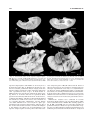
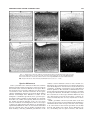
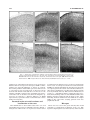
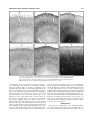
* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Get PDF file
Synaptic gating wikipedia , lookup
Sound localization wikipedia , lookup
Embodied cognitive science wikipedia , lookup
Affective neuroscience wikipedia , lookup
Emotional lateralization wikipedia , lookup
Metastability in the brain wikipedia , lookup
Neuroplasticity wikipedia , lookup
Neuroeconomics wikipedia , lookup
Sensory cue wikipedia , lookup
Human brain wikipedia , lookup
Orbitofrontal cortex wikipedia , lookup
Neural correlates of consciousness wikipedia , lookup
Aging brain wikipedia , lookup
Eyeblink conditioning wikipedia , lookup
Cortical cooling wikipedia , lookup
Time perception wikipedia , lookup
Feature detection (nervous system) wikipedia , lookup
Cerebral cortex wikipedia , lookup
THE JOURNAL OF COMPARATIVE NEUROLOGY 441:197–222 (2001) Architectonic Identification of the Core Region in Auditory Cortex of Macaques, Chimpanzees, and Humans TROY A. HACKETT,1,3* TODD M. PREUSS,2 AND JON H. KAAS3 Department of Hearing and Speech Sciences, Vanderbilt University, Nashville, Tennessee 37203 2 Cognitive Evolution Group, University of Louisiana at Lafayette, New Iberia, Louisiana 70560 3 Department of Psychology, Vanderbilt University, Nashville, Tennessee 37203 1 ABSTRACT The goal of the present study was to determine whether the architectonic criteria used to identify the core region in macaque monkeys (Macaca mulatta, M. nemestrina) could be used to identify a homologous region in chimpanzees (Pan troglodytes) and humans (Homo sapiens). Current models of auditory cortical organization in primates describe a centrally located core region containing two or three subdivisions including the primary auditory area (AI), a surrounding belt of cortex with perhaps seven divisions, and a lateral parabelt region comprised of at least two fields. In monkeys the core region can be identified on the basis of specific anatomical and physiological features. In this study, the core was identified from serial sets of adjacent sections processed for cytoarchitecture, myeloarchitecture, acetylcholinesterase, and cytochrome oxidase. Qualitative and quantitative criteria were used to identify the borders of the core region in individual sections. Serial reconstructions of each brain were made showing the location of the core with respect to gross anatomical landmarks. The position of the core with respect to major sulci and gyri in the superior temporal region varied most in the chimpanzee and human specimens. Although the architectonic appearance of the core areas did vary in certain respects across taxonomic groups, the numerous similarities made it possible to identify unambiguously a homologous cortical region in macaques, chimpanzees, and humans. J. Comp. Neurol. 441: 197–222, 2001. © 2001 Wiley-Liss, Inc. Indexing terms: comparative; primate; neuroanatomy; neurolinguistics; language; imaging; evolution; acetylcholinesterase; myelin The search for cortical regions that are largely or wholly devoted to auditory processing has been the subject of numerous investigations for over 125 years, leading to the identification of multiple auditory cortical fields in most mammals studied. The number of fields identified ranges from 1 (in marsupials) to over 12 (in primates). In cats, a single primary auditory field (AI) is surrounded by several nonprimary auditory fields. In monkeys two or three primary fields, including AI, are enveloped by an even greater number of nonprimary fields (for reviews, see Woolsey and Walzl, 1982; Brugge and Reale, 1985; Aitkin, 1990; Schreiner, 1992, 1998; Ehret, 1997; de Ribaupierre, 1997; Rouiller, 1997; Kaas et al., 1999; Kaas and Hackett, 2000). Currently, only the homology of AI has been well established across major taxonomic groups. Thus, the extent to which findings in one species can be generalized to another is uncertain. Extending findings from research © 2001 WILEY-LISS, INC. DOI 10.1002/cne.1407 animals to humans is especially problematic because experimental constraints limit direct comparisons