* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download ELECTROPHORESIS
Protein (nutrient) wikipedia , lookup
Magnesium transporter wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Gene expression wikipedia , lookup
Molecular evolution wikipedia , lookup
Immunoprecipitation wikipedia , lookup
Interactome wikipedia , lookup
Protein moonlighting wikipedia , lookup
History of molecular evolution wikipedia , lookup
List of types of proteins wikipedia , lookup
Biochemistry wikipedia , lookup
Circular dichroism wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Protein adsorption wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Proteolysis wikipedia , lookup
Capillary electrophoresis wikipedia , lookup
Protein mass spectrometry wikipedia , lookup
Community fingerprinting wikipedia , lookup
Gel electrophoresis of nucleic acids wikipedia , lookup
Western blot wikipedia , lookup
Agarose gel electrophoresis wikipedia , lookup
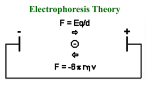
INTRODUCTION Electrophoresis is a migration of a charged particle in a medium under the influence of an applied electric field. The usual purposes of carrying out electrophoretic experiments are : 1. To determine the number, amount and mobility of components in a given sample or to separate them. 2. To obtain information about the electrical double layers surrounding the particles. TYPES OF ELECTROPHORESIS 1.PAPER ELECTROPHORESIS 1. In this the sample is applied as a circular support on a strip of whatman filter paper moistened with the buffer solution. 2. The ends of the paper are immersed in separate reservoirs containing buffer and in which the electrodes are fitted. 3. Upon passing electric current, the ions in the sample migrate towards oppositely charged electrodes. This method is suitable for separation of low molecular weight compounds such as amino acids and nucleotides. APPLICATIONS 1.This is single most critical procedure in electrophoresis process. 2.Special devices are available for this purpose. 3.There is no hard and fast rule for the time of application of the sample to the electrophoretogram.this can be applied before the paper has been equilibrated with the buffer or after it. 2. STARCH GEL ELECTROPHORESIS 1. Starch gels are prepared by heating and then cooling a solution of partially hydrolyzed starch in an appropriate buffer. 2. The Separation is accomplished due to differences in charge as well as the size of molecules because of molecular sieving effect of the gel. AGAROSE GEL ELECTROPHORESIS 1. Agarose gels are more porous and have a large pore size and therefore used to fractionate large macromolecules such as nucleic acids. 2. Agarose gels are cast by boiling agarose in presence of a buffer then poured into a mold and allowed to harden to form a matrix. 3. Higher the agarose larger is the pore size. 4. When the electric feild is applied across the gel, negatively charged molecules move towards oppositely charged electrodes on the basis of their molecular size. 5. Smaller DNA molecules will travel faster as compared to larger ones. Commonly used buffers are tris acetate, tris borate or tris phosphate at a concentration of 50mM over a pH range of 7.5-8.0. 3. POLYACRYLAMIDE GEL ELECTROPHORESIS 1. The components used in the formation of this gel are known to be neurotoxins. 2. The most commonly used components to synthesize the matrix are acrylamide monomer, N, N’-methylenebisacrylamide, ammonium persulphate and tetramethylenediamine(TEMED). 3. These free radicals can activate acryl amide monomer including them to react with other acrylamide monomer forming long chains. 4.This chains become cross-linked if the reaction is carried out in the presence of N-N’-methylenebisacrylamide. 5.TEMED acts as a catalyst of gel formation because of its ability to exist in free radical 0form. 6.The whole mixture then irradiated with UV light in the presence of oxygen. Buffer System 1. Gel electrophoresis can be carried out in buffers ranging from pH 3-10. 2. Ionic strength of the buffer system is generally kept low(0.01-0.1M) to reduce heat production depending upon the type of buffer systems used, polyacrylamide gel electrophoresis may be of the following kinds: (1) Native continuous polyacrylamide gel electrophoresis (2) Native discontinous polyacrylamide gel electrophoresis or native disc gel electrophoresis (3) SDS- polyacrylamide gel electrophoresis (reducing) (4) SDS- polyacrylamide gel electrophoresis (nonreducing) (1) Native continuous polyacrylamide gel electrophoresis In this same buffer ions are present throughout the sample, gel and electrode vessel reservoirs at constant pH such that the proteins have native conformation and biological activity and hence it is known as native continuous PAGE. In this system, protein sample is loaded directly onto the gel, referred to as resolving gel, in which separation the sample components occurs. (2) Disc gel electrophoresis Disc gel electrophoresis is a modification of a conventional zone electrophoresis which allows the sample to enter the gel as a sharp band, thereby helping further resolution. The two different porosity gels are used 1. Stacking gel ( high porosity) 2. Separating or running gel (low porosity). (3)SDS-PAGE(Non-reducing) 1. In this system proteins are dissociated into their sub units using ionic detergent such as sodium lauryl sulfate. 