* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
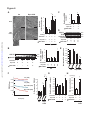
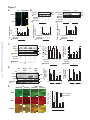
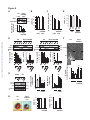
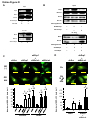
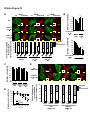
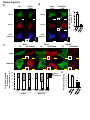
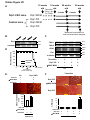
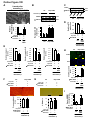
Download Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects
Cell membrane wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell encapsulation wikipedia , lookup
Signal transduction wikipedia , lookup
Cellular differentiation wikipedia , lookup
Cell culture wikipedia , lookup
Endomembrane system wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cell growth wikipedia , lookup
Cytokinesis wikipedia , lookup
Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects the Heart Against Energy Stress Yoshiyuki Ikeda1, Akihiro Shirakabe1, Yasuhiro Maejima1, Peiyong Zhai1, Sebastiano Sciarretta1,2, Jessica Toli1, Masatoshi Nomura3, Katsuyoshi Mihara4, Kensuke Egashira5,6, Mitsuru Ohishi7, Maha Abdellatif1, Junichi Sadoshima1 1 Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Department of Cell Biology and Molecular Medicine, Cardiovascular Research Institute, Rutgers New Jersey Medical School, Newark, New Jersey 07103, USA; 2IRCCS Neuromed, Pozzilli, Italy; 3 Department of Medicine and Bioregulatory Science, Kyushu University, Japan; 4Department of Molecular Biology, Graduate School of Medical Science, Kyushu University, Japan; 5Department of Cardiovascular Medicine, Kyushu University Hospital, Japan; 6Department of Cardiovascular Research, Development, and Translational Medicine, Graduate School of Medical Science, Kyushu University Hospital; 7Department of Cardiovascular Medicine and Hypertension, Graduate School of Medical and Dental Science, Kagoshima University, Japan Running title: Drp1 Mediates Autophagy Subject codes: [131] Apoptosis [138] Cell signaling/signal transduction [140] Energy metabolism Address correspondence to: Dr. Junichi Sadoshima Department of Cell Biology and Molecular Medicine Cardiovascular Research Institute Rutgers New Jersey Medical School 185 South Orange Ave., MSB G609 Newark, NJ 07103 Tel: 973-972-8619 Fax: 973-972-8919 [email protected] In September, 2014, the average time from submission to first decision for all original research papers submitted to Circulation Research was 14.29 days. DOI: 10.1161/CIRCRESAHA.116.303356 1 ABSTRACT Rationale: Both fusion and fission contribute to mitochondrial quality control. How unopposed fusion affects survival of cardiomyocytes (CMs) and left ventricular (LV) function in the heart is poorly understood. Objective: We investigated the role of Dynamin-related protein 1 (Drp1), a GTPase that mediates mitochondrial fission, in mediating mitochondrial autophagy, ventricular function, and stress resistance in the heart. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Methods and Results: Drp1 downregulation induced mitochondrial elongation, accumulation of damaged mitochondria, and increased apoptosis in CMs at baseline. Drp1 downregulation also suppressed autophagosome formation and autophagic flux at baseline and in response to glucose deprivation in CMs. The lack of lysosomal translocation of mitochondrially-targeted Keima indicates that Drp1 downregulation suppressed mitochondrial autophagy. Mitochondrial elongation and accumulation of damaged mitochondria were also observed in tamoxifen-inducible cardiac-specific Drp1 knockout (Drp1-CKO) mice. Following Drp1 downregulation, Drp1-CKO mice developed LV dysfunction, preceded by mitochondrial dysfunction, and died within 13 weeks. Autophagic flux is significantly suppressed in Drp1-CKO mice. Although LV function in cardiac-specific Drp1 heterozygous KO (Drp1-hetCKO) mice was normal at 12 weeks of age, LV function decreased more severely after 48 hours of fasting and the infarct size/area at risk after ischemia/reperfusion (I/R) was significantly greater in Drp1-hetCKO than in control mice. Conclusions: Disruption of Drp1 induces mitochondrial elongation, inhibits mitochondrial autophagy, and causes mitochondrial dysfunction, thereby promoting cardiac dysfunction and increased susceptibility to I/R. Keywords: Mitochondria, Drp1, heart, mitochondrial autophagy, ischemia/reperfusion. Nonstandard Abbreviations and Acronyms: Ad adenovirus Ad-shAtg7 adenovirus harboring Atg7 shRNA Ad-shDrp1 adenovirus harboring Drp1 shRNA Ad-shScr adenovirus harboring scramble shRNA Ad-tf-LC3 adenovirus harboring tandem fluorescent mRFP-GFP-LC3 MHC alpha myosin heavy chain CM cardiomyocyte COX IV cytochrome c oxidase subunit IV CsA cyclosporin A Drp1 dynamin-related protein 1 Drp1-CKO cardiac-specific conditional Drp1 knockout Drp1-hetCKO cardiac-specific heterozygous Drp1 knockout EM electron microscopic/microscopy GD glucose deprivation Keima-MLS Keima with mitochondrial localization signal I/R ischemia/reperfusion LV left ventricular Mfn Mitofusin MI/AAR infarct size/area at risk mPTP mitochondrial permeability transition pore DOI: 10.1161/CIRCRESAHA.116.303356 2 OCR PGC-1 ROS Tg-tf-LC3 TI oxygen consumption rate peroxisome proliferator-activated receptor-gamma coactivator 1 reactive oxygen species transgenic mice expressing mRFP-GFP-LC3 tamoxifen injection INTRODUCTION The heart muscle is characterized by a large volume of mitochondria due to its high energy demand . Mitochondria produce ATP primarily by utilizing the electrochemical gradient formed by electron transfer via the electron transport chain located on the inner mitochondrial membrane. However, electron leakage from the electron transport chain and production of O2- and H2O2, which arises from dismutation of O2-, occur constantly as byproducts of ATP synthesis, making mitochondria a major source of reactive oxygen species (ROS) in cardiomyocytes (CMs). Although ROS at physiological levels act as signaling molecules to induce adaptive responses 4, dysregulated ROS production in response to stress damages mitochondrial proteins, stimulating a feed-forward mechanism for ROS production, mitochondrial dysfunction, and cell death, including apoptosis triggered by cytochrome c release and necrosis triggered by mitochondrial permeability transition pore (mPTP) opening. To protect against these catastrophic events, cells have intrinsic quality control mechanisms to maintain the overall health of mitochondria, including fusion, fission and mitochondrial autophagy 5. 1-3 Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Mitochondria are dynamic organelles that constantly undergo fusion and fission 5 to adapt to changes in the cellular environment. Whereas mitochondrial fusion allows mitochondria to maintain membrane potential by fusing depolarized mitochondria to intact ones, fission allows the segregation of unrecoverable mitochondria so that they can be eliminated by autophagy or mitophagy, a specialized form of autophagy 6. Mitochondrial fusion is critically regulated by mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2), specialized proteins localized on the outer mitochondrial membrane, and by Opa1,a protein localized on the inner mitochondrial membrane, whereas mitochondrial fission is regulated by mitochondrial fission 1 (Fis1) and mitochondrial fission factor (Mff), localized on the outer mitochondrial membrane, and by recruitment of a cytoplasmic GTPase, Dynamin-related protein 1 (Drp1), to mitochondrial fission sites 7, 8. Although the presence of fission and fusion has not been documented in adult ventricular myocytes in an unequivocal manner, previous studies have suggested that mitochondrial quality control plays an essential role in protecting the heart against stress 2. For example, downregulation of Mfn1 and Mfn2 promotes cardiac dysfunction at baseline and in response to stress due to the lack of mitochondrial fusion 9, 10 . In contrast, Drp1-mediated mitochondrial fission appears to promote cell death during ischemia/reperfusion (I/R) 11. Since suppression of Drp1 induces mitochondrial fusion, these results have led to a general belief that mitochondrial fusion is protective 12, 13. However, experiments investigating the role of fission in the heart were conducted using mdivi-1, a chemical inhibitor of Drp1 14. Drp1 plays an essential role in mediating Parkin-induced mitochondria selective autophagy, namely mitophagy in MEF cells 15. Drp1 also mediates Bnip3-induced autophagy in adult CMs 16. However, whether Drp1 is involved in general autophagy that can remove mitochondria (which we here referred to as mitochondrial autophagy) at baseline and in response to stress in CMs awaits further investigation using specific interventions. We reasoned that loss-of-function experiments should be conducted using shRNA or a mouse model of genetic deletion of Drp1 17 in order to address the role of endogenous Drp1 in regulating mitochondrial autophagy and consequent CM survival and death. In this study, we asked 1) whether endogenous Drp1 plays a protective or detrimental role in the heart at baseline and in response to stress and 2) whether Drp1 mediates mitochondrial autophagy in response to energy stress in CMs. DOI: 10.1161/CIRCRESAHA.116.303356 3 METHODS An expanded Methods section is available in the online Data Supplement. Mouse models. Generation of Drp1 flox homo (fl/fl) mice has been described 17. Cardiac-specific conditional Drp1 knockout (Drp1-CKO) mice were generated by crossing Drp1 fl/fl mice and MHC-MerCreMer mice, and expression of Drp1 was downregulated by tamoxifen injection (20 mg/kg, ip) for 5 days. Cardiac-specific heterozygous Drp1 KO (Drp1-hetCKO) mice were generated by crossing Drp1 flox hetero (fl/+) mice and MHC-Cre mice. Transgenic mice expressing mRFP-GFP-LC3 (Tg-tf-LC3) have been described 18. All experiments involving animals were approved by the Rutgers–New Jersey Medical School’s Institutional Animal Care and Use Committee. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Keima with mitochondrial localization signal (Keima-MLS). Keima is a fluorescent protein that emits different colored signals at acidic and neutral pHs. Keima-MLS is a mitochondrially localized pH-indicator protein described by Katayama et al 19. We generated adenovirus harboring Keima-MLS. The method used to detect lysosomal delivery of Keima-MLS has been described 19 . Statistical analysis. Data are expressed as mean ± SEM. The difference in means between 2 groups was evaluated using the ttest. One-way ANOVA was used to compare multiple groups. Post-hoc comparisons of considered pairs were performed using the Bonferroni post-hoc test. P values of <0.05 were considered statistically significant. In figure legends, “n” indicates the number of experiments. RESULTS Drp1 downregulation stimulates apoptosis in CMs. To evaluate the role of endogenous Drp1 in regulating mitochondrial morphology in CMs, we constructed adenovirus harboring Drp1 shRNA (Ad-shDrp1) and confirmed that Ad-shDrp1 decreases Drp1 in CMs compared to adenovirus harboring scramble shRNA (Ad-shScr) (Online Figure IA). To observe the morphology of mitochondria, cultured CMs were co-transduced with adenovirus harboring mitochondrially targeted DsRed2 (mt-DsRed2). Ninety-six hours after transduction, mitochondria in AdshDrp1-transduced CMs were elongated compared to those in Ad-shScr-transduced CMs (Figure 1A). The proportion of CMs with elongated mitochondria, as defined by an average mitochondrion length greater than two sarcomere units (Online Figure IB), was significantly greater in Ad-shDrp1-transduced CMs than in Ad-shScr-transduced CMs (Figure 1A). On the other hand, CMs with foreshortened mitochondria, as defined by an average mitochondrion length smaller than one sarcomere unit (Online Figure IB), were markedly reduced in Ad-shDrp1-transduced CMs. These results suggest that Drp1 is required for mitochondrial foreshortening in CMs at baseline. Transduction with Ad-shDrp1 significantly increased the number of TUNEL-positive CMs (Figure 1B) and the amount of cleaved caspase 3 compared to transduction with Ad-shScr (Figure 1C), suggesting that endogenous Drp1 is essential in protection against apoptosis in CMs. To exclude the possibility that our timing prevented observation of a period during which Drp1 downregulation-induced fusion is protective, we evaluated CM viability at various time points after transduction of Ad-shDrp1 and Ad-shScr. Viability time-dependently decreased between 0 and 96 hours after transduction of Ad-shDrp1 into CMs, DOI: 10.1161/CIRCRESAHA.116.303356 4 and was significantly lower in Ad-shDrp1-transduced CMs than in Ad-shScr-transduced CMs after 72 hours (Figure 1D), indicating that Drp1 downregulation is persistently detrimental. Endogenous Drp1 mediates autophagy and mitochondrial quality control. We next examined the role of Drp1 in CM autophagy. Drp1 downregulation with Ad-shDrp1 significantly reduced LC3-II and increased p62/SQSTM1, a protein degraded by autophagy (Figure 2A). To evaluate autophagic flux, CMs were treated with chloroquine, which inhibits fusion of autophagosomes with lysosomes 20. Ad-shDrp1 significantly depressed chloroquine-induced accumulation of LC3-II and accumulation of p62/SQSTM1 did not change significantly after chloroquine treatment in the presence of Ad-shDrp1 (Figure 2A), suggesting that Drp1 downregulation suppresses autophagic flux in CMs at baseline. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 To further evaluate the effect of Drp1 downregulation upon autophagosome formation, CMs were transduced with Ad-GFP-LC3. The number of GFP-LC3 dots was low at baseline and there was no statistically significant difference between Ad-shScr- and Ad-shDrp1-transduced CMs (Figure 2B). However, there was significantly less chloroquine-induced accumulation of GFP-LC3 dots in Ad-shDrp1transduced CMs than in Ad-shScr-transduced CMs (Figure 2B), further supporting the idea that Drp1 downregulation suppresses autophagic flux at baseline. There were significantly more mitochondria, as evaluated with real-time PCR of cytochrome b DNA and immunoblotting of COX IV, in Ad-shDrp1-transduced CMs than in control CMs (Figure 2CD), suggesting that suppression of autophagy due to Drp1 downregulation leads to accumulation of mitochondria. Peroxisome proliferator-activated receptor-gamma coactivator 1 (PGC-1 expression was not significantly altered in Ad-shDrp1-transduced CMs (Online Figure IC), suggesting that mitochondrial biogenesis was not affected. Since suppression of autophagy may impair mitochondrial quality control, we evaluated the effect of Drp1 downregulation upon mitochondrial function. Mitochondrial ATP production was significantly lower in CMs transduced with Ad-shDrp1 than in those with Ad-shScr (Figure 2E). The effect of Drp1 downregulation upon mitochondrial membrane potential was evaluated with JC-1. Drp1 knockdown led to the appearance of green JC-1 staining, indicating depolarization of the mitochondrial membrane potential in CMs (Figure 2F). Furthermore, decreases in absorbance at 540 nm in mitochondrial swelling assays, indicative of mPTP opening, were significantly greater in CMs with Drp1 knockdown than in control CMs (Figure 2G), suggesting that mPTP opening is accelerated by Drp1 downregulation. Cyclosporin A (CsA) attenuated CM death as evaluated with CellTiter Blue® assays, suggesting that mPTP opening contributes to CM death in response to Drp1 downregulation (Figure 2H). We also evaluated the rate of oxidative phosphorylation in CMs, using a Seahorse analyzer (Online Figure IIA). We normalized the oxygen consumption rate (OCR) with either mtDNA content or cell viability in order to compensate for potential cell loss due to cell death. The basal OCR was significantly lower in Drp1-downregulated CMs than in control CMs (Online Figure IIB). The OCR-linked ATP synthesis, as evaluated with oligomycin treatment, and the maximum respiratory rate, as determined by FCCP uncoupling, were also significantly lower in Drp1-downregulated CMs than in control CMs (Online Figure IICD). The level of proton leak, determined by subtracting OCR-linked ATP synthesis from basal OCR, did not significantly differ between Drp1-downregulated and control CMs (Online Figure IIE). Together, these data indicate that Drp1 downregulation induces accumulation of mitochondria accompanied by mitochondrial dysfunction in CMs. DOI: 10.1161/CIRCRESAHA.116.303356 5 Drp1 mediates mitochondrial foreshortening in response to glucose deprivation. We next investigated the involvement of Drp1 in mitochondrial dynamics in response to energy stress. Drp1 was localized primarily in the cytosol in control CMs (Figure 3A). Glucose deprivation (GD), which is known to affect mitochondrial dynamics in other cell types 12, 13, induced modest mitochondrial accumulation of Drp1 in cultured CMs within 4 hours (Figure 3A), accompanied by a slight decrease in cytosolic Drp1, although the reduction did not reach statistical significance. GD-induced mitochondrial expression of Drp1 was also observed with anti-Drp1 immunostaining in mt-DsRed2 expressing CMs (Figure 3B). These results suggest that GD increases Drp1 translocation from the cytosol to mitochondria in CMs. