christou, E.A., Meyer, F.G., & Enoka, R.M. (2005). Frequency Modulation of Motor Unit Discharge Has Task-Dependent Effects on Fluctuations in Motor Output. J Neurophysiol, 94(4), 2878-2887. doi: 00390.2005 [pii] 10.1152/jn.00390.2005 Negro, F., Holobar, A., & Farina, D. (2009). Fluctuations in Isometric Muscle Force Can Be Described by One Linear Projection of Low-Frequency Components of Motor Unit Discharge Rates. J Physiol, 587(Pt 24), 5925-5938. doi: 10.1113/jphysiol.2009.178509 Noble, J.W., Eng, J.J., Kokotilo, K.J., & Boyd, L.A. (2011). Aging Effects on the Control of Grip Force Magnitude: An Fmri Study. Exp Gerontol, 46(6), 453-461. doi: S0531-5565(11)00031-3 [pii] 10.1016/j.exger.2011.01.004 Noteboom, J.T., Barnholt, K.R., & Enoka, R.M. (2001). Activation of the Arousal Response and Impairment of Performance Increase with Anxiety and Stressor Intensity. J Appl Physiol, 91(5), 2093-2101. Noteboom, J.T., Fleshner, M., & Enoka, R.M. (2001). Activation of the Arousal Response Can Impair Performance on a Simple Motor Task. J Appl Physiol, 91(2), 821-831. O'Reilly, J.X., Beckmann, C.F., Tomassini, V., Ramnani, N., & Johansen-Berg, H. (2010). Distinct and Overlapping Functional Zones in the Cerebellum Defined by Resting State Functional Connectivity. Cereb Cortex, 20(4), 953-965. doi: bhp157 [pii] 10.1093/cercor/bhp157 Oldfield, R.C. (1971). The Assessment and Analysis of Handedness: The Edinburgh Inventory. Neuropsychologia, 9(1), 97-113. Orr, E.L., Lacourse, M.G., Cohen, M.J., & Cramer, S.C. (2008). Cortical Activation During Executed, Imagined, and Observed Foot Movements. Neuroreport, 19(6), 625-630. Owen, A.M., McMillan, K.M., Laird, A.R., & Bullmore, E. (2005). N-Back Working Memory Paradigm: A Meta-Analysis of Normative Functional Neuroimaging Studies. Human Brain Mapping, 25(1), 46-59. doi: 10.1002/hbm.20131 Peinemann, A., Lehner, C., Conrad, B., & Siebner, H.R. (2001). Age-Related Decrease in Paired-Pulse Intracortical Inhibition in the Human Primary Motor Cortex. Neurosci Lett, 313(1-2), 33-36. Plow, E.B., Cunningham, D.A., Bonnett, C., Gohar, D., Bayram, M., Wyant, A., . . . Yue, G.H. (2013). Neurophysiological Correlates of Aging-Related Muscle Weakness. Journal of Neurophysiology, 110, 2563-2573. doi: 10.1152/jn.00205.2013 140 Prodoehl, J., Corcos, D.M., & Vaillancourt, D.E. (2009). Basal Ganglia Mechanisms Underlying Precision Grip Force Control. Neuroscience & Biobehavioral Reviews, 33(6), 900-908. Prodoehl, J., & Vaillancourt, D.E. (2010). Effects of Visual Gain on Force Control at the Elbow and Ankle. Exp Brain Res, 200(1), 67-79. doi: 10.1007/s00221-009-1966-3 Rajimehr, R., & Tootell, R. (2008). 1.30 - Organization of Human Visual Cortex. In R. H. Masland, T. D. Albright, T. D. Albright, R. H. Masland, P. Dallos, D. Oertel, S. Firestein, G. K. Beauchamp, M. C. Bushnell, A. I. Basbaum, J. H. Kaas & E. P. Gardner (Eds.), The Senses: A Comprehensive Reference (pp. 595-614). New York: Academic Press. Rao, S.M., Bandettini, P.A., Binder, J.R., Bobholz, J.A., Hammeke, T.A., Stein, E.A., & Hyde, J.S. (1996). Relationship between Finger Movement Rate and Functional Magnetic Resonance Signal Change in Human Primary Motor Cortex. J Cereb Blood Flow Metab, 16(6), 