* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Aldehydes and Ketones
Metal carbonyl wikipedia , lookup
Bottromycin wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Elias James Corey wikipedia , lookup
Kinetic resolution wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Discodermolide wikipedia , lookup
1,3-Dipolar cycloaddition wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Aldol reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
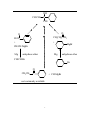
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
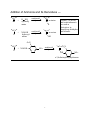
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Aldehydes and Ketones Preparation of Aldehydes — Oxidation of primary alcohols – RCH 2OH C 5H5 NH CrO 3Cl O RCH C 5H5 NH CrO 3 Cl is pyridinium chlorochromate, PCC The aldehyde that is the product is very easily oxidized to a carboxylic acid, RCOOH. Preparation of Ketones — Oxidation of secondary alcohols – H R1 C K 2C r 2O 7 R1 OH C O R2 R2 Unlike aldehydes, ketones are not easily oxidized. 1 Hydration of an alkyne – R1 C C R2 H2SO4, HgSO 4 H2O H R1 C H C R2 + R1 H O C C O H R2 Owing to the formation of mixtures if R1 ≠ R2, this reaction is most useful when R1 = R2 ... ...or when the alkyne has a terminal triple bond. H C C R H2SO 4, HgSO 4 H H C H2O C R H O An enol initially forms in this reaction, but it tautomerizes to the more stable ketone. Terminal alkynes, following Markovnikov’s rule, give methyl ketones rather than aldehydes. 2 Friedel-Crafts acylation for aryl ketones – O H + R C AlC l3 Cl O C R The aromatic ring cannot have, as a substituent, an amino group or a meta director. Structural Features of Aldehydes and Ketones Both contain the carbonyl δ+ δ− group and only carbons or hydrogens bonded to this sp 2 orbitals C O π group. In aldehydes at least one hydrogen is joined to the carbonyl carbon (formaldehyde has two). In ketones, only carbons are bonded to the carbonyl carbon. Since the carbon has a partial positive charge it is likely to be a site that is attacked by nucleophiles. And, since the oxygen bears a partial negative charge, it is likely to be a site of electrophilic attack. Since ordinary carbanions (R: −) and hydride ions (H: −) are very poor leaving groups (unlike halide ions, X−) nucleophilic substitution does not usually occur at the carbonyl carbon of aldehydes or ketones. 3 R1 Nu:- C O R2 Nu ∅ C O + R1: R2 4 Reactions of Aldehydes and Ketones — Oxidation — Aldehydes are easily oxidized to carboxylic acids, ketones are not. Aldehydes R C (Ar) O O 2 or CrO 3 or K 2Cr2O 7 H or KMnO 4, etc. Tollen's test for aldehydes: R C (Ar) O + Ag(NH3)2 H + R C (Ar) O OH - OH RCO - + Ago (Ar) O Fehling's test, Benedict's test: R C (not Ar) O + 2 CuO complexed with citrate or tartarate, in solution H RCOH O Ketones RCOH + R'CH2COH hot KMnO 4 O O or hot HNO 3 Vigorous conditions RCH2COH + R'COH required for reaction. O O RCH2 C CH2R' O + Cu2O red precipitate 5 Nucleophilic Additions — :Nu or :Nu - is a generic nucleophile. R, R' = alkyl, aryl, H :NuR R C O R' δ+ δ− Nu δ− C O δ− R' becoming tetrahedral: sp2 sp3 R R' Nu H+ C R R' O Nu C OH Since there is an increase in crowding on going from reactant to transition state (~120o to ~109o), some steric effects might be expected. This is one reason aldehydes (less crowded) are more reactive than ketones. 6 Nucleophilic additions may be acid catalyzed — R R C O + + H R' R C O H C O H R' R' More easily attacked by nucleophile than unprotonated carbonyl. However, when acid catalysis is employed one should usually be careful to avoid completely converting the nucleophile to its conjugate acid (which would be much less nucleophilic). Nucleophilic Addition of Water: Hydration — C O + H2O acid or base catalyst C OH OH The product here is known as a geminal diol. In most cases the equilibrium greatly favors the carbonyl compound. Formaldehyde and chloral (trichloroacetaldehyde) are two common exceptions. 