* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 33 Carbohydrates1
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Gene regulatory network wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Microbial metabolism wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Isotopic labeling wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Phosphorylation wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Photosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biosynthesis wikipedia , lookup
Paracrine signalling wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
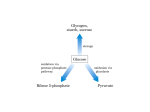
Lecture 33 Carbohydrate Metabolism 1 Key Concepts • Pentose Phosphate Pathway – – – – Enzymatic reactions in the oxidative phase Enzymatic reactions in the nonoxidative phase Glucose-6P dehydrogenase deficiency in humans NADPH vs. NAD+ in Metabolism • Gluconeogenesis and the Cori Cycle – Review of gluconeogenesis – The Cori Cycle provides glucose to muscle cells during exercise • What is the biochemical basis for favism and how is it related to malarial resistance? • How does fructose-2,6-bisphosphate control flux through gluconeogenesis and glycolysis? Three primary pathways in anabolic carbohydrate metabolism in nonphotosynthetic organisms: 1.pentose phosphate pathway 2.gluconeogenesis 3.glycogen metabolism Metabolism of ribose sugars in the pentose phosphate pathway is used to generate NADPH and to provide the carbohydrate component of nucleotides The major sources of carbon in gluconeogenesis are amino acids and glycerol in Overview of the Pentose Phosphate Pathway • Takes place entirely within the cytoplasm • Also known as: – the hexose monophosphate shunt – phosphogluconate pathway • The most important function: – to reduce two molecules of NADP+ to NADPH for each glucose-6phosphate (glucose-6P) that is oxidatively decarboxylated to ribulose-5-phosphate (ribulose-5P) – also responsible for producing ribose-5-phosphate (ribose-5P) from glucose-6P Overview of roles of NAD+ vs. NADP+ Two important conjugate redox pairs: NAD+/NADH vs. NADP+/NADPH NAD+ functions as the primary oxidant in the cell (accepts electrons) NADPH is the primary reductant in the cell (donates electrons) [NAD+]/[NADH] ratio is close to 1,000 [NADP+]/[NADPH] ratio is 0.01 Two Phases of Pentose Phosphate Pathway oxidative phase – generates NADPH nonoxidative phase – – interconverts C3, C4, C5, C6 and C7 sugar phosphates uses many of the same "carbon shuffle" reactions we saw in the Calvin cycle. Flux through Pentose Phosphate Pathway is Tightly Regulated Regulated in response to: – – – the NADP+/NADPH ratio energy needs of the cell requirements for nucleotide and coenzyme biosynthesis 1. When NADPH is needed, ribulose-5P is converted back into glucose6P to maintain flux through the pathway. 2. If ATP and NADPH are needed (which would be the case for most anabolic pathways), then some of the ribulose-5P is used to synthesis hexose phosphates for glycolysis. 3. If the cell needs to increase the rate of nucleotide and coenzyme biosynthesis, then most of the ribulose-5P is shunted toward ribose-5P synthesis. Pathway Questions 1. What does the pentose phosphate pathway accomplish for the cell? – The oxidative phase generates NADPH which is required for many biosynthetic pathways and for detoxification of reactive oxygen species. – The nonoxidative phase interconverts C3, C4, C5, C6 and C7 monosaccharides to produce ribose-5P for nucleotide synthesis, and also to regenerate glucose-6P to maintain NADPH production by the oxidative phase. Pathway Questions 2. What is the overall net reaction of the pentose phosphate pathway when it is utilized to generate the maximum amount of NADPH? 