* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download The Evolution of Membranes - University of Guelph Physics
Survey
Document related concepts
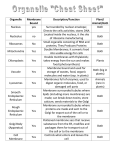
Cellular differentiation wikipedia , lookup
Extracellular matrix wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Tissue engineering wikipedia , lookup
SNARE (protein) wikipedia , lookup
Signal transduction wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell encapsulation wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
Cell membrane wikipedia , lookup
Lipid bilayer wikipedia , lookup
Endomembrane system wikipedia , lookup
Transcript
CHAPTER 2 The Evolution of Membranes M. BLOOM O.G. MOURITSEN Canadian Institute for Advanced Research & Department of Physics, University of British Columbia, 6224 Agricultural Road, Vancouver, B.C., Canada V6T 1Z1 Canadian Institute for Advanced Research & Department of Physical Chemistry, The Technical University of Denmark, DK-2800, Lyngby, Denmark 1995 Elsevier Science B.V. All rights reserved Handbook of Biological Physics Volume 1, edited by R. Lipowsky and E. Sackmann 65 Contents 1. 2. 3. 4. Introduction: optimization of physical properties via evolutionary processes . . . . . . . . . . . . . Magnetic crystals in magnetotactic bacteria: an example of optimization . . . . . . . . . . . . . . . . Pre-biotic membranes: did they exist? . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Molecular biology, geology and the classification of cells . . . . . . . . . . . . . . . . . . . . . . . . . . . . 67 68 70 72 4.1. Phylogenetic tree of procaryotes (eubacteria and archaebacteria) and eucaryotes from ribosomal RNA polynucleotide sequencing (‘molecular phylogeny’) . . . . . . . . . . . . . . . . 4.2. Geological information on cellular evolution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5. Archaebacterial membranes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6. Proteins in membranes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.1. Integral membrane proteins . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6.2. Laboratory experiments on model systems: an illustrative example . . . . . . . . . . . . . . . . . 7. The biosynthetic pathway for sterols and the plasma membranes of eucaryotic cells . . . . . . . 7.1. The insights of Konrad Bloch . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7.2. Influence of sterols on the physical properties of membranes . . . . . . . . . . . . . . . . . . . . . . 8. Lipid diversity and the physical properties of membranes . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.1. Lipid polymorphism . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8.2. Skin lipids – modification of lipids in situ to provide a water-resistant surface . . . . . . . 8.3. Essential fatty acids and brain lipids . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Acknowledgements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . References . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72 73 76 77 77 80 81 81 82 85 87 89 92 93 93 66 1. Introduction: optimization of physical properties via evolutionary processes Membranes play a crucial physical role in cells, defining as they do the boundary between the inside and outside of cells or organelles of cells. For this reason it is obviously appropriate to examine the evolution of membranes in relation to the evolution of cells. Still, although the study of cellular evolution is currently an extremely active and rapidly moving field, very little consideration has been given to the evolution of membranes. As an example, the word evolution does not appear in the index of a recent, comprehensive textbook on biomembranes [1]. In a symmetrical manner, the word membrane is not be found in an authoritative treatment of evolution in a textbook on biophysics [1a]. This is not really surprising in view of the fact that the systematic treatment of the evolution of physical properties of biological materials is a non-existent field. Modern biology, and especially evolutionary biology, is dominated by ‘molecular biology’, the study at the molecular level of the transfer of genetic information. Aside from structural proteins and relevant physical properties of proteins in general, the physical properties of biological materials are not programmed directly by the genes. The genetic code controls the synthesis of proteins, some of which act as enzymes for the synthesis or modification of other molecules. The physical properties are governed by the structures formed when these molecules aggregate in a manner dictated by the normal laws of physics and physical chemistry. Although the feedback leading to genetic control can be one or more stages removed from protein synthesis that is controlled directly by nucleic acid sequences, we assume that evolutionary processes still lead to optimization of the physical properties of biological materials because of the almost unimaginably long time scale available. This assumption is the motivation for our examination of membrane evolution in this chapter. The assumption of optimization of physical properties via evolutionary processes cannot be proven for cell membranes at this time. A schematic illustration of the essential features of the plasma membranes of eucaryotic cells is shown in fig. 1. The lipid bilayer core of biological membranes is fluid and leads to their characteristic, ultra-soft mechanical properties [2]. Because physical scientists have had little direct experience with such soft materials, we are still at an early stage in the characterization of their physical properties at the present time. We propose that information from evolutionary considerations be used to improve our perception and understanding of ‘Nature’s design’ in order to focus on potentially useful experimental and theoretical questions. In view of our relative lack of experience in dealing with the physical properties of soft, natural materials, it is useful to provide some clear-cut examples of optimization of physical properties of other types of biological materials via evolutionary 67 68 M. Bloom and O.G. Mouritsen Fig. 1. Schematic illustration of a biological membrane showing the geometrical relationship between the lipid bilayer and other membrane features (courtesy of Mr. Broo Sørensen of the Technical University of Denmark). The membrane depicted here is representative of the plasma membranes of eucaryotic cells. The rubber-like ‘cytoskeleton’ attached to the cytoplasmic (inner) surface of the bilayer exerts a strong influence on the mechanical properties of such a composite membrane. The glycocalyx carbohydrate network on the outside surface is generally believed to be responsible for cell–cell recognition and adhesion to other cells. The black objects extending from one side of the lipid bilayer to the other represent integral membrane proteins. processes. This is possible because biological systems also make hard, crystalline materials whose physical properties are extremely well understood and characterized using conventional condensed matter methods. Whenever such materials, made by cells, are carefully examined, they invariably prove to have been optimized via evolutionary processes. An illustrative example is given in section 2. 2. Magnetic crystals in magnetotactic bacteria: an example of optimization The study of bacterial swimming [3], chemotaxis [4–6] and magnetotaxis [7] provide wonderful examples of the optimization of physical properties of biological systems by evolutionary processes. In each case one is struck by the manner in which nature has found elegant solutions to formidable physical problems by exploiting fundamental physics in a subtle manner. Deformable bodies can swim under low Reynolds number conditions only by coupling translational motions to certain nontrivial cyclic changes in shapes [3, 8]. Chemotaxis leads to a variety of problems ranging from how to attain nearly perfect detection of molecules striking a surface in water with minimum coverage (≈ 0.1% for bacteria) of the surface by detectors [4] to the design of tiny motors [6]. However, we choose magnetotaxis as our example because, in solving the problem of directed bacterial swimming along the earth’s The evolution of membranes 69 magnetic field, nature has produced an optimal design of magnetic materials via evolutionary processes. Magnetotactic behaviour of bacteria was first discovered accidentally in 1975 by Richard Blakemore while a graduate student. Bacteria in water were observed (in the northern hemisphere) to swim persistently in a northerly direction. If observed in a droplet, they accumulated at its northern boundary. In the laboratory, the bacteria swam in the direction of an applied laboratory magnetic field. Observations of such bacteria in a transmission electron microscope always showed a series of opaque particles in a row. In fig. 2, a bacterium is shown with about 20 particles having an average diameter of about 50 nm. Each of these particles is a single crystal of magnetite (Fe3 O4 ), whose magnetic properties are well known, surrounded by a lipid bilayer membrane. These particles, called ‘magnetosomes’, have by now been thoroughly investigated [9, 10]. Fig. 2. Magnetotactic Bacterium is seen in a transmission electron microscope which reveals a striking feature of its internal structure: a chain of particles that are opaque to the electron beam, aligned more or less parallel to the long axis of the cell. The particles are called magnetosomes and consist of magnetite (Fe3 O4 ) single crystals of size ≈ 50 nm and surrounded by a lipid membrane. The chain functions as a compass, orienting the bacterium in the same direction as the lines of force of the earth’s magnetic field. The species shown here has a flagellum (a filamentous propeller) at each end (from ref. [7]). 