1250-1254. Rao, S.M., Binder, J.R., Hammeke, T.A., Bandettini, P.A., Bobholz, J.A., Frost, J.A., . . . Hyde, J.S. (1995). Somatotopic Mapping of the Human Primary Motor Cortex with Functional Magnetic Resonance Imaging. Neurology, 45, 919-924. Raz, N., Rodrigue, K.M., & Haacke, E.M. (2007). Brain Aging and Its Modifiers: Insights from in Vivo Neuromorphometry and Susceptibility Weighted Imaging. Ann N Y Acad Sci, 1097, 84-93. Reynolds, C.C., & Meltzer, C., F. (1999). In Vivo Assessment of Aging Changes in Serotonin Function. Neuropsychopharmacology, 21, 323-324. doi: doi:10.1016/S0893-133X(99)00058-5 Riley, Z.A., Maerz, A.H., Litsey, J.C., & Enoka, R.M. (2008). Motor Unit Recruitment in Human Biceps Brachii During Sustained Voluntary Contractions. J Physiol, 586(8), 2183-2193. doi: 10.1113/jphysiol.2008.150698 Roland, P.E., Larsen, B., Lassen, N.A., & Skinhoj, E. (1980). Supplementary Motor Area and Other Cortical Areas in Organization of Voluntary Movements in Man. Journal of Neurophysiology, 43, 118-136. Sale, M.V., & Semmler, J.G. (2005). Age-Related Differences in Corticospinal Control During Functional Isometric Contractions in Left and Right Hands. J Appl Physiol, 99(4), 1483-1493. Schaefer, S., & Schumacher, V. (2011). The Interplay between Cognitive and Motor Functioning in Healthy Older Adults: Findings from Dual-Task Studies and Suggestions for Intervention. Gerontology, 57(3), 239-246. doi: 10.1159/000322197 141 Schweizer, S., Grahn, J., Hampshire, A., Mobbs, D., & Dalgleish, T. (2013). Training the Emotional Brain: Improving Affective Control through Emotional Working Memory Training. J Neurosci, 33(12), 5301-5311. doi: 10.1523/JNEUROSCI.2593-12.2013 Seals, D.R., & Esler, M.D. (2000). Human Ageing and the Sympathoadrenal System. The Journal of Physiology, 528, 407-417. doi: 10.1111/j.1469-7793.2000.00407.x Seidler-Dobrin, R.D., & Stelmach, G.E. (1998). Persistence in Visual Feedback Control by the Elderly. Exp Brain Res, 119(4), 467-474. Semmler, J.G., Kornatz, K.W., Meyer, F.G., & Enoka, R.M. (2006). Diminished TaskRelated Adjustments of Common Inputs to Hand Muscle Motor Neurons in Older Adults. Experimental Brain Research, 172(4), 507-518. doi: 10.1007/s00221-0060367-0 Semmler, J.G., Tucker, K.J., Allen, T.J., & Proske, U. (2007). Eccentric Exercise Increases Emg Amplitude and Force Fluctuations During Submaximal Contractions of Elbow Flexor Muscles. Journal of Applied Physiology, 103, 979989. doi: 10.1152/japplphysiol.01310.2006 Sergio, L.E., Hamel-Paquet, C., & Kalaska, J.F. (2005). Motor Cortex Neural Correlates of Output Kinematics and Kinetics During Isometric-Force and Arm-Reaching Tasks. J Neurophysiol, 94(4), 2353-2378. Sherrington, C.S. (1925). Remarks on Some Aspects of Reflex Inhibition. Proceedings of the Royal Society of London. Series B, Containing Papers of aBiological Character, 97(686), 519-545. Silsupadol, P., Shumway-Cook, A., Lugade, V., van Donkelaar, P., Chou, L.S., Mayr, U., & Woollacott, M.H. (2009). Effects of Single-Task Versus Dual-Task Training on Balance Performance in Older Adults: A Double-Blind, Randomized Controlled Trial. Arch Phys Med Rehabil, 90(3), 381-387. doi: S0003-9993(08)01675-4 [pii] 10.1016/j.apmr.2008.09.559 