* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aldehydes and Ketones - University of Nebraska Omaha
Enantioselective synthesis wikipedia , lookup
Discodermolide wikipedia , lookup
Elias James Corey wikipedia , lookup
Ene reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Metal carbonyl wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Stille reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Petasis reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Hydroformylation wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Asymmetric induction wikipedia , lookup
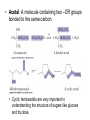
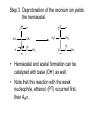
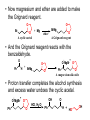
Aldehydes and Ketones Structure of Aldehydes and Ketones • Both aldehydes and ketones have a carbonyl group, a carbon atom doubly bonded to an oxygen atom, C=O. • Aldehydes have a carbonyl group bonded to an H atom. • Ketones have a carbonyl group bonded to two carbon atoms. • In this and several following chapters, we study the physical and chemical properties of classes of compounds containing the carbonyl group, C=O. • aldehydes and ketones (Chapter 12) • carboxylic acids (Chapter 13) • acid halides, acid anhydrides, esters, and amides (Chapter 14) Nomenclature of Aldehydes and Ketones • IUPAC nomenclature • The parent chain is the longest chain that contains the carbonyl group. • For an aldehyde, change the suffix from –e to –al; for a ketone change the suffix from –e to –one • For an unsaturated aldehyde or ketone, show the carbon-carbon double bond by changing the infix from –an– to –en–; the location of the suffix determines the numbering pattern. • For a cyclic molecule in which –CHO is bonded to the ring, add the suffix –carbaldehyde. • Some aromatic aldehydes and ketones have reserved names. (We need to know benzaldehyde, acetophenone, benzophenone.) Some examples Precedence of Functional Groups • When a molecule has several functional groups, which functional group uses a suffix and which functional group uses a prefix follows a preset order of precedence (priority). • Precedence in decreasing order (highest on top) (lower precedence prefix in parenthesis) • • • • • • • carboxylic acid aldehyde (oxo-) ketone (oxo-) alcohol (hydroxy-) thiol (mercapto-) [book has thiol and amine backwards] amine (amino-) alkene/alkyne (alkenyl/alkynyl) • Use prefixes to name functional group with lower precedence. • Common names • For aldehydes, the common name is derived from the common name of the corresponding carboxylic acid. • Know formaldehyde and acetaldehyde. • For ketones, name the alkyl or aryl groups bonded to the carbonyl carbon and add the word ketone. • Order branches according to molecular weight. • Know acetone. Physical Properties of Aldehydes and Ketones • Oxygen is more electronegative than carbon (3.5 versus 2.5); and therefore, a C=O group is polar. • Aldehydes and ketones are polar compounds and interact in the pure state by dipole-dipole interactions. • They have higher boiling points and are more soluble in water than nonpolar compounds of comparable molecular weight. • In liquid aldehydes and ketones, there are moderate intermolecular attractions between the carbon pole (+) of one molecule and the oxygen pole (-) of another molecule. • Hydrogen bonding is not possible between (purely) aldehyde or ketone molecules. • Aldehydes and ketones have lower boiling points than alcohols and carboxylic acids, compounds in which hydrogen bonding between molecules is possible. • Formaldehyde, acetaldehyde, and acetone are infinitely soluble in water. • Aldehydes and ketones become less soluble in water as the hydrocarbon portion of the molecule increases in size. Reactions of Aldehydes and Ketones • Nucleophilic addition to bond, AN • Grignard reactions (adding carbanion to carbonyl group) • Imine formation • Acetal and hemi-acetal formation • Keto-enol tautomerism • Racemization at an -carbon • Oxidation • Chromic acid oxidation of aldehydes • Nitric acid oxidation of ketones • Tollens test • Reduction • Catalytic reduction • Metal hydride reduction • Reductive amination AN Reactions with Aldehydes and Ketones • When