* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Environmental Microbiology
Gene therapy of the human retina wikipedia , lookup
Population genetics wikipedia , lookup
Genome (book) wikipedia , lookup
Pathogenomics wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genomic imprinting wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenomics wikipedia , lookup
Copy-number variation wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Frameshift mutation wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
History of genetic engineering wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Genome evolution wikipedia , lookup
Oncogenomics wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Heritability of IQ wikipedia , lookup
Helitron (biology) wikipedia , lookup
Gene expression profiling wikipedia , lookup
Designer baby wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Gene expression programming wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Quantitative trait locus wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Human genetic variation wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Nutriepigenomics wikipedia , lookup
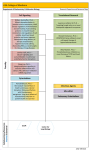
Broek, G. V. Bloemberg and B. Lugtenberg Environmental Microbiology (2005) 7(11), 1686–1697 doi:10.1111/j.1462-2920.2005.00912.x Minireview The role of phenotypic variation in rhizosphere Pseudomonas bacteria Daan van den Broek,*† Guido V. Bloemberg and Ben Lugtenberg Leiden University, Institute of Biology, Clusius Laboratory, Wassenaarseweg 64, 2333 AL Leiden, the Netherlands. Summary Colony phase variation is a regulatory mechanism at the DNA level which usually results in high frequency, reversible switches between colonies with a different phenotype. A number of molecular mechanisms underlying phase variation are known: slipped-strand mispairing, genomic rearrangements, spontaneous mutations and epigenetic mechanisms such as differential methylation. Most examples of phenotypic variation or phase variation have been described in the context of host–pathogen interactions as mechanisms allowing pathogens to evade host immune responses. Recent reports indicate that phase variation is also relevant in competitive root colonization and biological control of phytopathogens. Many rhizospere Pseudomonas species show phenotypic variation, based on spontaneous mutation of the gacA and gacS genes. These morphological variants do not express secondary metabolites and have improved growth characteristics. The latter could contribute to efficient root colonization and success in competition, especially since (as shown for one strain) these variants were observed to revert to their wild-type form. The observation that these variants are present in rhizosphere-competent Pseudomonas bacteria suggests the existence of a conserved strategy to increase their success in the rhizosphere. Introduction Phenotypic variation or phase variation has been defined by as a process of reversible, high-frequency phenotypic switching that is mediated by DNA mutations, reorganization or modification (Saunders et al., 2003). Phase variaReceived 15 March, 2005; accepted 25 July, 2005. *For correspondence. E-mail [email protected]; Tel. +31 338504076; Fax +31 338502035. †Present address: Meander Medisch Centrum, Utrechtseweg 160, 3818 ES Amersfoort, the Netherlands. © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd tion is used by several bacterial species to generate population diversity that increases bacterial fitness under certain circumstances and is important in niche adaptation, including immune evasion. Phase variation occurs at a high frequency of > 10−5 switches per cell per generation (Henderson et al., 1999) and can result in reversible ON or OFF switching of traits or in the variation of surface phenotypes. Phase variation is one of the mechanisms enabling pathogens to survive in the host by escaping the immune response (Craig and Scherf, 2003). This is illustrated by the fact that phase variation poses a problem in vaccine production due to the high frequency of variation in epitopes exposed by the pathogen (Maskell et al., 1993; Pedersen et al., 2004). Although phase variation, or antigenic variation, has primarily been associated with host–pathogen interactions, a number of reports describe phase and phenotypic variation in a broader context. These reports show that phenotypic variation is also involved in the production of exo-enzymes, production of secondary metabolites and affects colonization behaviour and biocontrol activity of rhizosphere bacteria (Chabeaud et al., 2001; Chancey et al., 2002; Sanchez-Contreras et al., 2002; van den Broek et al., 2003). This indicates that phase variation can have a much broader impact on the ecology of bacteria, affecting a high number of traits and processes, and therefore phase variation is not only relevant in host–pathogen interactions but also in more ecological and industrial processes. Genetic mechanisms of phase variation Phase variation is a phenomenon encompassing a variety of genetic mechanisms. These can be divided into programmed and unprogrammed variation (Borst, 2003). Programmed variation is characterized by two properties, (i) a family of genes encoding proteins with the same or similar function, which is combined with (ii) the ability to express only one of the gene family members at a time and alter the expression of these members from time to time (Borst, 2003). Programmed variation entails regulated DNA conversions as the result of slipped-strand mispairing (slipped-strand mispairing is, despite the fact that the variation is based on errors during DNA replication, considered to be programmed due to the requirement Phenotypic variation in rhizosphere Pseudomonas bacteria 1687 of a specific repeat tract) or genomic rearrangements (including inversions, deletions, recombinational events and gene conversions) but can also be epigenetic when based on differential methylation. Unprogrammed phase variation is based on DNA alterations through the accumulation of errors during DNA replication, imperfect DNA repair, the recombination between non-identical genes, or reassortment of gene segments if the genome is not present in one molecule (Borst, 2003). Programmed and unprogrammed phase variation are subject to regulation. Especially in a host–pathogen situation the regulation of phase variation is important to allow expression under relevant conditions. Phase variation itself is therefore often regulated by environmental factors. These environmental factors include temperature (Schwan et al., 1992; Gally et al., 1993; White-Ziegler et al., 2002), medium composition (Struve and Krogfelt, 1999; White-Ziegler et al., 2000) and stress conditions (Nicholson and Low, 2000; Hengge-Aronis, 2002; Yildiz et al., 2004). One of the effects of growth limitation or stress conditions is an increase in stationary-phase mutations (Moxon et al., 1994; Kivisaar, 2003; Lombardo et al., 2004). This is due to a downregulation of MMR (mismatch repair) (Tsui et al., 1997; Richardson and Stojiljkovic, 2001), the spontaneous mutations of MMR components (Horst et al., 1999; Shaver et al., 2002; Shaver and Sniegowski, 2003) and, possibly, the activity of error-prone polymerases like DinB (Strauss et al., 2000; McKenzie and Rosenberg, 2001). In Pseudomonas, the