* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Supplementary Information (doc 1146K)
Broca's area wikipedia , lookup
Embodied language processing wikipedia , lookup
Environmental enrichment wikipedia , lookup
Executive functions wikipedia , lookup
Cortical cooling wikipedia , lookup
Dual consciousness wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Neuroesthetics wikipedia , lookup
Neuroeconomics wikipedia , lookup
Human brain wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Limbic system wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Face perception wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Aging brain wikipedia , lookup
Neuroanatomy of memory wikipedia , lookup
Affective neuroscience wikipedia , lookup
Cognitive neuroscience of music wikipedia , lookup
Time perception wikipedia , lookup
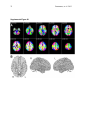
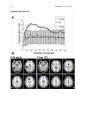
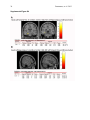
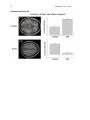
1 Pantazatos, et. al. 2013 Supplementary Material for Reduced anterior temporal and hippocampal functional connectivity during face processing discriminates individuals with social anxiety disorder from healthy controls and panic disorder, and increases following treatment. Authors: Spiro P. Pantazatos1,2,*,+, Ardesheer Talati3,7+, Franklin R. Schneier3,8, Joy Hirsch1,4,5,6,* 1 fMRI Research Center, Depts of 2Physiology and Cellular Biophysics, 3Psychiatry, 4 Neuroscience, 5Radiology, 6Psychology, Columbia University, New York, NY, USA; Divisions of 7 Epidemiology and 8Clinical Therapeutics, New York State Psychiatric Institute, New York, NY * To whom correspondence should be addressed: E-mail: [email protected], [email protected] This file includes Supplemental Methods Supplemental Results Supplemental Discussion Tables S1, S2, S3, S4 Supplemental Figures S1, S2, S3, S4, S5, S6 Supplemental References 2 Pantazatos, et. al. 2013 Supplemental Methods Subjects Primary Sample Potential subjects were first screened by a research assistant with the anxiety screening modules of the Schedule for Affective Disorders and Schizophrenia-Lifetime Version, Modified for Anxiety Disorders and Updated for DSM-IV (SADS-LA-IV (Mannuzza et al. 1986)); subjects who screened positive for SAD then participated in a full SADS-LA-IV interview (see below). Subjects with SAD were required to have a DSM-IV (Psychiatric Association 1994) diagnosis of the generalized subtype of social anxiety disorder (GSAD, characterized by fear of most social situations). Subjects in the comparison group were required to have a DSM-IV diagnosis of PD, either with or without agoraphobia. Subjects in both groups were required to have first onset by age 30, and have a first-degree relative with an anxiety disorder. HCs were required to have no lifetime history of any psychiatric disorder, with exceptions for past minor depressive disorder, adjustment disorders, or brief periods of substance abuse (not dependence) in adolescence or college. HCs also could not have a history of an anxiety disorder in any first-degree relative. Neither group could have a personal or family history of schizophrenia or bipolar disorder. All subjects were free of psychotropic medications for 10 weeks preceding the scan. Diagnostic assessments were administered by clinically trained mental health professionals with the SADS-LA-IV(Mannuzza et al. 1986). Training and monitoring procedures have been previously described (Talati et al. 2008). Family history was obtained with the Family History Screen (Weissman et al. 2000). Final diagnoses were made by an experienced clinician with the Best Estimate Procedure (Leckman et al. 1982). Replication Sample Exclusion criteria for GSAD participants included having a current Axis I disorder (other than secondary diagnoses of generalized anxiety disorder, dysthymia, or specific phobia), major 3 Pantazatos, et. al. 2013 depressive episode in the past year, substance abuse in the past 6 months, and clinically significant general medical conditions. HCs did not meet criteria for any lifetime Axis I disorder. Health status was confirmed by a physical examination including drug toxicology screen. All subjects were free of psychotropic medications for at least 4 weeks prior to study entry. Data from four GSAD patients were excluded from analyses (one subsequently revealed a recent history of major depression, one failed to follow imaging task instructions, and the functional scans of the others suffered from technical issues), yielding 14 GSAD patients. Secondary comorbid diagnoses in participants with GSAD consisted of current generalized anxiety disorder (N=3), past major depression (N=6), and past alcohol abuse (N=1). Six GSAD subjects had taken medication for anxiety or depression prior to the past 4 weeks. All subjects in both samples provided written informed consent after discussion of study procedures. Behavioral task Primary Sample Subjects performed a previously described task (Etkin et al. 2004; Pantazatos et al. 2012b) which consists of color identification of fearful, neutral, masked fearful and mask neutral faces (F, N, MF and MN respectively) with in a blocked paradigm (four 20 second blocks for each condition, 15 second baseline between each block). Stimuli: Black and white pictures of male and female faces showing fearful and neutral facial expressions were chosen from a standardized series developed by Ekman and Friesen (Pantazatos et al. 2012a). Faces were cropped into an elliptical shape that eliminated background, hair, and jewelry cues and were oriented to maximize inter-stimulus alignment of eyes and mouths. Faces were then artificially colorized (red, yellow, or blue) and equalized for luminosity. For the training task, only neutral expression faces were used from an unrelated set available in the lab. These faces were also cropped and colorized as above. 4 Pantazatos, et. al. 2013 Each stimulus presentation involves a rapid (200 ms) fixation to cue subjects to fixate at the center of the screen, followed by a 400 ms blank screen and 200 ms of face presentation. Subjects have 1200 ms to respond with a key press indicating the color of the face. Behavioral responses and reaction times were recorded. Unmasked stimuli consist of 200 ms of a fearful or neutral expression face, while backwardly masked stimuli consist of 33 ms of a fearful or neutral face, followed by 167 ms of a neutral face mask belonging to a different individual, but of the same color and gender. Each epoch consists of ten trials of the same stimulus type, but randomized with respect to gender and color. The functional run has 16 epochs (four for each stimulus type) that are randomized for stimulus type. To avoid stimulus order effects, we used two different counterbalanced run orders. Stimuli were presented using Presentation software (Neurobehavioral Systems, http://nbs.neuro-bs.com), and were triggered by the first radio frequency pulse for the functional run. The stimuli were displayed on VisuaStim XGA LCD screen goggles (Resonance Technology, Northridge, CA). The screen resolution was 800X600, with a refresh rate of 60 Hz. Prior to the functional run, subjects were trained in the color identification task using unrelated neutral face stimuli that were cropped, colorized, and presented in the same manner as the nonmasked neutral faces described above in order to avoid any learning effects during the functional run. After the functional run, subjects were shown all of the stimuli again, alerted to the presence of fearful faces, and asked to indicate whether they had seen fearful faces on masked epochs. Replication Sample Stimuli consisted of faces of both genders expressing neutral, high valence angry or happy expressions from the same standard series as above 26, during explicit and unattended viewing conditions. During the explicit processing condition, subjects were asked to judge the emotional facial expression (angry, neutral, happy) by