* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download pdf
Gene expression profiling wikipedia , lookup
Point mutation wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
X-inactivation wikipedia , lookup
Human genome wikipedia , lookup
Protein moonlighting wikipedia , lookup
Non-coding DNA wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Genetic code wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Hammerhead ribozyme wikipedia , lookup
Transfer RNA wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Short interspersed nuclear elements (SINEs) wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
RNA interference wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Messenger RNA wikipedia , lookup
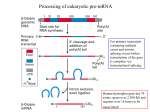
Polyadenylation wikipedia , lookup
RNA silencing wikipedia , lookup
Nucleic acid tertiary structure wikipedia , lookup
Alternative splicing wikipedia , lookup
History of RNA biology wikipedia , lookup
Non-coding RNA wikipedia , lookup
RNA-binding protein wikipedia , lookup