occurrence of phenotypic variants is clearly correlated to stress conditions. Culture conditions, medium composition and the scale of the cultures strongly influence the percentage of gac mutants in Pseudomonas fluorescens CHAO. The genetic stability and therefore the occurrence of gac mutants in this strain were correlated with stress conditions such as high electrolyte concentrations and certain mineral amendments (Duffy and Défago, 1995; 2000). A central role for stress in phenotypic variation of Pseudomonas is genetically supported by the relationship between the frequency of spontaneous mutation of gac and expression of the general stress response sigma factor RpoS (van den Broek et al., 2003; 2005a). Constitutive expression of the rpoS gene resulted in a 10-fold increase in the frequency of gac mutants in Pseudomonas sp. PCL1171 whereas mutation of rpoS reduced this frequency 20-fold (van den Broek et al., 2005a). A number of mechanisms of phase variation are known to play a role in specific host–pathogen interactions. Most of these species are human pathogens. We will shortly discuss the four well-known mechanisms of phase variation: slipped-strand mispairing, genomic rearrangements, differential methylation and unprogrammed phase variation via spontaneous mutation. Slipped-strand mispairing Slipped-strand mispairing uses short sequence repeats to regulate gene expression at the translational or transcriptional level. These repetitive DNA sequences are increasingly being identified in prokaryotes (Tomb et al., 1997; van Belkum et al., 1998; Aras et al., 2003) and can consist of homopolymeric repeat tracts or multimeric, heterogeneous repeats (Levinson and Gutman, 1987; Henderson et al., 1999). The stability of these repeat tracts is dependent on MMR and the sequence characteristics of the repeat tract (Levinson and Gutman, 1987; Lovett and Feschenko, 1996; Bayliss et al., 2002; Bayliss et al., 2004a,b; Fernandez-Lopez et al., 2004). Repeats associated with a single locus, present in the promoter region or within the coding region, can alter gene expression as a result of changes in the number of repeats (Fig. 1A). The number of repeats is varied via a RecA-independent mechanism through the formation of heteroduplex DNA (H-DNA), which is induced by superhelical coiling (Belland, 1991; Dybvig, 1993; Lovett and Feschenko, 1996). This H-DNA consists of a triple-stranded region, based on the formation of triple residue bonds within the repeat region, with as a result a single-stranded region, which will stimulate slipped-strand mispairing (Belland, 1991; Henderson et al., 1999). Altering the number of repeats will result in an incomplete gene product due to a shift in reading frame (Fig. 1A). For example, the regulation of expression of the Fig. 1. Model for phase variation via slipped-strand mispairing. A. Model for ON and OFF switching of traits via slipped-strand mispairing. Variations in the number of repeats () within the coding region of the gene results in a shift of reading frame in or out of frame. A shift out of frame will introduce premature stop codons (*). B. Model for volume control via slipped-strand mispairing. Variations in the number of repeats within the promoter region of the gene will vary promoter −10 and −35 spacing, thereby increasing (ON++) or decreasing (ON or OFF) promoter efficiency. © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 1688 D. van den Broek, G. V. Bloemberg and B. Lugtenberg opa (opacity) genes in Neisseria meningitides and Neisseria gonorhoeae species, switching loci ON and OFF is based on changes in the number of pentameric repeat elements (5′-CTTCT-3′) with which the expression state of the opa gene(s) changes. For example, 6, 9 or 12 repeats are equivalent to an Opa+ phenotype in which the gene is in frame (Fig. 1A). All other numbers (e.g. 7, 8 or 13) shift the gene out of frame, resulting in incomplete gene products and an Opa− phenotype (Stern et al., 1986) (Fig. 1A). An alternative slipped-strand mispairing mechanism regulates gene expression at the level of transcription (Sarkari et al., 1994). This regulation is mediated by the presence of repeats upstream of the encoding gene, which upon variation of the number of repeats, results in an increase or a decrease in expression by varying the promoter spacing. The expression of the opc promoter in N. meningitidis is attenuated by a polyC tract. A tract of 12 or 13 bases increases the expression of opc 10-fold (Opc++ phenotype) when compared with a promoter with a polyC tract of 11 or 14 bases (Opc+ phenotype) (Fig. 1B). When the number of repeats exceeds 15 or becomes less than 10 no expression of opc is detected anymore (Sarkari et al., 1994) (Fig. 1B). Slipped-strand mispairing as a regulatory mechanism is present in a wide range of bacteria regulating various traits. Examples of traits regulated via slipped-strand mispairing are presented in Table 1. Genomic rearrangements Genomic rearrangements combine a wide range of processes involved in phase variation. These include inversions, deletions, gene duplication and gene transfer using silent copies (recombinational deletion) (Borst, 2003). Control of expression of, for example, type 1 fimbriae in Escherichia coli is based on the presence of inverted repeats and the action of site-specific recombinases (Fig. 2). The presence of inverted repeats within the promoter region facilitates the inversion of the promoter switching expression ON or OFF (Abraham et al., 1985; McClain et al., 1991; 1993). Alternatively, when the promoter itself is flanked by inverted repeats, as described for H1 and H2 flagellin genes of Salmonella typhimurium, different sets of genes can be expressed. One orientation of the promoter will result in the expression of h2 and the repressor Rh1 of the h1 promoter. Upon inversion both h2 and rh1 are no longer expressed, lifting the repression of h1 by rh1 (Zieg et al., 1977). A second form of variation based on genomic rearrangements, for example, regulating variation of type IV pili in N. gonorrhoeae (Jonsson et al., 1992; Seifert, 1996) and the expression of surface proteins in Borrelia spp. (Barbour, 2003), uses deletions, gene duplications and gene transfer to create a large number of potential proteins to express. Although in many systems recA mutants are not yet available, those mutants analysed show that these rearrangements are dependent on the recA gene, and based on the deletion of one allele present in an active locus, which is subsequently replaced by transcriptionally inactive alleles present elsewhere on the genome (Koomey et al., 1987; Dybvig, 1993; Henderson et al., 1999; Meyer and Hill, 2003). This is often combined with the presence of highly variable and semivariable regions within these alleles, thereby increasing the variation potential of the gene product (Haas and Meyer, 1986; Meyer and Hill, 2003). This can enable bacteria to produce up to 107 variant proteins (Haas et al., 1992). Examples of traits regulated via genomic rearrangements are presented in Table 1. Differential methylation Phase variation of pap fimbriae and expression of antigen 43 in E. coli are dependent on a differential DNA methylation pattern and therefore represents an epigenetic mechanism of phase variation (van der Woude et al., 1996; Henderson et al., 1999). Methylation of GATC sites in the