* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Tricarboxylic Acid Cycle The First of the Final Common Pathways
Mitochondrion wikipedia , lookup
Butyric acid wikipedia , lookup
Biochemical cascade wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Electron transport chain wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Amino acid synthesis wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
The Tricarboxylic Acid Cycle
The First of the Final Common Pathways
Objectives:
I.
Describe that types of reactions available to the cell for breaking carbon-carbon bonds.
II. Describe the entry of pyruvate into the Citric Acid Cycle.
A. Its conversion into an acetate ion (acetate fragment) coupled to Coenzyme A.
1. Conversion of pyruvate into acetyl-CoA.
III. Describe the pyruvate dehydrogenase complex.
A. Subunit structure
B. Necessary cosubstrates and coenzymes.
1. Vitamins that these cosubstrates and coenzymes are derived from.
C. Reaction Mechanism
IV. What is the “fuel” of the TCA Cycle?
V. What are the products of one turn of the cycle?
VI. Summarize the reactions of the citric acid cycle.
A. How is the α-ketoglutarate dehydrogenase step similar to the reaction catalyzed by the
pyruvate dehydrogenase complex?
VII. State the reactants and products of the “first step” of the Citric Acid Cycle and the product(s) of
the “last step” of the Citric Acid Cycle.
VIII. Describe how the Tricarboxylic Acid Cycle provides precursors for energy generation under
aerobic conditions.
A. Generation of NADH and FADH2
IX. Given a step or the steps of the TCA cycle, indicate the type of reaction that has occurred and
possible enzyme involved.
X. Discuss the control points of the Tricarboxylic Acid Cycle
A. Describe how TCA Cycle intermediate concentrations control the rate of the TCA Cycle.
1. Reaction(s) that increase the intermediate concentration.
a) Describe the three most common ANAPLEUROTIC REACTIONS.
b) Allosteric control of the Pyruvate Carboxylase reaction.
2. Intermediates that are removed from the cycle to decrease the rate of the pathway.
a) Products formed from the intermediates removed.
3. Use of TCA Cycle intermediates as precursors for anabolic reactions.
B. Describe how acetate (acetyl-CoA) availability controls the rate of the TCA Cycle.
1. Describe how the rate of the pyruvate dehydrogenase complex is controlled.
2. Allosteric effectors and their effects.
3. Control by reversible covalent modification.
a) Allosteric effectors and their effects on Pyruvate Dehydrogenase Kinase and Pyruvate
Dehydrogenase Phosphatase.
C. Describe the allosteric enzymes within the Citric Acid Cycle.
1. Describe the allosteric modulators and their effects on the allosteric enzymes that control
The Citric Acid Cycle.
D. Discuss why The TCA Cycle is classified as an AMPHIBOLIC PATHWAY.
XI. Ask yourself “What If Questions”
1
©Kevin R. Siebenlist, 2016
Background
The two Final Common Pathways are both oxidative pathways. Although cells are marvelous machines
they are forced to function with a limited bag of tricks. Biological oxidations that require the cleavage of
carbon-carbon bonds are accomplished in the cell by one of two strategies. The first type of carbon bond
cleavage breaks the bond α to a carboxylic acid group provided that the α carbon is carrying a hydroxyl
group or carbonyl (ketone) group. This is an α-cleavage reaction. The second reaction type requires a
molecule with a carbonyl (ketone) or carboxylic acid group and it involves bond cleavage between the alpha
(α) and beta (β) carbons. The first step of the reaction catalyzed by the Pyruvate Dehydrogenase Complex,
the decarboxylation of pyruvate, is an oxidation reaction with an α-cleavage type. Two reactions during the
TCA cycle are oxidations that result in carbon-carbon bond breakage. One of the reactions employs
cleavage between the α- and β-carbons and the other reaction uses an α-cleavage.