a nucleophile adds to a carbonyl group (the electrophile), the resulting tetrahedral carbonyl addition intermediate (TCAI) has several different possible mechanistic routes. • These mechanistic routes are usually determined by how many electronegative atoms are attached to the tetrahedral carbon. AN Reactions Organized by Nucleophile 1. Very strong nucleophiles in very basic medium: addition of hydrides (reduction) and carbanions. • • AN occurs first, then proton transfer (PT) occurs as a separate step. Grignard reactions, metal hydride reductions 2. Moderate nucleophiles/bases: addition of nitrogen and cyanide nucleophiles. • • AN and PT occur very close in time. Imine formation 3. Weak nucleophiles/bases: addition of water and alcohols. • • PT occurs first, then AN Hemiacetal and acetal formation Grignard Reagents • Addition of carbon nucleophiles (carbanions) is one of the most important types of nucleophilic additions to a C=O group. • A new carbon-carbon bond is formed in the process. • Carbanions can be formed using Grignard reagents. Victor Grignard was awarded the Nobel Prize for chemistry in 1912 for their discovery and application to organic synthesis. Grignard reagents have the general formula RMgX, where R is an alkyl or aryl group and X is a halogen. • Grignard reagents are prepared by adding an alkyl or aryl halide to a suspension of Mg metal in diethyl ether. • Given the difference in electronegativity between carbon and magnesium (2.5 – 1.3), the C-Mg bond is polar covalent, with C– and Mg+. • In its reactions, a Grignard reagent behaves as a carbanion. • Carbanion: An anion in which carbon has an unshared pair of electrons and bears a negative charge. • A carbanion is an excellent nucleophile and adds readily to the carbonyl group of aldehydes and ketones. • Reaction with protic acids • Grignard reagents are very strong bases and react with Brønsted-Lowry acids to form alkanes. • Any compound containing an O-H, N-H, or S-H group reacts with a Grignard reagent by proton transfer. • Initially, this proton transfer is undesirable. • One can’t use water, alcohols or amines as solvents. • Reaction of a Grignard reagent with a carbonyl group yields an alcohol. • Reaction with formaldehyde gives a 1° alcohol. • Reaction with any aldehyde other than formaldehyde gives a 2° alcohol. • Reaction with a ketone gives a 3° alcohol. • Reactions with Grignard reagents are done in diethyl ether or THF. • Once the addition to carbonyl group is done, the reaction is completed by adding water to do a final proton transfer (and remove the magnesium halide ion). Imines • Imine: A compound containing a C=N bond; also called a Schiff base. • Formed by the reaction of an aldehyde or ketone with ammonia or a 1° amine and catalyzed with acid. • Schiff bases are important as intermediates in the synthesis and metabolism of amino acids. Addition of Alcohols • Hemiacetal: A molecule containing an –OH group and an -OR group bonded to the same carbon. • Hemiacetals are minor components of an equilibrium mixture except where a 5- or 6-membered ring can form. • Acetal: A molecule containing two –OR groups bonded to the same carbon. • Cyclic hemiacetals are very important in understanding the structure of sugars like glucose and fructose. Mechanism of Acid-Catalyzed Hemiacetal Formation Step 1. Add a proton: Proton transfer from HA to the carbonyl oxygen. H Cl H O O H3C C H3C CH 3 C CH 3 H3C Step 2. Oxygen on alcohol does nucleophilic attack on carbonyl carbon. O H3C H C O H CH 3 H2 C H3C CH 3 H O H C CH 3 O H2 C CH 3 O H C CH 3 Step 3. Deprotonation of the oxonium ion yields the hemiacetal. H3C H O H C CH 3 O H2 C H3C CH 3 O H C CH 3 O H2 C CH 3 • Hemiacetal and acetal formation can be catalyzed with base (OH-) as well. • Note that this reaction with the weak nucleophile, ethanol, (PT) occurred first, then AN. Mechanism for Acid-catalyzed Conversion of Hemiacetal to Acetal Step 1. Add a proton: Proton transfer from HA to the hemiacetal oxygen. Step 2. Break a bond to give a stabilized ion. (Leaving group is water.) Step 3. Reaction of an electrophile with a nucleophile to form a new covalent bond. Step 4. Deprotonation: Proton transfer