genome is dependent on deoxyadenosine methylase (dam), which binds to the GATC site and methylates adenosine at the N6 position (Palmer and Marinus, 1994). Normally, methylation provides the organism with a regulatory mechanism for DNA repair, protection from restriction endonucleases, and timing and targeting of cellular events (Marinus, 1996). Methylation of GATC sites within regions involved in gene regulation can inhibit or facilitate the binding of regulatory proteins at specific sites, and thus alter gene expression (Nou et al., 1995; van der Woude et al., 1996). A paradigm for regulation via differential methylation is presented in Fig. 3. Examples of genes controlled via differential methylation are presented in Table 1. Unprogrammed variation Random unprogrammed variation is based on the introduction of mutations due to imperfect replication and the subsequent removal of these mutations, coinciding with a switch back to the wild-type situation. One of the drawbacks of a mechanism stimulating diversification based on imperfect replication is a high mutational load. Higher organisms had to evolve a mechanism of mutation while controlling the mutation rate using mechanisms like mismatch repair pathways (Borst, 2003; Schofield and Hsieh, 2003), or, although still under discussion, specific errorprone DNA polymerases transcribing specific genomic regions (Moxon et al., 1994; McKenzie and Rosenberg, 2001; Kivisaar, 2003; Tegova et al., 2004). Most © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 Motility/flagella/biofilm formation Root colonization/flagella/exo-enzymes Root colonization Root colonization/motility/biofilm formation Motility and assimilation of certain sugars Virulence factors Secondary metabolism and exo-enzymes Bordetella bronchiseptica Pseudomonas sp. PCL1171 Pseudomonas aeruginosa Pseudomonas brassicacearum Pseudomonas fluorescens WCS365 P. fluorescens F113 Asospirillum lipoferum Capsule production Secondary metabolism/morphology Autoaggregation Pilus expression Fimbrial expression Surface lipoproteins Surface proteins Fimbrial expression Variable surface glycoproteins Flagellar expression Type IV fimbriae Fimbrial expression Major outer membrane protein Surface lipoprotein antigens DNA restriction and modification properties Adhesion/invasion LPS antigenicity, Lewis Y antigen LPS antigenicity Fimbrial expression Capsular polysaccharides Flagellum export Adhesion/invasion/neutrophil interaction Two component sensing Antigenicity of LOS ABC transporter ToxR regulon Hemoglobin binding outer membrane proteins Hemoglobin receptors Property affected Streptococcus pneumoniae Pseudomonas tolaasii E. coli E. coli Salmonella typhimurium Salmonella spp. Moraxella lacunata LPS, lipopoly saccharide; LOS, lipo-oligo saccharide; ABC, ATP-binding cassette. Mechanism unknown vir locus gacA/S (AY236957) Spontaneous mutations ON↔OFF vsp (AF396970 and AH008162) vsp/vlp (AF049852) cap3, cap8, tts (Z12159, AJ239004, AJ131985) pheN (U95300) pilE (AF043652) vsg genes Recombinational deletion ON↔OFF Spontaneous duplications ON↔OFF M. bovis Borrelia spp. hin (V01370) piv (M34367) ONa/OFFb↔ONb/OFFa agn43 (U24429) pap (X03391) pef (AB041905) N. gonorrhoeae/N. meningitides Trypanosome spp. fimA (Z37500) omp1 (U02462) vspA (L81118) hsd1 (AF003541) Site-specific inversion ON↔OFF Differential methylation ON↔OFF N. meningitides opc (A44611) Volume control OFF→ON+/ON++→OFF Escherichia coli Dichelobacter nodusus Moraxella bovis Mycoplasma pulmonis Helicobacter pylori Haemophilus influenza Neisseria gonorhoeae/N. meningitides N. meningitides Pseudomonas putida N. gonorhoeae/N. meningitides Bordetella spp. Haemophilus somnus Mycoplasma fermentans Vibrio cholerae N. gonorrhoeae N. meningitidis fucT2 (AF076779) lic1A,2A,3A (M37912-14) pilC (Z49120) siaD (M95053) flhB (AF031418) opa (P11296) bvgS (M25401) lob1 (U94833) p78 (AF100324) tcpH (X74730) hpuA (AF031495) hmbR, hpuAB (AF105339, U73112) Slipped-strand mispairing ON↔OFF Species Locus Mechanism Table 1. Examples of phase variable traits. Deziel et al. (2001) Chabeaud et al. (2001) Dekkers et al. (1998) Sanchez-Contreras et al. (2002) Vial et al. (2004) Monack et al. (1989) van den Broek (2005) Waite et al. (2003) Han et al. (1997) Owen et al. (1996) Blyn et al. (1990) Nicholson and Low (2000) Haas and Meyer (1986) Agur et al. (1989); Barry and McCulloch (2003) Lysnyansky et al. (1999) Barbour (2003) Zieg et al. (1977) Marrs et al. (1990); Heinrich and Glasgow (1997) Abraham et al. (1985) Moses et al. (1995) Lysnyansky et al. (1996) Dybvig and Yu (1994) Sarkari et al. (1994) Wang et al. (1999) Hood and Moxon (2003) Jonsson et al. (1991) Hammerschmidt et al. (1996) Segura et al. (2004) Stern et al. (1986) Stibitz et al. (1989) Inzana et al. (1997) Theiss and Wise (1997) Carroll et al. (1997) Chen et al. (1998) Lewis et al. (1999) Reference Phenotypic variation in rhizosphere Pseudomonas bacteria 1689 1690 D. van den Broek, G. V. Bloemberg and B. Lugtenberg these regions can consist of small deletions (50–500 bp), mismatches and duplications (Monack et al., 1989; Han et al., 1997; Waite et al., 2003; van den Broek et al., 2005b). Still unclear is the exact mechanism by which the capsule locus is switched ON again, which factors determine the switch OFF and the relevance of this mechanism in disease. Examples of spontaneous mutations in phase variation, switching genes ON and OFF are presented in Table 1. Phenotypic variation and Pseudomonas Fig. 2. Model for phase variation via a 314 bp invertible element. Inversion of a 314 bp promoter fragment will switch expression of fimA ON or OFF. The inversion is facilitated by two site-specific recombinases FimE and FimB, recognizing the two 9 bp inverted repeats (IR, the orientation is indicated with an arrow). FimE promotes the switch from ON to OFF, while FimB can invert the fragment in both directions. An Integration Host Factor (IHF) is required for efficient expression. As mutation of one of the subunits of the IHF locks the expression of fimA either in an ON or in the OFF configuration, the IHF is also involved in the inversion of the fimA promoter (Dorman and Higgins, 1987). Histone-like protein (H-NS) is directly involved in suppression of the fimB gene, suppressing the inversion from OFF to ON (Donato et al., 1997; O’Gara and Dorman, 2000). The Leucine Responsive protein (Lrp) stimulates expression of fimB and slightly decreases expression of fimE, stimulating inversion in both directions as shown by a decrease in the frequencies of inversion, upon mutation of lrp (Blomfield et al., 1993). organisms use this strategy to create diversity, for example, in antibody genes (Gearhart, 2002), but this mechanism has also been suggested to play a role in adaptive evolution in microorganisms (Moxon et al., 1994). In the context of phase variation, the mutations accumulating in Phase variation in Pseudomonas bacteria is still a relatively unexplored phenomenon, but an increasing number of interesting examples of phase variation and switching genes have been described. These examples can be divided into (i) mechanisms, of which the molecular basis is often unknown, affecting a specific trait such as variation of epitopes, expression of exo-enzymes, or flagella, and (ii) mechanism based on spontaneous mutation of gacA/S, which affects the expression of the secondary metabolism. Both