O
C
OH
O
C
C
C
C
C
C
O
Cleavage
Cleavage
O
O
C
O
C
C
O
O
Metabolism of Pyruvate
Pyruvate, produced by glycolysis or from other metabolic sources, is one of the key intermediates of
cellular metabolism. It can serve as a precursor in many biosynthetic pathways. For example, pyruvate can
serve as a precursor for glucose, oxaloacetate, and several of the amino acids. One of the reactions pyruvate
takes part in is the reaction catalyzed by the Pyruvate Dehydrogenase Complex. This enzyme complex
oxidatively decarboxylates pyruvate to acetate and then activates the acetate for subsequent reactions by
coupling it to Coenzyme A (CoA or CoA-SH) forming Acetyl-Coenzyme A (Ac-CoA or Ac-S-CoA).
Acetate carried by CoA (Acetyl-CoA) is also a key intermediate of metabolism. Acetyl-CoA is the
precursor for fatty acid, cholesterol, and isoprenoid biosynthesis. Acetyl-CoA is also the “fuel” for the first
of the two final common pathways. The TRICARBOXYLIC ACID CYCLE oxidizes the two carbon acetate
fragment into two molecules of CO2 and stores the energy released by the oxidations as high energy
electrons accepted by NAD → NADH and FAD → FADH2).
The conversion of pyruvate to acetate by the Pyruvate Dehydrogenase Complex is an irreversible process in
mammalian cells. Mammalian cells do not have the enzymatic machinery necessary to convert the two
carbon acetate fragment into a three carbon fragment. At this point the cell must make a “decision”: Is the
pyruvate from glycolysis going to be used as pyruvate for biosynthetic reactions or is it going to be
converted to Ac-CoA? The “decision” is made by the energy charge of the cell, the concentrations of
allosteric effectors controlling key metabolic enzymes, and the signal molecules present in the tissues.
2
©Kevin R. Siebenlist, 2016
Once converted to Ac-CoA will the acetate fragment be used for biosynthetic reactions or will it be oxidized
for energy production? The pyruvate dehydrogenase complex stands at this crossroads and controls the flux
of pyruvate/acetyl-CoA through the various metabolic pathways. For this reason the Pyruvate
Dehydrogenase Complex is one of the most tightly controlled enzyme complexes.
At this point in the discussion of Biochemistry, the pyruvate obtained from glycolysis will be passed
through the Pyruvate Dehydrogenase Complex and the resulting acetyl-CoA will serve as the “fuel” for the
TRICARBOXYLIC ACID CYCLE. The other fates of pyruvate and acetyl-CoA will be discussed as time goes
on.
The Pyruvate Dehydrogenase Complex
The Pyruvate Dehydrogenase Complex catalyzes the following overall reaction:
Pyruvate + CoA + NAD+ → Acetyl-CoA + CO2 + NADH
The Pyruvate Dehydrogenase Complex is a large multisubunit enzyme with a molecular mass of over
1,000,000 g/mole. It contains five different enzyme activities on four different polypeptide subunits. In
mammals there are 20-30 copies of Pyruvate Dehydrogenase (E1); 60 copies of Dihydrolipoyl
Transacetylase (E2); 20-30 copies of Dihydrolipoyl Dehydrogenase (E3); variable number of copies of
Pyruvate Dehydrogenase Kinase and Pyruvate Dehydrogenase Phosphatase. The number of kinase and
phosphatase subunits increase with starvation. The activities reside on the same polypeptide, similar to the
arrangement of kinase and phosphatase activities on Phosphofructokinase-2.
The enzyme complex requires two cosubstrates and three coenzymes for activity. From the overall reaction
the complex requires the cosubstrates NAD+ {niacin} and Coenzyme A {pantothenic acid}. E1 contains
Thiamine Pyrophosphate {thiamine} as a coenzyme. Each E2 subunit contains two Lipoic Acid molecules
as covalently linked prosthetic groups. The lipoic acids are linked to specific lysine side chains by amide
bonds. E3 contains Flavin Adenine Dinucleotide (FAD) {riboflavin}as a covalently linked coenzyme.
