* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Determining Compensatory Genes from Loss of Vacuolar
Oncogenomics wikipedia , lookup
Primary transcript wikipedia , lookup
Genomic imprinting wikipedia , lookup
Genetic engineering wikipedia , lookup
Point mutation wikipedia , lookup
Pathogenomics wikipedia , lookup
Human genome wikipedia , lookup
Protein moonlighting wikipedia , lookup
Ridge (biology) wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Non-coding DNA wikipedia , lookup
Public health genomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Biology and consumer behaviour wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Microevolution wikipedia , lookup
Genome (book) wikipedia , lookup
History of genetic engineering wikipedia , lookup
Gene expression profiling wikipedia , lookup
Designer baby wikipedia , lookup
Minimal genome wikipedia , lookup
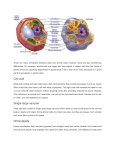
Determining Compensatory Mechanisms for Loss of Vacuolar Function in Saccharomyces cerevisiae Nick Netzel*, Jillian Wisby*, Pamela A. Marshall. Arizona State University at the West Campus, Glendale, AZ. *students contributed equally to this work Table 1: Up-regulated Genes of Interest Abstract wt Saccharomyces cerevisiae is dependent on its vacuole for proper sorting, degradation, and recycling of proteins, organelles, and other biomolecules that occur in the cell. For these processes to function properly, vacuolar docking from incoming vesicles must be achieved. Yeast mutants that have a characteristic loss of function vacuole are classified as vps mutants. The current study utilizes two similar vps mutants, vps33 and vps41, both of which are able to survive with a loss of function vacuole under laboratory conditions. The purpose of the current study is to compare these vps mutants via DNA microarray analysis and determine if similar genes are being expressed. Thus far, analysis has revealed vps33 and vps41 expressing similar compensatory genes which include four genes of interest (YLR243w being the most interesting), five genes resulting from stress, and three genes involved in mitochondrial activity. Due to YLR243W’s level of up-regulation in the mutants and its essential role in actin cytoskeleton formation, this gene will be a central focus in future studies. wt vps33 vps41 Table 2: Genes Associated with Stress Induced up-regulation Figure 2: Gel Confirming Successful RNA Extraction Introduction Cells must have specific ways to transport, store, and breakdown biological molecules. In humans, there are many DNA mutations that lead to malfunctions in these cellular transport processes and disease states. To better understand these processes we use a model organism, the yeast Saccharomyces cerevisiae. Central to S. cerevisiae’s protein sorting, storing and biomolecular breakdown is the vacuole. This characteristic vacuole is responsible for the major pathway in which degradation and recycling of proteins and other organelles occurs; the other pathway involves the peroxisome [1]. In comparison, human cells also have a peroxisome, but the lysosome is involved in the major pathway responsible for biomolecular breakdown [4]. Unlike human cells, yeast is easy and inexpensive to grow but can still yield important insights into human disease. In the laboratory, strains of yeast have been cultured which contain loss of function vacuoles yet still sustain the ability to survive. Yeast strains such as these are classified as vps mutants [2]. The specific mutants used in this study are vps33 and vps41 (also known as YLR396 and YDR080, respectively). Both mutant types prevent endosome vesicle docking to the yeast vacuole [6] (Figure 1). By taking advantage of the yeast vacuole and human lysosome relationship, improved treatment plans can be made for individuals suffering from lysosomal storage disorders. Lysosomal storage disorders are genetically inherited as the result of a defect in lysosomal proteins or a defect in protein transport to the lysosome. These disorders represent a group of at least 41 distinct genetic diseases [5]. The most common lysosomal storage disorder is Gaucher’s disease, which affects 1 in 57,000 births and is characterized by a deficiency of the enzyme