mechanisms of phenotypic variation often present themselves in the form of morphological variants. These morphological variants have been described as thick, small and opaque versus thin translucent colonies (Han et al., 1997; Bull et al., 2001; Chabeaud et al., 2001; Deziel et al., 2001; Chancey et al., 2002; Sanchez-Contreras et al., 2002; van den Broek et al., 2003). The determinants of these differences in colony morphology in Pseudomonas are not known. In addition to colony morphology, a number of other traits are affected by this phenotypic variation. Pseudomonas aeruginosa species regulates, in a temperaturedependent manner, the expression of the phosphocholine Fig. 3. Paradigm for phase variation of pap via differential methylation in E. coli. Differential methylation of two GATC sites, GATC1028 and GATC1130, regulates expression of papBA. Methylation of GATC1130 will inhibit papBA expression. The regulation is based on competition for binding sites as Lrp and methylation sites overlap. Binding of PapI to Lrp will reduce the affinity for binding to sites overlapping with GATC1130. The Lrp–PapI complex will preferentially bind to the sites overlapping GATC1028, probably after replication, and thus facilitate methylation of GATC1130 to enable papBA expression. Binding of CAP and PapB will lift HNS suppression (Forsman et al., 1992) and enable transcription of papI. In addition, binding of PapB upstream of the papI promoter will stimulate papI transcription (van der Woude et al., 1996). © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 Phenotypic variation in rhizosphere Pseudomonas bacteria 1691 Fig. 4. Work model for the genetic regulation of spontaneous mutations accumulating in gac (van den Broek, 2005). A schematic representation of the regulatory roles of GacA/S, RpoS and MutS in phase variation of PCL1171. Phase I cells harbour intact gacA and gacS genes that are required for the expression of rpoS, which, in combination with additional factors in stationary phase negatively regulates mutS expression (van den Broek et al., 2005a). Inefficient repair of mutations due to downregulation of mutS results in a decrease in the repair of spontaneous mutations, which in turn will result in the accumulation of mutations in gacA and gacS (indicated with an asterisk). As a result the cells will switch to the phase II phenotype. Mutation of gacA/S decreases rpoS expression and the subsequent increase in mutS expression, thus limiting the mutation rate. In addition, the mutation of rpoS is hypothesized to affect both the introduction and the repair of mutations in gacA/S (van den Broek, 2005). epitope of a 43 kDa protein through phase variation, which is hypothesized to play a role in its pathogenicity (Weiser et al., 1998). In addition, phase variation in this bacterium is described to regulate the expression of type IV pili, which affects swimming, swarming and twitching motility and, as a result biofilm formation in P. aeruginosa. This variation is hypothesized to be aimed at the diversification of cultures resulting in a prior presence of phenotypic forms well adapted to initiate the formation of biofilm as soon as environmental conditions are favourable for biform formation (Deziel et al., 2001). Recently phenotypic variants were shown to play an important role in competitive root colonization. An effect of phase variation on root colonization was suggested by the observation of a reduced competitive tomato root tip colonization upon mutation of sss encoding a site-specific recombinase in P. fluorescens WCS365. It was hypothesized that due to the mutation of sss, a fraction of the cells become locked in a configuration less fit for the rhizosphere (Dekkers et al., 1998). The link between phase variation, root colonization and sss was also studied in P. fluorescens F113. During root colonization of alfalfa by P. fluorescens F113, phenotypic variants were isolated. It was shown that the sss gene is responsible for the majority of the phenotypic variation, which is combined with a phenotypic selection for gac mutations. Three morphologically different variants, which showed a difference in colonization pattern, and in the production of cyanide, exo-protease and siderophores, were isolated (SanchezContreras et al., 2002; Martinez-Granero et al., 2005). The effect of phenotypic variation on root colonization has been described in more detail for the colonization of Arabidopsis thaliana by Pseudomonas brassicacearum NFM421. Pseudomonas brasicacearum shows two distinct morphological variants, designated phase I and phase II. Phase II cells of P. brassicacearum show an overproduction of flagellin by phase II bacteria, which results in a higher ability to swim and swarm compared with phase I bacteria. In root colonization experiments of P. brasicacearum, these phase II bacteria are found at the tip of the main root and on secondary roots, while the phase I bacteria are primarily localized at the basal parts of the root. Similarly, in Pseudomonas putida DOT-T1E the expression of the flhB gene, encoding a protein involved in flagellin export, is controlled in response to environmental changes via slipped-strand mispairing (Segura et al., 2004). Based on the role of these variants, phenotypic variation is suggested to be a strategy, increasing the colonization ability of P. brassicacearum (Achouak et al., 2004). In addition, in P. brasicacearum the expression of an extracellular alkaline protease, a serine protease homologue, and of a lipase is only expressed in a phase I morphology. Although the genes coding for the protease and lipase are organized in a single operon, the mechanism responsible for the ON and OFF switching of this operon has not yet been described (Chabeaud et al., 2001). Phenotypic variation can regulate a diversity of traits in Pseudomonas species. These variations can increase the population diversity and could have a positive effect on the success of a population. Similar effects are described for the second class of mechanisms responsible for phenotypic variation in Pseudomonas: spontaneous mutation of gacA/gacS. The gacA/gacS two-component regulatory system consists of a sensor kinase GacS and a response regulator GacA belonging to the FixJ family of transcrip- © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 1692 D. van den Broek, G. V. Bloemberg and B. Lugtenberg tional regulators (Laville et al., 1992). This two-component system regulates secondary metabolism and the production of exo-enzymes in Pseudomonas (Laville et al., 1992; Rich et al., 1994; Kitten et al., 1998; Blumer et al., 1999; van den Broek et al., 2003). Phenotypic variants as a result of spontaneous mutation of the gacA or gacS gene are known to occur in Pseudomonas species known for their biocontrol activity of phytopathogens. As a result spontaneous gac mutants are often isolated from the rhizosphere (Rich et al., 1994; Duffy and Défago, 1995; 2000; Bull et al., 2001; Chancey et al., 2002; SanchezContreras et al., 2002; van den Broek et al., 2003; Schmidt-Eisenlohr et al., 2003). Spontaneous gac mutants have been reported for Pseudomonas chlororaphis (Schmidt-Eisenlohr et al., 2003), Pseudomonas tolaasii (Han et al., 1997), P. fluorescens (Duffy and Défago, 2000; Bull et al., 2001), Pseudomonas aureofaciens 30–84 (Chancey et al., 2002) and Pseudomonas sp. PCL1171 (van