Mechanism of Pyruvate Dehydrogenase Action
The oxidative decarboxylation of pyruvate and the covalent attachment of the resulting acetate to coenzyme
A is divided into numerous steps. The first three steps of the reaction are catalyzed by the Pyruvate
Dehydrogenase (E1) subunit. The ketone group of pyruvate is covalently linked to the Thiamine
Pyrophosphate prosthetic group of Pyruvate Dehydrogenase (E1) and this enzyme catalyzes the
decarboxylation of pyruvate. The decarboxylation reaction is an α-cleavage reaction and it is non-oxidative;
the carbon atom attached to the thiamine pyrophosphate has not undergone a change in oxidation state.
Lipoic Acid covalently linked to Dihydrolipoyl Transacetylase (E2) swings into the active site of E1 and E1
catalyzes the oxidative transfer of the two carbon fragment from Thiamine Pyrophosphate to the oxidized
lipoyl (Lipoic Acid) group of E2. During this transfer, the carbonyl group of the two carbon fragment is
oxidized to a carboxylic acid group, the disulfide of Lipoic Acid is reduced, and the resulting acetate group
is covalently linked to one of the -SH groups of the reduced Lipoic Acid by a high energy thioester bond.
3
©Kevin R. Siebenlist, 2016
Dihydrolipoyl Transacetylase (E2) now catalyzes a transesterification reaction. The acetate group is
transferred from the -SH group of Lipoic Acid to the -SH group of Coenzyme A to form acetyl-Coenzyme A
and reduced Lipoic Acid. The high energy thioester bond between the acetate and lipoic acid is broken and
the energy is conserved in the thioester bond between acetate and CoA.
In the last step of the reaction the reduced lipoyl group of E2 swings to the active site of Dihydrolipoyl
Dehydrogenase (E3) where the reduced Lipoic Acid is oxidized to the cyclic disulfide. The FAD on E3 is
reduced to FADH2. To complete the reaction and regenerate the FAD for the next reaction cycle, the
electrons on FADH2 are passed to NAD+ forming FAD and the last product, NADH.
O
H 3C
HS-CoA
H3C
C
S
CoA
O
C
S
SH
Dihydrolipoyl
Transacetylase
SH
SH
O
C
Pyruvate
C
C
NH
O
O
H 3C
C
NH
E2
O
O
Thiamine
Pyrophosphate
(TPP)
FAD
HN
C
Pyruvate
Dehydrogenase
E1
OH
H 3C
C
TPP
O
H 3C
C
O
O
TPP
C
H
OH
S
Dihydrolipoyl
Dehydrogenase
E3
NADH
FADH2
S
NAD
CO2
The Tricarboxylic Acid Cycle
The TRICARBOXYLIC ACID CYCLE is the first of the two final common pathways responsible for the
complete oxidation of metabolites to CO2 and H2O with the concomitant production of ATP. The second of
the two final common pathways is the ELECTRON TRANSPORT / OXIDATIVE PHOSPHORYLATION (ET/
4
©Kevin R. Siebenlist, 2016
OXPHOS) pathway. Both of these pathways occur within the mitochondria of the cell.
The TRICARBOXYLIC ACID CYCLE was elucidated by HANS KREBS in 1933. This pathway is also called the
KREBS CYCLE in honor of Hans Krebs, the CITRIC ACID CYCLE, and/or the TCA CYCLE.
Metabolic intermediates derived from carbohydrates, lipids, and/or amino acids enter the TRICARBOXYLIC
ACID CYCLE where they are completely oxidized to CO2. Energy is released during the oxidative process.
Some of the energy is stored as high energy electrons carried by NADH and FADH2, some is stored as GTP
or ATP, and the remainder is released as heat.