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 After 1 hour of GD, the proportion of CMs with elongated mitochondria was increased, but that of CMs with foreshortened mitochondria was also increased slightly in Ad-shScr-transduced CMs (Figure 3C). A similar result was obtained in CMs transduced with adenovirus harboring LacZ (Ad-LacZ) (not shown). More than 50% of Ad-shDrp1-transduced CMs exhibited elongated mitochondria after 1 hour of GD. After 4 hours of GD, however, approximately 15% of Ad-shScr- or Ad-LacZ-transduced CMs exhibited foreshortened mitochondria, whereas more than 50% still showed elongated mitochondria and less than 1% exhibited foreshortened mitochondria in Ad-shDrp1-transduced CMs (Figure 3C).Thus, although GD induces transient mitochondrial elongation followed by foreshortening, Drp1 downregulation induces persistent increases in elongation irrespective of GD. These results suggest that Drp1 plays an essential role in mitochondrial foreshortening at baseline and during GD. Transduction with Ad-shDrp1 significantly increased TUNEL-positive CMs after 1 and 4 hours of GD compared to transduction with Ad-shScr (Figure 3D), suggesting that endogenous Drp1 protects CMs against apoptosis during GD. We evaluated the role of endogenous Drp1 in mediating autophagy in response to GD. Four hours of GD significantly increased the number of GFP-LC3 dots in Ad-shScr-transduced CMs, but this increase was significantly attenuated in Ad-shDrp1-transduced CMs (Figure 3E). To evaluate autophagic flux, CMs were co-transduced with adenovirus harboring tandem fluorescent mRFP-GFP-LC3 (Ad-tf-LC3) 21. mRFP (monomeric red fluorescent protein) retains its fluorescence but GFP loses its fluorescence in the acidic environment of lysosomes. In merged images, the red puncta that overlay green puncta and appear yellow indicate autophagosomes, whereas free red puncta indicate autolysosomes. After 4 hours of GD, the numbers of both yellow and free red dots were increased in Ad-shScr-transduced CMs, indicating stimulation of autophagic flux. However, the GD-induced increases were attenuated in Ad-shDrp1transduced CMs (Figure 3F), suggesting that Drp1 downregulation inhibits GD-induced autophagic flux. Atg7 increases autophagic flux in CMs 22, 23. Drp1 downregulation significantly reduced Atg7-induced increases in autophagosomes and autolysosomes at baseline and in response to GD in CMs (Figure 3G).Together, the data indicate that endogenous Drp1 plays an essential role in mediating mitochondrial foreshortening, autophagy, and cell survival during GD in CMs. Since Drp1 physically interacts with Bcl-xL in neurons 24 and Bcl-xL inhibits autophagy through its binding to Beclin1 {Pattingre, 2005 #3365}, we investigated the involvement of Bcl-xL in the suppression of autophagy by Drp1. Using co-immunoprecipitation assays, we confirmed that Drp1 physically interacts with Bcl-2 and Bcl-xL in CMs in the presence of Drp1 overexpression (Online Figure IIIA). Increased expression of Drp1 inhibited, whereas downregulation of Drp1 augmented, the physical interaction between Beclin1 and Bcl-2 or Bcl-xL (Online Figure IIIB). Downregulation of Drp1 decreased the number of GFP-LC3 dots at baseline and in response to 4 hours of GD. However, the number of GFPLC3 dots increased significantly when Drp1 was downregulated in the presence of Bcl-xL downregulation with or without chloroquine {Iwai-Kanai, 2008 #3728} (Online Figure IIICD). These results suggests that downregulation of Drp1 inhibits autophagy through a Bcl-xL-dependent mechanism, most likely by enhancing interaction between Beclin1 and Bcl-xL. DOI: 10.1161/CIRCRESAHA.116.303356 6 Prolonged treatment with mdivi-1 mimics the effect of Drp1 downregulation. Since previous studies showed that suppression of Drp1 by mdivi-1 protects CMs from cell death , we investigated the effect of mdivi-1 upon mitochondrial morphology and cell death. Single treatment with mdivi-1 at 50 or 100 M for 1 hour significantly increased the number of CMs with elongated mitochondria at baseline. Mdivi-1 at 100 M also prevented foreshortening of mitochondria after 4 hours of GD (Online Figure IVA). To compare the effects of Drp1 downregulation and mdivi-1 upon survival of CMs side-by-side, CMs were treated with chelerythrine (10 M), an inducer of apoptosis 25, in the presence or absence of either mdivi-1 or Ad-shDrp1. Although Ad-shDrp1 transduction for 96 hours decreased CM survival at baseline and in response to chelerythrine, mdivi-1 treatment for 1 hour increased CM survival at baseline and in response to chelerythrine compared to vehicle treatment (Online Figure IVB). Mdivi-1 treatment at 50 M did not significantly affect CM viability in response to GD, but 100 M significantly reduced it (Online Figure IVC). Taken together, these results suggest that a single treatment with mdivi-1 has direct cell-protective effects upon CMs independent of Drp1. However, a higher dose of mdivi-1 partially mimics the effect of Drp1 downregulation even after a single application. 11 Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Treatment of CMs with 50 M mdivi-1 every 24 hours for 1 week induced elongation of mitochondria at baseline and inhibited foreshortening of mitochondria even after 4 hours of GD (Online Figure IVD). Prolonged treatment with mdivi-1 time-dependently decreased CM viability compared to vehicle treatment (Online Figure IVE) and significantly suppressed GD-induced autophagic flux as evaluated with mRFP-GFP-LC3 (Online Figure IVF). Thus, prolonged treatment with mdivi-1 mimics the effect of Drp1 downregulation. Drp1 mediates autophagic removal of mitochondria. We investigated whether clearance of mitochondria is regulated by Drp1 using mitochondriatargeted Keima fluorescence 19. Keima has a bimodal excitation spectrum peaking at 438±12 and 550±15 nm, corresponding to neutral and acidic pH states, respectively 19. Because fusion of autophagosomes with lysosomes exposes the autophagosome contents to acidic pH, the maturation of autophagosomes to autolysosomes can be monitored by measuring Keima fluorescence 19. We confirmed that Keima with a mitochondria-localization signal (Keima-MLS) is expressed in CM mitochondria (Figure 4A). Puncta with a high ratio of excitation at 560/440 nm (high 560/440) colocalized with Alexa 488 Dextran, reflecting increased lysosomal localization of Keima-MLS, after treatment with 25 M of cyanide 3chlorophenylhydrazone (CCCP), a mitochondrial uncoupler, for 16 hours to induce mitochondrial autophagy 26 (Figure 4B, Online Figure VA), confirming that Keima-MLS works as expected in CMs. Puncta with high 560/440, indicating the presence of mitochondria in lysosomes, were significantly increased after 4 hours of GD in CMs transduced with Ad-shScr, but not in CMs transduced with AdshDrp1 (Figure 4C). This increase was abolished in the presence of Ad-shBeclin1-mediated Beclin1 downregulation (Online Figure VB), suggesting that it is mediated by autophagy and that Drp1 is necessary for stimulating autophagic mitochondrial degradation. Interestingly, downregulation of Beclin1 did not affect GD-induced increases in mitochondrial foreshortening (Online Figure VC) but significantly increased GD-induced cell death (Online Figure VD). Thus, although evidence suggests that unopposed fusion of mitochondria alone can induce cell death 27, suppression of autophagy alone may also induce CM death even when mitochondrial foreshortening is not affected. Atg7 overexpression, which is known to stimulate autophagy 22, 23, failed to increase puncta with high 560/440 in Drp1-downregulated CMs (Figure 4D), even though it increased autophagosomes and autolysosomes in this condition (Figure 3FG), nor did it inhibit Drp1 knockdown-induced cell death (Figure 4E). DOI: 10.1161/CIRCRESAHA.116.303356 7 To further elucidate the role of endogenous Drp1 in autophagy, CMs were subjected to GD in the presence or absence of Drp1 knockdown and electron microscopic (EM) analyses were conducted (Figure 4F). Drp1 downregulation significantly reduced the number of mitochondria and increased relative mitochondria mass at baseline (Figure 4G). Drp1 downregulation also decreased the total number of autophagosomes at baseline and in response to GD and decreased the number of autophagosomes selectively containing mitochondria (Figure 4H). These results suggest that endogenous Drp1 is important in mediating both general autophagy, including mitochondrial autophagy. Forced Drp1 overexpression induces apoptosis in CMs. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Adenovirus-mediated overexpression of Drp1 by 5 fold, which is higher than the level induced by GD, induced foreshortening of mitochondria in CMs at baseline (Online Figure VIAB). Under this condition, increases in apoptosis, decreases in mitochondrial DNA, and decreases in mitochondrial membrane potential were observed (Online Figure VIC-E). These results suggest that persistent and highlevel expression of Drp1 induces mitochondrial dysfunction and apoptosis in CMs. Drp1 overexpression significantly increased Keima-MLS puncta with high 560/440 in CMs both at baseline and after 4 hours of GD (Online Figure VIF), indicating stimulation of lysosomal removal of mitochondria. Interestingly, suppression of autophagy by Ad-shAtg7 attenuated the increased cell death induced by Drp1 overexpression (Online Figure VIG), suggesting that excessive activation of autophagy by Drp1 may induce cell death. Basal characterization of Drp1-CKO mice. To evaluate the role of endogenous Drp1 in vivo, we used loss-of-function mouse models. No homozygous mice were born during attempts to generate cardiac-specific Drp1 knockout mice using MHC-Cre mice. Therefore, in order to examine the effect of Drp1 on cardiac function in adult mice in vivo, we generated cardiac-specific conditional Drp1 knockout (Drp1-CKO) mice by crossing Drp1 flox homo (fl/fl) and MHC-MerCreMer mice, and Drp1 expression was downregulated in a tamoxifendependent manner. We used Drp1-CKO without tamoxifen injection (TI) and Drp1 fl/fl with or without TI as controls. Fifteen-week-old male mice were subjected to TI (20 mg/kg, ip) for 5 days. Four and 8 weeks after TI, we measured cardiac function and performed biochemical and histological analyses (Online Figure VIIA). Immunoblot analyses confirmed that cardiac Drp1 levels were significantly lower in Drp1-CKO mice than in control mice (Figure 5A) and that Drp1 was downregulated in a heart-specific manner in Drp1CKO mice (Online Figure VIIB). Cardiac levels of other proteins involved in mitochondrial dynamics, such as Mfn1, Mfn2, OPA1, and Fis1, were unaltered in Drp1-CKO mice compared to in control mice (Online Figure VIIC). Drp1-CKO mice started to die 8 weeks after TI and all died by 13 weeks after injection, whereas no control mice died during the observation period of 16 weeks following TI. Kaplan–Meier analysis revealed that the survival rate was significantly lower in Drp1-CKO mice than in control mice (Online Figure VIID). Four weeks after TI, the hearts of Drp1-CKO mice were enlarged compared to control hearts (Figure 5B). Postmortem assessment showed that both left ventricular (LV) weight/tibial length, an index of LV hypertrophy, and lung weight/tibial length, an index of lung congestion, were significantly greater in Drp1-CKO than in control mice 4 and 8 weeks after TI (Online Tables I and II). Wheat germ agglutinin (WGA) staining of LV sections 4 and 8 weeks after TI showed that CM crosssectional area was significantly greater in Drp1-CKO than in control mice (Figure 5C and Online Figure VIIE). Myocardial fibrosis, as evaluated with Picric Acid Sirius Red and Masson’s Trichrome staining, was also significantly greater in Drp1-CKO mice than in control mice (Figure 5D and Online Figure VIIFG). Echocardiographic measurements 4 and 8 weeks after TI showed that the LV diastolic dimension was significantly greater, and the LV ejection fraction (LVEF), an indicator of LV systolic function, was lower in Drp1-CKO mice than in control mice (Online Tables III and IV). Hemodynamic measurements at 4 weeks after TI showed that LV +dP/dt was decreased, whereas LV end-diastolic pressure was significantly elevated in Drp1-CKO compared to in control mice (Online Table II). We confirmed that MHCDOI: 10.1161/CIRCRESAHA.116.303356 8 MerCreMer alone did not influence cardiac function or histology in either the presence or absence of tamoxifen (Online Figure VIIF-H). Taken together, these results suggest that Drp1 downregulation induces LV dysfunction and cardiac hypertrophy at baseline. Drp1 downregulation induces mitochondrial elongation and dysfunction. