* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 1
Biochemistry wikipedia , lookup
P-type ATPase wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein moonlighting wikipedia , lookup
Mechanosensitive channels wikipedia , lookup
Membrane potential wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Protein adsorption wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Signal transduction wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Lipid bilayer wikipedia , lookup
Proteolysis wikipedia , lookup
Theories of general anaesthetic action wikipedia , lookup
SNARE (protein) wikipedia , lookup
List of types of proteins wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Model lipid bilayer wikipedia , lookup
Cell membrane wikipedia , lookup
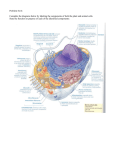
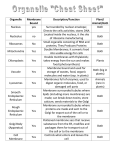
Lecture 19: Membrane Proteins Architecture of Membrane Proteins Fluid Mosaic Model Protein Targeting Membrane Proteins are Responsible for Most Membrane Functions Lipid bilayers can form closed compartments. Biological membranes have specific functions and these are carried out by membrane proteins. Approximately one-third of genomes code for membrane proteins. Lipid Bilayer Biological Membrane Different Membranes have Different Protein Compositions SDS-PAGE gel of three different membranes The different capabilities of membranes are conferred by membrane proteins associated with that membrane. Some membrane protein functions: Pumps and Channels: Transport or diffusion of substances across membranes. Receptors: Receive extracellular signals (eg hormones) and transmit information across membranes. Transducers: Interconvert energy from one form to another. Enzymes: Carry out chemical reactions. A: Erythrocyte plasma membrane B: Photoreceptor membranes of retina C: Muscle sarcoplasmic reticulum Bilayer-Protein Associations Membrane proteins can be associated with the lipid bilayer in various ways. The two sides of a membrane are different- membrane proteins are oriented. Proteins can span the membrane (transmembrane proteins) or be associated with one side through interactions with other proteins or with lipids. Peripheral membrane proteins (d,e) are loosely associated with membrane or other proteins through polar and charged interactions. These can be solubilized by disrupting such interactions, eg by salt or pH changes. Integral membrane proteins (a,b,c) are tightly associated with bilayer through hydrophobic interactions with lipids and can only be removed by detergents or organic solvents. They are insoluble in water. Structural Basis of Membrane Protein-Bilayer Interactions The absence of hydrogen-bonding groups inside the bilayer limits the types of structure that can exist there. Membrane proteins must satisfy their own hydrogen-bonding groups. Transmembrane a-helices: Some transmembrane proteins have alpha-helices that are sufficiently long to span the membrane. The outer surfaces of these helices interact with the lipid core of the membrane. Transmembrane b-strands: Membranes can also be spanned by beta-sheets in which case the outside of the sheet interacts with the lipids of the bilayer. Hydrophobic surfaces: Some proteins associate with the bilayer through hydrophobic areas on their surfaces which protrude into the bilayer but do not span it. Covalent attachment of hydrophobic “anchor”: Otherwise soluble proteins are sometimes tethered to membranes by covalent attachment of a hydrophobic group. Transmembrane a-Helices Transmembrane a-helices are the most common type of structure in membrane proteins. With a rise of 1.5 Angstrom/residue, approximately 30 helical residues are required to span a ~45 Angstrom membrane. The hydrocarbon core of the membrane is ~30 Angstroms, requiring 20 helical residues to span it. Transmembrane a-helices are comprised primarily of hydrophobic residues. Buried polar residues are unfavorable. Transmembrane helix Whole Bilayer ~40 Angstroms ~27 residues ~ 7-8 turns Hydrocarbon Core ~30 Angstroms ~20 residues ~5-6 turns Bacteriorhodopsin Bacteriorhodopsin absorbs light energy and uses it to pump protons across the plasma membrane of halophilic bacteria, moving ions from a lower concentration to an area of higher concentration. It consists of a bundle of 7 hydrophobic helices. Light energy H+ H+ Higher Concentration H+ Membrane Lower Concentration H+ Helical regions are in yellow- charged residues are pink. Transmembrane b-Sheets Another common architecture of membrane proteins is the b-barrel, in which the polypeptide backbone repeatedly crosses the bilayer as part of a b-sheet. With a 3.5 Angstrom distance between Ca positions, a b-strand would require a minimum of about 9 residues to cross the hydrophobic core of a lipid bilayer. However, in most barrels the strands are not oriented perpendicular to the membrane so more residues are required to span it. Transmembrane b-strand Whole Bilayer ~40 Angstroms 11-12 residues Hydrocarbon Core ~30 Angstroms ~9 residues Porin Higher Concentration Membrane Lower Concentration This porin is an example of a b-barrel membrane protein. It forms a continuous b-sheet of 16 transmembrane strands. The interior is a water-filled channel or pore that allows small molecules to diffuse passively through the membrane. The interior is lined with hydrophilic residues whereas the side of the sheet facing the lipids is hydrophobic. Hydrophobic residues in yellow. Hydrophobic Surfaces Proteins can associate with membranes through hydrophobic patches or protrusions that insert into the