During the TCA cycle, the equivalent of the two carbon acetate fragment is oxidized to two molecules of
CO2. The energy released during this oxidation is stored in three molecules of NADH, one molecule of
FADH2, and one molecule of GTP or ATP. The overall reaction of the TCA cycle is:
Acetyl-CoA + 3 NAD+ + FAD + GDP (or ADP) + PO4-3 + 2 H2O
CoA + 3 NADH + FADH2 + GTP (or ATP) + 2 CO2 + 2 H+
The Pathway
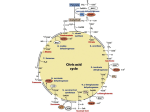
In the first reaction of the TCA cycle, acetyl-CoA, the activated form of acetate covalently linked to CoA,
reacts with oxaloacetate to form citrate and CoA-SH. This is a lyase reaction as well as an aldol
condensation. A hydrogen from the methyl group and the methylene carbon add across the carbon-oxygen
double bond of oxaloacetate. This irreversible reaction is catalyzed by Citrate Synthase.
The TCA cycle is an oxidative pathway. However, citrate does not contain an easily
oxidizable group. During the second step of the TCA cycle, the citrate molecule is
rearranged, isomerized to D-isocitrate. The tertiary hydroxyl group on carbon three of
citrate is moved to carbon two becoming a secondary alcohol. This freely reversible
reaction is catalyzed by the enzyme Aconitase. It is called Aconitase, after the enzyme
bound intermediate cis-aconitate.
O
O
C
CH2
O
C
O
C
CH
C
The first oxidation reaction occurs at the third step of the cycle. D-Isocitrate is oxidatively
O
O
+
decarboxylated to yield α-ketoglutarate, with the concomitant reduction of NAD to
NADH. This two step reaction involves oxidation of the secondary alcohol to a carbonyl
group followed by an α-β type elimination (decarboxylation) that expels the carboxyl group
on carbon 3 as CO2 (the central carboxylic acid group) resulting in the product α-ketoglutarate. This
irreversible reaction is catalyzed by the enzyme Isocitrate Dehydrogenase.
The α-ketoglutarate is now oxidized. The product of this reaction is succinyl-CoA. This reaction is
irreversible and is catalyzed by the α-Ketoglutarate Dehydrogenase Complex. Oxidation of α-ketoglutarate
to succinyl-CoA occurs by a mechanism analogous to the oxidation of pyruvate to acetyl-CoA, an αcleavage reaction followed by oxidation and coupling to CoA. The same five cosubstrates/coenzymes
5
©Kevin R. Siebenlist, 2016
O
O
NADH
O
C
C
NAD+
H3C
O
O
O
8
CH2
O
HO
Oxaloacetate
OH
HC
O
C
O
C
O
CH2
CH2
C
C
O
O
C
1
C
C
CoA-SH
S
Acetyl-CoA
CH2
O
CoA
C
O
O
O
Citrate
Malate
H2O
1 - Citrate Synthase
7
2
2 - Aconitase
O
O
O
3 - Isocitrate Dehydrogenase
C
C
H
HC
C
5 - Succinyl-CoA Synthetase
(Succinate Thiokinase)
C
O
CH2
4 - α-Ketoglutarate Dehydrogenase Complex
H
HO
6 - Succinate Dehydrogenase
O
6
8 - Malate Dehydrogenase
O
FADH2
O
FAD
O
CH2
CH2
CH2
CH2
C
5
O
O
C
CO2
O
4
CoA-SH
CH2
3
NADH
CO2
O
O
α -Ketoglutarate
CH2
CoA-SH
GDP+PO4–3 C
O
ADP+PO4–3 S
CoA
or
GDP
NAD+
C
O
Succinate
GTP
O
O
C
C
O
C
Nucleoside
Diphosphate
Kinase
O
CH
Isocitrate
7 - Fumarase
ATP
O
C
C
O
Fumarate
ADP
O
C
GTP
or
ATP
NAD+
NADH
Succinyl-S-CoA
6
©Kevin R. Siebenlist, 2016
(NAD+, Coenzyme A, thiamine pyrophosphate, lipoic acid, & FAD) are involved and two of the products
are identical, CO2 and NADH. At this point in the cycle two CO2 molecules have been released, so in
essence the acetate group that entered the cycle has been completely oxidized. Some of the energy released
during the oxidation reactions has been stored in two molecules of NADH and some energy remains in
succinyl-CoA. In the remaining steps of the pathway oxaloacetate is regenerated from succinyl-CoA and
the energy released during this process is trapped in a molecule of GTP/ATP, a molecule of NADH, and a
molecule of FADH2.