70 M. Bloom and O.G. Mouritsen Blakemore and Frankel [7] quickly realized that the biological reason for the evolution of magnetosomes was that it provided a mechanism for the bacteria to distinguish between up and down via the vertical component of the earth’s magnetic field! Consistent with this, it was found that Fe3 O4 crystals in magnetotactic bacteria in the southern hemisphere are magnetized in the opposite direction to those in the northern hemisphere. There are two biological advantages for bacteria to swim downward. One is to swim away from the higher concentrations of O2 (poisonous to anaerobic bacteria) near the surface of the water and the other is to swim towards the higher concentrations of food in the ooze at the bottom of the lake, river or ocean. Procaryotic cells, having a size L ≈ 1 µm, cannot distinguish between up and down via gravity with reliability as may be seen from the inequality satisfied by the ‘gravitational order parameter’ Sg = bmgL/kT 1, where b ≈ 0.1 is a buoyancy factor and mg is the weight of a bacterium. Sg is the ratio of the change of gravitational potential energy of a cell via shifts of its mass distribution over distances of order its size (i.e. as in shape changes while keeping the vertical position of one end of the cell fixed), to the fluctuations in the energy of the cell, kT , associated with equilibrium at a temperature T . By contrast, the magnetic order parameter Sm = M Be /kT ≈ 10 for the bacterium shown in fig. 2 having a magnetic moment M in the earth’s magnetic field Be ≈ 0.5 gauss. This result is obtained providing that the magnetite in the bacterium of fig. 2 has a value close to its saturation (maximum) magnetization per cm3 , i.e. M/V ≈ 500 erg/(gauss-cm3 ), leading to M ≈ 6.5 × 10−13 erg/gauss [7, 10]. The optimization of the magnetic material goes beyond just getting the ‘right answer’ for Sm . The membranes surrounding the magnetite particles are of such a size that the magnetosome diameters always fall within the window of sizes corresponding to mono-domain, ferrimagnetism, i.e. between 40 nm, below which the Fe3 O4 particles are ‘super-paramagnetic’ rather than ferrimagnetic, and 120 nm, above which they are multi-domained.The largest possible magnetic moment per atom is obtained within this window. This is a high class engineering solution to a materials problem and nature has found the solution using evolutionary processes. Space does not permit us to explore other subtle aspects of the magnetosome optimization [9, 10]. The intent of this brief description has been to illustrate that, in studying the physical properties of materials such as biological membranes in which physical scientists have had only limited experience, a consideration of how nature has optimized some important properties may provide a guide to useful, systematic laboratory experiments. We will review some such attempts for membranes in sections 6.2 and 7.2. 3. Pre-biotic membranes: did they exist? The origin of life is an unsolved problem and we confine ourselves to a few remarks on whether the type of membranes found in presently existing cells could have played a role in the very early stages of life. Readers interested in learning about more general aspects of pre-biotic aspects of evolution must look elsewhere (see, e.g., [10a]). The evolution of membranes 71 As discussed by Deamer [11], the first cellular systems to appear on the Earth, were presumably assembled from three molecular species: information-storing molecules capable of replication, enzyme-like catalysts structurally encoded by that information and able to enhance replication rates, and boundary-forming molecules which could encapsulate the system represented by the first two species. To these should be added molecules capable of storing energy and using this energy to convert common molecules into organized assemblies of biologically active molecules [12]. It is now generally agreed that under the conditions of volcanic and electrical activity that probably existed on pre-biotic Earth > 4 eons ago (1 eon = 109 years), the nucleotides and amino acids leading to polynucleotides and polypeptides, respectively, would likely have been generated spontaneously [13]. Since it is not clear whether molecules leading to encapsulation would also have been produced in the Earth’s pre-biotic atmosphere [14], an investigation was carried out on the physicochemical properties of amphiphilic compounds in carbonaceous meteorites believed to be similar to those bombarding the atmosphere of pre-biotic Earth [11, 14, 15]. The results of this study demonstrate that organic molecules capable of self-assembly into membranes could have been available on the primitive Earth through meteoric infall. The specific manner in which life on Earth originated is not known [16]. Even the ‘Central Dogma of molecular biology’, which asserts that replication information in living systems flows from polynucleotide to polynucleotide or polypeptide, but never from polypeptide, and which is taken as a starting point by almost all persons thinking about the origin of life, is really still open to question in this domain; see, e.g., the proposal by Dyson [17] for an evolutionary sequence from cells to enzymes (polypeptides) to genes (polynucleotides) and compare this with the arguments from a broad biological perspective [18] that “...the transition to vesicles led to the directed chemistry or vectorial chemistry necessary for biogenesis...” One reason for including this brief section in a chapter on the evolution of membranes is to point out that an extension of the studies initiated by Deamer [11] on extremely primitive, naturally occurring membranes to include their physical properties could ultimately contribute towards transferring some speculations concerning the origins of life into the experimental domain. For example, in our review of the evolution of cells in the next section, we will encounter the proposal that procaryotic and eucaryotic cells parted company from each other in pre-cellular times, each evolving independently from their common non-cellular, or ‘protocell’ progenote ancestor. Woese [19] has remarked that the nature of the common organismal ancestor is “...probably the most important, and definitely the least recognized, major question in biology today”. It may be fruitful to ask whether this proposal is consistent with the physical properties of the type of pre-biotic material brought to the Earth by meteorites. 4. Molecular biology, geology and the classification of cells 72 M. Bloom and O.G. Mouritsen Fig. 3. Universal phylogenetic tree determined from (r) RNA sequence comparisons, i.e. ‘molecular phylogeny’ [19]. Proposals have been made to root the tree in time on the basis of subsequent work using molecular phylogenic [24, 25] and geological techniques. Some of the proposed dates for important evolutionary developments are indicated in fig. 4 (from ref. [7]). 4.1. Phylogenetic tree of procaryotes (eubacteria and archaebacteria) and eucaryotes from ribosomal RNA polynucleotide sequencing (‘molecular phylogeny’) Though the distinction between procaryotic and eucaryotic cells has been recognized for more than a century, and codified more than 50 years ago, the phylogeny of cells entered a new era with the development and subsequent improvement of molecular biology techniques for the sequencing of polynucleotides. In particular, the sequencing of fragments of ribosomal (r) RNA has proven to be extremely informative [19]. Ribosomes are responsible for the production of proteins and are found in all cells. The quantitative study of correlations between nucleotide sequences of ribosomal (r) RNA for different cells can be used to define an empirical ‘evolutionary distance’ between the cells. It has led to a definitive classification of cells into three distinct kingdoms, the eucaryotes and two types of procaryotes, namely eubacteria and archaebacteria. A great triumph of the use of (r) RNA has been the identification of archaebacteria as a distinct kingdom. In the ‘phylogenetic tree’ shown in fig. 3, constructed from (r) RNA nucleotide sequences [19], there is a much larger ‘evolutionary distance’ between any two of the three kingdoms than between cells within each kingdom. There is broad agreement concerning the modern classification of cells, but some lively disagreements exist concerning the ancient evolutionary history of different types of cells [13, 19–25]. The evolution of membranes 73 It is well established that the fossil record of procaryotes goes back at least 3.5 eons while that of eucaryotes goes back no further than 1.9 eons [26]. The information available from the geological record will be discussed further in section 4.2. The conventional wisdom before the introduction of nucleotide sequencing of (r) RNA was that procaryotic cells were more primitive than eucaryotes and evolved into them after the ingestion of one procaryote into another that had lost the external peptidoglycan cell wall common to virtually all eubacteria – the endosymbiont hypothesis [13, 27]. There seems to be general agreement that the ancestors of the mitochondrial organelles found in most eucaryotes were, indeed, procaryotic cells as stated in the endosymbiont hypothesis. However, Woese and his collaborators have argued on the basis of (r) RNA sequencing that there is no reason to believe that either procaryotes or eucaryotes are more primitive or ancient than the other and that the three kingdoms of cells could well have parted company from their common ancestor very early in the evolutionary history of cells [19, 28], even as far back as 3.6–4.7 eons ago [29]. In their most recent work, Woese and his collaborators have argued that (r) RNA sequencing favours a ‘rooting’ of the phylogenetic tree shown in fig. 3 such that eubacteria and archaebacteria parted company from the putative common ancestor of all known cells, and that eucaryotes and archaebacteria diverged some time later in evolutionary history [24, 25]. A scenario with a different perspective has been developed by Cavalier-Smith [20, 21, 30]. He suggests that, sometime between approximately 1.0 and 1.5 eons ago, archaebacteria and eucaryotes evolved from eubacteria that had lost their ability to make the muramic acid required for the synthesis of murein, an essential ingredient in the eubacterial peptidoglycan cell wall. This cell wall, which is common to virtually all eubacteria, acts as an external armour plate that maintains the shape of the cell, protects it from osmotic shock, and so forth. Though Cavalier-Smith makes use of the same background data and observations as Woese, he places a greater emphasis on physical and morphological differences between the different types of cells. We do not attempt to judge here which of these two scenarios will ultimately prove to be closer to the truth. In fact they are not necessarily inconsistent with each other. However, because the arguments of Cavalier-Smith couple physical features of the different cell kingdoms explicitly to their molecular biology, they allow us to relate measurements of physical properties of cells and, in particular, of cell membranes to questions of evolutionary biology. A few of the physical and morphological differences between the different types of cells are listed in table 1. We emphasize that our brief list is, necessarily, a highly personal and intuitive selection. This is indicated by a perusal of table 6 of the paper by Cavalier-Smith [21], which lists forty-eight unique features of eucaryotes never found in procaryotes! 