* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Photodamaged Chloroplasts Are Targets of Cellular
Survey
Document related concepts
Cell encapsulation wikipedia , lookup
Cell culture wikipedia , lookup
Extracellular matrix wikipedia , lookup
Cell growth wikipedia , lookup
Cell membrane wikipedia , lookup
Cellular differentiation wikipedia , lookup
Signal transduction wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
Cytokinesis wikipedia , lookup
Chloroplast DNA wikipedia , lookup
Endomembrane system wikipedia , lookup
Chloroplast wikipedia , lookup
Programmed cell death wikipedia , lookup
List of types of proteins wikipedia , lookup
Transcript
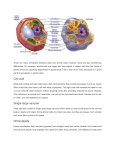
The Plant Cell, Vol. 29: 199, February 2017, www.plantcell.org ã 2017 American Society of Plant Biologists. All rights reserved. IN BRIEF Photodamaged Chloroplasts Are Targets of Cellular Garbage Disposal OPEN Autophagy, or “self eating,” is the process cells use to consume unwanted intracellular structures such as damaged organelles, excess membranes, and unneeded proteins (Mizushima and Komatsu, 2011). Typically, the unwanted structure becomes surrounded by an autophagosomal membrane, which then fuses with the membrane of either the vacuole (yeast and plants) or the lysosome (animals) to deliver its contents for destruction by hydrolytic enzymes (Nakatogawa et al., 2009). Mitochondria can be damaged by excess oxidation and thus may need to be destroyed. Mitophagy, the process by which cells consume defective mitochondria, is well characterized in both animals and yeast (Green et al., 2011; Youle and Narendra, 2011). Defects in mitophagy in humans can cause Parkinson’s-like symptoms, perhaps because impaired mitophagy results in accumulation of reactive oxygen species (ROS) and mutation of mitochondrial DNA. During photosynthesis, excess natural or artificiallightcancausephotooxidativedamage to chloroplasts. Izumi et al. (2017) now show thatchlorophagy, the intracellular destructionof chloroplasts, is induced by exposure to UV-B irradiation (280–315 nm) and high light. They also demonstrate that entire photodamaged chloroplasts are transported to the plant cell vacuole for destruction. Duringleaf senescence and energy starvation, chloroplast stroma proteins are degraded by a type of autophagosome known as the Rubisco-containing body (Ishida et al., 2008). Whether a similar process operates in autophagy of photodamaged chloroplasts is a key focus of this work. The authors first examine the relationship between UV-B-induced damage and autophagy in wild-type versus atg mutants, which are deficient in core proteins required for autophagy. Mutant plants irradiated with a UV-B treatment as short as 1 h had <40% the shoot fresh weight than similarly treated wild-type plants, and they showed a similar reduction in photosyntheticperformance.Thisdemonstrates that Arabidopsis plants are more damaged by UV-B when the autophagy process is defective. OPEN Articles can be viewed without a subscription. www.plantcell.org/cgi/doi/10.1105/tpc.17.00054 Chlorophagy is inhibited in autophagy mutants. UV-B irradiation of leaves has only a moderate effect on chloroplast ultrastructure in wild-type plants (left panels). In autophagy-deficient atg mutants, UV-B exposure leads to accumulation of grossly abnormal chloroplasts (right panels) that would otherwise be destroyed by chlorophagy. Bar 5 2 mm. (Reprinted from Izumi et al. [2017], Figure 5C.) Chloroplast location was monitored via expression of a chloroplast stroma-targeted GFP fusion protein (CT-GFP). Chloroplasts in untreated control leaves contained CT-GFP, but UV-B exposure caused the appearance of CT-GFP-deficient chloroplasts in the central area of mesophyll cells where the vacuole is typically found. Simultaneous expression of a vacuolar membrane marker protein and a stromal RFP fusion protein showed that RFP-deficient chloroplasts were indeed found inside the vacuole. This was not the case in atg mutant plants, showing that their movement to the vacuole depends on the presence of a functional autophagy pathway. In wild-type leaves, the number of chloroplasts in UV-B-exposed leaves decreased ;30% by 3 d following exposure. By contrast, no decrease was seen in UV-B-exposed atg mutant leaves, which instead accumulated abnormal chloroplasts. In untreated control plants, chloroplast morphology in atg mutant leaves was similar to wild-type morphology (see figure). However, following UV-B exposure, the chloroplasts of atg mutant leaves hadgrosslyalteredultrastructure andenhanced ROS production. This strongly suggests that, in normally functioning cells, chloro- phagy removes photodamaged chloroplasts following excessive UV-B exposure. The authors showed the involvement of ROS such as H2O2 in the initiation of autophagy following UV-B-induced photodamage to chloroplasts. Using concanamycin A, which inhibits the vacuolar H1-ATPase and thus suppresses hydrolytic enzymes, plus an autophagosomal membrane marker, the authors were able to stabilize and visualize tubular autophagosomal structures surrounding individual chloroplasts in the vacuoles of UV-B-exposed leaves. Further experiments described in the article showed that strong visible light as well as UV-B will induce chlorophagy and that photodamage-induced chlorophagy involves a pathway distinct from starvation-induced chlorophagy. These findings not only help to elucidate the process of chloroplast turnover in the plant cell but also provide insights into the process of autophagy and organelle turnover in cells. Gregory Bertoni Science Editor [email protected] ORCID ID: 0000-0001-7977-3724 REFERENCES Green, D.R., Galluzzi, L., and Kroemer, G. (2011). Mitochondria and the autophagyinflammation-cell death axis in organismal aging. Science 333: 1109–1112. Ishida, H., Yoshimoto, K., Izumi, M., Reisen, D., Yano, Y., Makino, A., Ohsumi, Y., Hanson, M.R., and Mae, T. (2008). Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiol. 148: 142–155. Izumi, M., Ishida, H., Nakamura, S., and Hidema, J. (2017). Entire photodamaged chloroplasts are transported to the central vacuole by autophagy. Plant Cell 29: 377–394. Mizushima, N., and Komatsu, M. (2011). Autophagy: renovation of cells and tissues. Cell 147: 728–741. Nakatogawa, H., Suzuki, K., Kamada, Y., and Ohsumi, Y. (2009). Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10: 458–467. Youle, R.J., and Narendra, D.P. (2011). Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 12: 9–14. Photodamaged Chloroplasts Are Targets of Cellular Garbage Disposal Gregory Bertoni Plant Cell 2017;29;199; originally published online February 10, 2017; DOI 10.1105/tpc.17.00054 This information is current as of August 9, 2017 Supplemental Data /content/suppl/2017/02/10/tpc.17.00054.DC1.html References This article cites 6 articles, 3 of which can be accessed free at: /content/29/2/199.full.html#ref-list-1 Permissions https://www.copyright.com/ccc/openurl.do?sid=pd_hw1532298X&issn=1532298X&WT.mc_id=pd_hw1532298X eTOCs Sign up for eTOCs at: http://www.plantcell.org/cgi/alerts/ctmain CiteTrack Alerts Sign up for CiteTrack Alerts at: http://www.plantcell.org/cgi/alerts/ctmain Subscription Information Subscription Information for The Plant Cell and Plant Physiology is available at: http://www.aspb.org/publications/subscriptions.cfm © American Society of Plant Biologists ADVANCING THE SCIENCE OF PLANT BIOLOGY