* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download powerpoint
Medical genetics wikipedia , lookup
Gene expression programming wikipedia , lookup
Non-coding DNA wikipedia , lookup
Primary transcript wikipedia , lookup
DNA methylation wikipedia , lookup
DNA vaccination wikipedia , lookup
No-SCAR (Scarless Cas9 Assisted Recombineering) Genome Editing wikipedia , lookup
Long non-coding RNA wikipedia , lookup
Cell-free fetal DNA wikipedia , lookup
Epigenetics in learning and memory wikipedia , lookup
Point mutation wikipedia , lookup
Bisulfite sequencing wikipedia , lookup
History of genetic engineering wikipedia , lookup
Vectors in gene therapy wikipedia , lookup
Skewed X-inactivation wikipedia , lookup
Transgenerational epigenetic inheritance wikipedia , lookup
Epigenetics in stem-cell differentiation wikipedia , lookup
Y chromosome wikipedia , lookup
Epigenomics wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Microevolution wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Epigenetics of diabetes Type 2 wikipedia , lookup
Behavioral epigenetics wikipedia , lookup
Therapeutic gene modulation wikipedia , lookup
Designer baby wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Mir-92 microRNA precursor family wikipedia , lookup
Epigenetic clock wikipedia , lookup
Epigenetics wikipedia , lookup
Cancer epigenetics wikipedia , lookup
Oncogenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Neocentromere wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Genome (book) wikipedia , lookup
X-inactivation wikipedia , lookup
Nutriepigenomics wikipedia , lookup
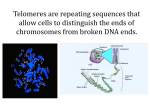
Telomeres and epigenetics The structure of mammalian telomeres Telomeres contain a double-stranded DNA region of TTAGGG repeats (green arrows) Telomeres are characterized by a 150–200-nt long singlestranded overhang of the G-rich strand (G-strand overhang; blue arrows) Telomerase recognizes the 3' OH at the end of the G-strand overhang, leading to telomere elongation. Telomerase enzyme (Cc) Ribonuleoprotein TERT, telomerase reverse transcriptase TERC, telomerase RNA component (single RNA molecule provides an AAUCCC (in mammals) template to guide the insertion of TTAGGG DKC1, dyskeratosis congenita 1 Components of telomeric proteins Two main protein complexes are bound to telomeres, the telomere repeat binding factor 1 and 2 complexes, TRF1 and TRF2 The components of the telomere repeat binding factor 1 (TRF1) (Ca) and 2 (TRF2) (Cb) complexes and are shown. human diseases in which expression of these components has been shown to be altered are indicated A telomere in a T-loop conformation Strand invasion of the Gstrand overhang is highlighted in red This conformation might prevent the access of telomerase to the 3' OH at the chromosome end. Telomerase and telomere length in ageing Most somatic cells show progressive telomere shortening owing to low or absent telomerase activity leads to critically short telomeres, which triggers a DNA damage response that results in chromosomal end-to-end fusions or cell arrest and apoptosis. thought to contribute to the onset of degenerative diseases including human premature ageing syndromes Telomerase and telomere length in tumourigenesis Tumour cells have shorter telomeres than normal cells However, telomerase is reactivated in more than 90% of all types of human tumours therapeutic strategies aimed at inhibiting telomerase will preferentially kill tumour cells and have no toxicity on normal cells. Premature ageing syndromes with short telomeres Ataxia telangiectasia (ATM) Werner syndrome (WRN); Bloom syndrome (BLM); Dyskeratosis congenita (DKC1, Terc); aplastic anaemia (Terc, Tert); Fanconi anaemia (FANC genes); Nijmegen breakage syndrome (NBS); and ataxia telangiectasia-like disorder (MRE11). Ataxia Telangiectasia Staggering gait, muscular unco-ordination Immunodeficiency Neurodegeneration premature aging Skin sensitivity to ionizing radiation susceptibility to certain types of cancer (breast cancer) A-T is fatal in the second or third decade of life Ataxia Telangiectasia Mutations in ATM gene located on chromosome 11q22-q23 encodes large protein kinase involved in cell cycle checkpoint and genotoxic stress responses Homozygous ATM mutant alleles rare ~ 1/40,000 heterozygous carriers ~ 1-2% Carriers exhibit intermediate sensitivity to radiation and predisposition to cancer (implications for radiotherapy) Werner syndrome (WRN short stature (common from childhood on) wrinkled skin baldness, cataracts, muscular atrophy tendency to diabetes mellitus gene WRN mapped to chromosome 8 predicted helicase belonging to the RecQ family FANCONI/BRCA1/BRCA2/ATM/NBS1 PATHWAY Epigenetics epigenetic modification of DNA in mammals is methylation of cytosine at position C5 in CpG dinucleotides Other main group is epigenetic posttranslational modification of histones Genomic imprinting defined as an epigenetic modification of a specific parental chromosome in the gamete or zygote that leads to differential expression of the two alleles of a gene in the somatic cells of the offspring. Differential expression can occur in all cells, or in specific tissues or developmental stages. About 80 genes are known to be imprinted Loss of imprinting (LOI) disruption of imprinted epigenetic marks through gain or loss of DNA methylation, or simply the loss of normal allele-specific gene expression. Epigenetics – differential imprinting Prader-Willi syndrome Angelman syndrome failure to thrive during infancy, hyperphagia and obesity during early childhood, mental retardation, and behavioural problems abnormal gait speech impairment, seizures, mental retardation inappropriate happy demeanor that includes frequent laughing, molecular defect involves a paternally imprinted domain at 15q11–q13 smiling, excitability defect lies within the maternally imprinted domain at 15q11–q13 Genetic causes Prader-Willi syndrome Angelman syndrome 70% have a deletion of the PWS/AS region on their paternal chromosome 15 70% have a deletion of the PWS/AS region on their maternal chromosome 15 25% have maternal uniparental disomy for chromosome 15 (the individual inherited both chromosomes from the mother, and none from the father) 7% have paternal uniparental disomy for chromosome 15 (the individual inherited both chromosomes from the father, and none from the mother) 5% have an imprinting defect <1% have a chromosome abnormality including the PWS/AS region 3% have an imprinting defect 11% have a mutation in UBE3A 1% have a chromosome rearrangement 11% have a unknown genetic cause DNA methylation and cancer Changes in methylation are early events in tumorigenesis In tumour cells, repeat-rich heterochromatin becomes hypermethylated and this contributes to genomic instability, a hallmark of tumour cells, through increased mitotic recombination events. De novo hypomethylation of CpG islands also occurs in cancer cells, and can result in the transcriptional silencing of growth-regulatory genes. Region of the genome showing repeat-rich, hypermethylated pericentromeric heterochromatin and tumour suppressor gene (TSG) associated with a hypomethylated CpG island (red). References 1) Telomeres and human disease by M Blasco Nature Reviews Genetics Aug 2005 vol 6 pp611 2) DNA methylation and human disease by KD Robertson Nature Reviews Genetics Aug 2005 vol 6 pp 597