4.2. Geological information on cellular evolution The fossil record is one of the most important sources of information on evolutionary history. It must, however, be treated with caution. As emphasized by Knoll [26] in his recent review of the geological record in relation to the early evolution of 74 M. Bloom and O.G. Mouritsen Table 1 Selected properties and functions of cells of one type essentially not present in the others. EUBACTERIA: Peptidoglycan cell wall. ARCHAEBACTERIA: Non-peptidoglycan, non-cellulosic cell wall. Distinctive lipids – e.g., isopranyl glycerol ether lipids with isoprenoid and hydroisopranoid hydrocarbons. EUCARYOTES: Compartmentalization – organelles. Cytoskeleton. Endo- and exo-cytosis. Sterols such as cholesterol for animals and ergosterol for plants. eucaryotes: “Fossil preservation is facilitated by mineralized hard parts and structures composed of organic materials that resist degradation. Many organisms have neither and so have little chance of fossilization. Those that do may only live in certain environments or become fossilized under specific sedimentary conditions. Thus, fossil presence and absence cannot be treated symmetrically.” In addition to supplying important information on fossils in rocks of independently assessed age, the geological record also provides vital information on chemical composition of the atmosphere and hydrosphere. Thus, the proliferation of diversity in eucaryotes and in animals can be reliably correlated with increased concentrations of O2 in the earth’s atmosphere [26, 31]. A crude plot of the variation of the earth’s atmospheric oxygen pressure with time [26] is shown in fig. 4 along with suggested correlations with important evolutionary developments as given by the fossil record and molecular phylogeny. Prior to the evolution of cyanobacterial photosynthesis (2.8 to 2.4 eons ago) the atmospheric oxygen pressure (pO2 ) was less than 10−10 atmospheres. Then, pO2 increased to about 1 to 2% PAL (present-day oxygen atmospheric level), an increase by a factor of 108 ! It is believed that pO2 remained at this level, controlled by high rates of oxygen consumption, until about 1.9 eons ago, when paleoweathering surfaces indicate a rise to > 15% PAL. There are indications of further dramatic increases of atmospheric oxygen concentration in the period preceding the appearance of animals, probably about 0.58 eons ago according to the fossil record [26, 31]. Molecular phylogeny suggests that the ancestor of mitochondria dates back to the first increase in pO2 or earlier. Knoll [26, 31] also mentions some evidence for the identification of certain fossils dated as far back as 1.9 eons ago with early eucaryotic cells. However, this identification is not yet definitive and the first pronounced burst of eucaryotic cell activity may have begun only 1.2 to 1.0 eons ago when the most obvious diversification in the paleontological record began. The review of evidence from molecular biology and geology on cellular evolution in this section reminds us that evolution should be considered as a historical science that can only be tested in a manner different from the usual ‘scientific method’, i.e. we can’t repeat the whole experiment. Here, we have paraphrased some comments by Stephen J. Gould [32] in which he makes ‘a plea for the high status of natural history’. The evolution of membranes 75 Fig. 4. A simplified plot of the atmospheric pressure of O2 on earth (pO2 ) as a function of time based on geological evidence [26] is shown on the left hand side of the diagram. Shown on the right hand side are suggested dates or time periods of some important developments in the evolution of cells. Prior to the evolution of cyanobacterial photosynthesis, pO2 was, perhaps as low as 10−10 atmos. It increased to concentrations capable of supporting aerobic respiration ≈ 2.8 to 2.4 eons ago. The increase in pO2 about 2 eons ago coincides with the first fossil records of eucaryotic cells and the increase about 1 eon (109 years) ago to the present atmospheric level (PAL) coincided with the proliferation of eucaryotic diversity according to the fossil record [26] and signaled the subsequent increase in animal phyla about 0.5 eons later [31]. It should be emphasized that the dates associated with the progenitor of modern eucaryotes (3.5 eons ago) and the first fossils of eucaryotes are not considered by the experts to be definitive at the time of writing. 76 M. Bloom and O.G. Mouritsen Workers in the field of molecular phylogeny are very cautious about assigning specific dates to the branches of the phylogenetic tree in fig. 3. For this reason, any connections made between studies of physical properties of membranes, cellular evolution using molecular phylogeny and the geological fossil record could be of importance. For example, if it can be shown whether or not the cytoskeleton can couple to the plasma membrane in the absence of sterols (As indicated in table 1 of section 4.1, all eucaryotic cells have both sterols and cytoskeletons!), then the first appearance of the cytoskeleton could be linked to that of sterols. These could, in turn, be linked to the geological evidence for oxygen concentration since the biosynthetic pathway for sterols requires O2 at many stages as shown by Konrad Bloch and to be discussed in section 7. 5. Archaebacterial membranes Archaebacteria were only identified as a separate kingdom of cells a relatively short time ago and the physical properties of their membranes have been studied less completely than those of eubacteria and eucaryotes. In the Cavalier-Smith [21] scenario for the evolution of archaebacteria, the integrity and form of the cell was maintained after the loss of the cell wall by the re-evolution of diverse types of cell walls [33], and the evolution of a unique class of archaebacterial lipids [34, 35] in response to the extreme conditions of salt concentration, temperature and pressure (e.g., as in deep sea hot springs) under which archaebacteria occupy their special niche. Unlike the straight-chain fatty acids and fatty acid ester-linked glycerol lipids that are characteristic of eubacteria and eucaryotes, archaebacterial lipids are distinguished by being isoprenoid and hydroisoprenoid hydrocarbon and isopranyl glycerol ether-linked lipids. Glycerol ether lipids are found in other types of cells in minor amounts, but only in archaebacteria have they been evolved as the fundamental glycerolipid component. While some ether lipids are structurally analagous to their counterparts in eubacteria and eucaryotes, in particular di-ether lipids, others such as the tetra-ether lipids are not. Some of these are about forty carbons long and have two polar ends. It would seem that such lipids would quite naturally span the lipid bilayer structure and this may well be their configuration in biological membranes. However, surprisingly little is known about the structure of archaebacterial membranes, partly, it seems, because of the small quantities of their pure lipids available for studies of physical properties [36]. One of the best structural studies using X-ray diffraction [37, 38] has led to the intriguing suggestion that the plasma membrane of the archaebacterium Sulfolobus solfataricus may be composed of two interlocking cubic phases [39]; see section 8.1. It appears that the study of the physical properties of archaebacterial lipids is still in its early stages and capable of yielding surprises. 6. Proteins in membranes The evolution of membranes 77 Fig. 4a. Illustration of some representative lipid sterol molecules found in eucaryotic and procaryotic (eubacterial and archaebacterial) cells. Four molecules are portrayed schematically from top to bottom. The molecules shown in (i), (ii) and (iii) are found in a single monolayer of bilayer membranes, while that shown in (iv) is believed to extend across the entire membrane. (i) The cholesterol molecule, representative of a class of sterol molecules found universally in the plasma (outer) membranes of eucaryotic cells, but not synthesized by procaryotic cells. (ii) The 1-palmitoyl-2-oleoyl-sn-glycerol-3phosphocholine (POPC) molecule illustrating the acyl chains (oleoyl above and palmitoyl below) and the headgroup (phospha-tidylcholine) coupled to the glycerol backbone by ester linkages. Such lipids are found in eubacteria and in eucaryotic cells in a variety of headgroups and a wide range of acyl chains of different lengths and degrees of saturation. (iii) A representative di-ether lipid, 2,3-Di-Ophytanyl-sn-glycerol, a basic lipid component of the membranes of all halophilic archaebacteria [35]. (iv) A representative tetra-ether lipid, Di-biphytanyl-diglycerol-tetraether, an isoprenoid ether, backbone of complex lipids of mathanogenic archaebacteria [35]. 