using a keypad, and reaction times were recorded. During the unattended processing condition, subjects were asked to identify gender of 5 Pantazatos, et. al. 2013 each face (male/female), responding via keypad. The stimuli were presented in a block design consisting of two 6 min and 48 sec. runs (one run unattended, one run explicit) each containing 4 blocks of angry (A), neutral (N) and happy (H) faces. Each block lasted 20 seconds, followed by 12-14 seconds of baseline (white crosshair against black backgroun d). Within each block, 10 stimuli (faces) were presented for 1 second, followed by 1 second crosshair between each stimulus presentation. At the start of each run, an instruction screen was presented for 10 seconds, with instructions for using the keypad. Subjects had been trained prior to the scanning session in the use of the keypad. Given that our primary sample performed an unattended face processing task (i.e. identification of colors overlaid on emotional faces), we conducted the replication analysis using the unattended condition from the replication sample. We note our replication sample was not prospectively designed to be a replication of our experimental paradigm, but rather it was an independent cohort of SAD and controls from a separate study (PI: Schneier) which performed a similar, though not identical, implicit face processing task. Due to a minor programming error, during the unattended runs, 11 baseline (pretreatment) subjects (6 controls, 5 cases) received a distribution (in no particular order) of 5/4/3 blocks of each condition, with 5 blocks tending to occur slightly more often for the A condition, and 3 blocks slightly more often for N (over all subjects, mean #blocks per condition: A-4.22, H3.91, N-3.88). Five (1 control, 4 cases) post-treatment runs were similarly affected (over all subjects, mean # blocks per condition: A-3.95, H-4.11, N-3.95). Node definition and functional connectivity estimation: Brain regions were parcellated according to bilateral versions of the Harvard-Oxford Cortical and sub-cortical atlases and the AAL atlas (cerebellum) and were trimmed to ensure no overlap with each other and to ensure inclusion of only voxels shared by all subjects (Supplemental Figure 1A). For each subject, time-series across the whole run (283 TRs) were extracted using Singular Value Decomposition (SVD) and custom modifications to the Volumes- 6 Pantazatos, et. al. 2013 of-Interest (VOI) code within SPM8 to retain the top 2 eigenvariates from each atlas-based region. Briefly, the data matrix for each atlas-based region is defined as A, an n x p matrix, in which the n rows represent the time points, and each p column represents a voxel within an atlas-based region. The SVD theorem states: Anxp= Unxn Snxp VTpxp, where UTU = Inxn and VTV = Ipxp (i.e. U and V are orthogonal). The columns of U are the left singular vectors (eigenvariates, or summary time courses of the region), S (the same dimensions as A) has singular values, arranged in descending order, that are proportional to total variance of data matrix explained by its corresponding eigenvariate, and is diagonal, and VT has rows that are the right singular vectors (spatial eigenmaps, representing the loading of each voxel onto its corresponding eigenvariate). Here we retain the top two eigenvariates (nodes) from each region. The above step resulted in a total of 248 nodes with an associated time course (i.e. eigenvariates) and spatial eigenmaps from the 124 initial atlas-based regions. Thus, each atlasbased region was comprised of two nodes. We note that this means it is possible that node 2 of a particular region shows functional connectivity that differentiates SAD diagnosis and node 1 of the same region has no differential connectivity. For clarity we therefore label each node using its Harvard-Oxford atlas label appended by either “_PC1” for the first eigenvariate and “_PC2” for the second. For display purposes, we calculated the MNI coordinates of the peak loading weight (locations averaged across subjects) for each eigenvariate from its associated eigenmap (Supplementary Figure 1B). Table S1 lists the average MNI coordinates for each node. For each subject, functional connectivity matrices (i.e. where cell i,j contains the Pearson correlation between region i and region j) were generated for non-masked (unattended) and masked (subliminal) fearful (F, MF) and neutral (N, MN) conditions (primary sample), and for unattended angry (A), happy (H) and neutral (N) conditions (replication sample), as well as over 7 Pantazatos, et. al. 2013 the full run (henceforth denoted as “Full”). The above time-series were segmented and concatenated according to conditions of interest, incorporating a lag of 6 or 7 s from the start of each block, before generating the correlation matrices. The lower diagonal of the above preprocessed correlation matrices were then used as input features to predict subjects’ diagnoses. SVM pattern analysis Support vector machines (SVM) are pattern recognition methods that find functions of the data that facilitate classification During the training phase, an SVM finds the hyperplane that separates the examples in the input space according to a class label. The SVM classifier is trained by providing examples of the form <x,c>, where x represents a spatial pattern and c is the class label. In particular, x represents the fMRI data (pattern of correlation strengths) and c is the condition or group label (i.e. c = 1 for SAD and c = −1 for control). Once the decision function is determined from the training data, it can be used to predict the class label of new test examples. Our intent is not to estimate or maximize the true accuracy of prediction given a completely new data set, but rather to test whether there exist information in the pattern of functional connections relevant and specific to SAD, and to approximate the optimal number of features that containing this information. We note that our approach in the primary sample (plotting AUC vs. number of top N features) is not biased, since for each number of top N features, and for each round of leave-one-out cross validation, the top N features were selected from a training set that was completely independent from the testing set. If there is a true signal present in the data, we expect, and in the current data observe, an initial rise in accuracy as more informative features are added to the feature set, and a dip in accuracy as less informative features (i.e. noise) are added to the feature set. 8 Pantazatos, et. al. 2013 For all binary classification tasks, a linear kernel SVM (Fu et al. 2008) with a filter feature selection (t-test) and leave-one-out cross validation was used. During each iteration of leaveone-out cross validation (primary sample), one subject was withheld from the dataset and 1) a 2-sample t-test was performed over the remaining training data 2) the features were ranked by absolute t-score and the top N were selected 3) these selected features were then used to predict the class of the withheld test examples during the classification stage. For classification in the replication sample, the SVM model was learned from the whole primary sample using the top 2 features identified in the analysis above, and this same model was used to predict SAD vs. controls in the replication sample. Prior to learning, the effects of age and gender were regressed out from the features using a general linear model, and features were z-scored. Classification performance is reported with “area under the curve” (AUC) (i.e. area under the receiver operator characteristic, or ROC curve (Hanley & McNeil 1982)). Since the predicted labels are binary (not continuous scores), AUC is identical to the arithmetic mean of sensitivity (true positive rate, or proportion of correctly classified cases) and specificity (true negative rate, or proportion of correctly classified controls). AUC vs. number of features that have been ranked by their