Singh, N.B., Arampatzis, A., Duda, G., Heller, M.O., & Taylor, W.R. (2010). Effect of Fatigue on Force Fluctuations in Knee Extensors in Young Adults. Philos Trans A Math Phys Eng Sci, 368(1920), 2783-2798. doi: 10.1098/rsta.2010.0091 Slifkin, A.B., & Newell, K.M. (1999). Noise, Information Transmission, and Force Variability. J Exp Psychol Hum Percept Perform, 25(3), 837-851. Sommervoll, Y., Ettema, G., & Vereijken, B. (2011). Effects of Age, Task, and Frequency on Variability of Finger Tapping. Percept Mot Skills, 113(2), 647-661. Sosnoff, J.J., & Newell, K.M. (2006a). The Generalization of Perceptual-Motor IntraIndividual Variability in Young and Old Adults. J Gerontol B Psychol Sci Soc Sci, 61(5), P304-310. 142 Sosnoff, J.J., & Newell, K.M. (2006b). Information Processing Limitations with Aging in the Visual Scaling of Isometric Force. Exp Brain Res, 170(3), 423-432. doi: 10.1007/s00221-005-0225-5 Spielberger, C.D. (2010). State-Trait Anxiety Inventory The Corsini Encyclopedia of Psychology: John Wiley & Sons, Inc. Spraker, M.B., Yu, H., Corcos, D.M., & Vaillancourt, D.E. (2007). Role of Individual Basal Ganglia Nuclei in Force Amplitude Generation. J Neurophysiol, 98(2), 821834. Springer, S., Giladi, N., Peretz, C., Yogev, G., Simon, E.S., & Hausdorff, J.M. (2006). Dual-Tasking Effects on Gait Variability: The Role of Aging, Falls, and Executive Function. Mov Disord, 21(7), 950-957. doi: 10.1002/mds.20848 Squire, L.R., Berg, D., Bloom, F.E., du Lac, S., Ghosh, A., & Spitzer, N.C. (2013). Fundamentals of Neuroscience (4th ed.): Academic Press. Stoodley, C.J., & Schmahmann, J.D. (2009). Functional Topography in the Human Cerebellum: A Meta-Analysis of Neuroimaging Studies. Neuroimage, 44(2), 489501. doi: S1053-8119(08)00972-5 [pii] 10.1016/j.neuroimage.2008.08.039 Stoodley, C.J., & Schmahmann, J.D. (2010). Evidence for Topographic Organization in the Cerebellum of Motor Control Versus Cognitive and Affective Processing. Cortex, 46(7), 831-844. Takahara, D., Inoue, K., Hirata, Y., Miyachi, S., Nambu, A., Takada, M., & Hoshi, E. (2012). Multisynaptic Projections from the Ventrolateral Prefrontal Cortex to the Dorsal Premotor Cortex in Macaques - Anatomical Substrate for Conditional Visuomotor Behavior. Eur J Neurosci, 36(10), 3365-3375. doi: 10.1111/j.14609568.2012.08251.x Talairach, J., & Tournoux , P. (1988). Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System : An Approach to Cerebral Imaging: Thieme Medical Publishers. Taylor, A.M., Christou, E.A., & Enoka, R.M. (2003). Multiple Features of Motor-Unit Activity Influence Force Fluctuations During Isometric Contractions. J Neurophysiol, 90(2), 1350-1361. doi: 10.1152/jn.00056.2003 00056.2003 [pii] Taylor, J.L., Butler, J.E., Allen, G.M., & Gandevia, S.C. (1996). Changes in Motor Cortical Excitability During Human Muscle Fatigue. J Physiol, 490 ( Pt 2), 519528. Taylor, J.L., & Gandevia, S.C. (2008). A Comparison of Central Aspects of Fatigue in Submaximal and Maximal Voluntary Contractions. J Appl Physiol, 104(2), 542550. doi: 10.1152/japplphysiol.01053.2007 143 Thickbroom, G.W., Phillips, B.A., Morris, I., Byrnes, M.L., & Mastaglia, F.L. (1998). Isometric Force-Related Activity in Sensorimotor Cortex Measured