6.1. Integral membrane proteins As depicted in fig. 1 and described elsewhere [2], the central core of any biological membrane is the lipid bilayer. There is an interaction between the lipids and proteins and this interaction must be controlled by the physical constraints imposed by the lipid bilayer medium. Such constraints include the fluidity and the hydrophobicity of the bilayer interior, which we will discuss in turn below. The fact that the lipid bilayers of almost all biological membranes are fluid under 78 M. Bloom and O.G. Mouritsen physiological conditions is almost certainly dictated by the requirement that most of the integral membrane proteins spanning the bilayer must undergo conformational transitions in order to function. Fluid membranes have the compliance to accommodate the modifications in protein geometry that must inevitably accompany such conformational changes. Membrane fluidity, in turn, implies zero shear restoring force. As a consequence, the shapes of cells are governed by the membrane superand sub-structure. Most procaryotic cells have exoskeletal cell walls to control and maintain their shapes while the shapes of eucaryotic cells are usually controlled by the subtle, and as yet imperfectly understood and characterized, interactions between the lipid bilayer of the plasma membrane and its associated cytoskeletal structure [40]. It may well be that, when the nature of the cytoskeleton-lipid bilayer aspect of lipid-protein interactions is more fully understood, it’s evolutionary development will turn out to be correlated with the proliferation of eucaryotic diversity depicted in fig. 4 as having probably occurred about 1 eon ago. In this section we confine ourselves to some remarks about the interaction between lipids and integral membrane proteins which are found in both eucaryotic and procaryotic cells and are shown schematically in fig. 1 as entities spanning the entire lipid bilayer. There is great biophysical interest in the determination of protein structure and in relating structure to the biological function of the protein. At this time, much many more structures have been worked out for cytoplasmic, i.e. water soluble, proteins than for integral membrane proteins. The reason for this is that the amphiphilic nature of integral membrane proteins has made it difficult to prepare the high quality single crystals required for X-ray diffraction measurements while the anisotropic nature of the membrane fluidity has made it impossible to use modern Nuclear Magnetic Resonance (NMR) high resolution methods of determining protein structure [2]. Although the number of integral membrane proteins for which high resolution structures are known is extremely small, some important general structural features have been established. The secondary structural elements within the bilayer are mainly α-helices composed of amino acids which have, on average, a relatively high degree of hydrophobicity. The 3D (tertiary) structure involves a clustering of these α-helices, stabilized by the ‘hydrophobic interactions’ acting in combination with coulomb forces between the small number of charged residues placed strategically within the hydrophobic region of the membrane. These features, already established with the first low resolution structure of Bacteriorhodopsin [41,42], and confirmed by subsequent high resolution studies [43, 44], have led to an important method of guessing membrane- embedded domains of integral membrane proteins via ‘hydropathy algorithms’ [45–47]. The hydrophobic matching of lipids and integral membrane proteins, established by diffraction methods, has been confirmed by nuclear magnetic resonance (NMR) experiments which show that the orientational order of lipid acyl chains in membranes, which is linearly related to hydrophobic thickness, is affected very little by the integral membranes that co-exist with the lipids in biological membranes [2]. As illustrated in fig. 5, an integral membrane protein whose hydrophobic length is either longer or shorter than the equilibrium hydrophobic thickness of its host lipid The evolution of membranes 79 Fig. 5. Schematic diagram illustrating the principles of of the ‘mattress model’ of lipid-protein interactions [48]. Cross-section of two lipid bilayers each containing amphiphilic transmembrane proteins or polypeptides. The lipid molecules are indicated schematically by a circular polar headgroup region and two flexible acyl chains. The proteins (or peptides) are shown as rod-shaped objects with hydrophilic ends and intermediate hydrophobic regions (cross-hatched). Two situations with a mismatch are illustrated; the protein is longer (a) and shorter (b) than the lipid bilayer thickness. bilayer membrane, would produce a mechanical strain on the bilayer. Consequences of such mismatch have been developed in the mattress model of lipid-protein interactions [48]. Manifestations of the effects and potential effects of interactions encompassed within the mattress model, which have been reviewed recently [49], include such phenomena as: (i) lateral molecular organization within the bilayer, (ii) membrane heterogeneity and the existence of domains having different lipid compositions, (iii) lipid selectivity by proteins. It can thus be seen that conformational transitions in proteins can trigger changes in the lipid bilayer medium and vice-versa [50]. This gives a link between membrane function and thermodynamics. In summary, the now well established matching of the hydrophobic regions of integral membrane proteins and their host bilayer lipids demonstrates the role of evolution in the optimization of a basic, biologically relevant, mechanical property of membranes; in this case the introduction of proteins into membranes in a strainfree manner. 80 M. Bloom and O.G. Mouritsen Fig. 6. Smoothed acyl chain orientational order profiles from depaked 2 H NMR spectra [51]. Plots a are from bilayers composed of a CHOL/POPC-d31 mixture containing 30 mol% CHOL, called B2 in the text; plots b are from bilayers of pure POPC-d31 , called B1 in the text. Both sets of plots are at a temperature of 25 ◦ C. For each set of plots, the (+) plots are for samples with no peptide added, the (•) plots are for samples with 3.5 and 6.3 mol% of peptide P16 in a and b, respectively, and the (4) plots are for samples with 3.5 and 4.8 mol% of peptide P24 in a and b, respectively. (From ref. [51].) 6.2. Laboratory experiments on model systems: an illustrative example We now illustrate how one can explore the effects of the hydrophobic matching condition by means of laboratory experiments on simple systems. Ultimately, such an exploration should lead to an improvement in the understanding of biological processes. The system considered is a POPC-d31 membrane (B1 ) in which the thirty one protons on the palmitic acid chain have been replaced by deuterons in order to carry out deuterium (2 H) NMR experiments to determine the orientational order parameter S (n) as a function of position on the acyl chain. It may be seen from fig. 6 that the introduction of 30 mol% cholesterol into the POPC bilayer (we call this cholesterol – containing membrane B2 ) results in a large increase in the magnitude of S (n). The hydrophobic thicknesses of B1 and B2 have been estimated as d(1) = 2.58 nm and d(2) = 2.99 nm, respectively [51], on the basis of a now well-established, semi-empirical relationship between d and hS (n)i for POPC. The physical origin of the increase in d when cholesterol is introduced into the lipid bilayer will be discussed in the next section. A qualitative test of the mattress model is provided by introducing synthetic amphiphilic peptides Pn = K2 GLn K2 A-amide of different hydrophobic lengths, corresponding to different values of n into B1 and B2 . In the experiments to be described, P16 and P24 were used. Since the polyleucine part of these peptides is known to The evolution of membranes 81 form a tight α-helix [52], the anticipated hydrophobic length of Pn is dn ≈ 0.15 n, corresponding to d16 ≈ 2.4 nm and d24 ≈ 3.6 nm. It is clear from fig. 6 that the addition of the short peptide P16 to the thin membrane (B1 ) or the long peptide to the thick membrane (B2 ) results in little change in d(1) and d(2), respectively, as expected from the mattress model if d16 is approximately matched to d(1) and d24 to d(2). Addition of the long (or short) peptide to the thin (or thick) membrane gives an increase (or decrease) in d(1) (or d(2)), again in agreement with the mattress model. 7. The biosynthetic pathway for sterols and the plasma membranes of eucaryotic cells 7.1. The insights of Konrad Bloch As discussed in section 4.1 (see table 1), cholesterol or structurally equivalent sterols are found in virtually all eucaryotic cells; yet, only a few procaryotic cells have been found that synthesize sterols. The biosynthetic pathway for cholesterol and other such sterols was worked out by Konrad Bloch [53–55]. He suggested that the evolution of sterols was intimately connected with the physical requirements imposed on the plasma membranes of eucaryotic cells, presumably because of their greater size and complexity. Since the biosynthesis of sterols involves O2 at several stages, and it is difficult to visualize an anaerobic pathway for sterols, this would imply that the evolution of the type of eucaryotic cells of the type that we know today could not have taken place before O2 appeared in appreciable quantities in the earth’s atmosphere (see fig. 4). It seems to us (see section 2) that this point of view may be at odds with some of the proposals on the relative ages of different kingdoms of cells that arise from molecular phylogeny related to (r) RNA nucleic acid sequencing. It would be interesting to search for ways of dating some of the important features of the plasma membranes of eucaryotic cells in relation to the appearance of O2 in the earth’s atmosphere. Did early eucaryotic cells already exist in the anaerobic earth atmosphere? If ‘yes’, did they have organelles and plasma membranes capable of endo- and exo- cytosis before sterols evolved? Bloch was especially impressed by the particular sequence of enzymatically controlled reactions that follow the cyclization of squalene to lanosterol. Squalene is the final molecule in the biosynthesis of sterols that is chemically accessible in an anaerobic environment. He writes [54] that “...selective demethylations occur at the sterol α-face streamlining