between species. One consequence is that both bodies of knowledge expand, but little connection is made between them. As Grant Sponsor: National Institutes of Health, NIDCD grants DC00249 and DC04318; Grant sponsor: the McDonnell-Pew Program in Cognitive Neuroscience; Grant number: JSMF 98-45; Grant sponsor: the James S. McDonnell Foundation; Grant number: JSMF 20002029; Grant sponsor: NINDS; Grant number: NS16446; Grant sponsor: the National Institute on Aging; Grant number: NS1P30 AG-13854-01. *Correspondence to: Troy A. Hackett, Ph.D., Vanderbilt University, 301 Wilson Hall, 111 21st Avenue South, Nashville, TN 37203. E-mail: [email protected] Received 9 March 2001; Revised 17 July 2001; Accepted 17 September 2001 Published online the week of November 12, 2001 198 T. HACKETT ET AL Fig. 1. Schematic view of the macaque left hemisphere showing the location and intrinsic connections of auditory cortex. The dorsal bank of the lateral sulcus has been removed (cut) to expose the superior temporal plane (LS ventral bank). The floor and outer bank of the circular sulcus (CiS) have been flattened to show the medial auditory fields. The core region (dark shading) contains three subdivisions (AI, R, RT). In the belt region (light shading) seven subdivisions are proposed (CM, CL, ML, AL, RTL, RTM, RM). The parabelt Abbreviations AChE AI AL AS ASC CiS CL CM CPB CS CSHG HG1 HG2 HSa HSp IPS LS LuS LuSMF ML N PS R RM RMRPB RT RTL RTM RTLRTMSI STG STS TTG acetylcholinesterase auditory area I (core) anterior lateral auditory belt arcuate sulcus caudal circular sulcus caudolateral auditory belt caudomedial auditory belt caudal parabelt circular sulcus Heschl’s gyrus first (anterior) gyrus of Heschl second (posterior) gyrus of Heschl Heschl’s sulcus (anterior) Heschl’s sulcus (posterior) intraparietal sulcus lateral sulcus lunate sulcus myelinated fibers middle lateral auditory belt Nissl substance principal sulcus rostral area (core) rostromedial auditory belt rostral parabelt rostrotemporal area (core) rostrotemporolateral auditory belt rostrotemporomedial auditory belt sulcus intermedius superior temporal gyrus superior temporal sulcus transverse temporal gyrus (of Heschl) region (RP, CP; no shading) occupies the exposed surface of the superior temporal gyrus (STG). The core fields project to surrounding belt areas (arrows). Inputs to the parabelt arise from the lateral and medial belt subdivisions. Connections between the parabelt and medial belt fields are not illustrated to improve clarity. Tonotopic gradients in the core and lateral belt fields are indicated by the letters H (high frequency) and L (low frequency). For abbreviations, see list. this trend continues, the need for studies that attempt to link these findings also grows. Toward this end, we have initiated comparative architectonic studies of auditory cortex in macaque monkeys, chimpanzees, and humans. Our goal is to identify features of auditory cortical organization that are common, and unique, to each taxonomic group. In recent years we have developed a model of auditory cortical organization in nonhuman primates based on a wide range of anatomical and physiological findings (Hackett et al., 1998a; Kaas et al., 1999; Kaas and Hackett, 2000). According to the model, primate auditory cortex consists of three major regions containing as many as 12 different fields (Fig. 1). Two or three cochleotopically organized primary or primary-like auditory areas (AI, R, RT) with independent parallel inputs from the ventral division of the medial geniculate complex (MGv) comprise the core region at a first level of processing. The core fields are surrounded by a belt region of possibly seven fields (CL, CM, RM, RTM, RTL, AL, ML) at a second level of processing, with major inputs from the core and the dorsal division of the medial geniculate complex (MGd). Cochleotopic organization is preserved in at least some of the belt fields (Rauschecker et al., 1995; Kosaki et al., 1997). The belt region is bordered laterally on the superior temporal gyrus by a parabelt region of two or more divisions (CP, RP) that are activated by inputs from the belt areas and the MGd, but not MGv or the core. Neurons in the belt