hydrocarbon core of the lipid bilayer. Polar part Hydrophobic part In general, the parts of membrane proteins that interact with the hydrocarbon core of membranes are covered with hydrophobic side-chains and the parts that are in contact with the surrounding aqueous solution are covered with hydrophilic side-chains. The lack of hydrogen bonding groups in the membrane interior makes it very unfavorable to have buried but unsatisfied hydrogen-bonding groups. Prostaglandin H2 synthase Prostaglandin H2 synthase-1 converts the fatty acid arachidonic acid (generated by hydrolysis of lipids) into Prostaglandin H2, which is a precursor of other prostaglandins involved in the inflammatory response, pain and fever. The enzyme is firmly attached to the membrane though an alpha-helical “knob” lined with hydrophobic residues that protrudes into the hydrocarbon core. Arachidonate can enter the active site of the enzyme through a hydrophobic channel in this “knob”. This enzyme is the site of action of aspirin, which acetylates a serine residue in the interior of this channel, blocking the entry of arachidonate and preventing the formation of prostaglandin H2. In turn the inflammatory response is inhibited. Covalent Attachment of Hydrophobic Anchors: Soluble protein Membrane anchor Otherwise soluble proteins can be tethered to the membrane by covalent attachment of hydrophobic groups to the protein. An example is the attachment of a palmitoyl group through a thioester bond to cysteine side-chains. Specific enzymes carry out these modifications by recognizing specific target sequences near the modification site. Detection of Transmembrane Helices by Sequence Analysis Membrane proteins comprise perhaps a third of all proteins. But analysis of the their structures is difficult. Of ~27,000 protein structures known, only about 30 are membrane proteins. To a greater extent than for globular proteins, we must rely on sequence analysis to predict structural features of membrane proteins. The location of transmembrane helices in a membrane protein can often be predicted from sequence information. Polarity Scales To quantify the likelihood of a given residue being found in a transmembrane helix, one way is to determine the difference in energy between a side-chain in the membrane interior and the same side-chain in water. The more hydrophilic the side-chain, the more favorable the transfer. Since many residues in a row must be hydrophobic in a transmembrane helix, summing the energy for all the residues in a contiguous segment gives a fairly reliable estimate of whether that segment is likely to contain a transmembrane helix. Plotting such a sum in a “sliding window” over the length of the chain gives a hydropathy plot. Glycophorin contains a single transmembrane helix. A hydropathy plot for glycophorin shows a significant peak near the sequence position of this transmembrane helix. By contrast a hydropathy plot for porin, which has no transmembrane helices, does not show a significant peak anywhere. Lipids Can Diffuse Freely in the Plane of the Membrane Lipids have high mobility in the plane of the membrane but rarely cross to the other side of the bilayer. Studies with fluorescently labeled lipids in which a local area is photobleached show that new fluorescent lipids rapidly diffuse into the bleached area. Membrane Fluidity Membranes must remain fluid and flexible for membrane proteins to function properly. However, at low temperatures, lipids can undergo a phase transition in which they “freeze” into an ordered and rigid state. Lipids with longer hydrocarbon chains tend to have higher transition temperatures, and unsaturated hydrocarbon chains with cis double bonds tend to have lower transition temperatures due less favorable packing. Bacteria control the fluidity of their membranes by regulating the fraction of lipids with longer hydrocarbon chains or cis double bonds. Animals regulate the cholesterol content of their membranes to achieve similar control over membrane fluidity. The Fluid Mosaic Model The fluid mosaic model proposes that biological membranes are asymmetric 2-dimensional fluids. The lipids are free to diffuse in the plane of the membrane and serve as a solvent for hydrophobic membrane proteins, which can also diffuse in the plane of the membrane. At the same time the membrane is a permeability barrier for polar and charged substances. Proteins are Targeted to Different Cellular Compartments by Signal Sequences The amino acid sequences of proteins contain specific sequences which are recognized by receptors and pores on particular membranes which lead to their being imported into a given compartment or embedded in that membrane. Lysozome Mitochondria Golgi complex Endoplasmic Reticulum Peroxisome Nucleus Plasma membrane Placing a nuclear localization sequence on a cytoplasmic protein causes it to be imported into the nucleus. Specific proteins recognize the localization sequences and bind them, initiating the import process. Proper targeting of proteins is necessary for them to perform their functions. Summary: Different membranes have different protein contents which confer their different capabilities. Membrane proteins associate with lipid bilayers in a variety of ways. In some cases the amino acid sequence can suggest structural features in such proteins. Proteins have internal sequence codes for the organelle to which they should be targeted. Key Concepts: Peripheral and Integral membrane proteins Architecture of membrane proteins Hydropathy plots Fluid mosaic model Targeting sequences