the sterol structure. Successive methyl group removal imposed by selection pressures rendered the sterol molecule progressively more and, ultimately, optimally competent for membrane function. The sterol pathway terminates with cholesterol, a molecule designed to optimize attractive van der Waal’s interaction with phospholipid chains in the membrane bilayer.” This raises the question as to whether “...one can discern in the contemporary sterol pathway and in the temporal sequence of modifying events a directed evolutionary process operating on a small molecule and, if so, whether each step of the sequence produces a molecule functionally superior to its precursor.” In these remarks, Bloch implicitly identifies sterols along the biosynthetic pathway to the end product (cholesterol for animal cells and ergosterol for many yeasts and 82 M. Bloom and O.G. Mouritsen fungi) as molecular fossils. This concept has also been explored for molecules such as triterpenes and hopanoids that are believed to play a similar structural role in procaryotic cells [56–58]. One unusual and instructive example of a procaryotic cell exhibiting ‘sterol fossils’ is the bacterium Methylococcus capsulatis, which synthesizes ‘methyl and dimethyl sterols’ of the type found along the biosynthetic pathway in eucaryotic cells, in the presence of O2 [58a]. Of particular interest in this paper is the measurement of the change in lipid and sterol composition with oxygen tensions. This may make it possible to correlate the evolution of certain enzymes responsible for sterol synthesis with the earth’s O2 concentration as provided by geological evidence and depicted in fig. 4. The concept of a molecular fossil for molecules along the biosynthetic pathway carries with it the possibility of an experimental laboratory program to identify the physical properties that are relevant to the evolutionary ‘optimization’ (see section 1), if, indeed it is a physical property that is being optimized. A study of the influence of various sterols along the biosynthetic pathway to cholesterol on membrane ‘microviscosity’ was carried out using steady state fluorescence depolarization by Bloch and co-workers [59] and their results, shown in fig. 7, exhibit a systematic increase of ‘microviscosity’ on progressing from lanosterol to cholesterol. In the light of these experiments and the many others carried out since we now review briefly the influence of sterols on the physical properties of membranes. Fig. 7. Effect of sterol molecules on the microviscosity of vesicles composed of lipids extracted from mycoplasma capricolum at 25 ◦ C using steady state fluorescence depolarization (from ref. [59]). Microviscosity is expressed in units of poise (g cm−1 s−1 ). The evolution of membranes 83 7.2. Influence of sterols on the physical properties of membranes Following Bloch’s suggestions that cholesterol (CHOL) and other structurally equivalent sterols provide some sort of optimization of physical properties of membranes, a more precise interpretation of the type of data shown in fig. 7, in conjunction with 2 H NMR, calorimetric and other measurements, has been developed [2, 60]. It turns out that the interpretation of steady state fluorescence depolarization simply in terms of ‘microviscosity’ is an oversimplification based on properties of isotropic fluids but not valid for anisotropic fluids such as membranes [60]. Nevertheless, the main proposals put forward by Bloch [53–55] are born out. As described in section 7.1, Bloch suggested that evolutionary pressure, giving rise to systematic enzymatically controlled modification of sterols, led ultimately to a final sterol (e.g., cholesterol or ergosterol or some other equivalent sterol) that optimized the physical properties of membranes. At this stage it is appropriate to treat this as a hypothesis that must be tested in the laboratory. We summarize some of the main features of the influence of CHOL as determined experimentally and reformulated in terms of our present understanding of membrane fluidity [2, 60]. It has been known for some time that CHOL in large concentrations eliminates the gel – liquid crystalline phase transition. However, as demonstrated [61] in a definitive study of DPPC/CHOL mixtures using 2 H NMR and calorimetry, and making use of data from a wide range of experimental techniques, the observation that CHOL increases the orientational order at temperatures T > Tc , i.e. above the normal phase transition temperature Tc for the pure lipid system, and decreases it at T < Tc , does not imply that the system becomes more ‘solid-like’ at T > Tc when CHOL is added. Rather, the effect of CHOL is to produce a new type of fluid phase, one in which the molecules behave as a fluid in two dimensions, i.e. the molecules diffuse and reorientate about the bilayer normal as in a normal fluid. Above all, the system exhibits no shear restoring force [2], but, at the same time, the acyl chains are orientationally ordered as in the gel phase of the pure phospholipid. As we shall explain below, this behavior can be understood in terms of the nature of the interactions between phospholipid and cholesterol molecules [62]. The theory has led to the following terminology for the various phases of the PC/CHOL mixtures. The gel phase is denoted by (so), the indices s standing for solid in two dimensions and o for orientationally ordered chains, the liquid-crystalline phase (ld) by the indices l for two-dimensional liquid and d for disordered chains and, finally, the new liquid ordered phase is denoted by (lo). We believe the (lo) to be the ‘biologically relevant’ phase. Phase diagrams for PC/CHOL mixtures are shown in fig. 8. The 2 H NMR studies of Vist and Davis [61] have been extended to three mono-unsaturated PC lipids, namely SEPC [63], PPetPC and POPC [63, 64]. This is appropriate since the plasma membranes of most eucaryotic cells often have high concentrations of unsaturated lipids. It seems that the phase diagram of PC/CHOL mixtures has a universal form. The 2 H NMR data clearly exhibit a ‘demixing’ between the so and lo phases below Tc . The demixing between the ld and lo phases in the DPPC/CHOL system has been inferred from calorimetry and a number of other measurements, but not from 84 M. Bloom and O.G. Mouritsen Fig. 8. Partial phase diagrams for several PC/CHOL mixtures. At low temperatures and low cholesterol (CHOL) concentrations, the system is in the gel or ‘solid-ordered’ (so) phase. As the temperature is raised, the system undergoes a transition to the liquid-crystalline or ‘liquid-disordered’ (ld) phase. At high CHOL concentrations, the system is in the ‘liquid-ordered’ (lo) phase and there is a demixing region at intermediate CHOL concentrations. The solid lines are theoretical predictions based on a model of the lipid-lipid and lipid–CHOL interactions [62] with the interaction parameters adjusted for the DPPC/CHOL system. It should be emphasized that the model parameters were not adjusted for optimum fit of the phase diagram. We show the theoretical phase diagram to emphasize its structure in relation to experimental observations. (a) The DPPC-d62 /CHOL system. The experiments were carried out on DPPC-d62 [61] which has a phase transition temperature about 4 ◦ C lower than for undeuterated DPPC. The experimental data points have been plotted at correspondingly higher temperatures to match them with the theoretical fits and to separate them more from the SEPC/CHOL data. (b) The SEPCd31 /CHOL system [64]. (c) The PPetPC-d31 /CHOL system [63, 64]. (d) The POPC-d31 /CHOL system [63, 64]. The evolution of membranes 85 NMR. It cannot be seen in the NMR spectra because of the rapid molecular exchange between the two co-existing liquid phases – or so it is believed [61, 63, 64]. Further experimental work is required on the (ld–lo) part of the phase diagram, which must be considered as tentative at this point from the experimental standpoint. In the theoretical interpretation of these phase diagrams, it is assumed that the interaction between pairs of phospholipid molecules depends on their mutual orientation and account is taken of the internal (conformational) energy of the acyl chains. In the pure lipid system, the model leads to a simultaneous discontinuity (so ↔ ld) in: (i) chain orientational order (o ↔ d, standing for order at low temperatures in the gel phase to disorder at high temperatures in the fluid phase) and (ii) two dimensional symmetry (s ↔ l, from the two-dimensional translational symmetry of the gel phase to the translationally disordered fluid phase). As described above, Konrad Bloch [53–55] has emphasized the role of evolution in producing, in CHOL, a molecule having a rigid ring structure in one face; the ‘α-face’ is ultra-smooth. This feature allows the lipid molecules in their vicinity to rotate rapidly. The effect of this is modeled theoretically [62] by assuming that this additional freedom reduces the orientation dependence of the interaction of a pair of phospholipid molecules that are both close to a CHOL molecule. Intuitively, the rotation would partially average out the orientation dependence of the interaction. The consequence of this feature of the lipid–CHOL interaction is to eliminate the gel to liquid crystalline phase transition at high (> 25%) CHOL concentrations and to produce, as a stable phase, a liquid with relatively high orientational order or, equivalently, a relatively thick membrane. The question still remains as to what biological advantages could be associated with the stabilization of the lo phase. In section 7.1, we quoted Bloch [54] as suggesting that the influence of cholesterol would be to optimize the van der Waals interaction. It is not clear whether the words that Bloch uses apply directly to the picture we have presented, but an important property of the lo phase is the enormous increase in mechanical strength over the ld phase [65], which must be at least partly due to the increased importance of the van der Waals interaction for the ordered chains in the lipid molecule. This increased strength is established while maintaining membrane fluidity and elimating the phase transition. The latter, in combination with increased bilayer thickness, reduces membrane permeability greatly [2]. 