* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry of Life
Survey
Document related concepts
Endomembrane system wikipedia , lookup
Protein moonlighting wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Protein adsorption wikipedia , lookup
Biosynthesis wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Circular dichroism wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Metalloprotein wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein structure prediction wikipedia , lookup
Transcript
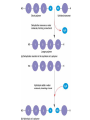
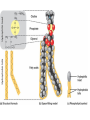
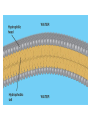
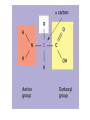
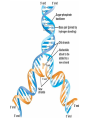
Acid Precipitation Normal rainfall pH around 5.6 – CO2 (atmos) + H2O → H2CO3 carbonic acid Rain, snow, or fog with a pH lower than pH 5.6 – – – Caused by sulfur oxides, nitrogen oxides in atmosphere-gaseous compounds which react with water in the air to form strong acids which fall with rain or snow Source of oxides-burning of fossil fuels (coal,oil, gas) in factories and automobiles Winds carry pollutants Acid Precipitation Damage to lakes, streams Washes away certain minerals from soil (e.g. Calcium, Magnesium which help buffer soil and are essential nutrients for plant growth Other minerals (e.g. aluminum) reach toxic concentrations when acidification increases solubility Acid Precipitation Upside Pollution control programs here, Canada, some European countries – – Decrease in oxide emissions over several decades Acid rain has decreased, but recovery is slow Chemistry of Life Part deux Chemistry of Life Outline I. Introduction II. Macromolecules-Large Organic Molecules A. Carbohydrates B. Lipids 1. glycerides 2. phospholipids C. Proteins D. Nucleic Acids Inorganic vs. Organic Inorganic compounds Organic compounds: Recall Carbon 6C – – – – 4 electrons in outer shell Can form up to 4 covalent bonds with other atoms Recall bonds can be single, double, triple Often bond with carbon atoms to create chains of carbons Carbon Chemistry Hydrocarbons: Examples: methane-simplest hydrocarbon CH4 Hydrocarbons undergo reactions that release relatively large amounts of energy Replace a H with a C and the backbone grows, keep going and you get long chains, branched chains, and ring structures No polarity to bonds, not soluble in water Isomers Functional Groups Groups of atoms attached to the carbon backbone Most chemical reactions that occur within organisms involve transferring a functional group from one molecule to another or breaking a carbon-carbon bond Behave consistently from one organic molecule to the next Macromolecules Large organic molecules 3 of these are – – Long molecules made up of similar or identical building blocks linked by covalent bonds Repeating units are called Assembly and Disassembly Chemical mechanism by which cells make and break polymers is the same even though monomers may be different for different macromolecules Enzymes: special class of proteins which catalyze (speed up) reactions between specific substances Assembly and Disassembly Condensation: monomer connection by reaction in which 2 molecules are covalently bonded to each other through loss of H2O (dehydration) Assembly and Disassembly Hydrolysis: “water breaking”-polymers broken down into monomers. H from water attaches to one monomer, OH attaches to adjacent monomer Carbohydrates Structure – – – – – MonosaccharideDisaccharidePolysaccharideC, H, O in 1:2:1 ratio Examples Function Important Carbohydrates Glucose: C6H12O6 forms ring structure in solution, major nutrient for cells, carbon skeletons serve as raw material for synthesis of other types of small organic molecules – – Water soluble Often linked to form disaccharide for transport Polysaccharides Glycogen: storage form of glucose in animals, long chains, highly branched therefore more insoluble in solution. Easily broken down via enzyme catalyzed hydrolysis to glucose Starch: storage form of glucose in plants Polysaccharides Cellulose: also made of glucose monomers but structurally different than starch. Component of tough walls that encase plant cells, not readily broken down Chitin: structural also, used by crustaceans, insects, spiders to build their exoskeletons – Lipids Insoluble in water (polar) but soluble in oil (nonpolar) Types of Lipids: Many C-H bonds Functions include Lipids Built from 2 subunits – – Glycerol: 3-C alcohol with 3 OH groups which forms the backbone of the lipid molecule Fatty acids: long chains of C-H (often 16-18 carbons longhence the insolubility) – Saturated: single bonds between carbons, chains lay flat, decreased reactivity. Solid at room T (lard, butter) Most animal fats Unsaturated: one or more double bonds which cause “kinks” in chains, therefore increasing reactivity. Liquid at room T (olive oil, cod liver oil) Most plant and fish fats 1-3 fatty acid chains per glycerol molecule Great energy storage Phospholipids Glycerol backbone with 2 fatty acid tails. Phosphate group PO4- attached Form lipid bilayer at cell surface Steroids Carbon skeleton of 4 rings Vary in functional groups attached and therefore vary in function Cholesterol: Proteins Account for almost 50% of dry weight of cells Instrumental for many cell functions including: – – – – Structural support and movement(bone,cartilage) Storage/transport molecules (hemoglobin) Hormones (insulin-sugar breakdown) Enzymes (control of cellular reactions Protein Chemistry R groups differ for each AA Amino acids joined together by special covalent bonds called peptide bonds Protein Structure Primary structure: specific and unique AA sequence Secondary structure: parts of AA chain bonded together via hydrogen bonds into helical structure (alpha helix) or sheet structure Tertiary structure: due to interactions of R groups-sheets often form fibers that have structural function, helical structures tend to have globular form-heavily influenced by R group Protein Structure Quaternary structure: Proteins that consist of more than one polypeptide chain. – Ex: Hemoglobin (carries O2 in body) globular protein with 4 chains, collagen fibrous protein of 3 chains forming a triple helix-suits function of connective tissue in skin, bone, tendons etc. Denaturation: change in 3D structure when chemical environment of protein changed – Exs. pH, salt concentration, temperature etc. Nucleic Acids Nucleotide monomer – – 5 C sugar-either ribose or deoxyribose N-containing base – Single ring Pyrimidine: C, T, U Double ring purine A, G Phosphate group Deoxyribonucleic Acid (DNA) Nucleotide has deoxyribose as 5C sugar Double helix structure (double strand of nucleotides) Lives in nucleus of cells Ribonucleic Acid (RNA) Nucleotide has ribose as 5C sugar Single chain nucleotide structure (single stranded) Directs production of proteins – – Once synthesized (via DNA), moves out of nucleus to protein synthesis machinery in cell and base sequence directs which AA’s are joined together 3 bases = particular AA