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 To examine how Drp1 deletion affects mitochondrial morphology in the heart, EM analysis was performed. At baseline, mitochondria in control mouse hearts were primarily rectangular or spherical in shape, whereas tubular mitochondria were observed less frequently. On the other hand, mitochondria in Drp1-CKO mice were mostly elongated/enlarged 4 and 8 weeks after TI (Figure 6A and Online Figure VIIIA). After 48 hours of fasting, mitochondria in control mouse hearts became smaller and spherical. In contrast, mitochondria in Drp1-CKO mice remained elongated even after fasting (Figure 6A). Autophagosomes containing mitochondria were observed in control mouse hearts after 48 hours of fasting, but not in Drp1-CKO mouse hearts (Figure 6A). Quantitative analysis revealed that mitochondrial mass was significantly greater in Drp1-CKO mouse hearts than in control mouse hearts at baseline and after fasting (Figure 6A and Online Figure VIIIA). These results suggest that endogenous Drp1 plays an essential role in mediating mitochondrial foreshortening at baseline and during fasting in the mouse heart in vivo. Four or 8 weeks after TI, Drp1 depletion increased the COX IV protein level (Figure 6B, Online Figure VIIIB), and mitochondrial DNA content, evaluated with real-time PCR of cytochrome b DNA, was significantly greater in Drp1-CKO mice than in control mice (Figure 6C). These results suggest that the mitochondrial content is increased by Drp1 downregulation. Mitochondrial biogenesis was evaluated by immunoblot analyses of PGC-1 and mitochondrial transcription factor A (TFAM). Cardiac protein expression of PGC-1 and TFAM did not differ between Drp1-CKO and control mice 4 and 8 weeks after TI, suggesting that Drp1 depletion did not affect mitochondrial biogenesis (Figure 6D and Online Figure VIIIC). However, mitochondrial ATP production was significantly attenuated in Drp1-CKO mouse hearts 4 and 8 weeks after TI compared to in control mouse hearts (Figure 6E and Online Figure VIIID). The activity of mitochondrial complexes I, II + III, and IV was also significantly attenuated in Drp1-CKO mouse hearts 8 weeks after TI compared to in control mouse hearts (Online Figure VIIIE). The extent of mPTP opening, as evaluated by the decrease in absorbance at 540 nm in mitochondrial swelling assays, was significantly greater in Drp1-CKO mouse hearts 4 weeks after TI than in controls (Figure 6F), suggesting that mPTP opening is accelerated in Drp1-CKO mice. The cardiac level of 4-Hydroxynonenal, a marker of oxidative stress, and mitochondrial production of H2O2, evaluated with Amplex® Red assays, were also significantly higher in Drp1-CKO mice 4 weeks after TI than in control mice (Figure 6GH). Thus, Drp1 depletion results in mitochondrial dysfunction and oxidative stress in the heart. Since the initial assessment of mitochondrial function was conducted using hearts harvested 4-8 weeks after TI, when both hypertrophy and LV dysfunction are obvious in Drp1-CKO mice, mitochondrial dysfunction could be secondary to pathological hypertrophy. We therefore also investigated an earlier time point. Echocardiographic analyses revealed no significant difference in LVEF between control and Drp1CKO mice 10 days after TI (Online Table V), nor was there a significant difference in CM cross-sectional area or cardiac fibrosis (Online Figure VIIIFG), confirming that this time point precedes the development of pathological hypertrophy. Nevertheless, mitochondrial function, as assessed by ATP production and mitochondrial swelling assays, was already severely attenuated in Drp1-CKO mice compared to in control mice 10 days after TI (Figure 6EF). Together with the observation that Drp1 downregulation directly induces mitochondrial dysfunction in cultured CMs (Figure 2E-J), these results suggest that Drp1 depletion induces mitochondrial dysfunction in the heart even before manifestation of hypertrophy and LV dysfunction. We investigated whether Drp1 downregulation in the heart affects apoptosis. There were significantly more TUNEL-positive nuclei in Drp1-CKO mouse hearts than in controls 4 and 8 weeks after DOI: 10.1161/CIRCRESAHA.116.303356 9 TI (Figure 7A and Online Figure VIIIH). Cleaved caspase-3 and cytochrome c release into the cytosolic fraction were also significantly elevated in Drp1-CKO mouse hearts 4 weeks after TI (Figure 7B), as was the serum HMGB1 level, an indicator of necrosis (Online Figure VIII I). These results suggest that endogenous Drp1 is required for protection against the mitochondrial mechanisms of apoptosis and necrosis in CMs. Autophagy is inhibited in Drp1-CKO mice. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 We next investigated the role of Drp1 in mediating autophagy in the heart in vivo. There was significantly less LC3-II and significantly more p62 in Drp1-CKO mouse hearts than in controls 4 weeks after TI (Figure 7C). To examine whether Drp1 downregulation attenuates autophagic flux, we evaluated the effect of chloroquine injection upon autophagosome accumulation 20. LC3-II accumulation was suppressed even in the presence of chloroquine, whereas p62/SQSTM1 accumulation did not change significantly after chloroquine treatment in the Drp1-CKO mouse heart (Figure 7D). To further evaluate the level of autophagic flux in CMs in vivo, we crossed Drp1-CKO and Drp1 fl/fl with cardiac-specific mRFP-GFP-LC3 (tf-LC3) transgenic mice. Both Drp1-CKO X tf-LC3 and Drp1 fl/fl X tf-LC3 (control tfLC3) were injected with tamoxifen for 5 days. Fasting increased the number of LC3 dots with both green and red color (appearing yellow in merged images), representing autophagosomes, as well as the number of dots with only red color, representing autolysosomes, in control tf-LC3 mice, indicating increased autophagic flux (Figure 7E). In contrast, the number of yellow and free red dots did not increase in response to fasting in Drp1-CKO X tf-LC3 mice (Figure 7E). Taken together, these results suggest that Drp1 downregulation suppresses autophagic flux at baseline. Drp1 depletion induces stress intolerance and enhances I/R injury. Although mitochondrial fission and fusion are essential for maintaining mitochondrial quality control, their role in cardiac development and stress resistance remains unknown. To address this question, we crossed Drp1 fl/fl mice with MHC-Cre mice. Although no mice with cardiac-specific homozygous Drp1 knockout were born, mice with cardiac-specific heterozygous Drp1 knockout (Drp1-hetCKO) were viable at 12 weeks, suggesting that Drp1 is required for normal prenatal development but that one functional allele is sufficient during this period. Cardiac Drp1 expression was 40% lower in Drp1-hetCKO mice than in control (Drp1 flox/+) mice (Figure 8A). LV function, assessed by LVEF, in Drp1-hetCKO mice was normal at 12 weeks of age (Figure 8B). Neither LV weight/tibial length nor lung weight/tibial length differed between 12-week-old Drp1-hetCKO and control mice (Online Table VI). Histological analyses showed that the CM cross-sectional area and myocardial fibrosis also did not differ between 12-week-old Drp1-hetCKO and control mice (Online Figure IXAB). However, ATP production was significantly lower in 12-week-old Drp1-hetCKO mice (Figure 8C), suggesting that mitochondrial dysfunction develops prior to histological and hemodynamic changes in Drp1-hetCKO mice. The fact that LV function is maintained at 12 weeks of age in Drp1-hetCKO mice allowed us to use these mice to examine the role of Drp1 during stress in the heart. Mitochondrial Drp1 was significantly increased in response to 48-hour fasting or I/R but not in Drp1-hetCKO mice (Figure 8D). To evaluate the role of endogenous Drp1 in protection against stress in vivo, 12-week-old Drp1-hetCKO and control mice underwent 48-hour fasting. The LVEF was significantly lower in Drp1-hetCKO mice than in control mice after fasting, suggesting that endogenous Drp1 acts to preserve LV function during fasting (Figure 8E). Similar results were observed in Drp1-CKO mice with tamoxifen treatment (Online Figure IXC). To evaluate the role of endogenous Drp1 in protection against I/R, 12-week-old Drp1-hetCKO and control mice were subjected to 30 minutes of myocardial ischemia followed by 24 hours of reperfusion. EM analyses showed that I/R increased the number of smaller and spherical mitochondria in control mice, suggesting that mitochondrial fission was induced. However, these changes were significantly attenuated in Drp1-hetCKO mice (Figure 8F), suggesting that endogenous Drp1 DOI: 10.1161/CIRCRESAHA.116.303356 10 mediates mitochondrial fission after I/R. Autophagosomes containing mitochondria were observed in control mouse hearts but not in Drp1-hetCKO mouse hearts after I/R (Figure 8F). There was also significantly less LC3-II and more p62 in Drp1-hetCKO mouse hearts than in control hearts at baseline and after I/R (Figure 8G), suggesting that autophagy is suppressed by heterozygous Drp1 downregulation. The infarct size/area at risk (MI/AAR) after I/R, as evaluated with Alcian Blue and tetrazolium chloride staining, was not affected by MHC-Cre alone (Online Figure IXD) but was significantly greater in Drp1-hetCKO mice than in control mice (55.2 ± 3.0 vs. 40.2 ± 1.6%, p<0.05, n=3 per group, Figure 8H). Similar results were observed in Drp1-CKO mice with tamoxifen treatment (Online Figure IXE).Taken together, these results suggest that inhibition of mitochondrial fission through Drp1 downregulation enhances myocardial injury in response to I/R. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 We also evaluated the effect of mdivi-1 upon I/R injury. One-time treatment with mdivi-1 just before I/R significantly reduced the MI/AAR (Online Figure XA), confirming previous observations by others 11. However, the same treatment also reduced the MI/AAR in Drp1-hetCKO mice, suggesting that short term treatment with mdivi-1 protects the heart through Drp-1-independent mechanisms (Online Figure XB). Although repetitive applications of mdivi-1 (1.2 mg/kg/day for 7 days) did not significantly reduce LV systolic function (Online Figure XC and Online Table VI), it significantly increased mitochondrial mass, as determined by EM (Online Figure XD), reduced mitochondrial function to a similar extent as heterozygous Drp1 downregulation, as determined by mitochondrial swelling assays and ATP production (Online Figures XEF), and significantly enhanced the MI/AAR after I/R (Online Figure XG), thereby mimicking the effect of Drp1-hetCKO. Thus, while the effects of long-term treatment with mdivi-1 are similar to those of Drp1 downregulation with regards to I/R injury enhancement, albeit weaker, one-time treatment with mdivi-1 appears to have protective effects, which are most likely independent of Drp1. DISCUSSION Our results suggest that endogenous Drp1 induces mitochondrial foreshortening at baseline and in response to stress in the heart and the CMs therein. Contrary to previous reports 11, 28, downregulation of endogenous Drp1 in CMs induces mitochondrial dysfunction and apoptosis despite significant induction of mitochondrial elongation, thereby inducing cardiac dysfunction at baseline and exacerbating myocardial injury in response to I/R. Using Keima-MLS, we show that Drp1 plays an essential role in mediating lysosomal removal of mitochondria in CMs. Thus, our results suggest that endogenous Drp1 contributes to mitochondrial quality control. Although it is generally believed that fused mitochondria function better, Drp1 downregulation significantly increased the number of CMs with depolarized mitochondria even at baseline. Although Drp1 is localized primarily in the cytosol in unstimulated CMs, a low level of mitochondrial turnover mediated by Drp1 appears essential to maintain mitochondrial function in CMs. Given that even heterozygous Drp1 downregulation induces mitochondrial dysfunction and heart failure in mice, it appears that endogenous Drp1 plays an essential role in mitochondrial quality control in the heart in vivo as well. Whether mitochondria undergo fusion or fission during stress may depend upon cell type and stress. In MEF cells 12, 13, fasting induces mitochondrial fusion induced by phosphorylation of Drp1 at Ser637 by protein kinase A and translocation of Drp1 to the cytoplasm, which allows mitochondria to maintain ATP synthesis and escape autophagic destruction 12, 13. On the other hand, in HL1 cells in vitro and CMs in the heart in vivo 11, 29, fasting and hypoxia stimulate mitochondrial fission. Regardless of whether fusion or fission is stimulated by stress, these studies showed that suppression of fission and/or stimulation of fusion through Drp1 downregulation, expression of dominant-negative Drp1, mdivi-1, or expression of Mfn1/2 promotes ATP production and cell survival. Here we show that mitochondria in CMs transiently undergo DOI: 10.1161/CIRCRESAHA.116.303356 11 elongation during GD, but that the number of mitochondria with foreshortening also increases thereafter, accompanied by accumulation of Drp1 in mitochondria. Drp1 downregulation in this scenario blunted foreshortening of mitochondria and exacerbated cell death, suggesting that the induction of foreshortening is adaptive in CMs. Our results suggest that endogenous Drp1 is important in mediating autophagy in CMs. Drp1 controls autophagic flux at least at the level of autophagosome formation, since there were fewer GFP-LC3 puncta when Drp1 was downregulated in the presence of chloroquine, an inhibitor of autophagosomelysosome fusion or autophagic flux 20. The suppressive effect of Drp1 downregulation upon global autophagy, rather than its specific effect upon mitochondria specific autophagy, was unexpected. We here show that Drp1 physically interacts with Bcl-2/Bcl-xL and that downregulation of Drp1 promotes interaction between Beclin1 and Bcl-2/Bcl-xL. Since Bcl-2 and Bcl-xL are endogenous inhibitors of Beclin1 {Pattingre, 2005 #3365}, increased interaction between Beclin1 and Bcl-2/Bcl-xL in the presence of Drp1 downregulation should lead to suppression of autophagy. In fact, the suppression of general autophagy by Drp1 downregulation was rescued by downregulation of Bcl-xL, indicating the critical role of the Bcl-2 family proteins in this process. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 We here show that a GD-induced increase in lysosomal localization of Keima-MLS 19 is attenuated in the presence of Drp1 downregulation. Given the mitochondrial localization of Keima-MLS, and that Keima-MLS puncta with high 560/440, indicating acidic pH, are localized in lysosomes and are abolished when Beclin1 is downregulated, increases in Keima-MLS puncta with high 560/440 presumably reflect autophagic degradation of mitochondria. Thus, the significant reduction in lysosomal Keima-MLS puncta, together with EM images showing a significant reduction in autophagosomes primarily containing mitochondria, in Drp1 knockdown CMs indicates that endogenous Drp1 plays an essential role in mediating GD-induced increases in mitochondrial autophagy. The Keima-MLS analysis was not sensitive enough to demonstrate a reduction in lysosomal removal of mitochondria at baseline when Drp1 is downregulated. However, given that dysfunctional mitochondria accumulate in Drp1-downregulated CMs, it is likely that Drp1 also mediates autophagic mitochondrial degradation at baseline. In this work, we used the term “mitochondrial autophagy” to describe the clearance of mitochondria by autophagy. Although our results suggest that Drp1 regulates mitochondrial clearance through general autophagy, whether or not Drp1 also affects mitochondria-selective autophagy, namely mitophagy, could not be evaluated due to technical limitations. To this end, specific assays to accurately evaluate the presence of mitophagy and/or specific interventions to modulate mitophagy appear essential. Conditional Drp1 downregulation leads to decreases in cardiac function within 4 weeks and all animals died within 13 weeks due to heart failure. Histological analyses showed that the Drp1 deficiency induces hypertrophy and fibrosis in the heart and increases CM apoptosis. The fact that conditional cardiacspecific combined downregulation of Mfn1 and Mfn2 (c-Mfn1/2-KO) also leads to rapid development of cardiac dysfunction within 2 weeks 9 indicates that both unopposed fission and unopposed fusion of mitochondria may cause cardiac dysfunction and suggests the critical importance of mitochondrial remodeling in the heart. There are some differences between the cardiac phenotypes of Drp1-CKO and c-Mfn1/2-KO mice . For example, neither cardiac hypertrophy nor the increased CM apoptosis observed in Drp1-CKO were apparent in c-Mfn1/2-KO mice. This suggests that ATP depletion may be more profound in the absence of fusion than in the absence of fission. 