2. The protein mixture is denatured by heating the sample at 100⁰C in the presence of excess SDS. 3. Under these conditions most of polypeptides bind SDS in a constant weight ratio (1.4g SDS per g polypeptide) . 4. The intrinsic charge of polypeptide are insignificant as compared to negative charges provided by the bound detergent and these migrate in polyacrylamide gels according to their size or molecular mass. (4) SDS-PAGE(Reducing) 1.It is similar to SDS-PAGE described above except that in addition to SDS. 2. In this method protein sample is boiled in presence of excess SDS and βmercaptoethanol to denature proteins their individual polypeptide. 3. SDS polypeptide have net negative charge and migrate towards anode on the basis of their size or molecular weight. SDS PAGE APPLICATIONS OF GEL ELECTROPHORESIS This technique is used in molecular biology 1.Determination of DNA sequences 2.Southern and northern blotting 3.Restriction mapping of DNA APPLICATIONS IN PROTEIN STUDY 1.Determination of molecular weight of proteins by electrophrosis. 2.Determination of subunit stoichiometry. OTHER TYPES OF ELECTROPHORESIS 1. ISOELECTRIC FOCUSING 1. It is an electrophoretic method for separation of proteins which differ in their isoelectric points. 2. The proteins are made to migrate in pH gradient maintained by mixture of low molecular weight ampholytes. 3.Proteins migrate depending upon their charge till they reach a region where the pH corresponds to respective isoelectric point at which pH protein posseses no net charge and hence get focused. APPLICATIONS OF ISOELECTROFOCUSING 1.Elecrofocusing has been used for separation and identification of serum proteins 2. It is used by food and agricutural industries. 3. It is used by forensic and human genetics laboratories. 4. It is used for research in enzymology, immunology, and membrane biochemistry etc. 2. IMMUNOELECTROPHORESIS 1. It is a agar/agarose gel electrophoresis which exploits the separation of proteins on the basis of their charge to mass ratio and their antigencity . 2. Protein mixtures are first separated by electrophoresis on agarose gel and then allowed to interact with specific antibody preparation. 3. Antibody diffuses through the gel and forms visible precipitate with electrophoresed proteins for which the antibody has affinity. 4. The technique is used in the determination of antigen concentration. As a antibody moves into gel, it encounters antibody and precipitates when antigen-antibody concentration and equivalent resulting in the gel. APPLICATIONS 1.It is used to diagnose multiple myeloma, a disease affecting the bone marrow. 2. It is used to identify certain proteiin molecules produced by the immune system. 3. It is used to measure the Ig present. 3.TWO DIMENSIONAL GEL ELECTROPHORESIS 1. This powerful technique combines the resolving power of isoelectric focusing with SDS gel electrophoresis. 2. The mixture is first subjected to isoelectric focusing in capillary tubes and are separated according to their charge. 3.Then sample is subjected to SDS gel electrophoresis which separates the proteins according to their molecular weights. 4. CAPILLARY ELECTROPHORESIS 1. The technique that has very high resolving power and speed. 2. CE involve electrophoresis of sample in small narrow capillary tubes and high voltage from 10-15 KV is applied. This technique is used to separate biological material including amino acids, proteins, nucleic acids as well as drugs or even metals. Very small amount of sample are required for analysis. APPLICATIONS OF CAPILLARY ELECTROPHORESIS 1. It is used in the isolation of the correct optical isomer. 2. CE plays an importan role in ensuring a constant product quality of therapeutic glycoproteins. 3. It is used in forensic area to detect performance enhancing drugs. PROTEIN (WESTERN) BLOTTING The transfer of proteins from an acrylamide gel onto a more stable and immobilzing support such as nitrocellulose filter is called as protein blotting or western blotting. Blotting of proteins bands allow them to become more accessible for detection and identification using a variety of methods such as glyco proteins etc and also filters can be stored for much longer period. The transfer of proteins from gels can be acheived by any of the methods such as capillary action, application of vaccum or electrophoretically.