6 Glucose-6P + 12 NADP+ + 12 H2O → 5 Glucose-6P + 12 NADPH + 12 H+ + 6 CO2 Pathway Questions 3. What are the key enzymes in the pentose phosphate pathway? Glucose-6P dehydrogenase (G6PD)– enzyme catalyzing the first reaction in the pathway which converts glucose-6P to 6phosphogluconolactone. This reaction is the commitment step in the pathway and is feedback-inhibited by NADPH. Defects in glucose6P dehydrogenase cause a dietary condition called favism. Transketolase and Transaldolase - together these two enzyme catalyze the reversible "carbon shuffle" reactions of the nonoxidative phase of the pathway. These are the same enzymes used in the Calvin Cycle to regenerate ribulose-5P from glyceraldehyde-3P. Pathway Questions 4. What are examples of the pentose phosphate pathway in real life? Glucose-6P dehydrogenase deficiency is the most common enzyme deficiency in the world and affects over 400 million people. A 90% decrease in enzyme activity results in the inability of red blood cells to produce enough NADPH to protect the cells from reactive oxygen species that are generated by anti-malarial drugs and by compounds in fava beans. http://www.g6pd.org/favism/english/index.mv Dish of Broad Beans by Giovanni Garzoni Enzymatic reactions in the oxidative phase Three enzymatic reactions: 1. Oxidation of glucose-6P by the enzyme glucose-6P dehydrogenase (G6PD) to 6-phosphogluconolactone is coupled to the reduction of NADP+ resulting in the formation of one molecule of NADPH. The commitment step in the pathway. 2. 6-phosphogluconolactone is hydrolyzed by lactonase to produce the open chain monosaccharide 6phosphogluconate 3. 6-phosphogluconate is then oxidized and 1 2 3 decarboxylated by 6-phosphogluconate dehydrogenase to generate ribulose-5P, CO2 and the second molecule of NADPH. Enzymatic reactions in the nonoxidative phase In cells that require high levels of NADPH for biosynthetic reactions, the ribulose-5P produced in the oxidative phase needs to be converted back into glucose-6P to maintain flux through the glucose-6P dehydrogenase reaction. The carbon shuffle reactions of the nonoxidative phase are used to regenerate glucose-6P using the same transketolase and transaldolase enzyme reactions that we saw in the Calvin cycle. Let’s Play: Count the Carbons! 1 1 2 2 3 3 4 5 6 4 5 Six C5 molecules (ribose-5P and xylulose-5P) are used to resynthesize five C6 molecules (glucose-6P) in the nonoxidative phase. Glucose-6P is a substrate for both the glycolytic pathway and the pentose phosphate pathway What controls the flux??? When the rates of NADPHdependent biosynthetic reactions are high in the cytosol, then the [NADP+]/[NADPH] ratio increases, leading to allosteric activation of glucose-6P dehydrogenase activity by NADP+ which increases flux through the pentose phosphate pathway. Increased levels of NADPH compete with NADP+ for binding to glucose-6P dehydrogenase, thereby reducing the activity of the enzyme. This results in decreased flux through the pentose phosphate pathway and the available glucose-6P is then metabolized by the glycolytic pathway as a source of energy for the production of ATP. Glucose-6P dehydrogenase deficiency in humans The pentose phosphate pathway is responsible for maintaining high levels of NADPH in red blood cells (erythrocytes) for use as a reductant in the glutathione reductase reaction. Glutathione is a tripeptide that has a free sulfhydryl group which functions as an electron donor in a variety of coupled redox reactions in the cell. Glutathione reductase uses two electrons from NADPH to maintain glutathione in the reduced state (GSSG → 2 GSH). Glucose-6P dehydrogenase deficiency in humans In erythrocytes, electrons from glutathione are used to keep cysteine residues in hemoglobin in the reduced state, and for reducing harmful reactive oxygen species and hydroxyl free radicals. When erythrocytes are exposed to chemicals that generate high levels of superoxide radicals, GSH is required to reduce these damaging compounds. An active pentose phosphate pathway in erythrocytes normally provides sufficient levels of NADPH to maintain the GSH:GSSG ratio at about 500:1. Discovery of G6PD deficiency – Mid 1950s Result of observations made 30 years earlier – the anti-malarial drug primaquine induced acute hemolytic anemia (red blood cell lysis) in a small percentage of people who had been given primaquine prophylatically. The biochemical basis for this druginduced illness was found to be lower than normal levels of NADPH in erythrocytes due to a G6PD deficiency. The same acute hemolytic anemia seen in individuals