The bond between succinate and CoA in succinyl-CoA is a high energy thioester bond. In animal cells the
energy stored in this bond is used to drive the formation of a GTP or ATP from GDP or ADP and PO4–3.
Succinate and the nucleoside triphosphate are the products of this reaction. This reversible reaction is
catalyzed by Succinyl-CoA Synthetase. This reaction is reversible and the enzyme was named by Krebs for
the reverse reaction. A better, more correct, name for this enzyme is Succinate Thiokinase. The
mitochondria contains two isoforms of this enzyme, one uses GDP/GTP pair and the other is specific for the
ADP/ATP pair. Mechanistically, the enzyme employs phosphate to cleave the high energy thioester bond
releasing CoA and forming a high energy mixed anhydride between the phosphate and the carboxyl group
of succinate. The high energy phosphate of this mixed anhydride is then passed from the intermediate to
GDP (ADP) forming GTP (ATP) and succinate. GTP (ATP) formation in this reaction is another example of
substrate level phosphorylation. The GTP is used by the mitochondria for the synthesis of its DNA, RNA,
and proteins. Alternatively it can be (is) converted to ATP by the action of Nucleosidediphosphate Kinase
(NDK) and made available to the entire cell.
NDK
⎯⎯⎯
⎯⎯
→ ADP + NTP
ATP + NDP ←
⎯
In the sixth step of this cyclic pathway a “trans” double bond is introduced into succinate to form fumarate.
This reaction is catalyzed by the Succinate Dehydrogenase Complex. The electrons removed from
succinate are initially accepted by a covalently bound FAD to form FADH2. To regenerate the enzyme these
electrons must be passed to a second electron acceptor. In the case of the Succinate Dehydrogenase
Complex, the secondary electron acceptor is the diffusible cosubstrate, Coenzyme Q (Ubiquinone or CoQ).
Electrons on the enzyme bound FADH2 are passed to CoQ forming FAD and reducing Coenzyme Q to
Coenzyme QH2 (CoQH2). Several steps are required for the movement of the electrons from FADH2 to
Coenzyme Q. These additional steps will be examined when the ELECTRON TRANSPORT / OXIDATIVE
PHOSPHORYLATION (ET/OXPHOS) pathway is discussed.
Fumarate is now reversibly hydrated to form malate. Water is added across the “trans” double bond in a
reaction catalyzed by the enzyme Fumarase (Fumarate Hydratase). Fumarase catalyzes a lyase type
reaction.
In the last step of this cyclic pathway, the secondary alcohol group on malate is oxidized to a carbonyl
group forming oxaloacetate. This reversible reaction is catalyzed by Malate Dehydrogenase. NAD+ is the
electron acceptor for this reaction and is reduced to NADH. Malate formation is favored in this reversible
reaction. The reaction is pulled toward oxaloacetate formation by the constant removal of oxaloacetate
during the Citrate Synthase reaction. With the (re)formation of oxaloacetate the cyclic pathway is
completed.
7
©Kevin R. Siebenlist, 2016
Regulation of The Citric Acid Cycle
Control by Intermediate Concentration
The CITRIC ACID CYCLE is a CATALYTIC CYCLE. Since the intermediates of the pathway are not consumed
during the cycle, low concentrations of TCA cycle intermediates (i.e., oxaloacetate) can catalyze the
complete oxidation of innumerable two carbon acetate fragments. One way to control the flux of
metabolites through the cycle is to control the level of TCA Cycle intermediates in the mitochondrial
matrix.
