* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download I - Decatur ISD
Signal transduction wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Restriction enzyme wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Metalloprotein wikipedia , lookup
Size-exclusion chromatography wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Proteolysis wikipedia , lookup
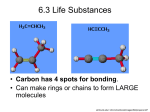
Biochemistry Notes Unit 2 Macromolecules A. What are they? 1. __________________________________ _______________________________________ 2. Made of smaller pieces called __________ that can be assembled like legos to form a variety of structures. A large chain of monomers is called a _________. Mono- ____________ Poly- _______________ B. Carbohydrates 1. Monomer: ____________________ 2. Polymer: _____________________ 3. Structure:______________________ ________________________________ 4. Uses: ___________________ 5. Examples: ________________ Carbohydrates include: Simple sugar: small sugar molecules in soft drinks Complex Carbohydrates: Long starch molecules in pasta and potatoes Monosaccharides _______________ is found in sports drinks _______________ is found in fruits Honey contains both glucose & fructose Galactose is called “milk sugar” Questions?? What does the suffix –ose mean? Where are glucose molecules found? Where would you find fructose? Disaccharides A disaccharide is a _______________ sugar They’re made by joining two monosaccharides Involves removing a water molecule (condensation) Common disaccharides include: Sucrose (______________________) Lactose (Milk Sugar) Maltose (______________________) Sucrose is composed of glucose + fructose Draw a model of a carbohydrate Biochemistry Notes Unit 2 Polysaccharides Complex carbohydrates Composed of many sugar monomers linked together Polymers of monosaccharide chains Questions?? What does the prefix poly- mean again? Mono-? Polymers of monosaccharide chains means what? Draw a model of a lipid Lipids 1. Monomer: __________ 2. Polymer: ___________ 3. Structure:______________________ ________________________________ 4. Uses: ________________________ 5. Examples: ____________________ Fats store energy, help to insulate the body, cushion and protect organs, and makes up the cell membrane (lipid bilayer) Lipids are molecules that consist of long hydrocarbon chains. Attaching the three chains together is usually a glycerol molecule. Lipids are ______________________ Cell membranes are made of lipids called _______________________________ Phospholipids have a head that is polar & attract water (________________) Phospholipids also have 2 tails that are nonpolar and do not attract water (__________________) Lipids are hydrophobic –”_____________” Do ______ mix with water Includes fats, waxes, steroids, & oils Most animal fats have a high proportion of saturated fatty acids & exist as solids at room temperature (butter, margarine, shortening) these are called ________________________ Most plant oils tend to be low in saturated fatty acids & exist as liquids at room temperature (oils) these are called ____________________________ Biochemistry Notes Unit 2 Draw a model of a protein Protein 1. Monomer: _______________ 2. Polymer: _____________________ 3. Structure:______________________ ________________________________ 4. Uses: ________________________ 5. Examples: ____________________ Proteins are building blocks of structures called _______________________. Proteins are what your DNA codes to make A peptide bond forms between amino acids by dehydration synthesis. ____________________________= the building up of large molecules by removing water molecules Enzymes A. Special proteins that speed chemical reactions 1. Chemical reactions require a certain _______________ to get started. 2. Enzymes decrease this energy, making reactions occur faster. B. Lock-and-Key Model 1. Enzymes are not used up by the reaction, but each can only work on one reaction (________________). 2. This is called the lock-and-key model of enzymes. An enzyme is like a _____ which can open exactly one _____. If you want to “unlock” another reaction, you need a different enzyme. C. Factors which affect enzymes 1. _____________--enzymes, like all proteins, change shape when exposed to heat or cold. Each has an optimal temperature range. 2. ____--all enzymes have an optimal range of pH. Example: stomach 3. _____________--having more enzymes makes the reaction faster. DRAW AN ENZYME SUBSTRATE COMPLEX MODEL & LABEL IT Biochemistry Notes Unit 2 Nucleic Acids 1. Monomer: _______________ 2. Polymer: ___________ 3. Structure:______________________ ________________________________ 4. Uses: ___________________ 5. Examples: _______________ Draw a NUCLEOTIDE Nucleic acids are polymers of nucleotides Identify the Parts of a Nucleotide Each DNA nucleotide has one of the following bases: (A) Guanine (G) (T) Cytosine (C) Nucleotide Monomers Form long chains called DNA Nucleotides are joined by sugars & phosphates on the side DNA Two strands of DNA join together to form a _________________ RNA – Ribonucleic Acid Ribose sugar has an extra –OH or hydroxyl group It has the base ____________ (U) instead of thymine (T) ATP A. B. C. D. E. F. ATP stands for _____________________ Cells use ATP as a __________________ Made of adenine with ___ phosphates Lots of energy is stored in the bond between _____________________________ When this bond is broken, tremendous energy is released. The pieces are then reassembled, storing more energy for another use. Questions?? Explain the ATP/ADP process.