8. Lipid diversity and the physical properties of membranes The modern era of membrane study essentially began with the fluid-mosaic model [1]. One class of questions that has recurred frequently since then concerns the large diversity of lipids found in biological membranes. When one takes into account the different polar head groups and acyl chains, a given membrane may often contain well over 100 unique species. Does each lipid have a specific functional role? Or, is the diversity of lipids in many cell types and the non-random differences in lipid composition between different cell types a manifestation of accidental historical 86 M. Bloom and O.G. Mouritsen development rather than the importance of specific lipid composition? At the present time, “...there is no satisfying explanation for the observed patterns” ([1], page 32). Some of the systematics are immediately understandable in a qualitative sense in terms of optimization of physical properties. With the appreciation of the importance of membrane fluidity, it soon became clear that many organisms actually adjust their lipid composition in response to changes in environmental parameters such as temperature in order to preserve membrane fluidity ([1], page 173). For example, it is well known that lipids with saturated acyl chains have higher melting temperatures than those with unsaturated chains. The mono-unsaturated lipid POPC has a transition temperature about 50 ◦ C lower than the saturated lipid DPPC. Many organisms systematically increase (or decrease) the concentration of unsaturated relative to saturated chains in their lipids when the growth temperature is decreased (or increased). The ability of organisms to make such compositional adjustments is presumably due to the enzymes responsible for the synthesis of unsaturated chains having higher activation energies, equivalent to higher temperature-derivatives of their activation rates, than those of enzymes responsible for the synthesis of saturated chains in lipids. This selection of enzymes driven, not simply on the basis of enzymatic efficiency but also by its temperature-derivative, must have occurred via evolutionary processes. In a similar vein, we have proposed in section 7 a scenario for the optimization of the physical properties of plasma membranes of eucaryotic cells, these membranes having to satisfy special mechanical considerations because of their relatively large size. In this case, nature seems often to require a preponderance of unsaturated lipids, mono-unsaturated for red blood cells and poly-unsaturated for the central nervous system (see section 8.3). Why mono-unsaturated in one case and poly-unsaturated in the other is not known – it would seem that both satisfy fluidity needs. These plasma membranes always have large concentrations of cholesterol (≈ 30 to 50 mol%). We suggested in section 7 that this is because sterols extend the fluid range, when alloyed with di-acyl lipids, while eliminating the phase transition completely, reducing the passive permeability of membrane and greatly increasing its mechanical strength. It is likely that many other examples of lipid diversity can be explained in terms of the optimization, via evolution, of the physical properties of membranes. We close this final section with brief accounts of three explicit cases that are presently being studied in many laboratories. Each of these cases focuses on different ways in which evolutionary forces have manifested themselves in the diversity of lipids. Lipid polymorphism brings us into contact with the role of symmetry and the breaking of symmetry in membranes as well as the influence of membrane curvature. Skin lipids give an example of lipids being modified in situ in order to play slightly different physical roles as they move from deep in the skin to the surface. Brain lipids bring us into contact with an important aspect of human evolution, brain development, and how that was made possible through the availability of new types of materials, essential fatty acids. These three topics are large ones and our treatments are brief and not intended as reviews. We hope that they will encourage further work from an evolutionary perspective on the manner in which specific lipids have been used to optimize physical properties of membranes of a variety of organisms. The evolution of membranes 87 8.1. Lipid polymorphism One of the areas of modern physics research closely coupled to membrane biophysics is the study of liquid crystals, especially, that of lyotropic liquid crystals, i.e. liquid crystals whose properties depend strongly on water concentration as well as on temperature. The lipid structure of prime importance to cell biology is the fluid bilayer. Lipids that spontaneously aggregate in a bilayer structure, in combination with proteins under physiological conditions, will usually but not always, form lamellar arrays having long range order when mixed with water. Such an aggregate, when fluid, is called the (lamellar) Lα phase and is comprised of a periodic arrangement of lipid bilayers of thickness dl alternating with water layers of thickness dw to define a one-dimensional liquid crystal of repeat distance equal to dl + dw . It has been known for many years that lipid-water mixtures can give rise to a variety of liquid-crystalline structures of different symmetries. Their long range order can be elucidated using standard methods such as X-ray diffraction. In addition to the Lα phase, two types of hexagonal phases are found, corresponding to two-dimensional arrays of hexagonally coordinated cylinders in which the lipid acyl chains are oriented inside (HI ) or outside (HII ) the cylinders. As illustrated in fig. 9, the molecular origin of the hexagonal phases can be understood qualitatively [39, 66] in terms of the shapes of the lipid molecules. Not shown in fig. 9, but discussed extensively in the paper from which this figure was taken [39], is the cubic liquid crystal phase consisting of cubically coordinated cylinders, mentioned previously (see section 5) as being potentially relevant to archaebacterial membranes [38]. Cubic phases are found, for some lyotropic liquid crystalline systems, in a narrow range of water concentrations between the low concentrations at which the Lα phase is stable and the higher concentrations at which the HII phase is stable [67]. Biological membranes are not liquid crystalline in the sense of having long range order so that one does not expect to see hexagonal phases in natural materials. Still, the study of hexagonal liquid crystalline phases has been of great value in focusing on potential structural and transitional roles of local cylindrical geometry in biological function. As an illustration of a potentially important structural role played by local cylindrical geometry, consider the tight junction, which is crucial to the function of epithelial sheets. To quote from a well-known cell biology text [13]: from page 29, “The epithelial sheet has much the same significance for the evolution of complex multicellular organisms that the membrane has for the evolution of single cells.” and from page 793, “Epithelia have at least one important function in common: they give us selective permeability barriers separating fluids on each side that have different chemical compositions. Tight junctions play a doubly important role in maintaining the selective-barrier function of cell sheets”. It is clear from reading further in the text of Alberts et al. [13] that, though the structure of the tight junction is not known with any confidence at this time, the authors favour a protein-based structure for the strands that produce the symmetry breaking required to couple the two bilayers and maintain the permeability barrier required of the tight junction. Nevertheless, it has been proposed, on the basis of 88 M. Bloom and O.G. Mouritsen Fig. 10. a: diagram of a cross-section of a tight junction illustrating the offset disposition of a pair of intramembrane cylinders. b: diagram illustrating the paired offset cylinders at a tight junction strand and the different possible planes of fracture through such a junction. c: proposed organization of the phospholipids at a tight junction strand. d: diagram of phospholipids combined with freeze-fracture micrograph to show how fractures through lipid micelles could produce images characteristic of tight junctions. (From ref. [68].) freeze fracture electron microscopy [68], see fig. 10, that a pair of intramembrane Fig. 9. Structure of different anisotropic liquid crystalline phases that membrane lipids can form. (A) Normal hexagonal (HI ) phase in which the acyl chains are in the interior regions of the hexagonally coordinated cylinders; (B) lamellar (Lα ) phase; and (C) reversed hexagonal (HII ) phase in which the acyl chains are in the exterior regions of the hexagonally coordinated cylinders. We do not show the cubic liquid crystal phases discussed extensively in the paper [66] from which this figure is taken. (From ref. [39].) The evolution of membranes 89 cylinders composed of lipids, i.e. of the type that form the building blocks of the HII phase, may well be the principal structural elements comprising tight junctions. If this proposal is correct, then the structure of tight junctions represents a solution via evolutionary methods to a physical symmetry problem, how to couple lamellar structures to each other in such a way as to maintain good control of permeability between them, while the system participates in biological activity. The proposed solution makes use of the range of possibilities opened up by the variety of molecular shapes of the lipid building blocks of membranes. Note that a protein based solution to this problem would involve the same type of symmetry considerations. There have been many candidates [39, 66, 67, 69, 70] proposed as probable biological manifestations of lipid polymorphism via transitional states of membranes. As in the case of structural examples such as the one described above, most of these candidates involve a change in local symmetry, transient in this case, which is made possible by local cylindrical symmetry of the boundary between the hydrophilic and hydrophobic regions of the lipid-water interface. The fusion of pairs of liposomes, budding of small vesicles from larger liposomes, endo- and exo-cytosis are all related to each other and are examples of changes in the topology of the membrane surfaces. In the transition from one vesicle to two or vice-versa, say, there must be a transitional state in which some part of the local membrane surface deviates appreciably from lamellar symmetry. The study of such changes of topology and shapes is currently an active and important area of both theoretical and experimental study [67, 69, 70]. Though most of the work underway at the present time is on model systems, we anticipate that these studies will have an impact on our understanding of physiological processes in the near future. 