9, 10 The reason for the opposite effects of Drp1 downregulation by genetic deletion and Drp1 suppression with mdivi-1 in response to I/R remains to be elucidated. One possibility is that our shRNA treatment may have induced stronger, more prolonged suppression of mitochondrial fission than a single DOI: 10.1161/CIRCRESAHA.116.303356 12 Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 dose of mdivi-1 at 50 M, the concentration used by others10. We observed modest cell death-promoting effects when CMs were treated with mdivi-1 at a higher concentration (100 M) or multiple times. A second possibility is that Drp1 downregulation may induce more potent suppression of general autophagy to even below physiological levels than mdivi-1. Although suppression of excessive autophagy may be salutary, suppression below physiological levels may be harmful. Third, mdivi-1 may more strongly suppress cell death than Drp1 downregulation by directly acting upon apoptosis mechanisms. Mdivi-1 blocks Bax/Bak-dependent release of both Smac/Diablo and cytochrome c in HeLa cells 14, and we found that mdivi-1 inhibited chelerythrine-induced apoptosis in CMs, which Drp1 downregulation did not. Furthermore, one-time treatment with mdivi-1 reduced I/R injury even in Drp1-hetCKO mice, suggesting that mdivi-1 most likely has a Drp1-independent anti-apoptotic function. Along this same line, mdivi-1 affects other molecules besides Drp1, including delayed rectifier K+ channels 27, raising the issue of specificity of the chemical inhibitor. Fourth, mitochondrial localization of Drp1 is positively regulated by protein kinase A 30, calcineurin 30, PUMA 31, Bax/Bak 32, ceramide 33, and O-linked--N-acetylglucosamine modification 34, and is negatively regulated by miR-499 35 and Pim1 36. Thus, some experimental conditions may induce excessive Drp1 activation/upregulation, which may in turn induce deleterious effects in CMs. In fact, Drp1 overexpression in CMs above the level caused by GD induced cell death. Drp1 suppression by mdivi-1 may be protective under such experimental conditions. We have shown previously that Beclin1 haploinsufficiency inhibits I/R injury and suppresses autophagy 37. Here we show that Drp1 haploinsufficiency exacerbates I/R injury, but is also accompanied by suppression of autophagy. Currently, mechanisms explaining the difference remain to be clarified. Drp1 downregulation may have a more pronounced effect upon general autophagy and/or mitochondrial autophagy than Beclin1 downregulation, thereby suppressing autophagy below physiological levels. Another possibility is that Drp1 downregulation may more globally affect mitochondrial quality control mechanisms, including inducing unopposed mitochondrial elongation and suppression of global autophagy, rather than being limited to suppression of autophagic mitochondrial degradation. Further investigation is required to address this issue. In summary, persistent Drp1 downregulation inhibits clearance of mitochondria by autophagy and causes mitochondrial dysfunction and consequent cell death in the heart and in the CMs therein, both at baseline and under stress conditions. Drp1 plays an important role in mediating mitochondrial foreshortening and autophagic mitochondrial degradation in CMs. ACKNOWLEDGEMENTS The authors wish to thank Christopher D. Brady and Daniela Zablocki for critical reading of the manuscript and Luke Fritzky for technical assistance. SOURCES OF FUNDING This work was supported in part by U.S. Public Health Service Grants HL102738, HL67724, HL69020, HL91469, AG23039, and AG27211. This work was also supported by the Fondation Leducq Transatlantic Networks of Excellence. IY has been supported by a Postdoctoral Fellowship from the Founders Affiliate, American Heart Association, and by a grant from the Rotary Foundation Ambassadorial Scholarship. DISCLOSURES None. DOI: 10.1161/CIRCRESAHA.116.303356 13 REFERENCES 1. 2. 3. 4. 5. 6. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. Gottlieb RA, Gustafsson AB. Mitochondrial turnover in the heart. Biochim Biophys Acta. 2011;1813:1295-1301. Dorn GW, 2nd. Mitochondrial dynamics in heart disease. Biochim Biophys Acta. 2012;1833:233241. Piquereau J, Caffin F, Novotova M, Lemaire C, Veksler V, Garnier A, Ventura-Clapier R, Joubert F. Mitochondrial dynamics in the adult cardiomyocytes: which roles for a highly specialized cell? Front Physiol. 2013;4:102. Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW, 2nd. Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circ Res. 2014;115:348-353. Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337:10621065. Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. Embo J. 2008;27:433-446. Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem.149:241-251. Okamoto K, Shaw JM. Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet. 2005;39:503-536. Chen Y, Liu Y, Dorn GW, 2nd. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ Res. 2011;109:1327-1331. Papanicolaou KN, Kikuchi R, Ngoh GA, Coughlan KA, Dominguez I, Stanley WC, Walsh K. Mitofusins 1 and 2 are essential for postnatal metabolic remodeling in heart. Circ Res. 2012;111:1012-1026. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012-2022. Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589-598. Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108:10190-10195. Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193-204. Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367-1380. Lee Y, Lee HY, Hanna RA, Gustafsson AB. Mitochondrial autophagy by Bnip3 involves Drp1mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2011;301:H1924-1931. Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958-966. Hariharan N, Zhai P, Sadoshima J. Oxidative Stress Stimulates Autophagic Flux during Ischemia/Reperfusion. Antioxid Redox Signal. 2010. DOI: 10.1161/CIRCRESAHA.116.303356 14 19. 20. 21. 22. 23. 24. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. Katayama H, Kogure T, Mizushima N, Yoshimori T, Miyawaki A. A sensitive and quantitative technique for detecting autophagic events based on lysosomal delivery. Chem Biol. 2011;18:10421052. Iwai-Kanai E, Yuan H, Huang C, Sayen MR, Perry-Garza CN, Kim L, Gottlieb RA. A method to measure cardiac autophagic flux in vivo. Autophagy. 2008;4. Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470-1482. Pattison JS, Osinska H, Robbins J. Atg7 induces basal autophagy and rescues autophagic deficiency in CryABR120G cardiomyocytes. Circ Res. 2011;109:151-160. Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, Sadoshima J. Rheb is a Critical Regulator of Autophagy during Myocardial Ischemia: Pathophysiological Implications in Obesity and Metabolic Syndrome. Circulation. 2012;125:1134-1146. Li H, Alavian KN, Lazrove E, Mehta N, Jones A, Zhang P, Licznerski P, Graham M, Uo T, Guo J, Rahner C, Duman RS, Morrison RS, Jonas EA. A Bcl-xL-Drp1 complex regulates synaptic vesicle membrane dynamics during endocytosis. Nat Cell Biol. 2013;15:773-785. Yamamoto S, Seta K, Morisco C, Vatner SF, Sadoshima J. Chelerythrine Rapidly Induces Apoptosis through Generation of Reactive Oxygen Species in Cardiac Myocytes. J Mol Cell Cardiol. 2001;33:1829-1848. Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211-221. Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW, 2nd. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res. 2014;114:257-265. Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. Kim H, Scimia MC, Wilkinson D, Trelles RD, Wood MR, Bowtell D, Dillin A, Mercola M, Ronai ZA. Fine-tuning of Drp1/Fis1 availability by AKAP121/Siah2 regulates mitochondrial adaptation to hypoxia. Mol Cell.44:532-544. Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939-944. Wang JX, Li Q, Li PF. Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein1. Cancer Res. 2009;69:492-500. Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439-450. Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77:387-397. Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR, 3rd, Hoshijima M, Dillmann W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287:30024-30034. Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71-78. Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, Joyo AY, Quijada P, Erhardt P, Magnuson NS, Konstandin MH, Sussman MA. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proc Natl Acad Sci U S A.110:5969-5974. DOI: 10.1161/CIRCRESAHA.116.303356 15 37. Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct Roles of Autophagy in the Heart During Ischemia and Reperfusion. Roles of AMP-Activated Protein Kinase and Beclin 1 in Mediating Autophagy. Circ Res. 2007;100:914-922. FIGURE LEGENDS Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Figure 1. Drp1 mediates mitochondrial fission and CM survival. A, Assessment of mitochondrial morphology using mitochondria-targeted DsRed2 (mt-DsRed2). TnI: Troponin-I. TnI staining indicates CM. The proportions of CMs with elongated and foreshortened mitochondria was quantified. Gray bar: cells with elongated/total cell number, black bar: cells with foreshortened/total cell number, white bar: cells with intermediate (mid) mitochondria/total cell number. * p<0.01 vs. foreshortened in Ad-shScr, # p<0.01 vs. elongated in Ad-shScr (n=4/group). Scale bar: 20 m. B, TUNEL staining of CMs. * p<0.01 vs. AdshScr (n=3/group). Scale bar: 200 m. C, Immunoblots for cleaved caspase 3 and -tubulin (n=3/group). D, CM viability was evaluated at multiple time points after transduction with Ad-shScr or Ad-shDrp1 using the CellTiter Blue assay. * p<0.01 vs. Ad-shScr 72 hours after transduction, # p<0.01 vs. Ad-shScr 96 hours after transduction (n=4/group). Figure 2. Drp1 plays an essential role in mediating autophagy and maintaining mitochondrial function in CMs at baseline. A, CMs were transduced with Ad-shScr or Ad-shDrp1 for 96 hours and then treated with or without chloroquine (10 μM) for 4 hours. Representative Immunoblots for Drp1, LC3 (long and short exposures), p62 and -tubulin in shScr- or shDrp1-transduced CMs and quantitative analyses are shown. Chl: chloroquine. * p<0.01 vs. Ad-shScr without chloroquine, # p<0.01 vs. Ad-shScr with chloroquine, † p<0.01 vs. Ad-shDrp1 without chloroquine (n=3/group). B, CMs were transduced with AdGFP-LC3 for 48 hours and Ad-shScr or Ad-shDrp1 for 96 hours. Some CMs were incubated with chloroquine (10 μM) for 4 hours. Representative images of fluorescent GFP-LC3 puncta in shScr- or shDrp1-transduced CMs and quantitative analysis of the GFP-LC3 puncta. Ctr: control. * p<0.01 vs. AdshScr Ctr, # p<0.01 vs. Ad-shScr Chl (n=5/group). In C-J, CMs were transduced with Ad-shScr or AdshDrp1 for 96 hours (n=3/group). C, Relative mitochondrial DNA content in CMs with Drp1 knockdown, evaluated by PCR for cytochrome b. * p<0.01 vs. Ad-shScr. D, Immunoblots for COX IV and -tubulin in CMs with Drp1 knockdown. * p<0.05 vs. Ad-shScr. E, Relative mitochondrial ATP production. * p<0.01 vs. Ad-shScr. F, Mitochondrial membrane potential, evaluated with JC-1. Red indicates mitochondria in which membrane potential is maintained, whereas green indicates depolarized mitochondria. The quantification of CMs with depolarized mitochondria is shown. * p<0.01 vs. shScr. Yellow scale bar: 500 m; white scale bar: 100 m. G, Mitochondrial membrane potential assessment with TMRE. Red staining indicates polarized mitochondria. Scale bar: 500 m. In H-I, some CMs were administered CsA (5 M) after transduction. H, Mitochondrial swelling induced by Ca2+. Each data curve in the left panel represents the average of 3 individual measurements. Right panel shows the decrease in optical density at 540 nm, indicating the extent of mPTP opening. * p<0.01 vs. shScr without CsA, # p<0.01 vs. shDrp1 without CsA (n=3/group). I, CM cell viability. * p<0.01 vs. shScr without CsA, # p<0.01 vs. shScr with CsA, † p<0.05 vs. shDrp1 without CsA (n=4/group). J, Assessment of oxidative stress using MitoSox Red. Scale bar: 200 m. Figure 3. Drp1 mediates mitochondrial fission, autophagy, and cell survival during GD. A, Immunoblots for Drp1, COX IV, and -tubulin in CM mitochondrial and cytosolic fractions. * p<0.01 vs. Ctr (n=3/group). B, Immunohistochemistry for Drp1 and mt-DsRed2. Green: Drp1, Red: mt-DsRed2. Scale DOI: 10.1161/CIRCRESAHA.116.303356 16 Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 bar: 50 m. C, Assessment of mitochondrial morphology using mt-DsRed2. Insets show representative mitochondria. Gray bar: cells with elongated/total cell number, black bar: cells with foreshortened/total cell number, white bar: cells with intermediate (mid) mitochondria/total cell number. * p<0.01 vs. foreshortened in Ad-shScr at baseline, # p<0.01 vs. foreshortened in Ad-shScr after 1 hour GD, † p<0.01 vs. foreshortened in Ad-shScr after 4 hours GD, ** p<0.05 vs. elongated in Ad-shScr at baseline, ‡ p<0.01 vs. elongated in Ad-shScr at baseline (n=4/group). Scale bar: 20 m. D, TUNEL staining of CMs with Drp1 knockdown. * p<0.01 vs. shScr Ctr, # p<0.01 vs. Ad-shDrp1 Ctr, † p<0.01 vs. Ad-shDrp1 after 1 hour GD (n=3/group). Scale bar: 200 m. E, Representative images of GFP-LC3 puncta. Scale bar: 50 μm. Bar graph indicates mean number of autophagosomes per cell. * p<0.01 vs. Ad-shScr Ctr. # p<0.01 vs. Ad-shDrp1 Ctr. † p<0.01 vs. Ad-shScr after 4 hours GD (n=5/group). Scale bar: 50 μm. F, Representative images of mRFP-GFPLC3 puncta. Free red puncta indicate autolysosomes, and red and green (yellow) puncta indicate autophagosomes. Scale bar: 50 μm. Bar graph indicates mean number of autophagosomes and autolysosomes per cell. * p<0.01 vs. Ad-shScr at baseline, # p<0.01 vs. Ad-shDrp1 at baseline (n=5/group). G, Representative images of mRFP-GFP-LC3 puncta. Scale bar: 50 m. Bar graph indicates mean number of autophagosomes and autolysosomes per cell. a p<0.01 vs. Ad-shDrp1 at baseline, b p<0.05 vs. Ad-shScr at baseline, c p<0.01 vs. Ad-shScr after 4 hours GD, d p<0.01 vs. Ad-shDrp1 at baseline, e p<0.01 vs. AdshDrp1 after 4 hours GD (n=3/group). Figure 4. Drp1 mediates mitochondrial autophagy during GD. A, CMs were transduced with Ad-LacZ or Ad-Keima-MLS and cytosolic and mitochondrial fractions were analyzed by immunoblot (n=3/group). B, CMs were treated with CCCP (25 M) for 16 hours. Representative images of Keima-MLS. Lysosomes visualized with Alexa 488 Dextran colocalized with puncta with a high ratio of red to green, detected at 560 nm and 440 nm, respectively (n=3/group). Scale bar: 20 μm. C-D, Representative images of KeimaMLS. Puncta with high 560/440 indicate mitochondrial autophagy. The ratio of the area of puncta with high 560/440 vs. the total cell area is shown. C, CMs were transduced with Ad-Keima-MLS and then with AdshScr or Ad-shDrp1. Some then underwent 4 hours of GD. * p<0.01 vs. Ad-shScr Ctr, # p<0.01 vs. AdshScr with GD (n=5/group). Scale bar: 20 μm. D, CMs were transduced with Ad-Keima-MLS and AdshDrp1, followed by Ad-LacZ or Ad-Atg7. Scale bar: 20 μm. E, CMs were transduced with Ad-shScr or Ad-shDrp1 followed by Ad-LacZ or Ad-Atg7. Cell viability was evaluated with the CellTiter Blue assay. * p<0.01 vs. Ad-shScr without Ad-Atg7, # p<0.01 vs. Ad-shScr with Ad-Atg7 (n=3/group). F, Representative EM images of CMs in vitro. Asterisks indicate elongated mitochondria. Open arrows indicate autophagic vacuoles without mitochondria. Closed arrows indicate autophagic vacuoles containing mitochondria. Scale bar: 2 m. G, Left panel shows the number of mitochondria per cell. * p<0.01 vs. AdshScr without GD. Right panel shows relative mitochondrial mass per cell. Mitochondrial mass in CMs transduced with Ad-shScr at baseline is expressed as 1. * p<0.01 vs. Ad-shScr at baseline, # p<0.01 vs. AdshScr with GD. H, Left panel shows the mean number of autophagic vacuoles per cell. * p<0.01 vs. AdshScr without GD, # p<0.01 vs. Ad-shScr with GD, † p<0.01 vs. Ad-shDrp1 without GD. Right panel shows the number of autophagic vacuoles containing mitochondria per cell. * p<0.01 vs. Ad-shScr with GD. Figure 5. Drp1-CKO mice develop cardiac hypertrophy and fibrosis. A, Immunoblot analysis of cardiac Drp1 in Drp1-CKO and control mice. MHC-MCM: Tg-MHC-MerCreMer. * p<0.01 vs. Ctr (n=3/group). B, Gross morphology and sagittal sections of control and Drp1-CKO mouse hearts stained with Hematoxylin-Eosin. Scale bar: 2 mm. C, Assessment of CM size using WGA staining. * p<0.01 vs.Ctr. Scale bar: 200 m. D, Picric Acid Sirius Red staining to assess cardiac fibrosis. * p<0.01 vs.Ctr. Scale bar: 500 m. In C-D, n=4/group. In A-D, heart samples were harvested 4 weeks after TI. Figure 6. Mitochondria are fused and dysfunctional in Drp1-CKO mice. A, EM images of Drp1-CKO and control mouse hearts. The inset shows mitochondrial autophagy seen only in control mouse hearts after 48 hours fasting. Asterisks indicate elongated mitochondria. Mitochondrial mass in control mouse hearts at DOI: 10.1161/CIRCRESAHA.116.303356 17 baseline is expressed as 1. * p<0.01 vs. Ctr without fasting, # p<0.01 vs. Ctr with fasting. Scale bar: 2 m. B, Immunoblots for COX IV and -tubulin in Drp1-CKO and control mice. * p<0.01 vs. Ctr. C, Relative mitochondrial DNA content in Drp1-CKO and control mouse hearts, evaluated by PCR for cytochrome b. * p<0.01 vs. Ctr. D, Immunoblots for PGC-1, TFAM, and -tubulin in Drp1-CKO and control mice. E, Relative cardiac ATP production in Drp1-CKO and control mice. * p<0.01 vs. Ctr 10 days after TI, # p<0.01 vs. Ctr 28 days after TI, † p<0.01 vs. Drp1-CKO 10 days after TI. F, Mitochondrial swelling induced by Ca2+. Each data curve in the left panel represents the average of 3 individual measurements. Right panel shows the decrease in optical density at 540 nm. * p<0.05 vs. Ctr 10 days after TI, # p<0.01 vs. Ctr 10 days after TI, † p<0.01 vs. Ctr 28 days after TI, ** p<0.01 vs. Drp1-CKO 10 days after TI. G, ELISA of 4Hydroxynonenal. 4-HNE: 4-Hydroxynonenal. 4-HNE concentration in control mouse hearts is expressed as 1. * p<0.05 vs Ctr. H, H2O2 production from isolated mitochondria was evaluated with the Amplex Red Assay. * p<0.01 vs Ctr. In A-H, n=3/group. In A-E and G-H, samples were harvested 4 weeks after TI. Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Figure 7. Apoptosis was increased, whereas autophagy was suppressed, in Drp1-CKO mice. A, TUNEL staining of hearts in Drp1-CKO and control mice. Arrow indicates TUNEL-positive nucleus. * p<0.01 vs. Ctr. Scale bar: 50 μm. B, Immunoblots for cleaved caspase 3, cytochrome c, COX IV, and tubulin. Expression normalized with -tubulin or COX IV in Drp1 fl/fl (control) mice is expressed as 1. * p<0.01 vs.Ctr. C and D, Immunoblots for LC3, p62, and -tubulin. * p<0.01 vs.Ctr. In D, some mice were treated with chloroquine (10 mg/kg, i.p.) and evaluated 4 hours later. * p<0.01 vs. Ctr without chloroquine, # p<0.01 vs. Ctr with chloroquine. E, Representative images of mRFP-GFP-LC3 puncta. Fst:fasting. Bar graph indicates mean number of autophagosomes and autolysosomes per cell. * p<0.01 vs. yellow dots of Ctr at baseline, # p<0.01 vs. free red dots of Ctr at baseline. Scale bar: 50 μm. In A-E, heart samples were harvested 4 weeks after TI (n=3/group). Figure 8. Drp1-hetCKO mice develop cardiac dysfunction and are more susceptible to I/R injury. A, Immunoblots for Drp1 and -tubulin. * p<0.01 vs. Ctr (n=3/group). B, LVEF at 12 weeks of age. There was no significant difference in LVEF between control and Drp1-hetCKO mice (n=4/group).C, Relative ATP production. * p<0.01 vs. Ctr, (n=3/group). D, Immunoblots for Drp1, COX IV, and -tubulin in mitochondrial and cytosolic fractions. Fst: fasting for 48 hours. I/R: 30 min ischemia and 24 hours reperfusion. * p<0.01 vs.Ctr at baseline, # p<0.01 vs. Ctr at baseline and Drp1-hetCKO at baseline and after Fst, † p<0.01 vs. Ctr sham, ** p<0.01 vs. Ctr sham and Drp1-hetCKO sham and after Fst (n=3/group). E, LVEF, as assessed by echocardiography. * p<0.01 vs. Ctr at baseline, Ctr after Fst and Drp1-hetCKO at baseline (n=3/group). F, EM images of Drp1-hetCKO and control mouse hearts. The inset shows mitochondrial autophagy seen only in control mouse hearts after I/R. Asterisks indicate elongated mitochondria. Mitochondrial mass in control mouse hearts without I/R is expressed as 1. * p<0.01 vs. Ctr without I/R, # p<0.01 vs. Ctr with I/R (n=3/group). Scale bar: 2 m. G, Immunoblots for LC3, p62, and tubulin. * p<0.01 vs. Ctr without I/R, # p<0.01 vs. Ctr with I/R (n=3/group). H, Representative images of tetrazolium chloride/Alcian Blue staining of LV sections after I/R. Statistical analyses of % area at risk (AAR) and MI/AAR are shown. * p<0.05 vs. control (n=3/group). DOI: 10.1161/CIRCRESAHA.116.303356 18 Novelty and Significance What Is Known? Combined genetic downregulation of mitofusin 1 and mitofusin 2, mitochondrial outer membrane proteins regulating mitochondrial fusion, causes mitochondrial fragmentation and dysfunction and heart failure in mice. Drp1 is a GTPase that mediates mitochondrial fission in non-cardiac cells. Pharmacological suppression of Drp1 with mdivi-1 attenuates myocardial injury in response to ischemia/reperfusion. What New Information Does This Article Contribute? Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Chronic downregulation of Drp1 induces elongation of mitochondria, mitochondrial dysfunction, heart failure and premature death in mice. Downregulation of Drp1 inhibits general autophagy and movement of mitochondrial proteins into lysosomes in cardiomyocytes. Chronic downregulation of Drp1 enhances myocardial injury in response to ischemia/reperfusion. Mitochondria have the ability to remove damaged parts through a process of fission and fusion and consequent degradation through autophagy. Using a loss-of-function mouse model, we show that chronic downregulation of Drp1, a GTPase known to induce mitochondrial fission, causes mitochondrial dysfunction, myocardial cell death, heart failure and the death of the animal. In vitro analyses show that genetic downregulation of Drp1 directly inhibits general autophagy in cardiomyocytes through Bcl-xLdependent mechanisms. Furthermore, using mito-Keima, a pH-sensitive protein, we show that endogenous Drp1 is essential for mitochondrial autophagy in response to glucose starvation in cardiomyocytes. Downregulation of Drp1 in turn causes accumulation of dysfunctional mitochondria and increased cell death. Furthermore, downregulation of Drp1 exacerbates ischemia/reperfusion injury in the mouse heart in vivo. These results suggest that endogenous Drp1 plays an important role in mediating mitochondrial autophagy and maintaining mitochondrial function in response to stress. DOI: 10.1161/CIRCRESAHA.116.303356 19 Figure 1 shDrp1 TnI 100 Elongated Mid Foreshortened 80 # 60 10 * 0 shScr shDrp1 t shScr Cells with elongated, foreshortened / total cell number (%) no C mtDsRed2 shScr shDrp1 o A ul di at st io rib n TUNEL positive ut Re nuclei (%) e. se D ac es h tr Pe oy e af r R Relative tecell viability e r u vie se w. . D caspase3 17kDa D-tubulin shScr TUNEL shDrp1 DAPI D 15 10 5 0 * (%) 100 䕔 䕦 rC irc 䕔 䕦 䕔 * 60 40 20 䕔 䕦 80 䕔 䕦 䕦 # shScr shDrp1 0 0 Fo Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 B 50kDa 24 48 72 (hours) 96 Figure 2 Chl - sh shScr sh Drp1 Drp1 - + + Drp1 LC3 I LC3 II 15kDa Short LC3 II p62 62kDa D-tubulin 50kDa * 1.6 1.2 0.8 #† 0.4 # 0.8 0.4 0 - + C * 80 60 2 0 E F 1.0 * 0.5 0 shScr shDrp1 * 20 0 shScr shDrp1 + CsA 0.22 0.20 0.18 0 shDrp1 0 4 8 12 16 20 Time (min) 20 H * 16 12 8 # 4 0 CsA 5PM * - # + - + shScr shDrp1 Relative cell viability shScr + CsA 0.24 % Decrease in OD540 Fo shScr shDrp1 0.26 1.2 #† 1.0 0.8 * 0.6 0.4 0.2 0 CsA 5PM - + - 50kDa 4 * 3 2 1 0 shScr shDrp1 40 G 17kDa D-tubulin shDrp1 60 rC irc Relative ATP production COX IV Ctr Chl Ctr Chl shScr * D # # 0 + shScr shDrp1 Relative Cox-IV expression 20 Chl 10PM 4Hr - shDrp1 1 40 OD540nm Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Ctr + shScr t shDrp1 Relative mt DNAo shDrp1 - Chl + no - ul di at st io rib n ut Re e. se D ac LC3 dots / cell es h tr Pe Green fluorescent oy e cell number / total cell number (%) af r R te ev r u ie se w. . D shScr * *# 1.2 0 Chl shScr B * 1.6 content Long 80kDa p62 / D-tubulin expression shScr LC3II / D-tubulin expression A + shScr shDrp1 Figure 3 Ctr GD 4Hr Drp1 COX IV D-tubulin 80kDa 17kDa 50kDa C Base mt-DsRed2 80 60 40 20 0 Fo TUNEL shScr Ctr GD 4Hr GD4Hr GD1Hr mt-DsRed2 TnI TnI E GD4Hr mt-DsRed2 Ctr GD4Hr shScr ** ‡ *# † *# ‡ ‡ #† † Base GD1Hr GD4Hr Base GD1Hr GD4Hr shScr shDrp1 GD1Hr GD4Hr shDrp1 † # 30 * * 25 #* 20 15 10 * 5 0 LC3 dots / cell 100 rC irc Cells with elongated, foreshortened / total cell number (%) DAPI 0 Elongated Mid Foreshortened Ctr shDrp1 0.2 Ctr GD 4Hr TUNEL positive nuclei (%) Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 shDrp1 TUNEL 0 Base 0.4 ul di at st io rib n ut Re e. se D ac es h tr Pe oy e af r R te ev r u ie se w. . D shScr D 1 0.6 Drp1 mt-DsRed2 Merge o TnI 2 B t GD 4Hr * 0.8 no Ctr 3 Drp1 / COX IV Cytosol Mitochondria Drp1 / D-tubulin A 30 20 #† 10 0 DAPI shScr shDrp1 shScr shDrp1 Figure 3 F shScr Baseline shDrp1 GD4Hr Baseline * 100 GD4Hr Free red puncta Yellow puncta 80 60 * 8 6 t RFP 40 no LC3 dots / cell GFP 2 # 0 Merge Free red puncta G shScr + Atg7 Baseline GD shDrp1 + Atg7 Baseline Base GD4Hr Base GD4Hr shScr shDrp1 de 120 Free red puncta de GD Yellow puncta 100 GFP 80 60 40 RFP a b a c Merge rC irc 20 Fo Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 ul di at st io rib n ut Re e. se D ac es h tr Pe oy e af r R te ev LC3 dots / cell r u ie se w. . D Yellow puncta o 4 0 Base GD4Hr Base GD4Hr shScr shDrp1 Atg7 A cytosol mitochondria B Ratio Alexa 488 560 nm 560/440 nm Dextran Merge 440 nm E CCCP 25 PM Keima COX IV D-tubulin 28kDa 17kDa 50kDa Relative cell viability Figure 4 1.0 *# 0.8 0.6 *# 0.4 0.2 0 Atg7 - - + + shScr shDrp1 C * 8 6 440 nm 560 nm 4 2 560 nm # # 0 GD - + High (560/440) signal area / cell area (%) shDrp1 shDrp1 + LacZ + Atg7 no GD4H䡎 ul di at st io rib n ut Re e. se D ac es h tr Pe oy e af r R te ev r u ie se w. . D 440 nm 0.8 0.4 0 shDrp1 - + + + Ratio Ratio F Baseline rC irc shScr * shDrp1 * GD 4Hr * * * 100 * 80 * * 60 40 20 0 GD Relative mitochondrial mass 2 120 * 1 0 - + shScr - + shDrp1 GD - + - + shScr shDrp1 Autophagic vacuole / cell H * # * 14 12 10 8 6 4 # 2 † *# 0 GD - + - + shScr shDrp1 Autophagic vacuole containing mitochondria / cell Fo * G Number of mitochondria / cell Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 High (560/440) signal area / cell area (%) shDrp1 Ctr GD4H䡎 o shScr Ctr t D 4 3 2 1 * 0 GD - * + - * + shScr shDrp1 Figure 5 B 50kDa Tamoxifen - + - + Drp1 fl/fl + + + + D-MHC MCM - - + + 0.8 0.4 0 Tamoxifen Drp1 fl/fl Ctr Drp1-CKO D-MHC MCM * + - + + - + + + + + t 80kDa Ctr 1.2 no Drp1 α-tubulin Relative Drp1 expression A C (Pm2) - Tamoxifen * 300 + 200 Drp1 fl/fl 㽢 D-MHC MCM Drp1 fl/fl 100 0 Tamoxifen - + - + Drp1 fl/fl + + + + D-MHC MCM - - + + Ctr D - rC irc Drp1 fl/fl 㽢 D-MHC MCM Drp1 fl/fl Fo Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 ul di at st io rib n ut Re e. se D ac es h tr Pe oy e area rR % Fibrosis aCross-sectional fte e r u vie se w. . D o Ctr Tamoxifen + * 12 8 4 0 Tamoxifen - + - + Drp1 fl/fl + + + + D-MHC MCM - - + + Ctr Figure 6 * 48Hr fasting 3 0 Tamoxifen 2 * 1 - Fasting + PGC-1D TFAM α-tubulin + + + + + + Drp1 fl/fl D-MHC MCM - - + + D-MHC MCM COX IV 17kDa D-tubulin + - * E 27kDa + + - Drp1-CKO 3 + + 1.2 1.0 * # 0.8 2 0.6 † 1 0.4 * # + Drp1 fl/fl + + D-MHC MCM - - - Ctr + + + + + 0 Tamoxifen Drp1 fl/fl D-MHC MCM rC irc ** Ctr (28D) Fo 0.50 Drp1-CKO (10D) Drp1-CKO (28D) 0.45 10 8 *† 6 4 2 0 0 0 4 8 + + + + + 0.2 0 12 16 20 1.8 1.6 1.4 1.2 1.0 0.8 0.6 0.4 0.2 0 Tamoxifen Drp1 fl/fl Time (min) D-MHC MCM Ctr Drp1 CKO Ctr G #† Ctr (10D) 0.55 + + - Ctr F 0.60 + - + - + + Ctr + + Drp1-CKO H * (Pmol/Pg) 2.5 H2O2 concentration - + + + Ctr 50kDa Tamoxifen OD540nm Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Drp1 fl/fl 4 90kDa 50kDa Tamoxifen ul di at st io rib n % Decrease in OD540 ut Re e. se Relative COX IV D acexpression es h tr Pe oy e Relative 4-HNE content af r R te ev r u ie se w. . D Relative ATP production + Ctr B D-MHC MCM + + + D + + Tamoxifen * + + - Drp1 fl/fl 0 * 2 no Base # 4 * 4 t Relative mitochondrial mass * C # * Drp1-CKO o Ctr Relative mtDNA content A + + + * 2.0 1.5 1.0 0.5 0 Tamoxifen + + + + D-MHC MCM + Ctr Drp1 CKO Drp1 fl/fl B mitochondria TUNEL DAPI 17kDa 50kDa Tamoxifen - + - + Drp1 fl/fl + + + + D-MHC MCM - - + + Cytochrome c COX IV D-tubulin Tamoxifen Drp1 fl/fl + D-MHC MCM - 0.2 0.1 + + * 12 8 4 0 Tamoxifen Drp1 fl/fl + D-MHC MCM - + + + + + - + + + + + p62 D-tubulin D-MHC MCM + + - + + 15kDa 1.2 62kDa 0.8 + + + + - + + + + + Drp1 fl/fl D-MHC MCM - Ctr + + - Chl - LC3 I - Ctr rC irc p62 E + Drp1 CKO + + + + + - Ctr Baseline Fasting 62kDa 50kDa *# 0 Chl - * 3 2 1 + Yellow puncta 10 * 0 Base Fst Base Fst Free red puncta 1 0 Chl - + Free red puncta 5 Merge * Yellow puncta 15 Ctr Drp1-CKO + + + * 2 Ctr 20 + + - * # 25 + + Ctr Drp1-CKO Baseline Fasting LC3 dots / area RFP - + + + 4 Ctr Drp1-CKO 30 GFP + + + + Ctr Drp1 fl/fl D-MHC MCM - # 1 + + 0 Tamoxifen + 2 + 15kDa LC3 II D-tubulin Drp1 CKO + + * 0 Tamoxifen Drp1 fl/fl + D-MHC MCM - expression Ctr + + Ctr Ctr D + - 1 * 0 Tamoxifen + + + + 2 0.4 Drp1 fl/fl 50kDa Ctr 50kDa Fo Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 LC-3 I LC-3 II + - 17kDa Ctr Ctr Tamoxifen 14kDa ul di at st io rib n ut Re e. se D ac LC3II / D-tubulin LC3II / D-tubulin es h expression expression tr Pe oy e af r R te ev r u ie se w. D p62 / D-tubulin . p62 / D-tubulin + + - Cytochrome c / D-tubulin expression % TUNEL positive nuclei * 0 Tamoxifen Drp1 fl/fl + D-MHC MCM - C Caspase3 / D-tubulin expression Ctr cytosol t Caspase3 D-tubulin no Drp1-CKO o Ctr expression Figure 7 A - + Drp1-CKO 80 80kDa 50kDa 1.0 60 40 20 * 0.5 0 Ctr 0 Ctr Drp1 hetCKO Drp1 hetCKO 0.8 * 60 40 20 0.4 0 0 Ctr (12W) (12W) 48Hr Fst Drp1 hetCKO Ctr (12W) (12W) 5 G * * p62 Drp1 80kDa 17kDa 48Hr Fst Drp1 hetCKO # 1.0 0.8 0.6 0.4 0.2 0 3 2 1 0 D-tubulin † † * * # 2 * 1 # *# *# 1 * 62kDa 0 I/R (12W) Infarct / AAR (%) (12W) I/R Drp1 hetCKO 40 AAR (%) Drp1 hetCKO 0 - + - + Ctr Ctr Drp1 hetCKO Sham * ** 10 8 6 4 2 0 2 50kDa Ctr 50kDa 15kDa Fo LC3 I LC3 II I/R F Drp1-hetCKO COX IV D tubulin 4 rC irc