and parabelt project to auditory-related fields in the temporal, parietal, and frontal lobes. Experimental evidence IDENTIFICATION OF THE AUDITORY CORE supporting this model is derived from numerous studies of monkeys and chimpanzees (Campbell, 1905; Beck, 1929; Walker, 1937; von Bonin, 1938; Ades and Felder, 1942; Bailey et al., 1943; Walzl and Woolsey, 1943; Walzl, 1947; von Bonin and Bailey, 1947; Bailey et al., 1950; Akert et al., 1959; Merzenich and Brugge, 1973; Jones and Burton, 1976; Imig et al., 1977; Fitzpatrick and Imig, 1980; Galaburda and Pandya, 1983; Aitkin et al., 1988; Luethke et al., 1989; Morel and Kaas, 1992; Morel et al., 1993; Jones et al., 1995; Kosaki et al., 1997; Rauschecker et al., 1997; Hackett et al., 1998a,b; Recanzone et al., 2000). Extension of this model to human auditory cortex can be done in a limited way by comparing anatomical features. Detailed architectonic parcellations of the human auditory cortex have appeared regularly for nearly a century (Campbell, 1905; Brodmann, 1909; Vogt and Vogt, 1919; Flechsig, 1920; von Economo and Koskinas, 1925; Beck, 1928; von Economo, 1929; von Economo and Horn, 1930; Poljak, 1932; Hopf, 1954; Blinkov, 1955; Braak, 1978; Galaburda and Sanides, 1980; Seldon 1981a,b, 1982; Ong and Garey, 1990; Rademacher et al., 1993; Rivier and Clarke, 1997; Clarke and Rivier, 1998; Morosan et al., 2001). Although these parcellations differ from one another in many respects, including nomenclature, common features include a centrally located region with anatomical features typical of primary sensory cortex (e.g., koniocortical cytoarchitecture, dense myelination) surrounded by a variable number of nonprimary fields with distinctive architectonic features. These findings suggest that the basic organizational scheme proposed for monkeys (i.e., core, belt) may also apply to humans. An important observation with relevance to the issue of homology is that the core, belt, and parabelt regions in macaques can be reliably identified on the basis of their structural architectonic features (e.g., cytoarchitecture, myeloarchitecture). In addition, various molecules (e.g., cytochrome oxidase, acetylcholinesterase, parvalbumin) are expressed at higher levels in the core than in the surrounding belt areas (Wallace et al., 1991; Morel et al., 1993; Jones et al., 1995; Hutsler and Gazzaniga, 1996; Rivier and Clarke, 1997; Hackett et al., 1998a; Clarke and Rivier,1998). Cytochrome oxidase, involved in the oxidative metabolism of cells, exhibits patterned expression reflecting the modular organization of primary sensory cortices (Wong-Riley, 1989) and is also related to the neurovascular events measured in functional imaging studies (Wobst et al., 2001). Acetylcholinesterase is linked to cholinergic activity in cortex (Mesulam and Geula, 1992) and is well known to modulate neuronal activity in primary auditory cortex (Edeline, 1999). The role of the calcium binding protein parvalbumin is less clear, but it has been associated with distinct subpopulations of ␥-aminobutyric acid (GABA)ergic neurons in sensory cortex (van Brederode et al., 1990) and is expressed at high levels in primary thalamocortical pathways to auditory cortex (Molinari et al., 1995; Hackett et al., 1998b). The coexpression of these molecules at high levels in the core suggests they may be used as markers of this region and adds support to the theory that the core is a functionally distinct region of auditory cortex. Furthermore, comparative studies of the architecture could reveal similarities and differences across taxonomic groups relevant to the organization and evolution of auditory cortex in primates. The purpose of the present study was to determine whether the architectonic criteria currently used to iden- 199 TABLE 1. Histologic Treatment of Macaque, Chimpanzee, and Human Brain Specimens1 Case M1–M4 M5–M6 Ch1 Ch2 Ch3 Ch4 Hu1 Hu2 Hu3 Fixation procedure Postmortem delay P 4% PBPF P 4% PBPF I 4% PBPF P 4% PBPF ⫹ 0.1% GA P 10% formalin I 10% formalin I 4% PBPF I 2% PBPF I 2% PBPF 0 0 ⬍12 hr 0 20 min 12 hr 6 hr ⬍24 hr 23 hr Plane of section Off-coronal Coronal Off-coronal Off-coronal Off-coronal Off-coronal Off-coronal Off-coronal Off-coronal