with Functional Mri. Exp Brain Res, 121(1), 59-64. Tombu, M., & Jolicoeur, P. (2003). A Central Capacity Sharing Model of Dual-Task Performance. J Exp Psychol Hum Percept Perform, 29(1), 3-18. Torta, D.M., & Cauda, F. (2011). Different Functions in the Cingulate Cortex, a MetaAnalytic Connectivity Modeling Study. NeuroImage, 56, 2157–2172. doi: 10.1016/j.neuroimage.2011.03.066 Tracy, B.L. (2007a). Force Control Is Impaired in the Ankle Plantarflexors of Elderly Adults. Eur J Appl Physiol, 101(5), 629-636. doi: 10.1007/s00421-007-0538-0 Tracy, B.L. (2007b). Visuomotor Contribution to Force Variability in the Plantarflexor and Dorsiflexor Muscles. Hum Mov Sci, 26(6), 796-807. doi: S01679457(07)00051-6 [pii] 10.1016/j.humov.2007.07.001 Tracy, B.L., Dinenno, D.V., Jorgensen, B., & Welsh, S.J. (2007). Aging, Visuomotor Correction, and Force Fluctuations in Large Muscles. Med Sci Sports Exerc, 39(3), 469-479. doi: 10.1249/mss.0b013e31802d3ad300005768-20070300000010 [pii] Tracy, B.L., & Enoka, R.M. (2002). Older Adults Are Less Steady During Submaximal Isometric Contractions with the Knee Extensor Muscles. J Appl Physiol, 92(3), 1004-1012. doi: 10.1152/japplphysiol.00954.2001 Tracy, B.L., Maluf, K.S., Stephenson, J.L., Hunter, S.K., & Enoka, R.M. (2005). Variability of Motor Unit Discharge and Force Fluctuations across a Range of Muscle Forces in Older Adults. Muscle Nerve, 32(4), 533-540. doi: 10.1002/mus.20392 Tracy, B.L., Mehoudar, P.D., & Ortega, J.D. (2007). The Amplitude of Force Variability Is Correlated in the Knee Extensor and Elbow Flexor Muscles. Exp Brain Res, 176(3), 448-464. doi: 10.1007/s00221-006-0631-3 Trinastic, J.P., Kautz, S.A., McGregor, K., Gregory, C., Bowden, M., Benjamin, M.B., . . . Crosson, B. (2014). An Fmri Study of the Differences in Brain Activity During Active Ankle Dorsiflexion and Plantarflexion. Brain Imaging and Behavior, 4(2), 121-131. doi: 10.1007/s11682-010-9091-2 Tseng, Y.W., Diedrichsen, J., Krakauer, J.W., Shadmehr, R., & Bastian, A.J. (2007). Sensory Prediction Errors Drive Cerebellum-Dependent Adaptation of Reaching. J Neurophysiol, 98(1), 54-62. doi: 10.1152/jn.00266.2007 Tzourio, C. (2007). Hypertension, Cognitive Decline, and Dementia: An Epidemiological Perspective. Dialogues Clin Neurosci, 9(1), 61-70. 144 Tzourio, C., Dufouil, C., Ducimetiere, P., & Alperovitch, A. (1999). Cognitive Decline in Individuals with High Blood Pressure: A Longitudinal Study in the Elderly. Neurology, 53, 1948-1952. Vaillancourt, D.E., Mayka, M.A., Thulborn, K.R., & Corcos, D.M. (2004). Subthalamic Nucleus and Internal Globus Pallidus Scale with the Rate of Change of Force Production in Humans. Neuroimage, 23(1), 175-186. Vaillancourt, D.E., Yu, H., Mayka, M.A., & Corcos, D.M. (2007). Role of the Basal Ganglia and Frontal Cortex in Selecting and Producing Internally Guided Force Pulses. Neuroimage, 36(3), 793-803. Van Cutsem, M., Feiereisen, P., Duchateau, J., & Hainaut, K. (1997). Mechanical Properties and Behaviour of Motor Units in the Tibialis Anterior During Voluntary Contractions. Can J Appl Physiol, 22(6), 585-597. doi: 10.1139/h97038 Van de Crommert, H.W.A.A., Mulder, T., & Duysens, J. (1998). Neural Control of Locomotion: Sensory Control of