den Broek et al., 2003; 2005b). In these mutants the growth characteristics (Schmidt-Eisenlohr et al., 2003; van den Broek et al., 2005a,b), the expression of secondary metabolites (Duffy and Defago, 2000; Chancey et al., 2002; van den Broek et al., 2003) and pathogenicity (Han et al., 1997) are affected. The presence of these mutants is therefore an important factor, for example, for the efficient biocontrol activity of these strains. Mechanisms influencing phase variation via gac Molecular characterization of these mutations showed that in both the gacA and the gacS gene mutations, which are random in nature as well as in distribution, accumulated. These mutations include base mismatches, small insertions (1 bp) and deletions (up to 12 bp) but also large rearrangements (Bull et al., 2001; van den Broek et al., 2005b). The observation that spontaneous mutations form the basis for phenotypic variation in Pseudomonas is supported by the possibility to complement these mutants using wild-type gacA or gacS genes and by the central role of MMR (van den Broek et al., 2003; 2005a). In Pseudomonas sp. PCL1171 the frequency of variation was directly correlated to the expression of MutS-dependent mismatch repair mechanisms. As the expression of mutS is regulated by RpoS in this strain, it was suggested that gac mutants are the result of stress-induced inefficient repair of replication-related mismatches (Fig. 4) (van den Broek et al., 2005a). The reason for the relatively high frequencies at which these mutants occur has been attributed to a positive selection for mutation of gac (Bull et al., 2001) or to the growth advantage of these mutants over wild-type bacteria (Schmidt-Eisenlohr et al., 2003; van den Broek et al., 2005a,b). The latter is observed as a decrease in the length of the lag-phase after reinoculation into fresh medium in combination with a slight increase in growth rate. This increase could be an effect of a reduced metabolic load of a gac mutant (Schmidt-Eisenlohr et al., 2003; van den Broek et al., 2005a,b). The effect on the lag-phase is the result of the need for intact gacA/S genes to fully express the rpoS gene (Schmidt-Eisenlohr et al., 2003) (Fig. 4). The combination of these growth characteristics is suggested to enable these derivatives to reinitiate growth more readily and to grow faster than the wild type, thereby providing a mixed population with a competitive advantage (Schmidt-Eisenlohr et al., 2003; van den Broek et al., 2005a) (Fig. 4). An additional effect of the spontaneous mutation of gac is the loss of secondary metabolism. Gac-negative subpopulations do not produce antimicrobial compounds such as hydrogen cyanide, protease, lipase, 2,4diacetylphloroglucinol, pyoluteorin or pyrrolnitrin (Rich et al., 1994; Han et al., 1997; Duffy and Defago, 2000; van den Broek et al., 2003; Schmidt-Eisenlohr et al., 2003) and are also limited in their cell-to-cell communication and biofilm formation (Schmidt-Eisenlohr et al., 2003). The presence of large populations of spontaneous gac mutants, as a result of the high frequency of switching or of inoculation with wild-type phase II cells, reduced the biocontrol efficiency of Pseudomonas sp. PCL1171 (van den Broek et al., 2003). This suggests that the presence of phenotypic variants could explain the variability of biocontrol efficiency in the field but only when it reduces the presence of wild-type bacteria on the root (van den Broek et al., 2003). The effect of a subpopulation of spontaneous gac mutants can therefore increase the competitive success as a result of more efficient growth and improved colonization abilities. However, these mutants lack secondary metabolites, which are often the basis for their biocontrol activity. The effect of this decrease in secondary metabolism on the biocontrol activity will usually be limited as this would require a significant fraction of bacteria to harbour a gac mutation. The presence of such significant populations of gac mutants in the rhizosphere was shown, especially in natural soils, not to be the result of the increased growth characteristics (Chancey et al., 2002; Schmidt-Eisenlohr et al., 2003). Under normal circumstances the presence of subpopulations of gac mutants can therefore be beneficial in the competitive environment of the rhizosphere and could in addition explain the relative success of Pseudomonas species in the rhizosphere. Reversibility of gac mutations Recent publications put the spontaneous mutation of gac in a new perspective. Pseudomonas sp. PCL1171 was described to switch reversibly between gac mutants and the wild type. This phase variation occurs at a frequency of 6.4 × 10−5 switches per cell per generation from phase © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 Phenotypic variation in rhizosphere Pseudomonas bacteria 1693 I to phase II and 8.2 × 10−2 switches per cell per generation for phase II to phase I (van den Broek et al., 2005a,b). This ability to restore the wild-type gac genes was also shown both on agar plates and at molecular level (van den Broek et al., 2005b). A similar observation was made for the pheN gene in P. tolaasii. This homologue of gacS was found to harbour a 661 bp reversible duplication (Han et al., 1997). Variation based on the introduction and subsequent removal of mutations is not dependent on specific DNA features, and spontaneous duplications and deletions have been described to control, for example, capsule genes in Streptococcus pneumonia (Waite et al., 2003), the pathogenicity and colony morphology in P. tolaasii and Ralstonia solanacearum (Han et al., 1997; Poussier et al., 2003), and virulence of Bordetella bronchiseptica (Monack et al., 1989). Presently the mechanism responsible for the exact restoration of the wild-type sequence is unknown for any of these cases. Interestingly, differences in the stability of phase II bacteria were observed, ranging from relatively instable phase II cells to a stable mutation in the form of a 307 bp deletion (van den Broek et al., 2005b). This suggests that phase II colonies growing on agar plates only represent the stable derivates. Although for none of the other Pseudomonas species reversion of the gac mutations has been reported, there is a possibility that this reversible phase variation via spontaneous mutation is a conserved strategy of rhizosphere Pseudomonas species, which improves their success in the heterogeneous and challenging environment of the rhizosphere (Dekkers et al., 1998; 2000; SchmidtEisenlohr et al., 2003; Achouak et al., 2004; van den Broek, 2005). The observation that gac mutations can be reversible (Han et al., 1997; van den Broek, 2005) makes the suggestion of a competitive advantage for mixed population more plausible. It can explain why gac mutants cannot out-compete their wild-type population despite their growth advantage and do not pose, under normal conditions, a threat to biocontrol efficacy (Chancey et al., 2002; Schmidt-Eisenlohr et al., 2003). Furthermore, it enables a gac mutant under less limiting conditions to switch back to its wild-type form to express its secondary metabolism and, as a result, become a biocontrol agent for plant pathogens, for example. Conclusions Recent work has shown that phenotypic variation is a phenomenon not only relevant in host–pathogen interactions. In contrast, research on Pseudomonas