with G6PD who are treated with primaquine explains the symptoms of favism. One of the active compounds in fava beans is called vicine, a toxic glycoside that induces oxidative stress in erythrocytes. Overview of Gluconeogenesis and the Cori Cycle • When dietary sources of glucose are insufficient, and glucose stores have been depleted, glucose is synthesized from non-carbohydrate compounds by a series of cytosolic reactions called the gluconeogenic pathway. • We have seen this pathway before. • Gluconeogenesis converts pyruvate to glucose using a set of reactions that require energy input in the form of ATP and GTP. • Importantly, gluconeogenesis is not simply the reversal of glycolysis. • The Cori Cycle provides glucose to muscle cells during exercise Common to Both Pathway Questions 1. What does gluconeogenesis accomplish for the organism? – The liver and kidney generate glucose from noncarbohydrate sources (lactate, amino acids, glycerol) for export to other tissues that depend on glucose for energy, primarily the brain and erythrocytes. – Plants use the gluconeogenic pathway to convert GAP, the product of the Calvin Cycle, into glucose which is used to make sucrose and starch. Pathway Questions 2. What is the overall net reaction of gluconeogenesis? 2 pyruvate + 2NADH + 4ATP + 2GTP + 6H2O → Glucose + 2NAD+ + 2H+ + 4ADP + 2GDP + 6Pi Pathway Questions 3. What are the key enzymes in gluconeogenesis? Pyruvate carboxylase – is a mitochondrial enzyme that catalyzes a carboxylation reaction converting pyruvate to oxaloacetate using a reaction mechanism involving a biotinyl "swinging arm" and ATP hydrolysis. Pyruvate carboxylase is dependent on allosteric activation by acetyl CoA. Phosphoenolpyruvate carboxykinase (PEPCK) – is localized to either the mitochondrial matrix or the cytosol (or both in the case of human liver cells) and catalyzes a phosphoryl transfer reaction that converts oxaloacetate to phosphoenolpyruvate (PEP) using the energy released by decarboxylation and GTP hydrolysis. Transcription of the PEPCK gene is regulated by hormones. Fructose-1,6-bisphosphatase-1 (FBPase-1) - catalyzes the dephosphorylation of fructose-1,6BP to form fructose-6P; this is the bypass reaction for PFK-1 in glycolysis. FBPase-1 is inhibited by the allosteric regulators F2,6BP and AMP which are also allosteric activators of PFK-1. Glucose-6-phosphatase - is an enzyme in liver and kidney cells (not present in muscle cells) that catalyzes the dephosphorylation of glucose-6P to form glucose which can be exported out of the cell. Glucose-6-phosphatase is located in the lumen of the endoplasmic reticulum. Review Control of Glycolysis vs. Gluconeogenesis You should understand: Reciprocal control of glycolysis and gluconeogenesis Regulation of PFK-1 and FBPase-1 Pyruvate carboxylase and phosphoenolypyruvate carboxykinase (PEPCK), are required to catalyze the bypass reaction that converts pyruvate to PEP. Pyruvate carboxylase is activated by acetyl CoA and has an important role in feeding OAA to the citrate cycle when acetyl CoA levels are high and the energy charge in the cell is low. Humans have two distinct PEPCK genes that encode mitochondrial and cytosolic PEPCK enzymes Moving NADH equivalents to cytosol Oxidation of lactate by lactate dehydrogenase Regulation of PFK-2/FBPase-2 The Cori Cycle Both pathways (glycolysis and gluconeogenesis) are active at the same time, but in different cells. Can be quite advantageous. The Cori cycle provides a mechanism to convert lactate produced by anaerobic glycolysis in muscle cells to glucose using the gluconeogenic pathway in liver cells. The Cori Cycle costs four high energy phosphate bonds, however, the benefit to the organism is that glycogen stores in the muscle can be quickly replenished following prolonged exercise. The reason athletes should "warm down" after exercise (same movement but under aerobic conditions) is to enhance circulation so that lactate will be cleared from the muscle and be used in the liver for glucose synthesis via the Cori Cycle. For more info (relatively accurate): http://www.cytosport.com/science/lacticacid.html Just don’t buy the guy’s juice!