t-score was plotted, and the performance significance was computed using nonparametric permutation tests (Golland & Fischl 2003) with 200 (1 to 40 features) or 10,000 (single reported peaks) iterations. The filter feature selection ranked features according to absolute t-score, which identifies the strongest differing features but discards directional information. Classification accuracy vs. every 5 features from the top 1 through 200 was first examined (the maximum number was chosen heuristically based on (Dosenbach et al. 2010). Other than a peak near 1 features, accuracies hovered near 50%. Therefore the range was changed to every single feature from top 1 through 40, the same range which was recently used in decoding supraliminal fear (Pantazatos et al. 2012b). Bonferroni correction was also applied for the number of total Top N comparisons (in this case 40). Confidence intervals for AUC estimate in the replication sample was also estimated using the 'bootstrap t' approach 9 Pantazatos, et. al. 2013 (Obuchowski & Lieber 1998) with 10,000 iterations using the Measures of Effect Size Toolbox (http://www.mathworks.com/matlabcentral/fileexchange/32398-measures-of-effect-size-toolbox). SVM learning and classification was done using the Spider v1.71 Matlab toolbox (http://people.kyb.tuebingen.mpg.de/spider/) using all default parameters (i.e. linear kernel SVM, regularization parameter C=1). Graphical neuro-anatomical connectivity maps of the top N features were displayed using Caret v5.61 software (http://brainvis.wustl.edu/wiki/index.php/Caret:About). We note that different features could have been selected during the feature selection phase of each round of cross-validation. Therefore in ranking the top N features, features were ranked by total number of times that feature was included in each round of cross-validation, and then among these features, features were sorted by absolute value of the average SVM weight. PsychoPhysiological Interaction (PPI) analysis (see Supplemental Figure 4) was conducted following a generalized PPI approach (McLaren et al. 2012). The PPI analysis measures the extent to which regions are differentially correlated during a given task or between subjects. We used ROI-based "seeds" for Left Hippocampus and Left Temporal Pole from the same atlas used in the main text, and extracted the 1st eigenvariate from each region to be used as the region's summary time course. The BOLD signal throughout the whole-brain was then regressed on a voxel-wise basis against the product of this time course and the vectors of the psychological variable of interest, (1*F + 1*N + 1*MF +1*MN), with the physiological and the psychological variables serving as regressors of no interest (F, N, MF and MN pyschological regressors were identical as in the main GLM analysis). Additional nuisance regressors included 9 motion parameters, and mean white and csf signal. Resulting beta maps for the N PPI condition were subsequently passed to 2nd level random effects analysis (one way ANOVA with 3 levels: controls, SAD and PD, with age and gender as additional nuisance covariates). Contrasts for control > SAD were then computed from this 2nd level model. 10 Pantazatos, et. al. 2013 Supplemental Results Provisionary results included 7 HCs completing pre-post scans 8 weeks apart. Additional analyses that incorporated 7 HCs confirmed that pre- to post-treatment FC increases in the SAD group were significantly greater than FC changes over 8 weeks in the control group (cases (post-pre) > controls (post-pre), t(17)=2.1, p=0.05, 2-sample t-test), and that FC did not change significantly in the control group (t(6)=1.56, p=0.17, one sample t-test). To further examine whether Left Hippocampus-Left Temporal Pole FC tracks social anxiety symptom severity longitudinally, regardless of diagnostic or treatment status, the extent to which changes in LSAS were associated with changes in this FC was tested. This analysis included controls, because we were primarily interested in longitudinal symptom change that is not necessarily specific to treatment, and in order to further test that small changes in LSAS in controls are accompanied by small changes in Left Hippocampus-Left Temporal Pole FC. Given that SAD subjects exhibited decreased FC relative to HCs at baseline, we hypothesized that, across both HCs and SAD subjects, increases in Left Hippocampus-Left Temporal Pole FC should be associated with decreases in symptom severity. This relationship was indeed observed for Left Hippocampus-Left Temporal Pole FC, with significant correlations observed for FC during angry and happy faces (pre-post ΔLSAS vs. post-pre ΔFC: angry R=0.55, p=0.008, happy R=0.58, p=0.004, neutral R=0.33 p=0.08, and full run R=0.37 p=0.06) (Supplementary Figure 6). These results held, particularly for FC during angry, happy and neutral faces, after removal of the top 1 and top 2 outliers (indicated as boxed 1s and 2s in Supplementary Figure 6) from each plot, (pre-post ΔLSAS vs. post-pre ΔFC top 1 removed: angry R=0.59, p=0.01, happy R=0.63, p=0.005, neutral R=0.35 p=0.15, full run R=0.39 p=0.10; top 2 removed: angry R=0.57, p=0.017, happy R=0.62, p=0.007, neutral R=0.51 p=0.018, and full run R=0.36 p=0.16). 11 Pantazatos, et. al. 2013 Changes in left hippocampus and left temporal pole activation following 8-weeks SSRI treatment. Pre-post activation differences in response to neutral and angry faces (vs. baseline) were also assessed in the left hippocampus and left temporal pole. Using left hippocampus as an ROI and thresholding at p<0.05 uncorrected, k=20, post > pre differences were observed at MNI [-14 -10 -22], t=2.14, k=21, while pre > post differences were observed at MNI [-32, -22, 14], t=2.43, k=40 for neutral faces, and MN [-36 -32 -10, t=3.53, k=54 and [-20 -10 -22], t=2.83, k=77 for angry faces. However no clusters survived cluster-extent correction at p<0.05 (k threshold=97). Using left temporal pole as an ROI, pre > post differences were observed at MNI=[-54, 6 -18],t=4.5, k=111 at p<0.05 uncorrected for neutral faces, which survived clusterextent correction at p<0.05 (k threshold=88), while post > pre cluster was observed for angry faces at MNI=[-30 14 -28], t=2.88, k=23, which was not significant. Overall these results suggest decreases in activation in left hippocampus and left temporal pole in response to angry and neutral faces following 8-weeks SSRI treatment. For angry and neutral faces, neither pre > post nor post > pre differences were observed in right anterior middle temporal gyrus at p<0.05 uncorrected, k=20, while left OFC, post>pre differences were observed for angry faces in [-30 12 -18], t=2.71, k=69 and pre>post differences for neutral faces in [-24 8 -18], t=2.64, k=32, but neither cluster reached significance at p<0.05 after cluster-extent thresholding (k threshold =103). In addition, pre-post change in Left Hippocampus and Left Temporal Pole grey matter volume were correlated with change in FC between these regions. Positive, albeit non-significant, associations were observed with change in Left Hippocampus volume with change in FC (r=0.39, p=0.11), change in Left Temporal Pole volume with change in FC (r=0.24, p=0.34), and change in Left Hippocampus volume with change in left Temporal pole volume (r=0.18, p=0.48). 