species has shown that phenotypic variation is a more broadly active phenomenon affecting phenomena such as root colonization, biocontrol activity, and the expression of exo-enzymes and secondary metabolites. The most important known mechanism responsible for the variation in Pseudomonas is spontaneous mutation of gacA/S, affecting the production of exo-enzymes and secondary metabolites. Overall, the different forms of phenotypic variation are relevant for rhizosphere bacteria to introduce a certain amount of diversity into a population by allowing the formation of specific subpopulations. These subpopulations can react rapidly to environmental changes or opportunities. The observation that these mechanisms are active in rhizosphere competent Pseudomonas bacteria suggests the existence of a conserved strategy to increase their success in the rhizosphere. Whether the variation via spontaneous mutation of gac is reversible for all these species remains to be seen, but this would substantially increase the importance of such mechanisms as it allows bacteria to switch between wild-type and mutant stages. References Abraham, J.M., Freitag, C.S., Clements, J.R., and Eisenstein, B.I. (1985) An invertible element of DNA controls phase variation of type 1 fimbriae of Escherichia coli. Proc Natl Acad Sci USA 82: 5724–5727. Achouak, W., Conrod, S., Cohen, V., and Heulin, T. (2004) Phenotypic variation of Pseudomonas brassicacearum as a plant root-colonisation strategy. Mol Plant–Microbe Interact 17: 872–879. Agur, Z., Abiri, D., and Van der Ploeg, L.H. (1989) Ordered appearance of antigenic variants of African trypanosomes explained in a mathematical model based on a stochastic switch process and immune-selection against putative switch intermediates. Proc Natl Acad Sci USA 86: 9626– 9630. Aras, R.A., Kang, J., Tschumi, A.I., Harasaki, Y., and Blaser, M.J. (2003) Extensive repetitive DNA facilitates prokaryotic genome plasticity. Proc Natl Acad Sci USA 100: 13579– 13584. Barbour, A.G. (2003) Antigenic variation in Borrelia: relapsing fever and Lyme borreliosis. In Antigenic Variation. Craig, A., and Scherf, A. (eds). London, UK: Academic press Elsevier, pp. 319–356. Barry, J.D., and McCulloch, R. (2003) Trypanosome antigenic variation – a heavy investment in the evasion of immunity. In Antigenic Variation. Craig, A., and Scherf, A. (eds). London, UK: Academic Press Elsevier, pp. 224–242. Bayliss, C.D., van de Ven, T., and Moxon, E.R. (2002) Mutations in polI but not mutSLH destabilize Haemophilus influenzae tetranucleotide repeats. EMBO J 21: 1465–1476. Bayliss, C.D., Dixon, K.M., and Moxon, E.R. (2004a) Simple sequence repeats (microsatellites): mutational mechanisms and contributions to bacterial pathogenesis. A meeting review. FEMS Immunol Med Microbiol 40: 11–19. Bayliss, C.D., Sweetman, W.A., and Moxon, E.R. (2004b) Mutations in Haemophilus influenzae mismatch repair genes increase mutation rates of dinucleotide repeat tracts but not dinucleotide repeat-driven pilin phase variation rates. J Bacteriol 186: 2928–2935. © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 1694 D. van den Broek, G. V. Bloemberg and B. Lugtenberg van Belkum, A., Scherer, S., van Alphen, L., and Verbrugh, H. (1998) Short-sequence DNA repeats in prokaryotic genomes. Microbiol Mol Biol Rev 62: 275–293. Belland, R.J. (1991) H-DNA formation by the coding repeat elements of neisserial opa genes. Mol Microbiol 5: 2351– 2360. Blomfield, I.C., Calie, P.J., Eberhardt, K.J., McClain, M.S., and Eisenstein, B.I. (1993) Lrp stimulates phase variation of type 1 fimbriation in Escherichia coli K-12. J Bacteriol 175: 27–36. Blumer, C., Heeb, S., Pessi, G., and Haas, D. (1999) Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc Natl Acad Sci USA 96: 14073– 14078. Blyn, L.B., Braaten, B.A., and Low, D.A. (1990) Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J 9: 4045–4054. Borst, P. (2003) Mechanisms of antigenic variation: an overview. In Antigenic Variation. Craig, A., and Scherf, A. (eds). London, UK: Elsevier Academic Press, pp. 1–15. van den Broek, D. (2005) Phase variation in Pseudomonas. PhD Thesis. Leiden, the Netherlands: Universiteit Leiden. van den Broek, D., Chin-A-Woeng, T.F.C., Eijkemans, K., Mulders, I.H., Bloemberg, G.V., and Lugtenberg, B.J.J. (2003) Biocontrol traits of Pseudomonas spp. are regulated by phase variation. Mol Plant–Microbe Interact 16: 1003– 1012. van den Broek, D., Chin-A-Woeng, T.F.C., Bloemberg, G.V., and Lugtenberg, B.J.J. (2005a) the role of RpoS and MutS in phase variation of Pseudomonas sp. PCL1171. Microbiology 151: 1403–1408. van den Broek, D., Chin-A-Woeng, T.F.C., Bloemberg, G.V., and Lugtenberg, B.J.J. (2005b) Molecular nature of spontaneous modifications in gacS which cause colony phase variation in Pseudomonas sp. PCL1171. J Bacteriol 187: 593–600. Bull, C.T., Duffy, B., Voisard, C., Defago, G., Keel, C., and Haas, D. (2001) Characterization of spontaneous gacS and gacA regulatory mutants of Pseudomonas fluorescens biocontrol strain CHAO. Antonie Van Leeuwenhoek 79: 327–336. Carroll, P.A., Tashima, K.T., Rogers, M.B., DiRita, V.J., and Calderwood, S.B. (1997) Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol 25: 1099–1111. Chabeaud, P., de Groot, A., Bitter, W., Tommassen, J., Heulin, T., and Achouak, W. (2001) Phase-variable expression of an operon encoding extracellular alkaline protease, a serine protease homolog, and lipase in Pseudomonas brassicacearum. J Bacteriol 183: 2117–2120. Chancey, S.T., Wood, D.W., Pierson, E.A., and Pierson, L.S., III (2002) Survival of GacS/GacA mutants of the biological control bacterium Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. Appl Environ Microbiol 68: 3308–3314. Chen, C.J., Elkins, C., and Sparling, P.F. (1998) Phase variation of hemoglobin utilization in Neisseria gonorrhoeae. Infect Immun 66: 987–993. Craig, A., and Scherf, A. (eds) (2003) Antigenic Variation. Amsterdam, the Netherlands: Academic Press Elsevier. Dekkers, L.C., Phoelich, C.C., van der Fits, L., and Lugten- berg, B.J. (1998) A site-specific recombinase is required for competitive root colonization by Pseudomonas fluorescens WCS365. Proc Natl Acad Sci USA 95: 7051–7056. Dekkers, L.C., Mulders, I.H., Phoelich, C.C., Chin-A-Woeng, T.F.C., Wijfjes, A.H., and Lugtenberg, B.J.J. (2000) The sss colonization gene of the tomato-Fusarium oxysporum f. sp. radicis-lycopersici biocontrol strain Pseudomonas fluorescens WCS365 can improve root colonization of other wild-type Pseudomonas spp. bacteria. Mol Plant–Microbe Interact 13: 1177–1183. Deziel, E., Comeau, Y., and Villemur, R. (2001) Initiation of biofilm formation by Pseudomonas aeruginosa 57RP correlates with emergence of hyperpiliated and highly adherent phenotypic variants deficient in swimming, swarming, and twitching motilities. J Bacteriol 183: 1195–1204. Donato, G.M., Lelivelt, M.J., and Kawula, T.H. (1997) Promoter-specific repression of fimB expression by the Escherichia coli nucleoid-associated protein H-NS. J Bacteriol 179: 6618–6625. Dorman, C.J., and Higgins, C.F. (1987) Fimbrial phase variation in Escherichia coli: dependence on integration host factor and homologies with other site-specific recombinases. J Bacteriol 169: 3840–3843. Duffy, B., and Défago, G. (1995) Influence of cultural conditions on spontaneous mutations in P. fluorescens CHAO. Phytopathology 85: 1146. Duffy, B.K., and