* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Soil_16s_RNA_Overview
Oncogenomics wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Extrachromosomal DNA wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Point mutation wikipedia , lookup
Transposable element wikipedia , lookup
Genetic engineering wikipedia , lookup
Gene desert wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Genomic imprinting wikipedia , lookup
Genomic library wikipedia , lookup
Ridge (biology) wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Public health genomics wikipedia , lookup
Non-coding DNA wikipedia , lookup
Genome (book) wikipedia , lookup
Gene expression profiling wikipedia , lookup
Designer baby wikipedia , lookup
Microsatellite wikipedia , lookup
Human genome wikipedia , lookup
Microevolution wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Helitron (biology) wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
History of genetic engineering wikipedia , lookup
Human microbiota wikipedia , lookup
Genome editing wikipedia , lookup
Pathogenomics wikipedia , lookup
Minimal genome wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
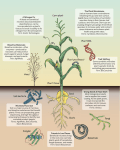
2006 Laboratory 2 Background Information I. Basic Facts About Microbes: Microbes are believed to be the common ancestors of all organisms (see Figure below; instruct.uwo.ca/ biology/284/intro.html). Microbes not only grow virtually everywhere but also are present in abundance. In contrast to the relatively small number of humans (6 x 109), populations of terrestrial and marine bacteria are immense, 5 x 1030 and 1.2 x 1029, respectively. In fact, the human body contains ten times more bacterial cells than human cells. Microbes carry out innumerable transformations of matter that are essential to life and thus have an enormous effect on climate and the geosphere. Much of our knowledge base in biology and molecular biology has derived from microbial studies. II. The Ubiquitous SAR 11 Clade: Previously Uncultivated Oceanic Microbes: When scientists realized in the early 1990’s that that only a small fraction (1%) of microbes in natural communities was known, these communities became the focus of many studies. Several years later, however, it is still shocking to realize the depth of our ignorance, which is well illustrated by the story of the SAR11 clade. When the technique of ribotyping (cloning and sequencing 16S rRNA genes) was first used to survey natural ecosystems, SAR 11 was one of the first groups of novel microbes to be discovered (Giovannoni et. al., 1990). We now know that 49 2006 Laboratory 2 Background Information the highest percentage of bacterial 16S ribosomal genes present in all oceanic and coastal waters are from members of the SAR11 clade, making this group “one of the most successful clades of organisms on the planet” (Morris et al, 2002; Giovannoni et. al., 2005). In some waters, SAR11 comprise up to 50% of the total surface bacterial community and approximately 25% of the subeuphotic zones. On average, members of the SAR11 clade account for about one third of all cells in surface waters. Both the great abundance and global distribution of SAR11 suggests that its members are instrumental in metabolizing oceanic dissolved organic matter (DOM), but the specific roles of these bacteria in biogeochemical cycles is still unknown (Malmstrom et al., 2004; Malmstrom et al, 2005). Although most members of the SAR11 clade remain uncultured, a few have recently been cultivated in the laboratory in seawater, though growth in synthetic medium remains elusive. As a result of several subsequent studies, SAR 11 was placed into the -subclass of Proteobacteria. However, members of the SAR11 group show less than 82% sequence similarity to cultivated Proteobacteria (Rappe et al., 2002.). One SAR11 isolate, Pelagibacter ubique. has the smallest genome (1.3 x 106 base pairs) of any known free-living cell in nature capable of independent replication (Rappe et al., 2002; Giovannoni et al. 2005). Although surprisingly the small P. ubique genome encodes almost all basic functions characteristic of -Proteobacteria, this genome contains little, if any, nonfunctional or redundant DNA and very short intergenic DNA regions, averaging only three bases in length (Giovannoni et al. 2005). It seems certain that many more surprises await from future studies of SAR 11. III. Nucleotide Sequence of 16S rRNA Genes: The basis for classification by ribotyping is the sequence of the 16S ribosomal RNA gene in prokaryotes and the 18S gene in eukaryotes. The 16S and 18S rRNA genes were selected for classification and identification of microbes because these genes are universal and essential; all living organisms must synthesize proteins to survive (Woese and Fox, 1977). These genes are also well suited for this purpose because they contain both conserved and variable regions, as is evident in the nucleotide sequence of the 16S gene shown in the Figure on the following pages. Sequences that are highly conserved are shown in brown, conserved regions are red, variable regions are black, highly variable sequences are blue and sequences that are > 75% variable are green. The map locations of some common PCR primers are also shown in the Figure, which was adapted from Baker et 50 2006 Laboratory 2 Background Information al, 2003. Conserved sequences are found in the 16S genes of all members of a domain, bacteria or archaea, and are used as universal PCR primers. In contrast, variable sequences are characteristic of certain genera, species or strains and are useful as more specific PCR primers. IV. References Baker, G.C., Smith, J.J., and D.A. Cowan. 2003. “Review and analysis of domain-specific 16S primers.” J. Microbiol. Meth. 55: 541 – 555. Giovannoni, S.J., Britschgi, T.B., Moyer, C.L. and K.G. Field. 1990. “Genetic diversity in Sargasso Sea bacterioplankton.” Nature 345: 60 – 63. Giovannoni, S.J., Tripp, H.J., Givan, S., Podar, M., Vergin, K.L., Baptista, D., Bibbs, L., Eads, J., Richardson, T.H., Noordewier, M., Rappe, M.S., Short, J.M., Carrington, J.C., and E.J. Mathur. 2005. “Genome streamlining in a cosmopolitan oceanic bacterium.” Science: 309: 1242 – 1245. Malmstrom, R.R., Kiene, R.P., Cottrell, M. T., and D. L. Kirchman. 2004. “Contribution of SAR11 bacteria to dissolved dimethysulfonioproprionate and amino acid uptake in the North Atlantic Ocean.” Appl. Environ. Microbiol. 70: 4129 - 4135. Malmstrom, R.R., Cottrell, M. T., Elifantz, H., and D. L. Kirchman. 2005. “Biomass production and assimilation of dissolved organic matter by SAR11 bacteria in the northwest Atlantic Ocean.” Appl. Environ. Microbiol. 71: 2979 - 2986. Morris, R.M., Rappe, M.S., Connn, S.A., Vergin, K.L., Siebold, W.A., Carlson, C.A., and S.J. Giovannoni. 2002. “SAR11 clase domoinates ocean surface bacterioplankton communities.” Nature 420: 806 – 810. Rappe, M.S. Connon, S.A., Vergin, K.L., and S.J. Giovannoni. 2002. “Cultivation of the ubiquitous SAR11 marine bacterioplankton clade.” Nature 418: 630 – 633 Woese, C. R. and G. E. Fox. 1977. “Phylogenetic Structure of the Prokaryotic Domain: The Primary Kingdoms.” Proc. Natl. Acad. Sci. USA. 74: 5088 - 5090. 51 2006 Laboratory 2 Background Information 27F 418F Helio 21Fa? Arch PRA624 6F QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. U518R A571F Sandell univ bact 968F 52 2006 Laboratory 2 Background Information QuickTime™ and a TIFF (Uncompressed) decompressor are needed to see this picture. 1391R 1492R 53