The concentrations of TCA cycle intermediates can be increased by reactions called ANAPLEUROTIC
REACTIONS. One of the three important anapleurotic reactions is the formation of oxaloacetate from
pyruvate. This reaction is catalyzed by the enzyme Pyruvate Carboxylase. Pyruvate Carboxylase is a
mitochondrial enzyme and the reaction, the addition of CO2 (HCO3–) to pyruvate to form oxaloacetate,
occurs in the mitochondrial matrix. Pyruvate Carboxylase, like most carboxylase enzymes, contains Biotin
as a covalently linked prosthetic group. This enzyme is an allosteric enzyme with an absolute allosteric
requirement for acetyl-CoA. Acetyl-CoA is a positive allosteric effector for the enzyme. When the
concentration of acetyl-CoA in the mitochondrial matrix is low this enzyme is for the most part inactive. As
the concentration of acetyl-CoA increases the activity of this enzyme increases dramatically. Thus, when
acetyl-CoA levels exceed the oxaloacetate supply, allosteric activation of Pyruvate Carboxylase by acetylCoA raises oxaloacetate levels, so that a greater amount of acetyl-CoA can enter the TCA cycle and be
metabolized.
CO2 (HCO3–)
+
ADP
+
PO4–3
ATP
+
H2 O
O
H3C
C
O
Biotin
O
O
C
C
O
Pyruvate
Carboxylase
O
C
H2
C
O
C
O
A second ANAPLEUROTIC REACTION is catalyzed by Phosphoenolpyruvate Carboxykinase. (PEP
Carboxykinase). This is a mitochondrial enzyme. The reversible reaction takes phosphoenolpyruvate
(PEP), CO2 and GDP and forms oxaloacetate and GTP. Some of the energy stored in PEP is used to
phosphorylate GDP to GTP and the remaining energy is used to form the new carbon-carbon bond.
O
O
P
O
O
H 2C
C
CO2 + GDP
GTP
O
O
O
C
C
Phosphoenolpyruvate
O
8
O
C
H2
C
O
C
O
Oxaloacetate
©Kevin R. Siebenlist, 2016
The third ANAPLEUROTIC REACTION is catalyzed by Malic Enzyme. The mitochondrial isoenzyme forms
malate by reductively carboxylating pyruvate. HCO3– is the source of the carboxyl group and in the
mitochondria, electrons are donated by NADH. There is a cytoplasmic isoform of Malic Enzyme. This
isoenzyme favors the formation of pyruvate from malate by oxidative decarboxylation using NADP as the
oxidizing agent.
–
HCO2
+
NADH
O
H 3C
C
O
O
OH
O
Malic Enzyme
C
Pyruvate
NAD
C
O
H2
C
C
H
Malate
O
C
O
NADP
NADPH
+
CO2
The level of TCA cycle intermediates can be / are decreased by a variety of means. Intermediates of the
TCA cycle are drawn off and used as precursors in many anabolic pathways. For example:
oxaloacetate → aspartate (and related amino acids)+ carbohydrates + nucleotides
citrate → fatty acids + steroids + isoprenes
α-ketoglutarate → glutamate + glutamine (and related amino acids) + nucleotides
succinyl-CoA → porphyrins (porphyrins + Fe+2 = heme)
Since the TCA cycle is central to many catabolic and anabolic pathways, the TCA cycle is more correctly
termed an AMPHIBOLIC PATHWAY. AMPHI means BOTH. The TCA cycle is both a catabolic and anabolic
pathway.
Control at the Pyruvate Dehydrogenase Complex
The TCA cycle is also controlled by the availability of “fuel” for the pathway. Fuel for the TCA cycle is the
acetate fragment carried by CoA. The flow of pyruvate, from glycolysis, to acetyl-CoA is tightly controlled
at the pyruvate dehydrogenase complex. This enzyme complex is under allosteric control and it is
controlled by reversible covalent modification.
The allosteric controls are at Dihydrolipoyl Transacetylase, (E2) and Dihydrolipoyl Dehydrogenase, (E3).
Dihydrolipoyl Transacetylase, the E2 subunit, is allosterically activated by Coenzyme A and allosterically
inhibited by Acetyl-CoA. The substrate and product of the reaction, respectively.