Défago, G. (2000) Controlling instability in gacS-gacA regulatory genes during inoculant production of Pseudomonas fluorescens biocontrol strains. Appl Environ Microbiol 66: 3142–3150. Dybvig, K. (1993) DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol 10: 465–471. Dybvig, K., and Yu, H. (1994) Regulation of a restriction and modification system via DNA inversion in Mycoplasma pulmonis. Mol Microbiol 12: 547–560. Fernandez-Lopez, L., Pineiro, E., Marcos, R., Velazquez, A., and Surralles, J. (2004) Induction of instability of normal length trinucleotide repeats within human disease genes. J Med Genet 41: e3. Forsman, K., Sonden, B., Goransson, M., and Uhlin, B.E. (1992) Antirepression function in Escherichia coli for the cAMP-cAMP receptor protein transcriptional activator. Proc Natl Acad Sci USA 89: 9880–9884. Gally, D.L., Bogan, J.A., Eisenstein, B.I., and Blomfield, I.C. (1993) Environmental regulation of the fim switch controlling type 1 fimbrial phase variation in Escherichia coli K-12: effects of temperature and media. J Bacteriol 175: 6186–6193. Gearhart, P.J. (2002) Immunology: the roots of antibody diversity. Nature 419: 29–31. Haas, R., and Meyer, T.F. (1986) The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell 44: 107–115. Haas, R., Veit, S., and Meyer, T.F. (1992) Silent pilin genes of Neisseria gonorrhoeae MS11 and the occurrence of related hypervariant sequences among other gonococcal isolates. Mol Microbiol 6: 197–208. Hammerschmidt, S., Muller, A., Sillmann, H., Muhlenhoff, M., Borrow, R., Fox, A., et al. (1996) Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): corre- © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 Phenotypic variation in rhizosphere Pseudomonas bacteria 1695 lation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol 20: 1211–1220. Han, B., Pain, A., and Johnstone, K. (1997) Spontaneous duplication of a 661 bp element within a two-component sensor regulator gene causes phenotypic switching in colonies of Pseudomonas tolaasii, cause of brown blotch disease of mushrooms. Mol Microbiol 25: 211–218. Heinrich, D.W., and Glasgow, A.C. (1997) Transcriptional regulation of type 4 pilin genes and the site-specific recombinase gene, piv, in Moraxella lacunata and Moraxella bovis. J Bacteriol 179: 7298–7305. Henderson, I.R., Owen, P., and Nataro, J.P. (1999) Molecular switches – the ON and OFF of bacterial phase variation. Mol Microbiol 33: 919–932. Hengge-Aronis, R. (2002) Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev 66: 373–395. Hood, D.W., and Moxon, E.R. (2003) Haemophilus influenzae. In Antigenic Variation. Craig, A., and Scherf, A. (eds). London, UK: Elsevier Academic Press, pp. 102–141. Horst, J.P., Wu, T.H., and Marinus, M.G. (1999) Escherichia coli mutator genes. Trends Microbiol 7: 29–36. Inzana, T.J., Hensley, J., McQuiston, J., Lesse, A.J., Campagnari, A.A., Boyle, S.M., and Apicella, M.A. (1997) Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus. Infect Immun 65: 4675–4681. Jonsson, A.B., Nyberg, G., and Normark, S. (1991) Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. EMBO J 10: 477–488. Jonsson, A.B., Pfeifer, J., and Normark, S. (1992) Neisseria gonorrhoeae PilC expression provides a selective mechanism for structural diversity of pili. Proc Natl Acad Sci USA 89: 3204–3208. Kitten, T., Kinscherf, T.G., McEvoy, J.L., and Willis, D.K. (1998) A newly identified regulator is required for virulence and toxin production in Pseudomonas syringae. Mol Microbiol 28: 917–929. Kivisaar, M. (2003) Stationary phase mutagenesis: mechanisms that accelerate adaptation of microbial populations under environmental stress. Environ Microbiol 5: 814–827. Koomey, M., Gotschlich, E.C., Robbins, K., Bergstrom, S., and Swanson, J. (1987) Effects of recA mutations on pilus antigenic variation and phase transitions in Neisseria gonorrhoeae. Genetics 117: 391–398. Laville, J., Voisard, C., Keel, C., Maurhofer, M., Défago, G., and Haas, D. (1992) Global control in Pseudomonas fluorescens mediating antibiotic synthesis and suppression of black root rot of tobacco. Proc Natl Acad Sci USA 89: 1562–1566. Levinson, G., and Gutman, G.A. (1987) Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol 4: 203–221. Lewis, L.A., Gipson, M., Hartman, K., Ownbey, T., Vaughn, J., and Dyer, D.W. (1999) Phase variation of HpuAB and HmbR, two distinct haemoglobin receptors of Neisseria meningitidis DNM2. Mol Microbiol 32: 977–989. Lombardo, M.J., Aponyi, I., and Rosenberg, S.M. (2004) General stress response regulator RpoS in adaptive mutation and amplification in Escherichia coli. Genetics 166: 669–680. Lovett, S.T., and Feschenko, V.V. (1996) Stabilization of diverged tandem repeats by mismatch repair: evidence for deletion formation via a misaligned replication intermediate. Proc Natl Acad Sci USA 93: 7120–7124. Lysnyansky, I., Rosengarten, R., and Yogev, D. (1996) Phenotypic switching of variable surface lipoproteins in Mycoplasma bovis involves high-frequency chromosomal rearrangements. J Bacteriol 178: 5395–5401. Lysnyansky, I., Sachse, K., Rosenbusch, R., Levisohn, S., and Yogev, D. (1999) The vsp locus of Mycoplasma bovis: gene organization and structural features. J Bacteriol 181: 5734–5741. McClain, M.S., Blomfield, I.C., and Eisenstein, B.I. (1991) Roles of fimB and fimE in site-specific DNA inversion associated with phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol 173: 5308–5314. McClain, M.S., Blomfield, I.C., Eberhardt, K.J., and Eisenstein, B.I. (1993) Inversion-independent phase variation of type 1 fimbriae in Escherichia coli. J Bacteriol 175: 4335–4344. McKenzie, G.J., and Rosenberg, S.M. (2001) Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr Opin Microbiol 4: 586–594. Marinus, M.G. (1996) Methylation of DNA. In Escherichia coli and Salmonelk: Cellular and Molecular Biology. Neidhardt, F.C., Curtiss, R., Ingraham, J.L., III, Lin, E.C.C., Low, K.B., Magasanik, B. et al. (eds). Washington, DC, USA: ASM Press, pp. 782–791. Marrs, C.F., Rozsa, F.W., Hackel, M., Stevens, S.P., and Glasgow, A.C. (1990) Identification, cloning, and sequencing of piv, a new gene involved in inverting the pilin genes of Moraxella lacunata. J Bacteriol 172: 4370–4377. Martinez-Granero, F., Capdevila, S., Sanchez-Contreras, M., Martin, M., and Rivilla, R. (2005) Two site-specific recombinases are implicated in phenotypic variation and competitive rhizosphere colonisation of Pseudomonas fluorescens. Microbiology 151: 975–983. Maskell, D., Frankel, G., and Dougan, G. (1993) Phase and antigenic variation – the impact on strategies for bacterial vaccine design. Trends Biotechnol 11: 506–510. Meyer, T.F., and Hill, S.A. (2003) Genetic variation in the pathogenic Neisseria species. In Antigenic Variation. Craig, A., and Scherf, A. (eds). London, UK: Academic Press Elsevier, pp. 142–164. Monack, D.M., Arico, B., Rappuoli, R., and Falkow, S. (1989) Phase variants of Bordetella bronchiseptica arise by spontaneous deletions in the vir locus. Mol Microbiol 3: 1719–1728. Moses, E.K., Good, R.T., Sinistaj, M., Billington, S.J., Langford, C.J., and Rood, J.I. (1995) A multiple site-specific DNA-inversion model for the control of Omp1 phase and antigenic variation in Dichelobacter nodosus. Mol Microbiol 17: 183–196. Moxon, E.R., Rainey, P.B., Nowak, M.A., and Lenski, R.E. (1994) Adaptive evolution of highly mutable loci in pathogenic