7 The mechanism for this reaction under basic conditions is as follows – HO HO C O C O H O H HO C O H + O H In this case – basic catalysis – a powerful nucleophile attacks the substrate. In acidic catalysis, as we shall see below, the nucleophile will be much weaker – water. But the substrate has been activated by protonation and is more susceptible to attack. 8 Under acidic conditions the following mechanism applies – H O H H O H H C O C O H C O H H O H H O C O H O C O H H H O H H H O H + H 9 Acetal Formation — Under acidic conditions an aldehyde or ketone will react with an alcohol to form a hemiacetal. The hemiacetal, in turn, will react with more alcohol to form an acetal. C O H ROH RO C OH hemiacetal 10 H ROH RO C OR + H2O acetal The mechanism is as follows – R O H R O H H C O C O H C O H R O H R O C O H O C O H H R O H R H O R + H hemiacetal OK. Now we have to get from the hemiacetal to the acetal. 11 R O R O C O H H H R O H H R O C O H R O H C O H H O R R H O C O H O R R R O C O R acetal H H O R Acetals are used to “protect” the carbonyl groups of aldehydes and ketones when one wants to have some other part of the molecule react without affecting the aldehyde or ketone functional group. They can be used this way because they are fairly unreactive and the carbonyl functional group can be regenerated from the acetal. For example if you wanted to convert a ketoacid to a ketoalcohol you could do the following: (1) convert the keto group to an acetal, (2) reduce the acid with LiAlH4, and (3) regenerate the keto group from the acetal. 12 Addition of Grignard Reagents — A powerful method for synthesis of alcohols. In the Grignard Synthesis smaller molecules —> larger molecules. Formation of Grignard reagent — R-X or Ar-X + Mg anhydrous C2H5OC2H5 or X = I, Br, Cl O 13 R-Mg-X or Ar-Mg-X Grignards react with aldehydes and ketones to give alcohols — H H RMgX or ArMgX + C O H formaldehyde H2O H3O + C O H aldehyde C OH 1o alcohol R H C OH 2o alcohol R R' R' + H R' R' + H2O H3O + H2O + H3O C O R'' ketone R'' C OH 3o alcohol R A pseudo-mechanism for this reaction — δ− δ+ R Mg X R C O δ+ δ− H2O Mg X C O R C O Mg X magnesium salt of an alcohol R C O H + Mg(OH)X 14 Synthesize 2-phenyl-2-butanol using a Grignard synthesis — The figure below shows three possible routes by which this synthesis can be accomplished. In practice, the route chosen would likely depend on the starting materials that may be at hand in the laboratory (all of these compounds could be purchased). In the scheme below, CH3MgBr could be made from CH3Br and Mg, but methyl bromide is not convenient to handle. It boils at 4oC, so it is a gas at room temperature. [Large quantities of methyl bromide are used as a soil and grain fumigant. Its use is quite controversial since it is somewhat toxic and an ozone depleting chemical. See, for example: http://www.epa.gov/docs/ozone/mbr/mbrqa.html] 15 OH CH3CH2 C CH3 O O H3CC CH3CH2CCH3 + + MgBr CH3CH2MgBr Mg anhydrous ether Mg anhydrous ether CH3CH2Br Br O CH3CH2 C + CH3MgBr not commonly available 16 It is possible to extend the Grignard synthesis to make quite complex alcohols from simple ones (you don’t win the Nobel prize for nothing). The basic scheme is as follows – alcohol 1 alkyl halide Grignard reagent aldehyde or ketone alcohol 2 more complicated alcohol which may become alcohol 1 or 2 in a subsequent Grignard synthesis, etc. 17 Formation of Cyanohydrins — R + C O + K -C N H3O R + R' R' C CN OH These compounds can be hydrolyzed by base or acid to give α-hydroxyacids or α,β-unsaturated acids, respectively. H CH3CH2CH2 C CN OH H3O+ H2O heat H CH3CH2CH C COOH H2O, KOH heat H CH3CH2CH2 H C COO- K+ HCl H2O OH CH3CH2CH2 C COOH OH 18 + KCl Addition of Ammonia and Its Derivatives — C O C O + + R NH2 a primary amine C weak acid catalyst N an imine Oximes, 2,4-DNPs, and semicarbazones are used as derivatives in identifying aldehydes and ketones. R C weak acid NH2OH catalyst hydroxylamine N an oxime OH O2 N C O + NH2NH NO 2 weak acid catalyst C O 2N N NH NO 2 a 2,4-dinitrophenylhydrazone 19