8.2. Skin lipids – modification of lipids in situ to provide a water-resistant surface The properties of skin lipids give an example of how nature has evolved a structure to provide protection of large scale biological organisms from potential environmental dangers, as in water loss, microbial invasion, ultraviolet irradiation, mechanical trauma, etc. We discuss here, very briefly one of these features, water loss, to make the point that as the cells making up human skin, for example, move from the inner (dermis) to the outer (epidermis) layers (see fig. 11), systematic modifications of the lipids take place in such a way as to ensure that, among other things, the outer (stratum corneum) layer of the epidermis is relatively water repellent. This can only be done with a significant change in cellular structure as ordinary cells are relatively water permeable. The detailed structure and thermodynamic phase properties of the epidermis and its associated epidermis has yet to be worked out. Those readers wishing to learn about recent work in this field as seen by the specialists in the field should consult the literature [71]. We are indebted to Dr. Neil Kitson for his assistance in providing the following extremely simplified description of a complex system. The epidermis, though only the outer layer of the skin, is itself a composite, but is composed mainly of cells derived from one type known as keratinocytes. There are other cells but no blood vessels; a complex vascular network is located immediately beneath the epidermis in the layer known as the dermis. 90 M. Bloom and O.G. Mouritsen Fig. 11. Schematic diagram of the stratum corneum in relation to the overall geometry of the epidermis. The upper part of the figure shows its location and thickness in relation to the outer layers of the skin, with an expanded sketch indicating the type of network of diffusive paths whereby water is believed to pass through the stratum corneum. The lower part of the figure shows the manner in which terminally differentiated epidermal cells (corneocytes) in the stratum corneum are surrounded by intercellular membranes described as ‘lamellar lipid sheets’. (This figure was provided courtesy of Dr. Russell O. Potts.) The evolution of membranes 91 The epidermis operates in a manner somewhat analagous to that of blood. In the formation of blood, a population of cells (‘stem cells’) spends its time making new cells. These new cells differentiate into ‘mature’ cells (red blood cells) which are biologically useful for short periods of time, ≈ 120 days in humans [13], and are then lost, destroyed or recycled. In the case of the epidermis, the stem cells live at the bottom of the epidermis, next to the dermis, and the maturing cells move up towards the skin surface. It takes them about two weeks to move from the bottom to the top of the epidermis where they are discarded as dust. The equivalent of the red blood cell is the ‘corneocyte’, and the equivalent of the circulating blood is the stratum corneum, the very top layer of the epidermis. Corneocytes are filled with keratins (rather than hemoglobin), are glued together with intercellular connections (called desmosomes), and have between them unusual lipid lamellae that provide the waterproofing. During the maturing process that occurs between the stem cells on the bottom of the epidermis and the stratum corneum on the top, the keratinocytes make small organelles (‘lamellar bodies’) filled with lipids and lipid metabolizing enzymes. These are eventually pushed out of the cell (exocytosis) and fuse with each other to form myelin-like layers around and between the cells. This intercellular lipid is usually referred to as the ‘stratum corneum lipid’ or the ‘intercellular matrix’ or ‘intercellular domains’. It is technically difficult to analyze the lipid composition at various levels of the epidermis. However, there is general agreement in the field now that, from the time they are synthesized in the lamellar bodies until they form mature intercellular membranes, three major classes of lipid are represented in approximately equal concentrations: sphingolipids, phospholipids and sterols. During the maturing process, enzymatic modifications take place so that a recent model membrane study [72] could reasonably be based on the assumption that a better approximation to the lipid composition just before the cells are discarded is a three-component equimolar mixture of free fatty acid, ceramide and cholesterol. The study of skin lipids may have a special twist with regard to evolution. After nature had evolved soft materials, it had to find a way of modifying them so as to provide the special protective features of the skins of animals while using the soft materials as a starting point. The precise way in which this has been done is still not really known, but should be amenable to study using modern physical methods. 8.3. Essential fatty acids and brain lipids The role of dietary fatty acids in the evolution of the human brain has been reviewed by Crawford [73] and discussed in relation to a broad evolutionary perspective in a book aimed at the general reader [74]. In this section we present a brief account of some of the arguments made in the review. An important feature of this discussion is that brain lipids contain a very large fraction of poly-unsaturated fatty acyl chains, which mammals are unable to synthesize because they lack the enzymes to introduce double bonds at carbon bonds beyond C-9 in the fatty acid chain [75]. In humans the poly-unsaturated lipids that seem to be of importance to the central nervous system are formed via elongation and desaturation from essential fatty acids (EFA), 92 M. Bloom and O.G. Mouritsen where the word essential means that they must be supplied in the diet and cannot be endogenously synthesized. The EFA’s occur as linoleic (n-6) and α-linoleic (n-3) acids, which are elongated and desaturated from 18-carbon chain lengths with two or three double bonds to 20- and 22-carbon chain lengths with four and six double bonds. Since the human brain evolved quite recently on the evolutionary time scale, it will be interesting to understand the physical role played by these poly-unsaturated fatty acids in brain lipids, and why nature evolved a brain system dependent on material derived from the local environment rather than being under the control of the cellular synthetic apparatus. The book by Crawford and Marsh [74] examines the consequences, in evolutionary ‘theory’, of important steps “being dictated by chemistry and physics responding to the prevailing conditions”. Such considerations would, for example, play a role in the relatively simple case of magnetotactic bacteria described in section 2 of this article, where the size distribution of the bacterial magnets is undoubtedly a reflection of the amount of iron available in the bacterial diet. As expressed by Crawford [73]: “Brain chemistry is characterized by two unique features. First, the brain maintains a constant flow of ionic and electrical information without which it dies. Its sophisticated communication network is achieved by transmembrane transfer systems with an outstandingly heavy investment in lipid biotechnology: some 60% of its structural material is lipid. Brain lipid is composed of cholesterol and phosphoglycerides that are rich in the preformed fatty acids, primarily arachidonic and docosahexaenoic (DHA), and not the parent EFAs. For instance, in the rat, the 20- and 22-carbon long-chain fatty acids are incorporated into the developing brain ten times more efficiently than are the parent EFAs. Most experts agree that both n-6 and n-3 families of fatty acids are required in the diet. It is also clear that the dietary supply of EFAs is limiting for brain growth”. Some of the physical implications of the correlations discussed by Crawford [73], deserve serious study from an evolutionary perspective: e.g., correlations between the use of DHA and the emergence of important biological features in human evolution such as the development of a photoreceptor, synaptic membranes and the role of inositol phosphoglyceride in its involvement with calcium transport. Such studies could lead to a better understanding of past and future human evolution. In addition, the inherent limitations in brain development now known to result from maternal malnutrition due to inadequate supplies of EFAs in the diet of babies in some third world countries provides a social motivation for serious study of how EFAs influence the physical aspects of fundamental processes in the central nervous system. Acknowledgements We wish to thank Neil Kitson and Jenifer Thewalt for helpful contributions to this article. References 1. 1a. Gennis, R.B., 1989, Biomembranes (Springer, New York). Kuhn, H. and Jürg Waser, 1983, Evolution, in: Biophysics, eds W. Hoppe, W. Lohmann, H. Markl and H. Ziegler (Springer, Berlin) p. 830. The evolution of membranes 2. 3. 4. 5. 6. 7. 8. 9. 10. 10a. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 93 Bloom, M., E. Evans and O.G. Mouritsen, 1991, Physical properties of the fluid lipid-bilayer component of cell membranes: A perspective, Q. Rev. Biophys. 