12 Pantazatos, et. al. 2013 Examining previous SAD-related and emotion-related FC reported in the literature In addition to the exploratory, data-driven approach above, we examined FC previously identified to be anomalous in SAD, in particular reduced aINS-dACC (31) and amygdala-dACC and amygdala-dlPFC (32) in SAD during fear. Using PPI analysis, a recent study observed less aINS-dACC FC during fearful (> happy) in gSAD relative to controls (Klumpp et al. 2012). All FC during both F and N conditions between bilateral Insula and Anterior Cingulate Gyrus was queried at p < 0.05 uncorrected, and the following was observed: Control > SAD, Left_Insular_Cortex_PC2-Left_Cingulate_Gyrus_anterior_division_PC1 t(33)=2.22/2.96 F/N, and Right_Insular_Cortex_PC2-Left_Cingulate_Gyrus_anterior_division_PC1 t(33)=1.82/Not significant F/N. The average peak location for Left Insula was anterior ([-36 16 2]), while peak MNI location for the right was middle insula ([42 -4 6]). These results are consistent with the aforementioned study. A related study (Prater et al. 2012) used PPI and observed less connectivity between amygdala-dACC and amygdala-dlPFC in SAD during fearful faces perception. As above, we interrogated FC between these regions during F and N conditions at p<0.05 uncorrected, Control > SAD and observed: Right_Amygdala_PC2-Right_Cingulate_Gyrus_anterior_division, t(33)=2.53/Not significant F/N and Right_Ventral_Frontal_Pole_PC1 - Right_Amygdala_PC1 = t(33) = 1.9208/Not significant F/N consistent with (Prater et al. 2012). However, many FC differences between amygdala and dlPFC/precentral gyrus were in the opposite direction (i.e. greater in SAD): Control > SAD, Right_Ventral_Frontal_Pole_PC1 - Right_Amygdala_PC2 = 1.94 Right_Ventral_Frontal_Pole_PC1 - Left_Amygdala_PC2 = -1.67, Left_Amygdala_PC1Left_Middle_Frontal_Gyrus_PC1, t(33)=-3.08, Left_Precentral_Gyrus_PC1Left_Amygdala_PC1, t=-2.63, Right, t=-1.72, Right_Ventral_Frontal_Pole_PC2 Left_Amygdala_PC1 , t=-2.5082. Although FC differences were mostly consistent with these studies, including the above connections (RAmygdala-RACC, Right_Ventral_Frontal_Pole_PC1-Right_Amygdala_PC1, Left 13 Pantazatos, et. al. 2013 Insula-dACC) with the top 2 connections identified in the main text did not improve classification performance (data not shown), while including only these connections resulted in poorer classification performance (AUC=0.53). It is important to note that FC was measure here using Pearson correlation, while these previous studies applied "seed" based regression analyses, which are different approaches for measure functional connectivity and their differences. See (Kim & Horwitz 2008) for further discussion. An additional analysis was conducted whereby the top 25 FC that discriminated supraliminal fearful from neutral faces (Pantazatos et al. 2012b) and top 25 FC that discriminated subliminal fearful from neutral faces (Pantazatos et al. 2012a) in a healthy sample were used as initial input feature set for predicting SAD vs. controls. Classifications were performed as in the main text from the top 1 to top 25 ranked features. For supraliminal conditions, the following peak SAD vs. Control AUCs were achieved: F: 0.62, N: 0.68, F-N: 0.74. For subliminal conditions, the following peak SAD vs. Control AUCs were achieved: MF: 0.69, MN: 0.64, MF-MN: 0.82. The relatively high discrimination achieved when using the differences between supraliminal and subliminal fearful and neutral faces (i.e. F-N and MF-MN) suggests that SAD subjects do not process fearful vs. neutral faces in the same way as healthy subjects, and instead use a different neural circuitry. However, it is important to note that this analysis is biased due to the fact that the primary control sample constituted half of the healthy subjects used in the above studies. Future studies using an additional independent control sample would be necessary to acquire unbiased results. Discriminating between SAD and healthy control subjects with patterns of spatial activity To compare the information content of patterns of interactivity (i.e. functional connections used above) vs. patterns of activity, SAD vs. Control classification was also conducted using beta estimates, which are considered summary measures of activation in response to each condition. This approach is conceptually similar to a recent study that used 14 Pantazatos, et. al. 2013 pattern classification of whole-brain activity (BOLD averaged over several TR’s of an event minus baseline activity immediately preceding the event) during sad face viewing to predict diagnosis (normal vs. clinically depressed)(Fu et al. 2008). In order to make featureselection/leave-one-out cross validation and SVM learning more computationally tractable, preprocessed functional data were resized from 2x2x2 mm voxel resolution to 4x4x4 mm resolution, and subject-specific GLM models were re-estimated, resulting in a reduction of total feature space per example from ~189,500 betas to ~23,500. Feature selection, leave-one-out cross validation and SVM learning proceeded exactly as above for FC data. When using the contrast F-N, we observed a peak AUC of 0.88 (p<0.0001 uncorrected) with 8 voxels (within cerebellum and middle occipital gyrus), and when using the F beta weights, a peak AUC=0.83, p=0.0008 uncorrected was observed with ~170 voxels (Supplementary Figure S3, Supplementary Table S2). However the AUC using F betas dropped to 0.49, and AUC using FN contrast dropped to 0.58 after regressing out the effects of age and sex prior to classification. Classification of SAD vs. PD using the same features as above was then attempted, and a decrease in classification performance was observed; when using F>N contrasts, AUC=0.59, p=0.03 uncorrected, and when using F beta weights, AUC=0.56 p=0.26 uncorrected, data not shown). Classification of SAD vs. PD using top 10:10:500 F-N contrast estimates over the whole-brain only achieved a peak AUC of 0.66 (data not shown). Thus, although peak classification performance for SAD vs. Controls using contrast estimates as features matched that of using pair-wise functional connectivity, under the current analysis these activation differences appear to be less specific to SAD. Standard GLM Activation analysis A standard GLM analysis was also run to identify SAD vs. Control differences in overall activation and differential activation to F, N, MF or MN conditions in the primary sample. For a 2way ANOVA 2nd level analysis was conducted (1st independent factor diagnosis: 2 levels, 2nd 15 Pantazatos, et. al. 2013 dependent factor face conditions: 4 levels-F, N, MF, MN). Omnibus F-tests were used to identify main effects of diagnosis and diagnosis X face condition interactions. Whole-brain results were thresholded at p<0.05 corrected using cluster-extent threshold using the 3dClusterSim program in AFNI (v.2011). Briefly, 1000 Monte Carlo simulations of whole-brain fMRI data were generated using the applied smoothing (8 mm fwhm), voxel size (2x2x2 mm) and whole-brain mask to determine the cluster size at which the false positive probability was below a desired alpha level of p < 0.05 corrected. For an uncorrected p-value threshold of 0.005, this yielded a cluster size required of 148. Greater activation to faces in the SAD group were observed in the fusiform, consistent with higher salience of faces in SAD, while greater activation to faces in the control group were observed in dorsolateral prefrontal cortex, consistent with reduced cognitive control during face processing in SAD (see Supplementary Table S4 and Supplemental Figure S5). These clusters were used to define ROIs to test for main effects of diagnosis in the replication group (in this case overall activation to A, H and N faces). Within these ROIs, no voxels survived a lenient threshold of p<0.05 uncorrected. In addition, the above ROIs were used as a mask to preselect voxels to be used in an additional, biased SVM analysis that used averages of these beta estimates across each face condition as well as beta estimates from each face condition as features. Performance was assessed across the range of top 10 through 650 (every 10) selected voxels. Despite biased feature selection, peak AUCs were still lower than those achieved using large-scale functional connectivity as features and unbiased feature selection (beta estimates averaged across all face conditions: 0.71, F: 0.67, N: 0.74, MF: 0.71, MN: 0.71, data not shown). Grey matter volume and pre-post activation analyses Voxel-based morphometry analyses (Ashburner & Friston 2000) were used to correlate pre-post changes in FC with pre-post changes in local grey matter (GM) volume. Intra-subject 