Sham Drp1 / COX IV Drp1 / D-tubulin Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Drp1 COX IV D tubulin 1.0 0.8 0.6 0.4 0.2 0 Ctr Drp1-hetCKO 30 20 10 0 Ctr Drp1 hetCKO - + - + Ctr Drp1 hetCKO * 60 40 20 0 Ctr Drp1 hetCKO I/R Relative mitochondrial mass Ctr ul di at LC3II / D-tubulin i expression s tr on ib R ut e Drp1 / D-tubulin e. se D ac es h tr Pe p62 / D-tubulin o COX IVe expression Drp1 /y af r R te ev r u ie se w. . D D H * o Relative Drp1 Expression Drp1 α-tubulin 80 1.2 t (12W) E C no (12W) B LVEF (%) Drp1 hetCKO Relative ATP production Ctr LVEF(%) Figure 8 A # * 2 # * 1 0 I/R * - Ctr + - + Drp1 hetCKO Downloaded from http://circres.ahajournals.org/ by guest on May 3, 2017 Endogenous Drp1 Mediates Mitochondrial Autophagy and Protects the Heart Against Energy Stress Yoshiyuki Ikeda, Akihiro Shirakabe, Yasuhiro Maejima, Peiyong Zhai, Sebastiano Sciarretta, Jessica Toli, Masatoshi Nomura, Katsuyoshi Mihara, Kensuke Egashira, Mitsuru Ohishi, Maha Abdellatif and Junichi Sadoshima Circ Res. published online October 20, 2014; Circulation Research is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2014 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7330. Online ISSN: 1524-4571 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circres.ahajournals.org/content/early/2014/10/20/CIRCRESAHA.116.303356 Data Supplement (unedited) at: http://circres.ahajournals.org/content/suppl/2014/10/20/CIRCRESAHA.116.303356.DC1 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation Research can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation Research is online at: http://circres.ahajournals.org//subscriptions/ Supplemental Materials and Methods Primary culture of neonatal rat ventricular myocytes Primary cultures of ventricular cardiomyocytes (CMs) were prepared from 1-day-old Crl:(WI) BR-Wistar rats (Harlan). A CM-rich fraction was obtained by centrifugation through a discontinuous Percoll gradient as described.1 Glucose deprivation To obtain starvation conditions, CMs were washed three times with phosphate-buffered saline (PBS) and incubated in glucose-free serum-free DMEM (11966-025, Invitrogen) at 37 °C as described.1 Chloroquine treatment To inhibit autophagic flux in vivo, chloroquine was injected (10 mg/kg) intraperitoneally as previously described.2 Four hours later, animals were euthanized for immunoblot detection of autophagy markers. To inhibit autophagic flux in vitro, cultured CMs were treated with 10 mM chloroquine for 4 hours. Adenovirus construction and transduction Adenoviruses harboring GFP-LC3 (Ad-GFP-LC3),2 tandem fluorescent mRFP-GFP-LC3 (Ad-tf-LC3),2 mt-DsRed2,3 wild-type Atg7,3 LacZ (Ad-LacZ),3 hemagglutinin (HA)-tagged Drp1,4 Beclin1 short hairpin (sh) RNA (Ad-shBeclin1),1 flag-tagged Beclin1,5 Bcl-xL shRNA (Ad-shBcl-xL),5 and Scramble shRNA (Ad-shScr)1 have been described. Adenoviruses harboring shRNA for Drp1 (Ad-shDrp1) and for Atg7 (Ad-shAtg7) were generated using the Admax system (Microbix) as previously described1 using the following hairpin-forming oligos: 5’CGGCAATTGAGCTAGCATATTTCAAGAGAATATGCTAGCTCAATGCCTTTTTA-3’ for 1 Ad-shDrp1 and 5’CGCGTCACAGCCCTGCCATATTCAAGAGATATGGCAGGGCTGTGACGCTTTTTA-3’ for Ad-shAtg7. Transductions with Ad-Drp1, Ad-Keima-MLS, Ad-mt-DsRed2, Ad-Atg7, Ad-tf-LC3, Ad-GFP-LC3, and Ad-LacZ were carried out for 48 hours. Knockdown adenoviruses, including Ad-shScr, Ad-shDrp1, Ad-shBeclin1, and Ad-shAtg7, were transduced for 96 hours. Adenoviruses were transduced at 15 MOI. Evaluation of fluorescent LC3 puncta The method of evaluating tandem fluorescent LC3 puncta using Ad-tf-LC3 has been described previously.2 CMs cultured on cover slips were transduced with Ad-GFP-LC3 or Ad-tf-LC3 at 15 MOI. Forty-eight hours after adenovirus transduction, the cells were washed with PBS, fixed with 4% paraformaldehyde (PFA), mounted with a reagent containing 4’,6-diamidino-2-phenylindole (DAPI) (Vectashield, Vector Laboratories), and viewed under a fluorescence microscope (Nikon Eclipse E800). The number of GFP and mRFP dots was determined by manual counting of fluorescent puncta from at least 4 different myocyte preparations with a 60X objective. At least 50 cells were scored in each experiment. The nuclear number was evaluated by counting the number of DAPIstained nuclei in the same field. The number of dots/cell was obtained by dividing the total number of dots by the number of nuclei in each microscopic field. For in vivo determination of the number of fluorescent LC3 dots, fresh heart slices were embedded in Tissue-Tek OCT compound (Sakura Finetechnical Co.) and frozen at -80°C. Sections 10 μm thick were obtained from the frozen tissue samples using a cryostat (CM3050 S; Leica), air-dried for 30 min, fixed by washing in 95% ethanol for 10 min, mounted using a reagent containing DAPI, and viewed under a fluorescence microscope. 2 Histological analysis Histological analysis was performed as described.1 In brief, heart specimens were fixed with formalin, embedded in paraffin, and sectioned at 6 m thickness. Interstitial fibrosis was evaluated by Masson’s Trichrome and Picric Acid Sirius Red (PASR) staining. The myocyte cross-sectional area was measured from images captured from wheat germ agglutinin (WGA)-stained sections. The outlines of 100–200 myocytes were traced in each section using NIH ImageJ. Immunohistochemistry The method of immunostaining has been described.3 CMs were stained with anti-Drp1 mouse monoclonal antibody (BD Transduction, 611112), anti-Troponin I rabbit polyclonal antibody (Santa Cruz, 15368), Alexa Fluor 488-conjugated goat anti-mouse IgG (Invitrogen), Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen), and Vectashield mounting medium with DAPI (Vector Laboratories). Analyses were performed using fluorescence microscopy (Zeiss). Electron microscopy Conventional electron microscopy was performed as described previously.1 In brief, CMs were fixed in Karnofsky’s fixative and then postfixed in 1% osmium tetraoxide, dehydrated in a graded series of acetone concentrations, and embedded in Sparr resin. Sections of 98 nm thickness were placed on copper grids that were double-stained with uranyl acetate and lead citrate. Discs were examined with a JEOL 1200 electron microscope. Mitochondrial mass was analyzed using Image J. The average mitochondrial mass was calculated from 50 mitochondria per slide on three different slides. 3 Evaluation of mitochondrial morphology Mitochondrial morphology was examined according to the modified methods described previously.6 At least 100 CMs transduced with mitochondria-targeted DsRed2 and immunostained with Troponin I were examined using confocal microscopy. Mitochondria whose length is shorter than one sarcomere (the distance between consecutive Zbands) are defined as foreshortened, those whose length is longer than 2 sarcomeres are defined as elongated, and those whose length is longer than one sarcomere and shorter than 2 sarcomeres are defined as intermediate (mid). Cells displaying either predominantly (>50%) elongated or (>50%) foreshortened mitochondria were classified as cells with elongated or foreshortened mitochondria, respectively. Cells containing <50% elongated and <50% foreshortened mitochondria were classified as intermediate (mid). Quantitative real-time PCR for mitochondrial DNA Total DNA was extracted from mouse hearts using the Quick-gDNA MiniPrep kit (ZYMO RESEARCH) according to the manufacturer’s protocol. The mtDNA content was quantified by real-time PCR of cardiac DNA as described.7 Primer sequences used for cytochrome b and β-actin are as follows: 5’-CCACTTCATCTTACCATTTATTATCGC-3’ (forward primer) and 5’-TTTTATCTGCATCTGAGTTTAA-3’ (reverse primer) for cytochrome b, and 5’-CTGCCTGACGGCCAGG-3’ (forward primer) and 5’CTATGGCCTCAGGAGTTTTGTC-3’ (reverse primer) for genomic β-actin. Subcellular fractionation Mitochondrial and cytosolic fractions were purified through a previously described procedure.7 Briefly, isolated mouse hearts were homogenized in 10 volumes of ice-cold 4 Buffer A [200 mM mannitol, 50 mM sucrose, 10 mM KCl, 1 mM EDTA, 10 mM HepesKOH (pH 7.4), 0.1% BSA, and a mixture of protease inhibitors]. Homogenates were centrifuged at 600 × g for 5 min at 4 °C. Supernatants were then centrifuged at 3,500 × g for 15 min at 4 °C. The pellets were resuspended in Buffer A and centrifuged at 1,500 × g for 5 min. The supernatants were centrifuged at 5,500 × g for 10 min at 4 °C, and then the pellets were suspended as the mitochondrial fraction in PBS containing protease inhibitors. The supernatant was further centrifuged at 100,000 × g for 60 min, and the resultant pellet and supernatant were used as microsomal and cytosolic fractions, respectively. ATP production assay The mitochondrial fraction of mouse hearts was prepared as described above. ATP production was measured with an ATP Bioluminescent Assay kit (Sigma). 25mg of mitochondria is incubated with ATP assay mix and MSH buffer containing 625M ADP and substrate (10mM pyruvate and 10mM malate). Mitochondrial complex activity assay The mitochondrial fraction was prepared from mouse hearts as described above. Electron transport chain complex activities were measured, using MitoCheck Complex I, II-III and IV Activity Assay Kit (Cayman Chemical Company, USA) according to the method described previously.8 Briefly, complex I was assayed by monitoring the rotenone-sensitive ubiquinone-1 (Q1)-stimulated NADH oxidation, complex II+III by measuring the rate of reduced cytochrome c formation using succinate as substrate, and complex IV by measuring ferrocytochrome c oxidation with or without KCN. Mitochondrial complex activities were normalized by the weight of mitochondria. 5 Mitochondrial swelling assay The mitochondrial swelling assay was performed as described.7 In brief, 50 g of isolated mitochondria from mice or 30 g of isolated mitochondria from CMs were suspended in a swelling buffer [250 mM sucrose, 10 mM MOPS, 5 EGTA, 2 mM MgCl2, 5 mM KH2PO4, 5 mM pyruvate, and 5 mM malate] and incubated with 150 M calcium chloride (CaCl2) in a final volume of 200 L in a 96-well plate for 20 min. Absorbance was read at 540 nm. Evaluation of mitochondrial membrane potential In order to evaluate mitochondrial membrane potential/integrity, cultured CMs were stained with tetramethylrhodamine ethyl ester (TMRE) and 5,5’,6,6’- tetrachloro1,1’,3,3’-tetraethylbenzimidazolocarbocyanine iodide (JC-1) using MitoPT® TMRE and MitoPT® JC-1 (ImmunoChemistry Technologies), respectively, according to the manufacturer’s instructions. Mitochondrial flux analyses using the Seahorse system To measure the rate of oxidative phosphorylation in intact CMs, a Seahorse XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA) was used according to the methods described previously.9 CMs were plated at a density of 120,000 cells/well in 24-well Seahorse assay plates. CMs were transduced with AdshScr or Ad-shDrp1 for 96 hours prior to measurement. One hour prior to the beginning of measurements, the medium was replaced with XF medium supplemented with 17.5 mM glucose and 1 mM pyruvate and incubated for 1 hour in a 37°C incubator without CO2. Oxygen consumption rate (OCR) was measured three times at baseline, followed by injection with oligomycin (1 μM) to measure the ATP-linked OCR. Carbonylcyanide6 p-triflouromethoxyphenylhydrazone (FCCP, 3 μM), an uncoupler, was used to determine maximal respiration, and rotenone (1 μM) and antimycin A (1 μM) were injected to determine the non-mitochondrial respiration. Three independent experiments were performed. OCR was normalized by the amount of mtDNA or the rate of CM cell viability in each well. H2O2 measurement H2O2 production was measured with an Amplex Red H2O2 assay kit (Molecular Probes; Invitrogen) as described.10 Assays for measurement of oxidative stress Cardiac tissue homogenates were assessed for 4-Hydroxynonenal (4-HNE) content (Cell Biolabs, Inc, San Diego, CA, USA) as described.11 Cell viability Cell viability was measured by CellTiter-Blue (CTB) assays (Promega) as described.1 In brief, CMs (1 X 105 per 100 μl) were seeded onto 96-well dishes. After 24 hours, the cells were incubated with complete medium or glucose-free medium in the presence or absence of chelerythrine chloride (10 mM) or adenovirus vectors. Viable cell numbers were measured on the indicated days by the CTB assay. The CTB assays were performed according to the supplier's protocol. The experiments were conducted in triplicate at least three times. Evaluation of apoptosis DNA fragmentation was detected in situ with the use of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL), as described.1 Nuclear density was determined by manual counting of DAPI-stained nuclei in 6 fields for each animal with 7 the 40x objective, and the number of TUNEL-positive nuclei was counted by examining the entire section with the same power objective. Immunoblot analysis The methods used for preparation of cell lysates from in vitro and in vivo samples and for immunoblot analyses have been described previously.2 For in vitro samples, protein lysates were prepared from myocytes cultured in 6 cm culture dishes using boiled (95 °C for 2 min) 2X SDS sample buffer containing 4% SDS, 20% glycerol, 120 mM Tris-HCl (pH 6.8), 0.01% bromophenol blue, and 5% beta-mercaptoethanol. The protein samples were immediately boiled again at 95 °C for 3 min. Heart tissue homogenates were prepared using RIPA buffer containing 50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.1% SDS, 1% Igepal CA-630, and 0.5% sodium deoxycholate with protease inhibitors (Sigma, P8340) at a 1:400 dilution. The antibodies used include Drp1 (BD Transduction, 611112), Mfn1 (abcam, ab57602), Mfn2 (Sigma, M6319), OPA1 (BD Transduction, 612608), Fis1 (Santa Cruz, sc98900), LC3 (BML, M186-3), p62 (ORIGENE, TA307334), PGC-1 (Santa Cruz, sc-13067), TFAM (Sigma, SAB1401383), COX IV (Cell Signaling, 4844S), cytochrome c (Cell Signaling, 4272S), cleaved caspase 3 (Cell Signaling, 9664S), Keima (BML, M182-3), and -tubulin (Sigma, T6199). Densitometric analyses were performed using Scion Image software (Scion). Immunoprecipitation Immunoprecipitation was performed according to the methods described previously.5 In brief, CMs were lysed with IGEPAL CA-630 buffer (50 mM Tris-HCl (pH 7.4), 1% IGEPAL CA-630, 10 mM EDTA, 150 mM NaCl, 50 mM NaF, 1 μM leupeptin and 0.1 μM aprotinin). Primary antibody was covalently immobilized on protein A/G agarose using 8 the Pierce Crosslink Immunoprecipitation Kit according to the manufacturer's instructions (Thermo Scientific). Samples were incubated with immobilized antibody beads for at least 2 h at 4 °C. After immunoprecipitation, the samples were washed with TBS five times. They were then eluted with glycine-HCl (0.1 M, pH 3.5) and the immunoprecipitates were subjected to immunoblotting using specific primary antibodies and a conformation-specific secondary antibody that recognizes only native IgG (Cell Signaling). Hemodynamic analysis Echocardiography and measurement of LV +dP/dt were performed as described,12 using ultrasonography (Acuson Sequoia C256; Siemens Medical Solutions) and a highfidelity microtip pressure transducer catheter (1.4 Fr, Model SPR-839; Millar Instruments, Houston, TX), respectively. I/R surgery and assessment of area at risk and infarct size Myocardial I/R was achieved by temporarily occluding the left anterior descending coronary artery (LAD) and then releasing the occlusion as described.1 The duration of ischemia was 30 min, and that of reperfusion was 24 hours. To demarcate the ischemic area at risk (AAR), Alcian blue dye (1%) was perfused into the aorta and coronary arteries. Hearts were excised, and LVs were sliced into 1 mm thick cross sections. The heart sections were then incubated with a 1% triphenyltetrazolium chloride solution at 37 °C for 10 min. The infarct area (pale), the AAR (not blue), and the total LV area from both sides of each section were measured using Adobe Photoshop (Adobe Systems Inc.), and the values obtained were averaged. The percentage of area of infarction and AAR of each section were multiplied by the weight of the section and then totaled from 9 all sections. AAR/LV and infarct area/AAR were expressed as a percentage as previously reported.1 Mdivi1 treatment CMs or mice were administered with mdivi1 (Sigma) as described.6 10 References 1. Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914-922. 2. Hariharan N, Maejima Y, Nakae J, Paik J, Depinho RA, Sadoshima J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ Res. 2010;107:1470-1482. 3. Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, Sadoshima J. Activation of NADPH Oxidase 4 in the Endoplasmic Reticulum Promotes Cardiomyocyte Autophagy and Survival During Energy Stress Through the Protein Kinase RNA-Activated-Like Endoplasmic Reticulum Kinase/Eukaryotic Initiation Factor 2α/Activating Transcription Factor 4 Pathway. Circ Res. 2013;113:1253-1264. 4. Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958-966. 5. Maejima Y, Kyoi S, Zhai P, Liu T, Li H, Ivessa A, Sciarretta S, Del Re DP, Zablocki DK, Hsu CP, Lim DS, Isobe M, Sadoshima J. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat Med. 2013;19:1478-88. Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. 11 Circulation. 2010;121:2012-2022. 6. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565-15570. 7. Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P, Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554-564. 8. Yu H, Tigchelaar W, Koonen DP, Patel HH, de Boer RA, van Gilst WH, Westenbrink BD, Silljé HH. AKIP1 expression modulates mitochondrial function in rat neonatal cardiomyocytes. PLoS One. 2013;8:e80815. 9. Matsushima S1, Kuroda J, Ago T, Zhai P, Ikeda Y, Oka S, Fong GH, Tian R, Sadoshima J. Broad suppression of NADPH oxidase activity exacerbates ischemia/reperfusion injury through inadvertent downregulation of hypoxia-inducible factor-1α and upregulation of peroxisome proliferator-activated receptor-α. Circ Res. 2013 12;112:1135-49. 10. Ikeda Y, Fujita S, Miyata M, Shinsato T, Kubozono T, Kuwahata S, Hamada N, Miyauchi T, Yamaguchi T, Torii H, Hamasaki S, Tei C. Effect of Waon therapy on oxidative stress in chronic heart failure. Circ J. 2011;75:348-356. 11. Maejima Y, Galeotti J, Molkentin JD, Sadoshima J, Zhai P. Constitutively active MEK1 rescues cardiac dysfunction caused by overexpressed GSK-3α during aging and hemodynamic pressure overload. Am J Physiol Heart Circ Physiol. 12 2012;303:H979-88. 13 Online table I. Measurement of organ weight parameters in Drp1-CKO mice 4 weeks after tamoxifen injection Drp1 flox/flox MHC-MerCreMer Tamoxifen ip n BW (g) Tibia length (mm) LV weight (mg) LV weight/Tibia length (mg/mm) RV weight (mg) RV/Tibia length (mg/mm) Lung weight (mg) Lung weight/Tibia length (mg/mm) Liver weight (mg) Liver weight/Tibia length (mg/mm) + 4 26.0±3.4 19.6±0.3 84±4 4.3±0.2 15±3 0.8±0.2 166±4 8.5±0.3 983±111 50.3±5.8 + + 4 28.2±3.9 19.4±0.1 86±3 4.4±0.2 14±3 0.7±0.1 162±12 8.4±0.7 945±73 48.8±3.7 + + 4 24.1±0.5 19.6±0.4 83±2 4.2±0.2 12±3 0.6±0.1 163±16 8.3±0.8 935±123 47.9±6.7 + + + 8 26.4±4.1 19.5±0.6 105±13 * 5.4±0.8 * 21±4 * 1.1±0.2 * 191±16 * 9.8±0.8 * 1089±294 56.3±16.5 BW: Body weight, LV: Left ventricle, RV: Right ventricle. * p<0.01 vs. Drp1 flox/flox without Tamoxifen ip, Drp1 flox/flox with Tamoxifen ip or Drp1 flox/flox X MHCMerCreMer without Tamoxifen ip. Online table II. Measurement of organ weight parameters in Drp1-CKO mice 8 weeks after tamoxifen injection Drp1 flox/flox MHC-MerCreMer Tamoxifen ip n BW (g) Tibia length (mm) LV weight (mg) LV weight/Tibia length (mg/mm) RV weight (mg) RV/Tibia length (mg/mm) Lung weight (mg) Lung weight/Tibia length (mg/mm) Liver weight (mg) Liver weight/Tibia length (mg/mm) Ctr + + 4 26.3±2.6 20.2±0.9 87.5±7.6 4.34±0.44 13.0±2.4 0.64±0.09 158.0±13.9 7.9±1.0 1079±141 53.6±8.7 Drp1-CKO + + + 4 27.5±2.3 20.4±0.4 112.3±5.3 # 5.51±0.32 # 25.0±5.1 # 1.23±0.26 # 199.0±21.0 * 9.8±1.1 * 1340±91 * 65.7±4.1 * BW: Body weight, LV: Left ventricle, RV: Right ventricle. * p<0.05 vs. Ctr, # p<0.01 vs. Ctr. Online table III. Measurement of hemodynamic parameters in Drp1-CKO mice 4 weeks after tamoxifen injection Drp1 flox/flox MHC-MerCreMer Tamoxifen ip n HR (beat/min) LVEDP (mmHg) LVSP (mmHg) dP/dt Max (mmHg/sec) dP/dt Min (mmHg/sec) LVDd (X10-2mm) LVDs (X10-2mm) LVEF (%) IVS (X10-2mm) LVPW (X10-2mm) + 4 495±34 2.5±1.0 88.0±5.7 7250±100 7200±650 308±28 169±26 83.7±3.3 80.5±1.3 79.8±1.7 + + 4 495±34 2.5±1.0 89.5±4.4 7500±890 7500±380 328±39 179±22 82.0±1.9 79.5±1.7 80.5±2.1 + + 4 488±30 3.5±1.9 88.5±4.1 7400±520 7500±500 317±21 176±11 82.8±1.9 79.8±1.7 80.0±0.8 + + + 8 484±61 12.0±2.1 * 75.5±8.7 * 5200±520 * 5000±1100 * 393±44 * 307±38 * 52.4±5.9 * 84.0±2.6 * 84.5±3.3 * HR: Heart rate, LVEDP: Left ventricular end diastolic pressure, LVSP: Left ventricular systolic pressure, LVDd: Left ventricular diastolic dimension, LVDs: Left ventricular systolic dimension, LVEF: Left ventricular ejection fraction, IVS: Interventricular septum, LVPW: Left ventricular posterior wall. * p<0.01 vs. Drp1 flox/flox without Tamoxifen ip, Drp1 flox/flox with Tamoxifen ip or Drp1 flox/flox X MHC-MerCreMer without Tamoxifen ip. Online table IV. Measurement of hemodynamic parameters 8 weeks after tamoxifen injection Drp1 flox/flox MHC-MerCreMer Tamoxifen ip n HR (beats/min) LVDd (X10-2mm) LVDs (X10-2mm) LVEF (%) Ctr + + 4 498±79 319±15 175±15 83.2±4.4 Drp1-CKO + + + 4 493±54 409±11 * 339±24 * 41.9±12.9 * HR: Heart rate, LVDd: Left ventricular diastolic dimension, LVDs: Left ventricular systolic dimension, LVEF: Left ventricular ejection fraction. * p<0.01 vs. Ctr. Online table V. Measurement of hemodynamic parameters 10 days after tamoxifen injection Drp1 flox/flox MHC-MerCreMer Tamoxifen ip n HR (beats/min) LVDd (X10-2mm) LVDs (X10-2mm) LVEF (%) Ctr + + 5 504±14 318±11 178±15 81.9±4.8 Drp1-CKO + + + 6 502±12 319±25 177±16 82.0±5.9 HR: Heart rate, LVDd: Left ventricular diastolic dimension, LVDs: Left ventricular systolic dimension, LVEF: Left ventricular ejection fraction. Online table VI. Measurement of organ weight parameters in Drp1-hetCKO mice Drp1 flox/+ MHC-Cre Age (Week) n BW (g) Tibia length (mm) LV weight (mg) LV weight/Tibia length (mg/mm) RV weight (mg) RV/Tibia length (mg/mm) Lung weight (mg) Lung weight/Tibia length (mg/mm) Liver weight (mg) Liver weight/Tibia length (mg/mm) + 12 4 22.1±2.4 18.2±0.3 73.5±5.7 4.04±0.27 13.0±3.8 0.71±0.21 147.0±7.4 8.1±0.5 882±88 48.5±4.9 BW: Body weight, LV: Left ventricle, RV: Right ventricle. + + 12 4 22.4±2.5 18.2±0.6 74.0±9.5 4.06±0.40 13.0±3.8 0.73±0.26 146.5±7.0 8.0±0.2 883±94 48.4±3.9 Online table VII. Measurement of organ weight parameters in mdivi-1-treated mice Age (Week) n BW (g) Tibia length (mm) LV weight (mg) LV weight/Tibia length (mg/mm) RV weight (mg) RV/Tibia length (mg/mm) Lung weight (mg) Lung weight/Tibia length (mg/mm) Liver weight (mg) Liver weight/Tibia length (mg/mm) Ctr 12 4 26.4±1.3 18.3±0.2 88.8±7.7 4.84±0.40 18.3±5.1 1.00±0.28 145.0±18.6 7.9±1.0 858124 48.3±5.1 BW: Body weight, LV: Left ventricle, RV: Right ventricle. mdivi-1 12 4 25.1±2.3 18.8±0.6 88.0±2.7 4.69±0.23 18.3±3.8 0.97±0.18 1461.5±11.5 7.5±0.6 878±152 47.2±8.4 Online Figure I A B shScr shDrp1 80kDa a-tubulin 50kDa Relative Drp1 Expression Drp1 TnI Foreshortened mitochondria 1.0 0.5 0 * shscr shDrp1 Elongated mitochondria mt-DsRed2 Merge C shScr shDrp1 PGC1-a 90kDa a-tubulin 50kDa Online Figure I. Experimental validations. A, Construction of Ad-shDrp1. Representative immunoblots for Drp1 and α-tubulin are shown. * p<0.01 vs. Ad-shScr. The experiment was repeated 3 times. B, Definition of foreshortened and elongated mitochondria. Mitochondria whose length is shorter than one sarcomere (the distance between consecutive Zbands) are defined as foreshortened, those whose length is longer than 2 sarcomeres are defined as elongated, and those whose length is longer than one sarcomere and shorter than 2 sarcomeres are defined as intermediate (mid). TnI: Troponin-I, mt-DsRed2: mitochondria-targeted DsRed2. C, Immunoblot for PGC-1α and α-tubulin in CMs transduced with Ad-LacZ or Ad-shDrp1. The experiment was repeated 3 times. E 300 100 * 0 C 400 300 200 100 * 0 600 400 200 0 90 60 30 0 * Relative basal OCR / mt DNA 400 43.5 1.2 1.0 0.8 0.6 0.4 0.2 1.2 1.0 0.8 0.6 0.4 0.2 0.5 0 1.5 1.0 0.5 0 54.4 65.3 * 0 # 0 1.5 1.0 * 76.2 Relative basal OCR / cell viability 32.6 Relative ATP linked OCR / cell viability 21.8 FCCP Relative maximum OCR / cell viability 200 10.9 Relative ATP linked OCR / mt DNA OCR (pMol/min) Oligomycin Relative proton leak / cell viability Basal OCR (pMol/min) 0 Relative maximum OCR / mt DNA ATP linked OCR (pMol/min) A Relative proton leak / mt DNA Maximum OCR (pMol/min) D Proton leak (pMol/min) Online Figure II Rotenone Antimycin A 600 shScr 500 shDrp1 400 300 200 100 0 Time (min) 87.0 97.9 B 1.2 1.0 0.8 0.6 0.4 0.6 0.4 0.5 0 1.5 1.0 0.5 0 * 0.2 0 1.2 1.0 0.8 * 0.2 0 1.5 1.0 * Online Figure II. Downregulation of Drp1 inhibits mitochondrial function in CMs as assessed with a Seahorse system. A, Representative changes in OCR in CMs in response to oligomycin, FCCP, and rotenone plus antimycin A. Each data point represents the mean of 4 replicates. B-E, Bar graphs showing basal OCR (B), ATPlinked OCR (C), maximum OCR (D) and proton leak (E). Experimental data were normalized with mtDNA (middle columns) or cell viability (left columns). In all graphs, data from CMs transduced with Ad-shScr is expressed as 1. *p<0.05, #p<0.01 vs. Ctr, The experiment was repeated 3 times. Online Figure III A B Input Input Drp1 Drp1 Beclin1 Bcl-2 Bcl-2 Bcl-xL Ad-HA-Drp1 Ad-LacZ – + Bcl-xL + – IP: HA Ad-Flag-Beclin1 + + + Ad-HA-Drp1 – + – Ad-sh-Drp1 – – + IP: Flag Bcl-2 Bcl-2 Bcl-xL Ad-HA-Drp1 – Bcl-xL + Ad-HA-Drp1 + – + + + – Ad-shDrp1 – – + Ad-Flag-Beclin1 C shScr shDrp1 shDrp1 + shBcl-xL D shBcl-xL shScr Ctr GD 4Hr Chl 10mM 4Hr † 60 50 40 † † * # * # * # † * # 30 20 10 0 † * # † Number of GFP-LC3 dots / cell Number of GFP-LC3 dots / cell GD (-) GD4Hr # * 170 150 130 * 40 * 20 0 Chl - + shScr Baseline shDrp1 + shBcl-xL - + shDrp1 + shBcl-xL Online Figure III. Drp1 physically interacts with Bcl-2/Bcl-xL, thereby inhibiting interaction between Beclin1 and Bcl-2/Bcl-xL. Downregulation of Drp1 inhibits autophagy by stimulating interaction between Beclin1 and Bcl-2/Bcl-xL. A, CMs were transduced with Ad-HA-Drp1 or Ad-LacZ. Forty-eight hours after transduction, lysates were extracted for immunoprecipitation with HA antibody, followed by probing with Bcl-2 or Bcl-xL antibodies. Representative images are shown. B, CMs were transduced with Ad-Flag-Beclin1 either in the absence or presence of Ad-HA-Drp1 or Ad-sh-Drp1. Seventy-two hours after transduction, lysates were extracted for immunoprecipitation with Flag antibody, followed by probing with Bcl-2 or Bcl-xL antibodies. Representative images are shown. In A and B, experiments were repeated 3 times. C, Representative images of GFP-LC3 puncta. Scale bar: 50 μm. Bar graph indicates mean number of autophagosomes per cell. * p<0.01 vs. shScr without GD, # p<0.01 vs. shDrp1 without GD, † p<0.01 vs. shDrp1 with GD. D, Representative images of GFP-LC3 puncta in CMs incubated with chloroquine (10 μM) or vehicles for 4 hours. Scale bar: 50 μm. Chl: chloroquine. Bar graph indicates mean number of autophagosomes per cell. * p<0.01vs. shScr without Chl, # p<0.01 vs. shScr with Chl, † p<0.01 vs. shDrp1+shBcl-xL without Chl. In C and D, 50 myocytes per group were evaluated in each experiment and experiments were repeated 5 times. A Base TnI GD1Hr mt-DsRed2 TnI GD4Hr mt-DsRed2 TnI mt-DsRed2 B Ctr mdivi-1 (50 mM) 100 80 a b 60 40 Elongated Mid Foreshortened g g ab 20 c c 0 Ctr gh e ad cf mdivi-1 g gh cf cf * 0.8 0.6 0.4 0.2 0 1.2 1.0 * 0.8 0.6 * † *# 0.4 0.2 0 Chelerythrine - + - + 10mM shScr shDrp1 mdivi-1 (50 mM) (DMSO) (100 mM) D 1.2 Ctr * 1.0 (DMSO 1W) 0.8 0.6 0.4 mdivi-1 0.2 (50mM, 1W) 0 GD - - + + - + Relative cell viability (%) DMSO mdivi-1 mdivi-1 50mM 100mM (Ctr) 120 100 80 60 40 20 ■ ▲ * Ctr # mdivi-1 0 Base 1 24 48 72 96 (hours) Cells with elongated, foreshortened / total cell number (%) Relative cell viability 1.0 Relative cell viability Cells with elongated, foreshortened / total cell number (%) (100 mM) E *†† 1.2 Chelerythrine - + - + 10mM DMSO mdivi-1 (Ctr) 50 mM mdivi-1 C Relative cell viability Online Figure IV 100 Elongated Mid Foreshortened 80 f 60 e i eh i eh d ac d bc cd Base GD1Hr GD4Hr i eg 40 20 ac a 0 Base GD1Hr GD4Hr Ctr mdivi-1 (DMSO, 1W) (50mM, 1W) Online Figure IV F Ctr Baseline mdivi-1 4Days GD Baseline Free red puncta 120 Yellow puncta GD d 100 GFP LC3 dots / cell RFP 80 60 d e f a 40 a b c 20 Merge e 0 Base Ctr GD b Base GD mdivi-1 Online Figure IV. GD induces mitochondrial fission in CMs treated with mdivi-1.A, Assessment of mitochondrial morphology using mt-DsRed2. CMs were treated with mdivi-1 (50 M) or DMSO (vehicle) as control. Insets show typical mitochondrial morphology. Gray bar: cells with elongated/total cell number; black bar: cells with foreshortened/total cell number; white bar: cells with intermediate (mid)/total cell number . Base: baseline, Ctr: DMSO (control). a p<0.01 vs. Ctr foreshortened at baseline, b p<0.01 vs. Ctr foreshortened after 1 hour GD, c p<0.01 vs. Ctr foreshortened after 4 hours GD, d p<0.01 vs. mdivi-1 foreshortened at baseline, e p<0.01 vs. mdivi-1 after 1 hour GD, f p<0.01 vs. mdivi-1 foreshortened after 4 hours GD, g p<0.01 vs. Ctr elongated at baseline, h p<0.01 vs. Ctr elongated after 4 hours GD (n=4/group). Scale bar: 20 m. B, Upper panel: Cell viability of CMs with DMSO as a control or 50 M mdivi-1. * p<0.01 vs. DMSO without chelerythrine, † p<0.01 vs. DMSO with 10 M chelerythrine (n=4/group). Lower panel: Cell viability of CMs in Ad-shScr- or Ad-shDrp1transduced CMs. * p<0.01 vs. Ad-shScr without chelerythrine, # p<0.01 vs. Ad-shScr with 10 M chelerythrine, † p<0.01 vs. Ad-shDrp1 without chelerythrine (n=4/group). C, Cell viability of CMs with DMSO as a control or mdivi-1 at 2 different doses (50 M and 100 M). *p<0.05 vs Ctr or 50 M mdivi-1 with GD. D, Assessment of mitochondrial morphology using mt-DsRed2. CMs were treated with mdivi-1 or the same volume of DMSO as control for 4 days. Insets show representative mitochondria. Gray bar: cells with elongated/total cell number; black bar: cells with foreshortened/total cell number; white bar: cells with intermediate (mid)/total cell number. a p<0.01 vs. Ctr foreshortened at baseline, b p<0.05 vs. Ctr foreshortened at baseline,.c p<0.01 vs. Ctr foreshortened after 1 hour GD, d p<0.01 vs. Ctr foreshortened after 4 hours GD, e p<0.01 vs. Ctr elongated at baseline, f p<0.05 vs. Ctr elongated at baseline, g p<0.01 vs. Ctr elongated after 1 hour GD, h p<0.05 vs. Ctr elongated after 1 hour GD, i p<0.01 vs. Ctr elongated after 4 