* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Lecture_14.pps
Magnesium transporter wikipedia , lookup
Interactome wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Polyadenylation wikipedia , lookup
Secreted frizzled-related protein 1 wikipedia , lookup
Gene expression wikipedia , lookup
Biochemical cascade wikipedia , lookup
Biochemistry wikipedia , lookup
Paracrine signalling wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Protein purification wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Signal transduction wikipedia , lookup
Western blot wikipedia , lookup
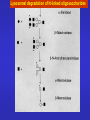
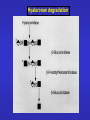
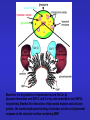
ESSENTIALS OF GLYCOBIOLOGY LECTURE 14 DEGRADATION AND TURNOVER OF GLYCOCONJUGATES Hud Freeze USUAL TURNOVER • Most glycans are extracellular or on cell surface • Membrane recycling • Receptor and non-receptor mediated endocytosis To Endosome Lysosome Lysosomal exoglycosidases degrade glycans at low pH Specific lysosomal transporters carry neutral hexose, acetylatedaminohexose (GlcNAc, GalNAc), and Anionic sugars (GlcA, Sia) to the “cytosol”. SUGAR CHAIN DEGRADATION ENZYMES Most Are: Lysosome/Endosome Low pH optimum, Sugar/anomeric specificity Exo-glycosidases Targeted to lysosome through P-lectins and Man-6-P But Some Are: Non-lysosomal Active near neutral pH Endoglycosidases Targeted as membrane bound molecules Not in the lysosome Special Features for Degradation of Different Glycoconjugates TYPE FEATURE Glycoproteins N-linked O-linked ER/Golgi/Cytoplasm/ Lysosome Unexpected Products Proteoglycans Endoglycosidases Unique ORDER Non-glycohydrolase enzymes Glycosphingolipids Assisting proteins Special Problems for N-Linked Sugar Chains • N-glycosylation occurs in ER-Topology for lysosomal degradation is wrong • ~50% of ER proteins misfold and are degraded what happens to the sugar chain? To glycopeptides? • Protein synthetic rate and glycosylation rate must be coordinated • Competition for lectin-based chaperones lots of Man( ) Released What happens to the released mannose? OLIGOSACCHARIDE HOUSE-KEEPING CENTRAL MANNOSE METABOLISM IN CELLS AND MORE Mannose in plasma comes from Oligosaccharide turnover in cells Cells also produce mannose From glucose Glc Glc-6-P Fru-6-P Man-6-P Golgi cytosol Lysosome Glycans ER Lysosomal degradation of N-linked oligosaccharides Lysosomal degradation of N-linked oligosaccharides Enzymatic defects are usually found by accumulation of Partially degraded oligosaccharides in urine O-LINKED OLIGOSACCHARIDE DEGRADATION Same enzymes used for N-linked oligosaccharide degradation a-GalNAc’ase deficiency--produce GalNAc terminated Oligosaccharides? a Ser/Thr Excretion of GalNAc-a-Ser/Thr? No!! Why Not? The oligosaccharides are larger size! How to explain this? a Ser/Thr Partially degraded polysaccharides accumulate in tissues and urine. Structural analysis of glycans used to work out pathway Hyaluronan degradation HEPARAN SULFATE DEGRADATION HEPARAN SULFATE DEGRADATION CHONDROITIN SULFATE DEGRADATION GSL Degradation Needs Assistants LIFE CYCLE OF GM2 ACTIVATOR PROTEIN Model for the degradation of membrane-bound GlcCer by glucocerebrosidase and SAP-C and Cer by acid ceramidase and SAP-D, respectively. Besides the interaction of lysosomal enzyme and activator protein, the model emphasizes binding of activator protein and lysosomal enzymes to the vesicular surface containing BMP. REMEMBER THAT •Different types of glycans have unique degradation pathways •Mutations in different degradative enzymes lead to rare diseases •Limiting glycan synthesis in genetic disorders reduces pathology •Tissue specific differences in salvage/de novo biosynthesis may be important for health and lead to pathology