to A– gives the acetal and regenerates the acid catalyst. • The formation of acetals is an equilibrium reaction and is reversible. • To form an acetal, experimental conditions are used that remove water as it is formed (such as high heat), thus driving the equilibrium reaction toward acetal formation. • By manipulating the equilibrium (using a large excess of water), an acetal can be converted back to the original aldehyde or ketone. Acetals as a Carbonyl-Protecting Group • One way to synthesize the hydroxyaldehyde on the right is by a Grignard reaction. • But the aldehyde of the bromoaldehyde must be protected; often by converting it to a cyclic acetal. (or else the Grignard reagent will react with the other end of the molecule) • Now magnesium and ether are added to make the Grignard reagent. O O Br O A cyclic acetal BrMg + Mg ether O A Grignard reagent • And the Grignard reagent reacts with the benzaldehyde. O - H + BrMg Ph O MgBr O + O O Ph A magnesiu m alk oxid e O • Proton transfer completes the alcohol synthesis and excess water undoes the cyclic acetal. - + O MgBr Ph OH O O HCl, H2 O Ph O H + HO OH Keto-Enol Tautomerism • Enol: A molecule containing an -OH group bonded to a carbon of a carbon-carbon double bond • The keto form predominates at equilibrium for most simple aldehydes and ketones. • Interconversion of keto and enol forms is catalyzed by both acid and base. • Mechanism for acid catalysis Step 1. Add a proton to the carbonyl oxygen. Step 2 Take a proton away from the -carbon to A-. Racemization at an -Carbon • When an enantiomerically pure aldehyde or ketone with at least one -hydrogen is treated with a trace of acid or base, it gradually becomes a racemic mixture; it loses all optical activity. • Racemization occurs because of the formation of the achiral enol that is intermediate between the two enantiomers. Oxidation • Aldehydes are one of the most easily oxidized of all functional groups. • Ketones are not normally oxidized by H2CrO4. • Instead, chromic acid is used to oxidize 2° alcohols to ketones. • They are oxidized by HNO3 at higher temperatures. • Oxidation is via the enol and a multi-step mechanism. • Adipic acid is one of the starting materials for the synthesis of nylon 66. Silver Mirror Test for Aldehydes • Tollens’ reagent: Prepared by adding NaOH to AgNO3 (aq) to precipitate Ag2O, then adding ammonia to form the silver-ammonia complex ion. • Tollens’ reagent is specific for the oxidation of aldehydes. If silver deposits on the walls of the container as a silver mirror when Tollens’ reagent is mixed with an unknown substance, the substance must be an aldehyde. Reduction • Aldehydes are reduced to 1° alcohols. • Ketones are reduced to 2° alcohols. • Reductions are done in two ways. • Catalytic Hydrogenation • Reaction with Metal Hydrides Catalytic Reduction • Catalytic reductions are generally carried out at from 25°C to 100°C and 1 atm to 5 atm of H2. • A carbon-carbon double bond may also be reduced under these conditions. Metal Hydride Reductions • Reductions that are more specific than catalytic reduction involve metal hydrides. • The most common metal hydrides that are used for the reduction of aldehydes and ketones are NaBH4 and LiAlH4. • Both reagents are sources of hydride ion, H:–, a very strong nucleophile. • Reductions with NaBH4 are most commonly carried out in aqueous methanol, in pure methanol, or in ethanol. • One mole of NaBH4 reduces four moles of aldehyde or ketone. • The key step in metal hydride reductions is transfer of a hydride ion (a nucleophile) to the C=O group (an electrophile) to form the new covalent bond of a tetrahedral carbonyl addition compound. • Metal hydride reducing agents do not normally reduce carbon-carbon double bonds, and selective reduction of either C=O or C=C is often possible. • If reduction of the carbon-carbon double bonds is desired instead, the carbonyl group can be “protected” by forming a cyclic acetal. Reductive Amination • Reductive amination: The formation of an imine followed by its reduction to an amine. • Reductive amination is a valuable method for the conversion of ammonia to a 1° amine, and conversion of a 1° amine to a 2° amine.