16 Pantazatos, et. al. 2013 realignment, bias correction, segmentation, and DARTEL normalization, and modulation of anatomical data were conducted using batch processing for longitudinal data within VBM8 toolbox (no smoothing was applied). Processed post-images were subtracted from processed pre-images to create a pre-post subtraction image for each subject. Briefly, the coordinate of the peak loading factor from the first PC spatial eigenmap within Left Hippocampus and Left Temporal Pole was identified for each subject, and the estimates of local GM volume pre-post value was extracted from a 6 mm radius sphere about the coordinate. These values were then correlated with pre-post changes in Left-Hippocampus-Left Temporal Pole FC. These analyses included the same subjects as in the analysis of pre-post changes in FC except for one case subject whose anatomical data was discarded due to technical issues with the scan (total 18 subjects: 11 cases, 7 controls). See methods, main text for description of GLM analysis. For pre-post activation analyses, cluster-extent correction was applied using the 3dClusterSim program in AFNI (v.2011). Briefly, 1000 Monte Carlo simulations of whole-brain fMRI data were generated using the applied smoothing (8 mm fwhm), voxel size (2x2x2 mm) and ROI masks (Left Hippocampus and Left Temporal Pole) to determine the cluster size at which the false positive probability was below a desired alpha level of p < 0.05 (i.e. an effective threshold of p < 0.05 corrected for multiple comparisons). Supplemental Discussion A study using effective connectivity (Granger causality analysis) of a few a priori selected regions (amygdala, visual and association cortex) suggested increased connectivity between amygdala and visual cortex, and decreased connectivity with OFC during resting-state in SAD (Liao et al. 2010), while atlas-based functional connectivity analysis of resting-state data suggested decreased functional connectivity between frontal and occipital lobes in SAD (Ding et al. 2011). More recent work has focused on condition-dependent functional connectivity (i.e. 17 Pantazatos, et. al. 2013 while viewing fearful faces or during perception of scrutiny) and/or during rest of amygdala, anterior cingulate, frontal cortex and other regions using PPI analysis, which assess FC between an a priori selected seed region and the rest of the brain (Prater et al. 2012; Klumpp et al. 2012; Giménez et al. 2012; Pannekoek et al. 2012). When we focused on specific FC differences of the amygdala and insula, we observed some consistency with the above studies (Prater et al. 2012; Klumpp et al. 2012) . However, these were not the strongest observed FC differences observed in the current data. One possible explanation for this speaks to an advantage of the current approach in that it assesses the multivariate pattern of the strongest FC differences selected from among many thousands of estimated pair-wise FCs, and these previous approaches may have missed this FC because they did not employ hippocampus nor temporal pole as seed regions. However, it is also important to point out that significance FC differences estimates using correlation varies much more for different scanning and hemodynamic parameters relative to FC differences estimated using regression approaches (i.e. PPI) (Kim & Horwitz 2008), and SAD vs. Control comparison using PPI analysis seeded with either Left Hippocampus or Left Temporal Pole in the current data produced only moderately significant results (current approach, peak T-value = 5.39; PPI peak T-value = 3.43, see Supplementary Table S3, Supplementary Figure S4). Finally, we note that our findings that activity (fearful vs. neutral faces contrast) in middle occipital gyrus distinguished SAD vs. controls Supplemental Figure 3, is consistent with (Doehrmann et al. 2012), in which greater response to CBT treatment correlated significantly with greater pretreatment activation (angry vs. neutral faces contrast) in middle occipital gyrus. In addition, positive, albeit non-significant, correlations between increases in Left Hippocampus-Left Temporal Pole FC with increases in GM volume in each of these structures were observed, suggesting increases in grey matter volume may be associated with increased functional connectivity between these regions. Future studies with larger sample sizes are needed to confirm whether this is indeed a true association. 18 Pantazatos, et. al. 2013 It may seem counterintuitive that the most predictive FC was during neutral faces in primary sample. However this is consistent with evidence suggesting that SAD is characterized by negative interpretation bias, particularly when presented with ambiguous social cues (i.e. neutral faces) (Winton et al. 1995; Yoon & Zinbarg 2007). Other studies demonstrate abnormal reactivity to emotional, and in particular harsh (i.e. angry, disgust), faces (Klumpp et al. 2010). In the current study, case vs. control and pre-post treatment differences in Left Hippocampus-Left Temporal Pole FC during neutral faces in the replication sample was observed on a trend level (Table 3, Neutral column: SAD>Control, p=0.09, SAD pre>post p=0.10). However the strongest effects in this sample were observed for this FC during angry faces (Table 3, Angry column: SAD > Control, p=0.027, pre>post p=0.007). One possible interpretation is that angry faces (relative to neutral) are more salient in SAD, and larger differences in Left Hippocampus-Left Temporal Pole FC might have observed in the primary sample if angry faces had been used. Alternatively, neutral (relative to angry) faces could be a more salient in SAD, but the signal was not apparent in the replication sample due to a minor technical issue that caused slightly fewer blocks of neutral face conditions relative to angry (see Methods: Replication Sample, last paragraph). Future studies using a balanced block design with both angry and neutral faces can facilitate a direct comparison that should help resolve this ambiguity. Interestingly, we observed increased FC between Left Hippocampus-Left Temporal Pole concomitant with symptom improvement following 8-weeks SSRI treatment, yet there was a trend-level decrease in activity in each of these structures in response to angry and neutral faces following treatment (see Supplementary Results). Previous PET and SPECT studies have also shown reduced perfusion and cerebral blood flow (rCBF) in these regions following 8weeks SSRI treatment. PET imaging during a public speaking paradigm in SAD subjects demonstrated that regardless of treatment approach (SSRI citalopram or behavioral therapy), improvement was accompanied by a decreased rCBF-response to public speaking bilaterally in the amygdala, hippocampus, and the periamygdaloid, rhinal, and parahippocampal cortices 19 Pantazatos, et. al. 2013 (Furmark et al. 2002), while a related SPECT study demonstrated reduced cerebral perfusion in left hippocampus following 8 or 12 weeks of citalopram in a combined group of SAD, obsessive compulsive disorder and post-traumatic stress disorder patients (Carey et al. 2004). A related SPECT study observed reduced perfusion in anterior and lateral temporal cortex in SAD subjects following 8-weeks citalopram treatment (Van der Linden et al. 2000), while in a recent fMRI BOLD study, temporal pole activity during successful understanding of others' mental states correlated with neuroticism (Jimura et al. 2010). Taken together, these results suggest that while increased activation of hippocampus and temporal pole may be associated with increased social anxiety symptom severity, increased functional connectivity between these two structures is associated with decreased symptom severity. Limitations The estimate of AUC=0.89 in the primary group is not an estimate of the true accuracy given new data of our machine learning approach, since we did not know a priori how many top N features to select. Although significantly above chance, classification