bacteria. Curr Biol 4: 24–33. Nicholson, B., and Low, D. (2000) DNA methylationdependent regulation of pef expression in Salmonella typhimurium. Mol Microbiol 35: 728–742. Nou, X., Braaten, B., Kaltenbach, L., and Low, D.A. (1995) Differential binding of Lrp to two sets of pap DNA binding sites mediated by PapI regulates Pap phase variation in Escherichia coli. EMBO J 14: 5785–5797. © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 1696 D. van den Broek, G. V. Bloemberg and B. Lugtenberg O’Gara, J.P., and Dorman, C.J. (2000) Effects of local transcription and H-NS on inversion of the fim switch of Escherichia coli. Mol Microbiol 36: 457–466. Owen, P., Meehan, M., Loughry-Doherty, H., and Henderson, I. (1996) Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol Med Microbiol 16: 63–76. Palmer, B.R., and Marinus, M.G. (1994) The dam and dcm strains of Escherichia coli – a review. Gene 143: 1–12. Pedersen, M.K., Hoiby, E.A., Froholm, L.O., Hasseltvedt, V., Lermark, G., and Caugant, D.A. (2004) Systemic pneumococcal disease in Norway 1995–2001: capsular serotypes and antimicrobial resistance. Epidemiol Infect 132: 167– 175. Poussier, S., Thoquet, P., Trigalet-Demery, D., Barthet, S., Meyer, D., Arlat, M., and Trigalet, A. (2003) Host plantdependent phenotypic reversion of Ralstonia solanacearum from non-pathogenic to pathogenic forms via alterations in the phcA gene. Mol Microbiol 49: 991–1003. Rich, J.J., Kinscherf, T.G., Kitten, T., and Willis, D.K. (1994) Genetic evidence that the gacA gene encodes the cognate response regulator for the lemA sensor in Pseudomonas syringae. J Bacteriol 176: 7468–7475. Richardson, A.R., and Stojiljkovic, I. (2001) Mismatch repair and the regulation of phase variation in Neisseria meningitidis. Mol Microbiol 40: 645–655. Sanchez-Contreras, M., Martin, M., Villacieros, M., O’Gara, F., Bonilla, I., and Rivilla, R. (2002) Phenotypic selection and phase variation occur during alfalfa root colonization by Pseudomonas fluorescens F113. J Bacteriol 184: 1587–1596. Sarkari, J., Pandit, N., Moxon, E.R., and Achtman, M. (1994) Variable expression of the Opc outer membrane protein in Neisseria meningitidis is caused by size variation of a promoter containing poly-cytidine. Mol Microbiol 13: 207–217. Saunders, N.J., Moxon, E.R., and Gravenor, M.B. (2003) Mutation rates: estimating phase variation rates when fitness differences are present and their impact on population structure. Microbiology 149: 485–495. Schmidt-Eisenlohr, H., Gast, A., and Baron, C. (2003) Inactivation of gacS does not affect the competitiveness of Pseudomonas chlororaphis in the Arabidopsis thaliana rhizosphere. Appl Environ Microbiol 69: 1817–1826. Schofield, M.J., and Hsieh, P. (2003) DNA mismatch repair: molecular mechanisms and biological function. Annu Rev Microbiol 57: 579–608. Schwan, W.R., Seifert, H.S., and Duncan, J.L. (1992) Growth conditions mediate differential transcription of fim genes involved in phase variation of type 1 pili. J Bacteriol 174: 2367–2375. Segura, A., Hurtado, A., Duque, E., and Ramos, J.L. (2004) Transcriptional phase variation at the flhB gene of Pseudomonas putida DOT-T1E is involved in response to environmental changes and suggests the participation of the flagellar export system in solvent tolerance. J Bacteriol 186: 1905–1909. Seifert, H.S. (1996) Questions about gonococcal pilus phaseand antigenic variation. Mol Microbiol 21: 433–440. Shaver, A.C., and Sniegowski, P.D. (2003) Spontaneously arising mutL mutators in evolving Escherichia coli populations are the result of changes in repeat length. J Bacteriol 185: 6076–6082. Shaver, A.C., Dombrowski, P.G., Sweeney, J.Y., Treis, T., Zappala, R.M., and Sniegowski, P.D. (2002) Fitness evolution and the rise of mutator alleles in experimental Escherichia coli populations. Genetics 162: 557–566. Stern, A., Brown, M., Nickel, P., and Meyer, T.F. (1986) Opacity genes in Neisseria gonorrhoeae: control of phase and antigenic variation. Cell 47: 61–71. Stibitz, S., Aaronson, W., Monack, D., and Falkow, S. (1989) Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature 338: 266–269. Strauss, B.S., Roberts, R., Francis, L., and Pouryazdanparast, P. (2000) Role of the dinB gene product in spontaneous mutation in Escherichia coli with an impaired replicative polymerase. J Bacteriol 182: 6742–6750. Struve, C., and Krogfelt, K.A. (1999) In vivo detection of Escherichia coli type 1 fimbrial expression and phase variation during experimental urinary tract infection. Microbiology 145: 2683–2690. Tegova, R., Tover, A., Tarassova, K., Tark, M., and Kivisaar, M. (2004) Involvement of error-prone DNA polymerase IV in stationary-phase mutagenesis in Pseudomonas putida. J Bacteriol 186: 2735–2744. Theiss, P., and Wise, K.S. (1997) Localized frameshift mutation generates selective, high-frequency phase variation of a surface lipoprotein encoded by a mycoplasma ABC transporter operon. J Bacteriol 179: 4013–4022. Tomb, J.F., White, O., Kerlavage, A.R., Clayton, R.A., Sutton, G.G., Fleischmann, R.D., et al. (1997) The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388: 539–547. Tsui, H.C., Feng, G., and Winkler, M.E. (1997) Negative regulation of mutS and mutH repair gene expression by the Hfq and RpoS global regulators of Escherichia coli K-12. J Bacteriol 179: 7476–7487. Vial, L., Pothier, J.F., Normand, P., Moenne-Loccuz, Y., Bally, R., and Wisniewski-Dye, F. (2004) Construction of a recA mutant of Asospirillum lipoferum and involvement of recA in phase variation. FEMS Microbiol Lett 236: 291– 299. Waite, R.D., Penfold, D.W., Struthers, J.K., and Dowson, C.G. (2003) Spontaneous sequence duplications within capsule genes cap8E and tts control phase variation in Streptococcus pneumoniae serotypes 8 and 37. Microbiology 149: 497–504. Wang, G., Rasko, D.A., Sherburne, R., and Taylor, D.E. (1999) Molecular genetic basis for the variable expression of Lewis Y antigen in Helicobacter pylori: analysis of the alpha (1,2) fucosyltransferase gene. Mol Microbiol 31: 1265–1274. Weiser, J.N., Goldberg, J.B., Pan, N., Wilson, L., and Virji, M. (1998) The phosphorylcholine epitope undergoes phase variation on a 43-kilodalton protein in Pseudomonas aeruginosa and on pili of Neisseria meningitidis and Neisseria gonorrhoeae. Infect Immun 66: 4263–4267. White-Ziegler, C.A., Villapakkam, A., Ronaszeki, K., and Young, S. (2000) H-NS controls pap and daa fimbrial transcription in Escherichia coli in response to multiple environmental cues. J Bacteriol 182: 6391–6400. White-Ziegler, C.A., Black, A.M., Eliades, S.H., Young, S., and Porter, K. (2002) The N-acetyltransferase RimJ © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697 Phenotypic variation in rhizosphere Pseudomonas bacteria 1697 responds to environmental stimuli to repress pap fimbrial transcription in Escherichia coli. J Bacteriol 184: 4334– 4342. van der Woude, M.W., Braaten, B., and Low, D. (1996) Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol 4: 5–9. Yildiz, F.H., Liu, X.S., Heydorn, A., and Schoolnik, G.K. (2004) Molecular analysis of rugosity in a Vibrio cholerae O1 El Tor phase variant. Mol Microbiol 53: 497–515. Zieg, J., Silverman, M., Hilmen, M., and Simon, M. (1977) Recombinational switch for gene expression. Science 196: 170–172. © 2005 Society for Applied Microbiology and Blackwell Publishing Ltd, Environmental Microbiology, 7, 1686–1697