* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Mitochondrion wikipedia , lookup
Gene regulatory network wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Microbial metabolism wikipedia , lookup
Paracrine signalling wikipedia , lookup
Isotopic labeling wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemistry wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biosynthesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
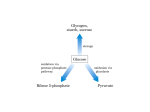
Other Pathways of Carbohydrate Metabolism Aulanni’am Laboratory of Biochemistry FMIPA Brawijaya University Other pathways of carbohydrate metabolism • 1. Gluconeogenesis • 2. The Glyoxylate pathway • 3. The Pentose Phosphate Pathway 1. The Gluconeogenesis Pathway Gluconeogenesis requires 3 bypass reactions. 1st bypass - pyruvate is converted to PEP in 2 steps. Pyruvate is converted to oxaloacetate before conversion to phosphoenolpyruvate. 1. Pyruvate carboxylase A Biotin prosthetic group is required Acetyl-CoA is an allosteric activator 2. PEP carboxykinase (PEPCK) Pyruvate Carboxylase has a biotin prosthetic group Two phase reaction mechanism of pyruvate carboxylase Bicarbonate PEP Carboxykinase catalyzes the GTP-driven decarboxylation of oxaloacetate to form PEP and GDP. Gluconeogenesis requires metabolite transport between mitochondria and cytosol • Generation of oxaloacetate from pyruvate or citric acid cycle intermediates occurs only in the mitochondrion. • Enzymes that convert PEP to glucose are cytosolic. • Either oxaloacetate must leave the mitochondrion for conversion to PEP, or the PEP formed must enter the cytosol. • Oxaloacetate must be converted to malate for which transport systems exist. The malate dehydrogenase route results in transport of reducing equivalents from the mitochondrion to the cytosol. • Cytosolic NADH is required for gluconeogenesis. Gluconeogenesis requires metabolite transport between mitochondria and cytosol • The difference between the 2 routes involves transport of NADH. • The malate dehydrogenase route (Route 2) results in transport of NADH. • The aspartate aminotransferase route (Route 1) does not involve NADH. • Since cytosolic NADH is required for gluconeogenesis, route 2 is usually required. • However, if lactate is the gluconeogenic precursor, it is oxidized to pyruvate generating cytosolic NADH. Therefore, either route may then be used. Transport of PEP and oxaloacetate from the mitochondrion to the cytosol. 2nd and 3rd bypass reactions. Hydrolytic reactions bypass PFK and Hexokinase. At these steps, instead of generating ATP by reversing the glycolytic reactions, FBP and G6P are hydrolyzed, releasing Pi in exergonic processes catalyzed by FBPase and glucose-6-phosphatase. B. Regulation of gluconeogenesis • If glycolysis and gluconeogenesis were uncontrolled, the net effect would be a futile cycle wastefully hydrolyzing ATP and GTP. • However, the pathways are reciprocally regulated so as to meet the needs of the organism. • Glycolysis and Gluconeogenesis are controlled by allosteric interactions and covalent modifications. Regulation of glycolysis and gluconeogeneis by Allosteric Interactions and Covalent Modifications 1. Hexokinase/glucose-6-phosphatase 2. PFK/FBPase 3. Pyruvate kinase/pyruvate carboxylase-PEPCK C. The Cori Cycle Through the intermediacy of the bloodstream, liver and muscle participate in a metabolic cycle known as the Cori cycle. Hormonal regulation of [F2,6P] activates gluconeogenesis in liver in response to low blood sugar. The Cori cycle. Lactate produced by muscle glycolysis is transported by the bloodstream to the liver, where it is converted to glucose by gluconeogenesis. The bloodstream carries glucose back to the muscles, where it may be stored as glycogen. 2, The Glyoxylate Pathway (in plants but not animals). Two essential enzymes for The glyoxylate pathway not found in the citric acid cycle in animals are: (1) isocitrate lysase (2) malate synthase The overall reaction of the glyoxylate cycle is the formation of oxaloacetate from 2 molecules of acetyl-CoA. This enables germinating seeds to convert stored triacylglycerols, through acetyl-CoA, to glucose. The glyoxylate cycle and its relationship to the citric acid cycle. Electron micrograph of a germinating cucumber seed, showing a glyoxysome, mitochondria, and surrounding lipid bodies Glyoxylate cycle reactions (in glyoxysomes) proceed simultaneously with, and mesh with those of the citric acid cycle (in mitochondria). 