performance in the replication sample for the features selected in the primary sample was lower (primary sample/replication sample AUC=0.89/0.71). There are several possible reasons for this. Firstly the task in the replication sample was slightly different (identify gender vs. color), and eleven subjects received only 3 blocks of neutral face blocks (as opposed to 4) due to a minor programming error (see methods). Secondly, the peak MNI location for Left Orbitofrontal Cortex was very inferior, bordering the dorsal tip of the left temporal pole. Since parcellation was done in 3D, and smoothing was applied, it is possible this signal originated from the left dorsal temporal pole. Future studies should derive functional connectivity from 2D cortical surface maps that are then registered across subjects, in order to ensure that regions are more precisely labeled. Previous simulations have raised concerns regarding the use of atlas-based approaches 20 Pantazatos, et. al. 2013 for parcellating the brain (Smith et al. 2011). Because the spatial ROIs used to extract average time-series for a brain region do not likely match well the actual functional boundaries, BOLD time-series from neighboring nodes are likely mixed with each other. While this hampers the ability to detect true functional connections between neighboring regions, it has minimal effect on estimating functional connectivity between distant regions. This perhaps explains why in this study most of the functional connections that discriminated between SAD and controls were long-distance. Future experiments using non-atlas based approaches would likely lead to better estimates of shorter-range functional connections. We also note that the current atlas-based approach may have under-sampled the prefrontal cortex, and that possible future improvements could break up the prefrontal regions into smaller pieces in order to sample more nodes from this area. Finally, we note that using Pearson correlation, it is possible that any association between two brain regions is the result of a spurious association with a third brain region. Conclusion Here we applied a whole-brain, data-driven approach that combines pattern analysis with atlas-based condition-dependent FC to identify a subset of FC features that can discriminate individual SAD subjects from both HCs and PD subjects. These features discriminated SAD subjects in an independent replication sample, which performed a similar emotional face viewing task, with significantly higher than chance performance. Finally, the most discriminative feature at baseline normalized following effective SSRI treatment and also correlated with change in symptom severity. We propose a valuable, exploratory approach to identify FC-based emerging biomarkers for psychiatric diagnosis and treatment effects. 21 Pantazatos, et. al. 2013 Supplemental Table S1 ROI X Y Z Name 1 -32 -56 -26 Cerebelum_34567b_L_PC1 2 -14 -38 -22 Cerebelum_34567b_L_PC2 3 32 -56 -24 Cerebelum_34567b_R_PC1 4 12 -42 -18 Cerebelum_34567b_R_PC2 5 -4 -48 -34 Cerebelum_8910_L_PC1 6 -6 -66 -32 Cerebelum_8910_L_PC2 7 6 -48 -36 Cerebelum_8910_R_PC1 8 10 -64 -34 Cerebelum_8910_R_PC2 9 -40 -64 -28 Cerebelum_Crus1_L_PC1 10 -18 -74 -30 Cerebelum_Crus1_L_PC2 11 40 -62 -28 Cerebelum_Crus1_R_PC1 12 28 -74 -34 Cerebelum_Crus1_R_PC2 13 -8 -80 -30 Cerebelum_Crus2_L_PC1 14 -6 -72 -32 Cerebelum_Crus2_L_PC2 15 8 -78 -32 Cerebelum_Crus2_R_PC1 16 6 -70 -32 Cerebelum_Crus2_R_PC2 17 2 0 -12 Hypothalamus_PC1 18 -6 -4 -8 Hypothalamus_PC2 19 -8 12 -6 Left_Accumbens_PC1 20 -10 12 -4 Left_Accumbens_PC2 21 -20 0 -20 Left_Amygdala_PC1 22 -24 -8 -14 Left_Amygdala_PC2 23 -52 -60 26 Left_Angular_Gyrus_PC1 24 -48 -56 44 Left_Angular_Gyrus_PC2 25 -8 10 8 Left_Caudate_PC1 26 -12 4 18 Left_Caudate_PC2 27 -46 -14 10 Left_Central_Opercular_Cortex_PC1 28 -56 -20 16 Left_Central_Opercular_Cortex_PC2 22 Pantazatos, et. al. 2013 29 0 40 6 Left_Cingulate_Gyrus_anterior_division_PC1 30 0 10 34 Left_Cingulate_Gyrus_anterior_division_PC2 31 0 -52 24 Left_Cingulate_Gyrus_posterior_division_PC1 32 0 -28 42 Left_Cingulate_Gyrus_posterior_division_PC2 33 -2 -80 28 Left_Cuneal_Cortex_PC1 34 0 -84 24 Left_Cuneal_Cortex_PC2 35 -4 62 24 Left_Dorsal_Frontal_Pole_PC1 36 -38 46 18 Left_Dorsal_Frontal_Pole_PC2 37 -28 -72 52 Left_Dorsal_Lateral_Occipital_Cortex_superior_division_PC1 38 -34 -72 52 Left_Dorsal_Lateral_Occipital_Cortex_superior_division_PC2 39 -2 50 -10 Left_Frontal_Medial_Cortex_PC1 40 -4 42 -12 Left_Frontal_Medial_Cortex_PC2 41 -42 18 2 Left_Frontal_Operculum_Cortex_PC1 42 -36 18 8 Left_Frontal_Operculum_Cortex_PC2 43 -36 16 -22 Left_Frontal_Orbital_Cortex_PC1 44 -32 16 -16 Left_Frontal_Orbital_Cortex_PC2 45 -46 -16 6 Left_Heschls_Gyrus_H1_and_H2_PC1 46 -44 -22 10 Left_Heschls_Gyrus_H1_and_H2_PC2 47 -16 -16 -22 Left_Hippocampus_PC1 48 -22 -26 -12 Left_Hippocampus_PC2 49 -54 16 14 Left_Inferior_Frontal_Gyrus_pars_opercularis_PC1 50 -54 14 20 Left_Inferior_Frontal_Gyrus_pars_opercularis_PC2 51 -52 30 8 Left_Inferior_Frontal_Gyrus_pars_triangularis_PC1 52 -50 28 18 Left_Inferior_Frontal_Gyrus_pars_triangularis_PC2 53 -50 -40 -18 Left_Inferior_Temporal_Gyrus_posterior_division_PC1 54 -42 -14 -24 Left_Inferior_Temporal_Gyrus_posterior_division_PC2 55 -50 -58 -16 Left_Inferior_Temporal_Gyrus_temporooccipital_part_PC1 56 -50 -50 -18 Left_Inferior_Temporal_Gyrus_temporooccipital_part_PC2 57 -44 0 -6 Left_Insular_Cortex_PC1 58 -36 16 2 Left_Insular_Cortex_PC2 23 Pantazatos, et. al. 2013 59 -2 -72 8 Left_Intracalcarine_Cortex_PC1 60 -6 -82 6 Left_Intracalcarine_Cortex_PC2 61 0 0 54 Left_Juxtapositional_Lobule_Cortex_Supp_Motor_cortex_PC1 62 -4 -6 48 Left_Juxtapositional_Lobule_Cortex_Supp_Motor_cortex_PC2 63 -38 -78 -18 Left_Lateral_Occipital_Cortex_inferior_division_PC1 64 -50 -70 0 Left_Lateral_Occipital_Cortex_inferior_division_PC2 65 0 -76 0 Left_Lingual_Gyrus_PC1 66 -2 -88 -22 Left_Lingual_Gyrus_PC2 67 -48 16 40 Left_Middle_Frontal_Gyrus_PC1 68 -40 34 38 Left_Middle_Frontal_Gyrus_PC2 69 -52 -8 -16 Left_Middle_Temporal_Gyrus_anterior_division_PC1 70 -56 -8 -14 Left_Middle_Temporal_Gyrus_anterior_division_PC2 71 -58 -36 -6 Left_Middle_Temporal_Gyrus_posterior_division_PC1 72 -58 -24 -10 Left_Middle_Temporal_Gyrus_posterior_division_PC2 73 -56 -54 6 Left_Middle_Temporal_Gyrus_temporooccipital_part_PC1 74 -56 -56 -4 Left_Middle_Temporal_Gyrus_temporooccipital_part_PC2 75 -30 -78 -20 Left_Occipital_Fusiform_Gyrus_PC1 76 -24 -74 -14 Left_Occipital_Fusiform_Gyrus_PC2 77 2 -98 4 Left_Occipital_Pole_PC1 78 -28 -98 -2 Left_Occipital_Pole_PC2 79 -20 -6 -2 Left_Pallidum_PC1 80 -24 -12 0 Left_Pallidum_PC2 81 -2 52 2 Left_Paracingulate_Gyrus_PC1 82 0 28 36 Left_Paracingulate_Gyrus_PC2 83 -16 -18 -26 Left_Parahippocampal_Gyrus_anterior_division_PC1 84 -20 -8 -30 Left_Parahippocampal_Gyrus_anterior_division_PC2 85 -16 -30 -22 Left_Parahippocampal_Gyrus_posterior_division_PC1 86 -12 -36 -14 Left_Parahippocampal_Gyrus_posterior_division_PC2 87 -50 -32 16 Left_Parietal_Operculum_Cortex_PC1 88 -50 -34 24 Left_Parietal_Operculum_Cortex_PC2 24 Pantazatos, et. al. 2013 89 -46 -2 -14 Left_Planum_Polare_PC1 90 -48 -4 -2 Left_Planum_Polare_PC2 91 -58 -26 10 Left_Planum_Temporale_PC1 92 -62 -32 16 Left_Planum_Temporale_PC2 93 -40 -26 58 Left_Postcentral_Gyrus_PC1 94 -58 -12 30 Left_Postcentral_Gyrus_PC2 95 -52 4 34 Left_Precentral_Gyrus_PC1 96 0 -24 48 Left_Precentral_Gyrus_PC2 97 0 -58 22 Left_Precuneous_Cortex_PC1 98 0 -76 56 Left_Precuneous_Cortex_PC2 99 -24 6 -2 Left_Putamen_PC1 100 -28 -8 4 Left_Putamen_PC2 101 -2 8 -14 Left_Subcallosal_Cortex_PC1 102 -2 10 -6 Left_Subcallosal_Cortex_PC2 103 -2 54 36 Left_Superior_Frontal_Gyrus_PC1 104 -2 18 54 Left_Superior_Frontal_Gyrus_PC2 105 -42 -44 60 Left_Superior_Parietal_Lobule_PC1 106 -28 -46 62 Left_Superior_Parietal_Lobule_PC2 107 -48 -2 -16 Left_Superior_Temporal_Gyrus_anterior_division_PC1 108 -54 -2 -10 Left_Superior_Temporal_Gyrus_anterior_division_PC2 109 -60 -34 6 Left_Superior_Temporal_Gyrus_posterior_division_PC1 110 -56 -18 -6 