hours GD (n=4/group). Scale bar: 20 m. E, Time course of cell viability in CMs treated daily with DMSO or 50 M mdivi-1, as evaluated with the CellTiter Blue assay (n=4/group). * p<0.01 vs. Ctr 72 hours after transduction, # p<0.01 vs. Ctr 96 hours after transduction. F, Representative images of mRFP-GFP-LC3 puncta. Scale bar: 50 m. Bar graph indicates mean number of autophagosomes and autolysosomes per cell. a p<0.01 vs. Ctr at baseline, b p<0.01 vs. Ctr with 4 hours GD, c p<0.01 vs. mdivi-1 at baseline, d p<0.01 vs. Ctr at baseline, e p<0.01 vs. Ctr with 4 hours GD, f p<0.01 vs. mdivi-1 at baseline (n=3/group). B CCCP 25 mM 440 nm shScr + GD 440 nm 560 nm 560 nm Ratio 560/440 nm Ratio 560/440 nm C shBeclin1 + GD TnI Base mt-DsRed2 TnI GD1Hr mt-DsRed2 TnI High (560/440) signal area / cell area (%) Online Figure V A Ctr 12 9 6 * 3 0 GD4Hr mt-DsRed2 shScr 100 80 e 60 f f e h eg 40 ab ab 20 cd cd Base GD1Hr 0 Base GD1Hr shScr GD4Hr GD4Hr shBeclin1 Elongated Mid Foreshortened D Relative cell viability Cells with elongated, foreshortened / total cell number (%) shBeclin1 1.2 1.0 0.8 0.6 0.4 0.2 0 * Online Figure V. Detection of CCCP-induced mitophagy using mitochondriatargeted Keima. A, Representative images of fluorescent Keima puncta in CMs with or without 25 M CCCP treatment after transduction with adenovirus harboring mitochondria-targeted Keima (Ad-Keima-MLS). Inset shows punctum with high 560/440 ratio. Scale bar: 20 m. B, CMs were transduced with Ad-Keima-MLS and either AdshScr or Ad-shBeclin1. Some were then subjected to 4 hours of GD. Inset shows foreshortened mitochondria. * p<0.01 vs. Ad-Scr with GD (n=5/group). Scale bar: 20 μm. C, Assessment of mitochondrial morphology using mt-DsRed2. CMs were transduced with Ad-shBeclin1 or Ad-shScr as control for 4 days. Insets show representative mitochondria. Gray bar: cells with elongated/total cell number; black bar: cells with foreshortened/total cell number; white bar: cells with intermediate (mid)/total cell number. a p<0.01 vs. shScr foreshortened at baseline, b p<0.01 vs. shScr foreshortened after 1 hour GD, c p<0.01 vs. shScr foreshortened after 4 hours GD, d p<0.01 vs. shBeclin1 elongated after 4 hours GD, e p<0.01 vs. shScr elongated at baseline, f p<0.01 vs. shScr elongated after 1 hour GD, g p<0.01 vs. shScr elongated after 4 hours GD, h p<0.01 vs. shBeclin1 elongated at baseline (n=4/group). Scale bar: 20 m. D, Cell viability of CMs transduced with either Ad-shScr or Ad-shBeclin1 for 4 days followed by 4 hours GD. * p<0.01 vs. shScr with 4 hours GD (n=4/group). B LacZ a-tubulin 50kDa Relative Drp1 Expression Drp1 80kDa Drp1 TnI * 4 mtDsRed2 2 0 Cells with elongated, foreshortened / total cell number (%) Online Figure VI A LacZ Drp1 100 60 * 40 20 0 LacZ Drp1 LacZ Drp1 LacZ D Drp1 LacZ Drp1 E Relative mt DNA content C Elongated Mid Foreshortened # 80 TUNEL DAPI 0.8 * 0.4 0 * 8 4 0 LacZ Drp1 * 60 40 20 0 LacZ Drp1 Relative cell viability 12 Green fluorescent cell number / total cell number (%) % TUNEL positive nuclei LacZ Drp1 G † 1.0 *# 0.8 0.6 0.4 0.2 0 shAtg7 - + LacZ Ctr 440 nm 560 nm Ratio GD4Hr + Drp1 Drp1 Ctr † GD4Hr High (560/440) signal area / cell area (%) LacZ F - * # 10.0 8.0 * * 6.0 4.0 2.0 0 Ctr GD Ctr GD LacZ Drp1 Online Figure VI. Forced overexpression of Drp1 induces mitochondrial dysfunction and apoptosis in CMs. A, Construction of Ad-Drp1. Representative immunoblots for Drp1 and -tubulin are shown. * p<0.01 vs. Ad-LacZ. B, Assessment of mitochondrial morphology using mt-DsRed2. The proportions of CMs with elongated and foreshortened mitochondria were quantitated. TnI staining indicates CM. Gray bar: cells with elongated /total cell number; black bar: cells with foreshortened /total cell number; white bar: cells with intermediate (mid)/total cell number. * p<0.01 vs. foreshortened in Ad-LacZ, # p<0.01 vs. elongated in Ad-LacZ (n=4/group). Scale bar: 20 m. C, TUNEL staining of CMs with overexpression of Drp1. * p<0.01 vs. Ad-LacZ (n=3/group). Scale bar: 200 m. D, Mitochondrial membrane potential was evaluated with JC-1. Red color indicates mitochondria in which membrane potential is maintained, whereas green color indicates depolarized mitochondria. Quantification of CMs with depolarized mitochondria is shown. * p<0.01 vs. Ad-LacZ (n=3/group). Yellow scale bar: 500 m; white scale bar: 100 m. E, Relative mitochondrial DNA content in CMs with Drp1 overexpression, as evaluated by PCR for cytochrome b. * p<0.01 vs. Ad-LacZ (n=3/group). F, Representative images of Keima fluorescent puncta after transduction with Ad-Keima-MLS. A high 560/440 ratio indicates mitophagy. Ctr: control. The proportions of the high ratio (560/440) signal area to the total cellular area are shown. * p<0.01 vs. Ctr of Ad-LacZ, # p<0.01 vs. GD of Ad-LacZ, † p<0.01 vs. Ctr of Ad-Drp1 (n=5/group). Scale bar: 20 μm. G, Cell viability in Ad-LacZ- or Ad-Drp1-transduced CMs, as evaluated with the CellTiter Blue assay. * p<0.01 vs. Ad-LacZ without AdshAtg7, # p<0.01 vs. Ad-LacZ with Ad-shAtg7, † p<0.01 vs. Ad-Drp1 without Ad-shAtg7 (n=8/group). Online Figure VII 15 weeks old A 16 weeks old Tamoxifen (20mg / ip) for 5 days Drp1-CKO mice 20 weeks old 24 weeks old 4 weeks 4 weeks Drp1 MCM Drp1 fl/fl Control mice Drp1 MCM Drp1 fl/fl B Measured hemodynamics and harvested tissue samples C Drp1 80kDa Mfn-1 75kDa α-tubulin 50kDa Mfn-2 86kDa 100kDa OPA1 D 86kDa Survival rate (%) 100 17kDa Fis1 80 α-tubulin 60 40 Drp1-CKO 20 Control 0 0 10 50kDa Tamoxifen - + - + Drp1 fl/fl + + + + a-MHC MCM - - + + 30 (weeks) 20 Ctr Tamoxifen ip (15-16 weeks old) E Drp1 fl/fl a-MHC MCM Tamoxifen Ctr + + Drp1-CKO + + + F - Tamoxifen + Drp1 fl/fl × a-MHC MCM (mm2) * 400 300 200 100 0 Drp1 fl/fl a-MHC MCM Tamoxifen + + + + + 8 weeks after tamoxifen ip * % Fibrosis Cross-sectional area Drp1 fl/fl 16 12 8 4 0 Tamoxifen Drp1 fl/fl a-MHC MCM + - + + Ctr + + + + + Online Figure VII G Ctr + + Drp1 fl/fl a-MHC MCM Tamoxifen H Drp1-CKO + + + - Tamoxifen + a-MHC MCM WT * Cross-sectional area % Fibrosis 20 15 10 5 0 Drp1 fl/fl + + a-MHC MCM - + Tamoxifen + + 8 weeks after tamoxifen ip I 100 0 - + - + a-MHC MCM - - + + J Tamoxifen + 80 a-MHC MCM WT 2 % Fibrosis 200 Tamoxifen LVEF (%) - (mm2) 1 0 Tamoxifen - + - + a-MHC MCM - - + + 60 40 20 0 Tamoxifen - + - + a-MHC MCM - - + + Online Figure VII. Basal characterization of Drp1-CKO mice. A, A scheme of the experimental protocol. MCM: MerCreMer B, Immunoblots for Drp1 and -tubulin in Drp1-CKO mice (n=3/group). C, Immunoblots for factors related to mitochondrial dynamics in Drp1-CKO and control mice (n=3/group). D, Kaplan–Meier curve of Drp1CKO and control mice. p<0.05 vs. control (n=8/group). E, Assessment of CM crosssectional area in control and Drp1-CKO mice using WGA staining 8 weeks after tamoxifen injection. Scale bar: 200 m. * p<0.01 vs. Ctr (n=3/group). F, Masson’s trichrome staining of sections from Drp1-CKO and control mice. * p<0.01 vs. controls (n=4/group). Scale bar: 500 m. G, Assessment of CM fibrosis in control and Drp1-CKO mice using Picric Acid Sirius Red (PASR) staining 8 weeks after tamoxifen injection. Scale bar: 500 m. * p<0.01 vs. Ctr (n=3/group). H, Assessment of CM size in TgMHC-MerCreMer and wild-type mice with or without tamoxifen using WGA staining. Scale bar: 200 m. There was no significant difference between the 4 groups of mice (n=4/group). WT: wild-type. I, Assessment of fibrosis in Tg-MHC-MerCreMer and wildtype mice with or without tamoxifen using PASR staining. Scale bar: 500 m. There was no significant difference between the 4 groups (n=4/group). J, LV ejection fractions (LVEF), as evaluated with echocardiography 4 weeks after tamoxifen injection (n=4/group). In B, C and F, heart samples were harvested 4 weeks after tamoxifen injection. In E and G, heart samples were harvested 8 weeks after tamoxifen injection. B C tamoxifen ip Ctr Drp1-CKO Ctr Tamoxifen ip * * * Drp1-CKO 4W 8W 4W PGC1-a 90kDa a-tubulin 50kDa 8W COX IV 17kDa a-Tubulin 50kDa Ctr * 6 Relative COX IV expression Relative Mitochondrial mass D 4 2 0 + + Drp1 fl/fl a-MHC MCM Tamoxifen + + + *# *# 3 2 Drp1-CKO 1.2 Relative ATP production Online Figure VIII A 8 weeks after 0.8 * 0.4 0 1 Tamoxifen + + - + + + Ctr Drp1 CKO Drp1 fl/fl 0 4W Ctr Drp1 CKO 8W Ctr 4W a-MHC MCM 8W Drp1-CKO * Tamoxifen + + - Drp1 fl/fl a-MHC MCM 1.2 1.0 0.8 0.6 0.4 0.2 0 * Tamoxifen + + + Drp1 fl/fl a-MHC MCM Ctr Drp1 CKO + + - + + + Ctr Drp1 CKO 1.2 1.0 0.8 0.6 0.4 0.2 0 * Ctr Drp1-CKO TUNEL Tamoxifen Drp1 fl/fl a-MHC MCM + + - + + + Ctr Drp1 CKO DAPI 8 weeks after tamoxifen ip 8 weeks after tamoxifen ip 8 weeks after tamoxifen ip H % TUNEL positive nuclei 1.2 1.0 0.8 0.6 0.4 0.2 0 Relative complex IV activity Relative complex I activity E Relative complex II+III activity 8 weeks after tamoxifen ip * 2.0 1.5 1.0 0.5 0 + + + + + Ctr Drp1 CKO Drp1 fl/fl F Ctr Drp1-CKO + + + + + Drp1 fl/fl a-MHC MCM Tamoxifen G Drp1 fl/fl a-MHC MCM Tamoxifen Ctr Drp1-CKO + + + + + a-MHC MCM Tamoxifen 8 weeks after tamoxifen ip I Serum HMGB1 concentration (mm2) 200 % Fibrosis Cross-sectional area (ng/ml) 100 0 Drp1 fl/fl a-MHC MCM Tamoxifen + + + + + 10 days after tamoxifen ip 1.5 1 0.5 0 Drp1 fl/fl a-MHC MCM Tamoxifen + + + + + 10 days after tamoxifen ip * 12 8 4 0 Drp1 fl/fl a-MHC MCM Tamoxifen + + Ctr + + + Drp1 CKO 4 weeks after tamoxifen ip Online Figure VIII. Hypertrophy and mitochondrial dysfunction in Drp1-CKO mice. A, Electron microscope images of Drp1-CKO and control mouse hearts 8 weeks after tamoxifen injection. Asterisks indicate elongated mitochondria. Mitochondrial mass in control mouse hearts is expressed as 1. * p<0.01 vs. Ctr (n=3/group). Scale bar: 2 m. B, Immunoblots for COX IV and -tubulin in Drp1-CKO and control mice. * p<0.01 vs. Ctr 4weeks after tamoxifen ip, # p<0.01 vs. Ctr 8weeks after tamoxifen ip (n=3/group).C, Immunoblot for PGC-1 and -tubulin in Drp1-CKO and control mice whose hearts were harvested 4 or 8 weeks after tamoxifen injection. D, Relative cardiac ATP production in Drp1-CKO and age-matched control mice 8 weeks after tamoxifen injection. * p<0.01 vs. Ctr (n=3/group). E, Respiratory chain complex activity in mitochondria from mouse hearts. Relative respiratory chain complex I, II+II, and IV activities are shown. The activity in mitochondria from Ctr mouse hearts is expressed as 1. *p<0.05 vs. Ctr (n=4/group). F, Assessment of CM size in control and Drp1-CKO mice using WGA staining 10 days after tamoxifen injection. Scale bar: 200 m. There was no significant difference between the 2 groups (n=3/group). G, Assessment of CM fibrosis in control and Drp1-CKO mice using PASR staining 10 days after tamoxifen injection. Scale bar: 500 m. There was no significant difference between the 2 groups (n=3/group). H, TUNEL staining of hearts in Drp1-CKO and control mice 8 weeks after tamoxifen injection. Arrows indicate TUNEL-positive nuclei. * p<0.01 vs. Ctr. Scale bar: 50 μm. I, Serum HMGB1 concentration in Drp1-CKO and age-matched control mice 4 weeks after tamoxifen injection was assessed by ELISA. * p<0.05 vs. Ctr (n=3/group). In A-E and H, samples were harvested 8 weeks after tamoxifen injection. In B and I, samples were harvested 4 weeks after tamoxifen injection. Ctr B Drp1 hetCKO Ctr Drp1 hetCKO (mm2) 200 100 0 % Fibrosis * # 40 20 WT 6000 * *# 4000 40 2000 20 0 Ctr E Drp1 CKO AAR (%) 40 30 20 10 Ctr Drp1 CKO Ctr Drp1-CKO Infarct / AAR (%) Ctr 60 a-MHC Cre 8000 0 0 Drp1 hetCKO 0.4 AAR (%) LVEF (%) * Ctr 0.8 D LV +dP/dt (mmHg/sec) C 60 Drp1 hetCKO 1.2 0 80 Ctr * 40 20 0 Ctr Drp1 CKO Drp1 CKO Infarct / AAR (%) A Cross-sectional area Online Figure IX 40 20 0 Online Figure IX. Basal characterization of Drp1-hetCKO mice. A, Assessment of CM size using WGA staining. Ctr: control. (n=3/group). Scale bar: 200 m. B, PASR staining to assess cardiac fibrosis. (n=3/group). Scale bar: 500 m. C, LV ejection fraction, as evaluated with echocardiography, and +dP/dt, as evaluated by hemodynamic measurement. * p<0.01 vs. Ctr at baseline, # p<0.01 vs. Drp1-hetCKO at baseline. Fst: fasting (n=4/group). D, Representative images of heart sections with I/R injury. WT: wild-type. Statistical analysis of % area at risk (AAR) and ratio of infarct size to AAR are shown. There was no significant difference between Tg-MHC-Cre and wild-type mice (n=3/group). E, Representative images of TTC/Alcian Blue staining of LV sections after I/R. All of the mice underwent I/R surgery 10 days after tamoxifen injection. Statistical analyses of AAR and infarct size/AAR are shown. * p<0.05 vs. Ctr (n=3/group). Online Figure X A Ctr (DMSO) B mdivi-1 50mM Drp1-hetCKO mdivi-1 - C + 10 0 30 20 10 0 30 20 10 0 mdivi-1 - + Ctr mdivi-1 Ctr (DMSO 1week) (1 week) (Drp1 fl/+) LVEF (%) 0 20 0 Drp1hetCKO OD540nm Ctr (DMSO 1W) 0.55 Drp1-hetCKO 0.50 mdivi1 (1W) 0.45 0 0 4 8 12 16 20 Time (min) AAR (%) 40 30 20 10 0 Ctr mdivi-1 (DMSO, 1W) (50mM, 1W) Infarct / AAR (%) G 50 40 30 20 10 0 * % Decrease in OD540 * Ctr (Drp1 fl/+) * * † 6 4 # 2 0 + 50mM * 0.60 40 40 * E 60 20 * mdivi-1 - 50mM D 60 Relative mitochondrial mass 20 40 * F Relative ATP production 30 40 Infarct / AAR (%) AAR (%) 40 AAR (%) Infarct / AAR (%) 80 2 * # 1 0 1.2 1.0 0.8 0.6 0.4 0.2 0 * # Online Figure X. Chronic treatment with mdivi-1 leads to mitochondrial dysfunction and exacerbates I/R injury. A, Representative images of TTC/Alcian Blue staining of LV sections after I/R. Mice were injected with mdivi-1 at 1.2 mg/kg or the same volume of DMSO once 30 minutes prior to myocardial ischemia. Statistical analyses of % area at risk (AAR) and infarct size/AAR are shown. * p<0.05 vs. Ctr (DMSO ip once) (n=3/group). B, Representative images of TTC/Alcian Blue staining of LV sections after I/R. Drp1-hetCKO mice were treated with mdivi-1 at 1.2 mg/kg or the same volume of DMSO once 30 minutes prior to myocardial ischemia. Statistical analyses of AAR and infarct size/AAR are shown. * p<0.05 vs. Drp1-hetCKO without mdivi-1 (n=3/group). C, LVEF of mice treated with mdivi-1 at 1.2 mg/kg or the same volume of DMSO. There was no significant difference in LVEF between control and mdivi-1-treated mice (n=3/group). D, Electron microscope images of hearts from mdivi1-treated, Drp1-hetCKO and control mice. Mdivi-1 at 1.2 mg/kg or the same volume of DMSO was injected intraperitoneally for 1 week in the mdivi-1-treated and control groups, respectively. Asterisks indicate elongated mitochondria. Mitochondrial mass in control (injected with DMSO) mouse hearts is expressed as 1. * p<0.05 vs. Ctr (DMSO), # p<0.01 vs. Ctr (Drp1 fl/+) (n=3/group). Scale bar: 2 m. E, Mitochondrial swelling induced by Ca2+. Each data curve in the left panel represents the average of 3 individual measurements. Right panel shows the decrease in optical density at 540 nm. * p<0.01 vs. Ctr (DMSO ip for 1 week), # p<0.01 vs. mdivi-1 ip for 1 week, † p<0.01 vs. Ctr (Drp1 fl/+) (n=3/group). F, Relative cardiac ATP production in mdivi-1 treated, Drp1-hetCKO and control mice. Mdivi-1 at 1.2 mg/kg or the same volume of DMSO was injected intraperitoneally for 1 week in the mdivi-1-treated and control groups, respectively. * p<0.01 vs. Ctr (DMSO), # p<0.01 vs. Ctr (Drp1 fl/+) at 12 weeks of age (n=3/group). G, Representative images of TTC/Alcian Blue staining of LV sections after I/R. Mice underwent treatment with mdivi-1 at 1.2 mg/kg or the same volume of DMSO for 1 week. Statistical analyses of % area at risk (AAR) and infarct size/AAR are shown. * p<0.05 vs. Ctr (DMSO ip for 1 week) (n=3/group).