3. The Pentose Phosphate Pathway p.617 • Many endergonic reactions, notably the reductive biosynthesis of fatty acids and cholesterol, as well as photosynthesis, require NADPH in addition to ATP. • Whereas NADH participates in utilizing the free energy of metabolite oxidation to synthesize ATP, NADPH is involved in utilizing the free energy of metabolite oxidation for otherwise endergonic reductive biosynthesis. • Cells normally maintains the [NAD+]/[NADH] ratio near 1000 which favors metabolite oxidation. The Pentose Phosphate Pathway • The [NADP+]/[NADPH] ratio is near 0.01 which favors metabolite reduction. • NADPH is generated by oxidation of G6P via the pentose phosphate pathway. • About 30% of glucose oxidation in liver occurs via the pentose phosphate pathway. • Ribose-5-phosphate (R5P) produced by this pathway is required for nucleotide biosynthesis. Pentose phosphate pathway There are 3 stages: (1) Oxidative reactions (reactions 1-3) that form NADPH and ribulose-5-phosphate (Ru5P). (2) Isomerization and epimerization reactions (reactions 4 and 5) that transform Ru5P either to ribose-5phosphate (R5P) or to xylulose-5-phosphate (Xu5P). (3) A series of C-C bond cleavage and formation reactions (reactions 6-8) that convert 2 Xu5P and 1 R5P to 2 F6P and 1 glyceraldehyde-3-phosphate (GAP). The overall reaction of the pathway is: 3G6P + 6NADP+ + 3H2O 6NADPH + 6H+ + 3CO2 +2F6P +GAP 1 2 3 (1) Oxidative reactions (reactions 1-3) that form NADPH and ribulose-5-phosphate (Ru5P). (2) Isomerization and epimerization reactions (reactions 4 and 5) that transform Ru5P either to ribose-5phosphate (R5P) or to zylulose-5-phosphate (Xu5P). (3) A series of C-C bond cleavage and formation reactions (reactions 6-8) that convert 2 Xu5P and 1 R5P to 2 F6P and 1 glyceraldehyde-3-phosphate (GAP). These reactions require transketolase and transaldolase. Transketolase transfers 2C units. Transaldolase transfers 3C units. Ru5P 2. 3. NONOXIDATIVE REACTIONS BY TRANSKETOLASE AND TRANSALDOLASE CONVERT PENTOSE PHOSPHATES BACK INTO HEXOSE PHOSPHATES Pentose Phosphate Pathway The glucose-6-phosphate dehydrogenase reaction (reaction 1). This enzyme is specific for NADP+ and Is strongly inhibited by NADPH. The phosphogluconate dehydrogenase reaction (reaction 3). Ribulose-5-phosphate isomerase and ribulose-5-phosphate epimerase reactions both involve endiolate intermediates. Carbon-carbon bond cleavage and formation reactions • Transketolase catalyzes the transfer of C2 units • TPP is a cofactor in the transfer of C2 units. • A C2 unit is transferred from Xu5P to R5P yielding GAP and sedoheptulose-7-phosphate (S7P) (a 7 carbon sugar). Transketolase utilizes the coenzyme thiamine pyrophosphate (TPP) to stabilize the carbanion formed on the cleavage of the C2-C3 bond of Xu5P. Transaldolase Catalyzes the Transfer of C3 Units. • Transaldolase catalyzes the transfer of a C3 unit. • A C3 unit is transferred from S7P to GAP yielding erythrose-4-phosphate (E4P) and F6P. • The reactions occurs by an aldol cleavage. Transaldolase contains an essential Lys residue that forms a Schiff base with S7P to facilitate an aldol cleavage reaction. Carbon-carbon bond formations and cleavages that convert 3 C5 sugars to 2 C6 and 1 C3 sugar in the pentose phosphate pathway. (6) Transketolase (2 carbon transfer) (7) Transaldolase (3 carbon transfer) (8) Transketolase (2 carbon transfer) A schematic diagram showing the pathway leading from 6 pentoses (5C) to 5 hexoses (6C). Pentose Phosphate Pathway Control of the pentose phosphate pathway • When the need for NADPH exceeds that of R5P in nucleotide biosynthesis, excess R5P is converted to glycolytic intermediates. GAP and F6P are consumed through glycolysis and oxidative phosphorylation or recycled by gluconeogenesis to form G6P. In the latter case, 1 G6P can be converted, via 6 cycles of pentose phosphate pathway and gluconeogenesis, to 6 CO2 and 12 NADPH. • When R5P is needed more than NADPH, F6P and GAP can be diverted from the glycolytic pathway for use in synthesis of R5P by the reversal of the transaldolase and transketolase reactions. • Flux through the pathway and thus the rate of NADPH production is controlled by rate of glucose-6-phosphate dehydrogenase reaction (the first committed step). The activity is regulated by the NADP+ concentration (substrate availability). Glucose-6-phosphate dehydrogenase deficiency • NADPH is required for many reductive processes in addition to biosynthesis. Erythrocyte membrane integrity requires reduced glutathione (GSH) to eliminate H2O2 and organic hydroperoxides. Peroxides are eliminated by glutathione peroxidase using GSH and yielding glutatione disulfie (GSSG). • GSH is regenerated by NADPH reduction of GSSG catalyzed by glutathione reductase. Therefore, a steady supply of NADPH is vital for erythrocyte integrity. Glucose-6-phosphate dehydrogenase deficiency • Primaquine, an antimalarial agent, causes hemolytic anemia in glucose-6-phosphate dehydrogenase mutants. • Under most conditions, the erythrocytes have sufficient enzyme activity for normal function. • However, primaquine and similar agents stimulate peroxide formation, thereby increasing the demand for NADPH to a level that mutant cells cannot meet. • About 400 million people are deficient in G6PD, which makes this condition the most common human enzymopathy.