Left_Superior_Temporal_Gyrus_posterior_division_PC2 111 0 -78 12 Left_Supracalcarine_Cortex_PC1 112 -12 -66 16 Left_Supracalcarine_Cortex_PC2 113 -58 -32 40 Left_Supramarginal_Gyrus_anterior_division_PC1 114 -60 -32 32 Left_Supramarginal_Gyrus_anterior_division_PC2 115 -62 -44 20 Left_Supramarginal_Gyrus_posterior_division_PC1 116 -56 -46 38 Left_Supramarginal_Gyrus_posterior_division_PC2 117 -32 -6 -36 Left_Temporal_Fusiform_Cortex_anterior_division_PC1 118 -40 -8 -30 Left_Temporal_Fusiform_Cortex_anterior_division_PC2 25 Pantazatos, et. al. 2013 119 -28 -40 -22 Left_Temporal_Fusiform_Cortex_posterior_division_PC1 120 -36 -24 -22 Left_Temporal_Fusiform_Cortex_posterior_division_PC2 121 -36 -60 -22 Left_Temporal_Occipital_Fusiform_Cortex_PC1 122 -24 -52 -14 Left_Temporal_Occipital_Fusiform_Cortex_PC2 123 -40 10 -26 Left_Temporal_Pole_PC1 124 -44 6 -18 Left_Temporal_Pole_PC2 125 0 -4 2 Left_Thalamus_PC1 126 0 -18 12 Left_Thalamus_PC2 127 -44 48 2 Left_Ventral_Frontal_Pole_PC1 128 -2 58 -4 Left_Ventral_Frontal_Pole_PC2 129 -50 -68 30 Left_Ventral_Lateral_Occipital_Cortex_superior_division_PC1 130 -32 -86 22 Left_Ventral_Lateral_Occipital_Cortex_superior_division_PC2 131 0 -24 -8 Midbrain_PC1 132 2 -36 -10 Midbrain_PC2 133 -14 -32 -26 Pons_PC1 134 -16 -32 -28 Pons_PC2 135 8 12 -6 Right_Accumbens_PC1 136 12 16 -8 Right_Accumbens_PC2 137 20 0 -20 Right_Amygdala_PC1 138 26 -4 -18 Right_Amygdala_PC2 139 58 -54 28 Right_Angular_Gyrus_PC1 140 54 -50 44 Right_Angular_Gyrus_PC2 141 10 12 8 Right_Caudate_PC1 142 12 6 18 Right_Caudate_PC2 143 50 -8 6 Right_Central_Opercular_Cortex_PC1 144 44 -12 16 Right_Central_Opercular_Cortex_PC2 145 2 38 2 Right_Cingulate_Gyrus_anterior_division_PC1 146 2 36 16 Right_Cingulate_Gyrus_anterior_division_PC2 147 2 -50 20 Right_Cingulate_Gyrus_posterior_division_PC1 148 2 -22 36 Right_Cingulate_Gyrus_posterior_division_PC2 26 Pantazatos, et. al. 2013 149 4 -80 32 Right_Cuneal_Cortex_PC1 150 2 -72 22 Right_Cuneal_Cortex_PC2 151 34 54 20 Right_Dorsal_Frontal_Pole_PC1 152 0 62 24 Right_Dorsal_Frontal_Pole_PC2 153 34 -70 52 Right_Dorsal_Lateral_Occipital_Cortex_superior_division_PC1 154 14 -82 46 Right_Dorsal_Lateral_Occipital_Cortex_superior_division_PC2 155 0 50 -10 Right_Frontal_Medial_Cortex_PC1 156 10 48 -10 Right_Frontal_Medial_Cortex_PC2 157 44 20 0 Right_Frontal_Operculum_Cortex_PC1 158 38 18 8 Right_Frontal_Operculum_Cortex_PC2 159 42 22 -12 Right_Frontal_Orbital_Cortex_PC1 160 32 18 -24 Right_Frontal_Orbital_Cortex_PC2 161 46 -14 4 Right_Heschls_Gyrus_H1_and_H2_PC1 162 44 -22 14 Right_Heschls_Gyrus_H1_and_H2_PC2 163 18 -18 -20 Right_Hippocampus_PC1 164 26 -22 -14 Right_Hippocampus_PC2 165 56 16 8 Right_Inferior_Frontal_Gyrus_pars_opercularis_PC1 166 54 16 24 Right_Inferior_Frontal_Gyrus_pars_opercularis_PC2 167 52 26 2 Right_Inferior_Frontal_Gyrus_pars_triangularis_PC1 168 54 26 18 Right_Inferior_Frontal_Gyrus_pars_triangularis_PC2 169 46 -36 -20 Right_Inferior_Temporal_Gyrus_posterior_division_PC1 170 44 -26 -20 Right_Inferior_Temporal_Gyrus_posterior_division_PC2 171 54 -54 -20 Right_Inferior_Temporal_Gyrus_temporooccipital_part_PC1 172 56 -52 -10 Right_Inferior_Temporal_Gyrus_temporooccipital_part_PC2 173 46 -4 -2 Right_Insular_Cortex_PC1 174 42 -4 6 Right_Insular_Cortex_PC2 175 2 -74 8 Right_Intracalcarine_Cortex_PC1 176 10 -80 6 Right_Intracalcarine_Cortex_PC2 177 2 0 54 Right_Juxtapositional_Lobule_Cortex_Supp_Motor_cortex_PC1 178 6 -10 50 Right_Juxtapositional_Lobule_Cortex_Supp_Motor_cortex_PC2 27 Pantazatos, et. al. 2013 179 42 -72 -22 Right_Lateral_Occipital_Cortex_inferior_division_PC1 180 48 -70 8 Right_Lateral_Occipital_Cortex_inferior_division_PC2 181 2 -76 0 Right_Lingual_Gyrus_PC1 182 8 -92 -14 Right_Lingual_Gyrus_PC2 183 48 18 40 Right_Middle_Frontal_Gyrus_PC1 184 34 28 48 Right_Middle_Frontal_Gyrus_PC2 185 48 2 -24 Right_Middle_Temporal_Gyrus_anterior_division_PC1 186 58 -6 -22 Right_Middle_Temporal_Gyrus_anterior_division_PC2 187 62 -18 -12 Right_Middle_Temporal_Gyrus_posterior_division_PC1 188 62 -30 -6 Right_Middle_Temporal_Gyrus_posterior_division_PC2 189 60 -48 6 Right_Middle_Temporal_Gyrus_temporooccipital_part_PC1 190 62 -44 -4 Right_Middle_Temporal_Gyrus_temporooccipital_part_PC2 191 30 -74 -20 Right_Occipital_Fusiform_Gyrus_PC1 192 24 -70 -12 Right_Occipital_Fusiform_Gyrus_PC2 193 6 -100 6 Right_Occipital_Pole_PC1 194 16 -92 32 Right_Occipital_Pole_PC2 195 20 -2 -2 Right_Pallidum_PC1 196 24 -10 -2 Right_Pallidum_PC2 197 0 50 -2 Right_Paracingulate_Gyrus_PC1 198 4 26 38 Right_Paracingulate_Gyrus_PC2 199 18 -20 -26 Right_Parahippocampal_Gyrus_anterior_division_PC1 200 6 -6 -24 Right_Parahippocampal_Gyrus_anterior_division_PC2 201 18 -28 -22 Right_Parahippocampal_Gyrus_posterior_division_PC1 202 16 -34 -16 Right_Parahippocampal_Gyrus_posterior_division_PC2 203 54 -28 18 Right_Parietal_Operculum_Cortex_PC1 204 42 -32 20 Right_Parietal_Operculum_Cortex_PC2 205 46 0 -12 Right_Planum_Polare_PC1 206 48 -2 -2 Right_Planum_Polare_PC2 207 60 -24 12 Right_Planum_Temporale_PC1 208 52 -28 14 Right_Planum_Temporale_PC2 28 Pantazatos, et. al. 2013 209 58 -14 40 Right_Postcentral_Gyrus_PC1 210 54 -14 42 Right_Postcentral_Gyrus_PC2 211 54 8 34 Right_Precentral_Gyrus_PC1 212 48 -8 54 Right_Precentral_Gyrus_PC2 213 2 -56 24 Right_Precuneous_Cortex_PC1 214 4 -70 62 Right_Precuneous_Cortex_PC2 215 26 6 0 Right_Putamen_PC1 216 28 -4 6 Right_Putamen_PC2 217 0 26 -4 Right_Subcallosal_Cortex_PC1 218 2 16 -8 Right_Subcallosal_Cortex_PC2 219 2 54 36 Right_Superior_Frontal_Gyrus_PC1 220 4 20 54 Right_Superior_Frontal_Gyrus_PC2 221 38 -48 58 Right_Superior_Parietal_Lobule_PC1 222 30 -46 62 Right_Superior_Parietal_Lobule_PC2 223 50 -2 -16 Right_Superior_Temporal_Gyrus_anterior_division_PC1 224 60 -2 -6 Right_Superior_Temporal_Gyrus_anterior_division_PC2 225 50 -12 -8 Right_Superior_Temporal_Gyrus_posterior_division_PC1 226 62 -12 -4 Right_Superior_Temporal_Gyrus_posterior_division_PC2 227 2 -78 12 Right_Supracalcarine_Cortex_PC1 228 18 -64 16 Right_Supracalcarine_Cortex_PC2 229 64 -28 32 Right_Supramarginal_Gyrus_anterior_division_PC1 230 56 -28 48 Right_Supramarginal_Gyrus_anterior_division_PC2 231 62 -40 34 Right_Supramarginal_Gyrus_posterior_division_PC1 232 64 -44 16 Right_Supramarginal_Gyrus_posterior_division_PC2 233 34 -6 -36 Right_Temporal_Fusiform_Cortex_anterior_division_PC1 234 38 -2 -40 Right_Temporal_Fusiform_Cortex_anterior_division_PC2 235 30 -34 -24 Right_Temporal_Fusiform_Cortex_posterior_division_PC1 236 38 -28 -22 Right_Temporal_Fusiform_Cortex_posterior_division_PC2 237 34 -56 -22 Right_Temporal_Occipital_Fusiform_Cortex_PC1 238 36 -52 -26 Right_Temporal_Occipital_Fusiform_Cortex_PC2 29 Pantazatos, et. al. 2013 239 40 12 -26 Right_Temporal_Pole_PC1 240 48 8 -10 Right_Temporal_Pole_PC2 241 2 -4 2 Right_Thalamus_PC1 242 10 -22 6 Right_Thalamus_PC2 243 38 54 0 Right_Ventral_Frontal_Pole_PC1 244 0 58 0 Right_Ventral_Frontal_Pole_PC2 245 50 -66 28 Right_Ventral_Lateral_Occipital_Cortex_superior_division_PC1 246 34 -84 26 Right_Ventral_Lateral_Occipital_Cortex_superior_division_PC2 247 2 -46 -18 Vermis_PC1 248 0 -54 -34 Vermis_PC2 30 Pantazatos, et. al. 2013 Supplemental Table S2 SAD vs. Controls F betas, top 170 features Region x y z Fastigium Cerebelum_4_5_R Cerebelum_Crus1_L Sub-Gyral Frontal_Sup_Orb_R Midbrain Temporal_Mid_R Extra-Nuclear Lingual_R Frontal_Mid_Orb_L Temporal_Mid_L Frontal_Inf_Tri_L Occipital_Sup_L SupraMarginal_L Lateral Ventricle Insula_L Cuneus Frontal_Inf_Tri_L Posterior Cingulate Sub-Gyral Cuneus_L Cingulate Gyrus Extra-Nuclear Frontal_Inf_Tri_L Cingulum_Mid_L Precentral_L SupraMarginal_R Precentral_R Postcentral_R Frontal_Sup_Medial_L Paracentral_Lobule_L F>N contrast values, top 8 features Region 10 14 -26 42 18 10 66 6 22 -38 -58 -50 -22 -54 -6 -22 -18 -42 -2 30 -10 -10 -10 -34 -6 -42 62 38 30 -2 -6 -60 -52 -88 -36 32 -28 -20 0 -52 56 -48 40 -64 -24 16 24 -80 24 -32 16 -88 -16 0 32 -32 8 -24 -20 -28 28 -24 -30 -22 -22 -14 -14 -10 -10 -10 2 -2 10 10 26 14 14 14 14 22 22 22 26 30 26 26 34 42 42 58 46 54 58 x y z Cerebelum_6_R