the Central Pattern Generator and Its Relation to Treadmill Training. Gait & Posture, 7(3), 251-263. doi: http://dx.doi.org/10.1016/S0966-6362(98)00010-1 van Duinen, H., Renken, R., Maurits, N.M., & Zijdewind, I. (2008). Relation between Muscle and Brain Activity During Isometric Contractions of the First Dorsal Interosseus Muscle. Hum Brain Mapp, 29(3), 281-299. Voelcker-Rehage, C., & Alberts, J.L. (2007). Effect of Motor Practice on Dual-Task Performance in Older Adults. J Gerontol B Psychol Sci Soc Sci, 62(3), P141-148. Voelcker-Rehage, C., Stronge, A.J., & Alberts, J.L. (2006). Age-Related Differences in Working Memory and Force Control under Dual-Task Conditions. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn, 13(3-4), 366-384. Wang, J., Korczykowski, M., Rao, H., Fan, Y., Pluta, J., Gur, R.C., . . . Detre, J.A. (2007). Gender Difference in Neural Response to Psychological Stress. Soc Cogn Affect Neurosci, 2(3), 227-239. Wasmund, W.L., Westerholm, E.C., Watenpaugh, D.E., Wasmund, S.L., & Smith, M.L. (2002). Interactive Effects of Mental and Physical Stress on Cardiovascular Control. J Appl Physiol, 92(5), 1828-1834. doi: 10.1152/japplphysiol.00019.2001 West, R.L. (1996). An Application of Prefrontal Cortex Function Theory to Cognitive Aging. Psychological Bulletin, 120(2), 272-292. doi: http://dx.doi.org/10.1037/0033-2909.120.2.272 Whelan, P.J. (1996). Control of Locomotion in the Decerebrate Cat. Progress in Neurobiology, 49(5), 481-515. doi: http://dx.doi.org/10.1016/03010082(96)00028-7 145 Wong, S.W., Kimmerly, D.S., Masse, N., Menon, R.S., Cechetto, D.F., & Shoemaker, J.K. (2007). Sex Differences in Forebrain and Cardiovagal Responses at the Onset of Isometric Handgrip Exercise: A Retrospective Fmri Study. J Appl Physiol, 103(4), 1402-1411. Woollacott, M., & Shumway-Cook, A. (2002). Attention and the Control of Posture and Gait: A Review of an Emerging Area of Research. Gait Posture, 16(1), 1-14. doi: S0966636201001564 [pii] Yogev-Seligmann, G., Hausdorff, J.M., & Giladi, N. (2008). The Role of Executive Function and Attention in Gait. Mov Disord, 23(3), 329-342; quiz 472. doi: 10.1002/mds.21720 Yoon, T., Keller, M.L., De-Lap, B.S., Harkins, A., Lepers, R., & Hunter, S.K. (2009). Sex Differences in Response to Cognitive Stress During a Fatiguing Contraction. J Appl Physiol, 107(5), 1486-1496. doi: 00238.2009 [pii] 10.1152/japplphysiol.00238.2009 Yoon, T., Schlinder Delap, B., Griffith, E.E., & Hunter, S.K. (2007). Mechanisms of Fatigue Differ after Low- and High-Force Fatiguing Contractions in Men and Women. Muscle Nerve, 36(4), 515-524. Yoon, T., Schlinder Delap, B., Griffith, E.E., & Hunter, S.K. (2008). Age-Related Muscle Fatigue after a Low-Force Fatiguing Contraction Is Explained by Central Fatigue. Muscle Nerve, 37(4), 457-466. Yoon, T., Vanden Noven, M., Nielson, K.A., & Hunter, S.K. (2014). Brain Areas That Modulate Force Steadiness and Intensity During Isometric Ankle Dorsiflexion in Men and Women. Experimental Brain Research, Accepted. Zijdewind, I., van Duinen, H., Zielman, R., & Lorist, M.M. (2006). Interaction between Force Production and Cognitive Performance in Humans. Clin Neurophysiol, 117(3), 660-667. doi: 10.1016/j.clinph.2005.11.016