24, 293–297. Purcell, E.M., 1977, Life at low Reynolds number, Am. J. Phys. 45, 3–11. Berg, H.C. and E.M. Purcell, 1977, Physics of chemoreception, Biophys. J. 20, 193–219. Berg, H.C., 1983, Random Walks in Biology (Princeton Univ. Press, Princeton, NJ). Berg, H.C., 1988, A physicist looks at bacterial chemotaxis, in: Cold Harbor Symposia on Quantitative Biology LIII, 1–9. Blakemore, R.P. and R.B. Frankel, 1981, Magnetic navigation in bacteria, in: Sci. Am., December, pp. 58–65. Shapere, A. and F. Wilczek, 1987, Self propulsion at low Reynolds number, Phys. Rev. Lett. 58, 2051–2054. See also 1989, J. Fluid Mech. 198, 557–585 and 587–599. Gorby, Y.A., T.J. Beveridge and R.P. Blakemore, 1988, Characterization of the bacterial magnetosome membrane, J. Bacteriol. 170, 834–841. Mann, S. and R.B. Frankel, 1989, Magnetite biomineralization in unicellular microorganisms, in: Biomineralization – Chemical and Biochemical Perspectives, eds S. Mann, J. Webb and R.J.P. Williams (VCH Verlagsgesellschaft GmbH, Weinheim, Germany) p. 389. Wächterhäuser, G., 1988, Microbiol. Rev. 52, 452–484. Deamer, D.W., 1985, Surface structures are formed by organic components of the Murchison carbonaceous chondrite, Nature 317, 792–794. Morowitz, H.J., B. Heinz and D.W. Deamer, 1988, The chemical logic of a minimum protocell, Origins Life 18, 281–287. Alberts, A., D. Bray, J. Lewis, M. Raff, K. Roberts and J.D. Watson, 1989, Molecular Biology of the Cell, 2nd edition (Garland, New York). Deamer, D.W., 1986, Role of amphiphilic compounds in the evolution of membrane structure on the early Earth, Origins Life 17, 3–25. Deamer, D.W. and R.M. Pashley, 1989, Amphiphilic components of the Murchison carbonaceous chondrite: Surface properties and membrane formation, Origins Life 19, 21–38. Morgan, J., 1991, In the beginning, Sci. Am. 257(2), 116–125. Dyson, F.J., 1985, Origins of Life (Cambridge Univ. Press, Cambridge). Morowitz, H.J., 1992, Beginnings of Cellular Life (Yale Univ. Press, New Haven, CT). Woese, C.R., 1987, Bacterial evolution, Microbiol. Rev. 51, 221–271. Cavalier-Smith, T., 1975, The origin of nuclei and of eucaryotic cells, Nature 256, 463–468. Cavalier-Smith, T., 1987, The origin of eucaryote and archaebacterial cells, Ann. NY Acad. Sci. 503, 17–54. Lake, J.A., 1989, Origin of the Eukaryotic nucleus: eukaryotes and eocytes are genotypically related, Can. J. Microbiol. 35, 109–118. Rivera, M.C. and J.A. Lake, 1992, Evidence that eukaryotes and eocyte prokaryotes are immediate relatives, Nature 257, 74–76. Woese, C.R., O. Kandler and M.L. Wheelis, 1992, Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria and Eucharya, Proc. Nat. Acad. Sci. USA 87, 4576–4579. Wheelis, M.L., O. Kandler and C.R. Woese, 1992, On the nature of global classification, Proc. Nat. Acad. Sci. U.S.A. 89, 2930–2934. Knoll, A.H., 1992, The early evolution of eukaryotes: A geological perspective, Science 256, 622–627. Margulis, L., 1975, Origin of Eucaryotic Cells (Yale Univ. Press, New Haven, CT). Woese, C.R. and R.S. Wolfe, 1985, Archaebacteria: The Urkingdom, in: The Bacteria, Vol. 8, eds C.R. Woese and R.S. Wolfe (Academic Press, Orlando, FL) p. 459. Ragan, M.A., 1988, Ribosomal RNA and the major lines of evolution: A perspective, Biosystems 21, 177–188. Cavalier-Smith, T., 1981, The origin and early evolution of the eucaryotic cell, in: Molecular and Cellular Aspects of Microbial Evolution, eds M.J. Carlile, J.F. Collins and B.E.B. Moseley (Cambridge Univ. Press, Cambridge) p. 33. Knoll, A.H., 1991, End of the proterozoic eon, in: Sci. Am., Oct. 1991, pp. 64–73. 94 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. 52. 53. 54. 55. 56. 57. 58. M. Bloom and O.G. Mouritsen Gould, S.J., 1989, Wonderful Life: The Burgess Shale and the Nature of History (Norton and Co., New York). Kandler, O. and H. König, 1985, Cell envelopes of Archaebacteria, in: The Bacteria, Vol. 8, eds C.R. Woese and R.S. Wolfe (Academic Press, Orlando, FL) p. 413. Langworthy, T.A., 1985, Lipids of Archaebacteria, in: The Bacteria, Vol. 8, eds C.R. Woese and R.S. Wolfe (Academic Press, Orlando, FL) p. 459. de Rosa, M., A. Gambacorta and A. Gliozzi, 1986, Structure, biosynthesis, and physicochemical properties of archaebacterial lipids, Microbiol. Rev. 50, 70–80. Lo, S.-L. and E.L. Chang, 1990, Purification and characterization of a liposomal-forming tetraaether lipid fraction, Biochem. Biophys. Res. Commun. 167, 228–243. Gulik, A., V. Luzzati, M. de Rosa and A. Gambacorta, 1985, Structure and polymorphism of bipolar isopranyl ether lipids from archaebacteria, J. Mol. Biol. 182, 131–149. Luzzati, V.I., A. Gulik, M. de Rosa and A. Gambacorta, 1987, Lipids from Sulfolobus solfataricus. Life at high temperatures and the structure of membranes, Chem. Scr. B 27, 211–219. Lindblom, G. and L. Rilfors, 1989, Cubic phases and isotropic structures formed by membrane lipids – possible biological relevance, Biochim. Biophys. Acta 988, 221–256. Sackmann, E., 1990, Molecular and global structure and dynamics of membranes and lipid bilayers, Can. J. Phys. 68, 999–1011. Henderson, R. and P.N.T. Unwin, 1975, Three-dimensional model of purple membrane obtained by electron microscopy, Nature (London) 257, 28–32. Henderson, R., 1981, Membrane protein structure, in: Membranes and Intermolecular Communications, eds R. Balian, M. Chabre and P.F. Devaux (North-Holland, Amsterdam) p. 231. Deisenhofer, J. and H. Michel, 1989, The photosynthetic reaction center from the purple bacterium Rhodopseudomonas viridis, Science 245, 1463–1473. Henderson, R., J.M. Baldwin, T.A. Ceska, F. Zemlin, E. Beckmann and K.H. Downing, 1990, Model for the structure of Bacteriorhodopsin based on high-resolution electron cryo-microscopy, J. Mol. Biol. 213, 899–929. Kyte, J. and R.F. Doolittle, 1982, A simple method for displaying the hydropathic character of a protein, J. Mol. Biol. 157, 105–132. Guy, H.R., 1985, Amino side chain partition energies and distribution of residues in soluble proteins, Biophys. J. 47, 61–70. Eisenberg, D., 1984, Three-dimensional structure of membrane and surface proteins, Annu. Rev. Biochem. 53, 595–623. Mouritsen, O.G. and M. Bloom, 1984, Mattress model of lipid-protein interactions in membranes, Biophys. J. 46, 141–153. Mouritsen, O.G. and M. Bloom, 1993, Models of lipid–protein interactions in membranes, Annu. Rev. Biophys. Biophys. Chem. 22, 145–171. Sackmann, E., 1984, Physical basis of trigger processes and membrane structures, in: Biological Membranes, Vol. 5, ed. D. Chapman (Academic Press, Orlando, FL) p. 105. Nezil, F.A. and M. Bloom, 1992, Combined influence of cholesterol and synthetic amphiphilic peptides upon bilayer thickness in model membranes, Biophys. J. 61, 1176–1183. Davis, J.H., D.M. Clare, R.S. Hodges and M. Bloom, 1983, Interaction of a synthetic amphiphilic polypeptide and lipids in a bilayer structure, Biochemistry 22, 5298–5305. Bloch, K., 1965, The biological synthesis of cholesterol, Science 150, 19–28. Bloch, K., 1983, Sterol structure and membrane function, CRC Crit. Rev. Biochem. 14, 47–92. Bloch, K., 1985, Cholesterol, evolution of structure and function, in: Biochemistry of Lipids and Membranes, eds D.E. Vance and J.E. Vance (Benjamin Cummings, New York) p. 1. Rohmer, M., P. Bouvier and G. Ourisson, 1979, Molecular evolution of biomembranes: Structural equivalents and phylogenetic precursors to sterols, Proc. Nat. Acad. Sci. USA 76, 847–851. Ourisson, G., P. Albrecht and M. Rohmer, 1984, The microbial origin of fossil fuels, Sci. Am. 250(8), 44–51. Krajewski-Bertrand, M.-A., A. Milon, Y. Nakatani and G. Ourisson, 1992, The interaction of various cholesterol ‘ancestors’ with lipid membranes: A 2 H-NMR study on oriented bilayers, Biochim. Biophys. Acta 1105, 213–220. The evolution of membranes 95 58a. Jahnke, L.L. and P.D. Nichols, 1986, J. Biol. Chem. 167, 238–242. 59. Dahl, C.E., J.S. Dahl and K. Bloch, 1980, Effect of alkyl-substituted precursors of cholesterol on artificial and natural membranes and on the viability of ‘Mycoplasma capricolum’, Biochemistry 19, 1462–1467. 60. Bloom, M. and O.G. Mouritsen, 1988, The evolution of membranes, Can. J. Chem. 66, 706–712. 61. Vist, M.R. and J.H. Davis, 1990, Phase equilibria of Cholesterol/DPPC mixtures: 2 H-NMR and differential scanning calorimetry, Biochemistry 29, 451–464. 62. Ipsen, J.H., G. Karlstrom, O.G. Mouritsen, H.W. Wennerström and M.J. Zuckermann, 1987, Phase equilibria in the phosphatidylcholine-cholesterol system, Biochim. Biophys. Acta 905, 162–172. 63. Thewalt, J.L. and M. Bloom, 1993, Phosphatidylcholine: Cholesterol phase diagrams, Biophys. J. 63, 1176–1181. 64. Thewalt, J.L., C.E. Hanert, F.M. Linseisen, A.J. Farrall and M. Bloom, 1992, Lipid-sterol interactions and the physical properties of membranes, Acta Pharm. 42, 9–23. 65. Needham, D. and R.S. Nunn, 1990, Cohesive properties (elastic deformation and failure) of lipid bilayer membranes containing cholesterol, Biophys. J. 58, 997–1009. 66. Cullis, P.R., M.J. Hope, B. de Kruijff, A.J. Verkleij and C.P.S. Tilcock, 1985, Structural properties and functional roles of phospholipids in biological membranes, in: Phospholipids and Cellular Regulation, Vol. 1, ed. J.F. Kuo (CRC Press, Boca Raton, FL). 67. Gruner, S.M., 1989, Stability of lyotropic phases with curved interfaces, J. Phys. Chem. 93, 7562–7570. 68. Kachar, B. and T.S. Reese, 1982, Evidence for the lipidic nature of tight junction strands, Nature 296, 464–466. 69. Lipowsky, R., 1991, The conformation of membranes, Nature 349, 475–481. 70. Lipowsky, R., 1992, The physics of flexible membranes, Solid State Phys. 32, 19–44. 71. Elias, P.M., 1989, The stratum corneum as an organ of protection: old and new concepts, Curr. Probl. Dermatol. 18, 10–21. 72. Thewalt, J., N. Kitson, C. Araujo, A. MacKay and M. Bloom, 1992, Models of stratum corneum intercellular membranes: The sphingolipid headgroup is a determinant of phase behavior in mixed lipid dispersions, Biochem. Biophys. Res. Commun. 188, 1247–1252. 73. Crawford, M.A., 1992, The role of dietary fatty acid in biology: Their place in the evolution of the human brain, Nutr. Rev. 50, 3–11. 74. Crawford, M.A. and D.E. Marsh, 1989, The Driving Force (Heinemann, London). 75. Stryer, L., 1981, Biochemistry, 2nd edition (Freeman, San Francisco), p. 403.