Middle Occipital Gyrus_R Extra-Nuclear 10 26 34 -76 -64 -8 -22 14 26 Cluster size 2 12 2 1 3 1 3 2 8 7 17 2 13 14 2 3 1 19 1 1 1 6 3 1 1 11 1 12 6 13 1 SVM weight Cluster size 1 6 1 T-value 0.11766 0.14319 0.093878 0.16173 -0.1472 0.13876 0.079332 0.14802 0.0921 -0.08209 -0.12757 -0.11533 0.14933 0.19237 -0.12109 -0.12363 0.1218 -0.23092 -0.069371 -0.064564 0.03237 -0.14666 -0.17125 -0.071431 -0.098303 0.076975 -0.24465 -0.15092 -0.1556 -0.11001 0.1246 -1.5561 1.2832 1.5478 31 Pantazatos, et. al. 2013 Supplemental Table S3 Whole brain results corresponding to Supplemental Figure 4: PPI analysis, Left Temporal Pole (top) and Left Hippocampus (bottom) as seed, N condition, Control > SAD LTP, P-value = 0.001, Cluster threshold = 4 Region x y z Cluster size T-value Pons 0 -32 -30 11 -3.6385 Cerebelum_6_L -10 -60 -20 9 -3.4956 Fusiform_L -34 -58 -20 15 -3.9095 ParaHippocampal_L -20 -18 -20 4 3.4287 Midbrain 4 -24 -10 13 3.7302 Temporal_Mid_L -58 -56 -4 30 3.8853 Temporal_Mid_R 58 -46 2 21 3.856 Hippocampus_L -36 -32 2 4 3.3426 Sub-Gyral 32 -42 8 7 3.6794 Cingulum_Mid_L -8 -22 44 13 -3.7999 Precentral_L -50 0 40 6 -3.5892 LHipp, P-value = 0.001, Cluster threshold = 4 Region x y z Cluster size T-value Thalamus_R 8 -16 8 13 -4.0177 Sub-Gyral 28 22 16 24 4.2935 Frontal_Inf_Tri_L -52 26 14 6 3.5373 Precuneus_L -8 -48 14 8 -3.8489 Temporal_Sup_R 40 -34 18 6 3.6271 Sub-Gyral -32 22 18 4 3.5652 Cingulum_Post_R 12 -48 22 4 3.5628 Sub-Gyral 32 -4 30 4 3.887 32 Pantazatos, et. al. 2013 Supplemental Table S4 SAD vs. control activation differences at p<0.05 corrected (p=0.005, cluster threshold > 148), based on F-test from 2-way ANOVA (1st factor diagnosis: 2 levels, 2nd factor face conditions: 4 levels-F, N, MF, MN). For directionality of results see supplemental figure S5. No significant diagnosis X face condition interactions were observed at this threshold. 2-way ANOVA Main effect of diagnosis Region L/R x y z Cluster size F-value(1,130) Z score DLPFC L -50 6 60 152 28.28 4.91 Fusiform L -32 -76 -14 503 23.04 4.45 33 Supplemental Figure S1 Pantazatos, et. al. 2013 34 Supplemental Figure S2 Pantazatos, et. al. 2013 35 Supplemental Figure S3 Pantazatos, et. al. 2013 36 Supplemental Figure S4 Pantazatos, et. al. 2013 37 Supplemental Figure S5 Pantazatos, et. al. 2013 38 Supplemental Figure S6 Pantazatos, et. al. 2013 39 Pantazatos, et. al. 2013 References Ashburner J & Friston KJ. 2000. Voxel-based morphometry--the methods. Neuroimage, 11(6 Pt 1), 805-21. Carey PD, Warwick J, Niehaus DJ, van der Linden G, van Heerden BB et al. 2004. Single photon emission computed tomography (SPECT) of anxiety disorders before and after treatment with citalopram. BMC Psychiatry, 4, 30. Ding J, Chen H, Qiu C, Liao W, Warwick JM et al. 2011. Disrupted functional connectivity in social anxiety disorder: a resting-state fMRI study. Magn Reson Imaging, 29(5), 701-11. Doehrmann O, Ghosh SS, Polli FE, Reynolds GO, Horn F et al. 2012. Predicting Treatment Response in Social Anxiety Disorder From Functional Magnetic Resonance Imaging. Arch. Gen. Psychiatry, 1-11. Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD et al. 2010. Prediction of individual brain maturity using fMRI. Science (New York, N.Y.), 329(5997), 1358-1361. Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R et al. 2004. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron, 44(6), 1043-1055. Fu CH, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF et al. 2008. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biological psychiatry, 63(7), 656-662. Furmark T, Tillfors M, Marteinsdottir I, Fischer H, Pissiota A et al. 2002. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitivebehavioral therapy. Arch. Gen. Psychiatry, 59(5), 425-33. Giménez M, Pujol J, Ortiz H, Soriano-Mas C, López-Solà M et al. 2012. Altered brain functional connectivity in relation to perception of scrutiny in social anxiety disorder. Psychiatry research. Golland P & Fischl B. 2003. Permutation tests for classification: towards statistical significance in image-based studies. Inf Process Med Imaging, 18, 330-41. Hanley JA & McNeil BJ. 1982. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology, 143(1), 29-36. Jimura K, Konishi S, Asari T & Miyashita Y. 2010. Temporal pole activity during understanding other persons' mental states correlates with neuroticism trait. Brain Res, 1328, 104-12. Kim J & Horwitz B. 2008. Investigating the neural basis for fMRI-based functional connectivity in a blocked design: application to interregional correlations and psycho-physiological interactions. Magnetic resonance imaging, 26(5), 583-593. 40 Pantazatos, et. al. 2013 Klumpp H, Angstadt M, Nathan PJ & Phan KL. 2010. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Res, 183(2), 167-9. Klumpp H, Angstadt M & Phan KL. 2012. Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol, 89(1), 273-6. Leckman JF, Sholomskas D, Thompson WD, Belanger A & Weissman MM. 1982. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch. Gen. Psychiatry, 39(8), 879-83. Liao W, Qiu C, Gentili C, Walter M, Pan Z et al. 2010. Altered effective connectivity network of the amygdala in social anxiety disorder: a resting-state FMRI study. PLoS ONE, 5(12), e15238. Mannuzza S, Fyer AJ, Klein DF & Endicott J. 1986. Schedule for Affective Disorders and Schizophrenia—Lifetime Version modified for the study of anxiety disorders (SADSLA): rationale and conceptual development. Journal of psychiatric research. McLaren DG, Ries ML, Xu G & Johnson SC. 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage, 61(4), 1277-86. Obuchowski NA & Lieber ML. 1998. Confidence intervals for the receiver operating characteristic area in studies with small samples. Acad Radiol, 5(8), 561-71. Pannekoek JN, Veer I, van Tol MJ, van der Werff S, Demenescu LR et al. 2012. Resting-state functional connectivity abnormalities in limbic and salience networks in social anxiety disorder without comorbidity. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. Pantazatos SP, Talati A, Pavlidis P & Hirsch J. 2012a. Cortical functional connectivity decodes subconscious, task-irrelevant threat-related emotion processing. NeuroImage, 61(4), 1355-1363. Pantazatos SP, Talati A, Pavlidis P & Hirsch J. 2012b. Decoding unattended fearful faces with whole-brain correlations: an approach to identify condition-dependent large-scale functional connectivity. PLoS Comput. Biol, 8(3), e1002441. Prater KE, Hosanagar A, Klumpp H, Angstadt M & Phan KL. 2012. ABERRANT AMYGDALA-FRONTAL CORTEX CONNECTIVITY DURING PERCEPTION OF FEARFUL FACES AND AT REST IN GENERALIZED SOCIAL ANXIETY DISORDER. Depress Anxiety. 41 Pantazatos, et. al. 2013 Psychiatric Association American. 1994. Diagnostic and statistical manual of psychiatric disorders. Washington, DC. Smith SM, Miller KL, Salimi-Khorshidi G, Webster M, Beckmann CF et al. 2011. Network modelling methods for FMRI. NeuroImage, 54(2), 875-891. Talati A, Ponniah K, Strug LJ, Hodge SE, Fyer AJ & Weissman MM. 2008. Panic disorder, social anxiety disorder, and a possible medical syndrome previously linked to chromosome 13. Biol. Psychiatry, 63(6), 594-601. Van der Linden G, van Heerden B, Warwick J, Wessels C, van Kradenburg J et al. 2000. Functional brain imaging and pharmacotherapy in social phobia: single photon emission computed tomography before and after treatment with the selective serotonin reuptake inhibitor citalopram. Prog. Neuropsychopharmacol. Biol. Psychiatry, 24(3), 419-38. Weissman MM, Wickramaratne P, Adams P, Wolk S, Verdeli H & Olfson M. 2000. Brief screening for family psychiatric history: the family history screen. Arch. Gen. Psychiatry, 57(7), 675-82. Winton EC, Clark DM & Edelmann RJ. 1995. Social anxiety, fear of negative evaluation and the detection of negative emotion in others. Behav Res Ther, 33(2), 193-6. Yoon KL & Zinbarg RE. 2007. Threat is in the eye of the beholder: social anxiety and the interpretation of ambiguous facial expressions. Behaviour research and therapy, 45(4), 839-847.