Dihydrolipoyl Dehydrogenase, the E3 subunit, is allosterically activated by NAD+ and allosterically
inhibited by NADH. The substrate and product of the reaction, respectively.
9
©Kevin R. Siebenlist, 2016
Control by reversible covalent modification involves the fourth subunit, the Pyruvate Dehydrogenase
Kinase / Pyruvate Dehydrogenase Phosphatase of the Pyruvate Dehydrogenase Complex. The Pyruvate
Dehydrogenase Kinase transfers phosphate from ATP to the E1 subunit, (Pyruvate Dehydrogenase), and the
Pyruvate Dehydrogenase Phosphatase removes the phosphate. When E1 is phosphorylated by the kinase,
the enzyme is inhibited, when it is dephosphorylated by the phosphatase E1 is activated.
ATP, NADH,
Acetyl-CoA
—
Pyruvate
Dehydrogenase
Phosphatase
Catalyzes the
Removal of PO4–3
Acetyl-CoA
—
+
Insulin,
PEP, AMP,
Ca+2 ,Mg+2
E1
(—) O3PO
Pyruvate, ADP
Ca2+, Mg2+
—
Pyruvate
Dehydrogenase
Kinase
+
E2
NADH
—
E3
+
+
NAD
Coenzyme A
Catalyzes the
Addition of PO4–3
ATP, NADH
Acetyl-CoA
The kinase / phosphatase of the Pyruvate Dehydrogenase Complex are under allosteric control. Pyruvate
Dehydrogenase Kinase subunits are allosterically activated by elevated levels of NADH and Acetyl-CoA.
The products of the Pyruvate Dehydrogenase Complex activate the Pyruvate Dehydrogenase Kinase, the
active Protein Kinase phosphorylates the Pyruvate Dehydrogenase (E1) subunit, and phosphorylation of E1
inhibits that activity of this subunit and thereby slows (inhibits) the activity of the enzyme complex. The
Pyruvate Dehydrogenase Kinase is inhibited by ADP, Pyruvate, Ca+2, and Mg+2.
The Pyruvate Dehydrogenase Phosphatase is likewise highly regulated. It is stimulated by insulin,
10
©Kevin R. Siebenlist, 2016
Phosphoenolpyruvate, AMP. Muscle-specific isoforms of the enzyme are also stimulated by elevated
concentrations of Ca+2 and Mg+2. It is inhibited by ATP, NADH and Acetyl-CoA.
Control by Allosteric Enzymes Within the TCA Cycle
Finally, the Krebs cycle is controlled by allosteric enzymes within the pathway. Three enzymes within the
TCA cycle catalyze irreversible reactions and are sites for control. These three enzymes are Citrate
Synthase, Isocitrate Dehydrogenase, and the α-Ketoglutarate Dehydrogenase Complex.
+ ADP
Citrate Synthase
– ATP & NADH (Allosteric Inhibitors)
Citrate & Succinyl-CoA (Competitive Feedback Inhibitors)
+ Ca+2, NAD, & ADP
Isocitrate Dehydrogenase
– NADH & ATP
+ Ca+2
-Ketoglutarate Dehydrogenase
– NADH & Succinyl-CoA
The mechanism for the control of Citrate Synthase is complex and still open to question. In vitro ATP and
NADH allosterically inhibit the enzyme. Citrate and Succinyl-CoA act as Competitive Feedback Inhibitors,
competing with the normal substrates for binding at the substrate / active site. ADP acts as an allosteric
activator. However, it is unclear whether these “effectors” ever achieve a concentration sufficiently high in
the mitochondrial matrix to inhibit or activate the enzyme.
Isocitrate Dehydrogenase is allosterically activated by Ca+2, NAD and ADP, and allosterically inhibited by
NADH and ATP.
α-Ketoglutarate Dehydrogenase Complex is allosterically activated by elevated levels of Ca+2 and is
allosterically inhibited by NADH and succinyl-CoA.
11
©Kevin R. Siebenlist, 2016