glucocerebrosidase, causing fatty tissue to build up in vital organs such as the spleen and liver [3]. Symptoms of this and other lysosomal storage disorders (Tay-Sachs and Krabbe disease) include an enlarged spleen and liver, liver malfunction, skeletal disorders, and bone lesions. Results/ Discussion Figure 3: DNA Microarrays of vps33 (left) and vps41 (right). Our hypothesis is that changes in gene expression compensate for the loss of vacuolar function in vps33 and vps41. We used DNA microarray technology to determine the changes in gene expression that allow mutant strains lacking vacuole function to thrive in the lab. We have successfully compared the complete genomes of two different mutant strains of Saccharomyces cerevisiae, vsp33 and vsp41, to a wild type strain using DNA microarray technology. Repeated trials have consistently shown up-regulation of 12 different genes in both mutant strains (Tables 1 & 2). Analysis using the S. cerevisiae database has shown 4 of the selected genes to be be of possible significance in genetic compensation for the loss of vacuole function. The most interesting upregulation is YLR243W. As it turns out, this gene’s protein product interacts with other proteins that play a part in actin cytoskeleton formation, allowing for regulation of endocytosis during times of osmotic stress [7]. At this point it is hypothesized that the other 8 genes under consideration are likely linked to stress induced up-regulation due to experimental growth conditions. Further studies should investigate the relationship between YLR243W up-regulation and actin cytoskeleton formation. Future studies will include increased cellular stress by the addition of 100 and 200 mM calcium. Since the yeast vacuole is a primary storage center for divalent cations, we expect to see gene compensation more definitively. Literature Cited 1. Bryant, N.J., and Stevens, T.H. 1998. Vacuole biogenesis in Saccharomyces cerevisiae: protein transport pathways to the yeast vacuole. Microbiology and Molecular Biology Reviews 62: 240-247. Materials and Methods Vacuole X Nucleus Autophagy Golgi ER Endosome Secretory Vesicle Figure 1: Biomolecular Sorting, Storage, and Breakdown in Yeast. Both vps33 and vps41are not able to bind incoming vesicles to the vacuole. The red “X” indicates where vps mutants are hindered which leads to a total loss of vacuolar function in cell strains lacking these genes. -RNA Extraction S. cerevisiae RNA was extracted using Ambion’s RiboPureYeast Kit and purified using Qiagen’s PCR Purification Kit -Gel Electrophoresis Agarose gel electrophoresis was executed to visually ensure RNA was extracted (Figure 2) -Microarray Preparation DNA microarrays were produced using a Genisphere 3DNA Array 350 Labeling Kit (Figure 3) -Analysis Initial DNA microarray analysis was performed utilizing MicroArray Genome Imaging and Clustering Tool (MAGIC Tool) Up-regulated genes were then cross referenced with the Saccharomyces Genome Database (Tables 1 and 2) [7] 2. Herman, P.K., and Emr, S.D. 1990. Characterization of VPS34, a gene required for vacuolar protein sorting and vacuole segregation in Saccharomyces cerevisiae. Molecular and Cellular Biology 10: 6742-6754. 3. Hollak, C.E., van Weely, S., van Oers, M.H., and Aerts, J.M. Marked elevation of plasma chitotriosidase activity: a novel hallmark of Gaucher disease. Journal of Clinical Investigation 93: 1288-1292. 4. Luzio, J., Poupon, V., Lindsay, M., Mullock, Piper, R., and Pryor, P. 2003. Membrane dynamics and the biogenesis of lysosomes. Molecular Membrane Biology 20: 141-154. 5. Meikle, P.J., Hopwood, J.J., Clague, A.E., and Carey, W.F. 1999. Prevalence of lysosomal storage the American Medical Association 281: 249-254. disorders. Journal of 6. Rieder, S., and Emr, S.D. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Molecular Biology of the Cell 8: 2307-2327. 7. Saccharomyces Genome Database <http://www.yeastgenome.org/> Acknowledgements NN was supported by the SRP Life Sciences Scholarship. Special thanks to the Genome Consortium for Active Teaching for training (PAM), support, and subsidized micrarrays.
















![[pdf]](http://s1.studyres.com/store/data/008791587_1-e65c6aed4cb40504aeeddda921f62bfc-150x150.png)