A A A A A A A B 1 M, macaque; Ch, chimpanzee, Hu, human; P, perfusion; I, immersion; PBPF, phosphate-buffered paraformaldehyde; GA, glutaraldehyde; off-coronal A, perpendicular to the superior temporal plane and midline; off-coronal B, perpendicular to the superior temporal plane and long axis of the first transverse temporal gyrus. All cortical blocks are left hemisphere. tify the core region in monkeys could be used to identify a homologous region in chimpanzees and humans. A preliminary report of these findings was previously published in abstract form (Hackett et al., 1998c). MATERIALS AND METHODS Tissue specimens The brains of six macaque monkeys (three Macaca mulatta and three M. nemestrina), four chimpanzees (Pan troglodytes), and three humans (Homo sapiens) were obtained post mortem for use in these studies. Of the macaque brains, four were included in previous experiments of auditory cortex in which the auditory core had been identified by microelectrode mapping and/or tracer injections (Morel et al., 1993; Hackett et al., 1998a); thus, nonarchitectonic verification of the boundaries of the core region was available for these cases only. Chimpanzee brains were obtained from the New Iberia Research Center (New Iberia, LA) and the Yerkes Regional Primate Center (Atlanta, GA). All animals died of natural causes or were euthanized for veterinary reasons. Chimpanzees were adult males, age range 20 –33 years (estimated), and presumed wild-caught. One human brain (Hu1) was obtained from the Vanderbilt University Medical Center Department of Pathology from an adult male (45 years) who died of non-Hodgkin’s type lymphoma. The other two human brains were normal controls provided to the University of Louisiana at Lafayette by the Northwestern Alzheimer’s Disease Center. The present analyses were limited to the left temporal lobe; thus hemispheric differences were not addressed. Histological processing All macaque monkey brains were perfused transcardially immediately after death. Perfusates consisted of the following solutions delivered in succession: 500 ml phosphate-buffered saline (pH 7.4, room temperature); 500 ml 4% paraformaldehyde dissolved in 0.1 M phosphate buffer (4°C, pH 7.4); and 500 ml 4% paraformaldehyde ⫹ 10% sucrose in 0.1 M phosphate buffer (4°C, pH 7.4; Table 1). Immediately after perfusion the brains were removed, separated from the thalamus and brainstem, and cut into blocks. The blocks containing the temporal lobe were immersed in 30% sucrose in 0.1 M phosphate buffer (4°C, pH 7.4) overnight and then cut in a coronal or semicoronal plane (Off-coronal type A, perpendicular to 200 T. HACKETT ET AL Fig. 2. Dorsolateral views of the superior temporal plane. A,B: Macaque cases M1 and M3. C,D: Chimpanzee cases Ch1 and Ch4. E,F: Human cases Hu1 and Hu2. Single asterisks denote location of HG1 (first gyrus of Heschl). Double asterisk in E denotes HG2 (second gyrus of Heschl). In human case Hu2 (F), only one HG is present. Solid white lines indicate plane of section. Dashed straight white lines designate sulcal landmarks. a, anterior; m, medial. For other abbreviations, see list. Scale bars ⫽ 5 mm. superior temporal plane and midline) at 40 or 50 m on a freezing microtome (Fig. 2). Chimpanzee brains were perfused at variable postmortem delays ranging from zero minutes (Ch2) to 20 minutes (Ch3) or fixed by immersion after a postmortem delay of up to 12 hours (Table 1). Human brains were obtained at postmortem delays ranging from 6 to 23 hours and were not perfused. These specimens were fixed by immersion for 24 – 48 hours (4°C), as indicated in Table 1 and then cut into blocks. Temporal lobe blocks were sunk in 30% sucrose (4°C) and then cut on a freezing microtome. Chimpanzee and two human cases (Hu1 and Hu2) were cut at 50 m in a plane perpendicular to the superior temporal plane and long axis of the circular sulcus (Off-coronal A), as indicated in Figure 2. One human case (Hu3) was cut perpendicular to the superior temporal plane and long axis of the first trans- verse temporal gyrus of Heschl (Off-coronal B). Series of adjacent sections were processed for 1) anatomical tracers (macaque experimental cases only); 2) acetylcholinesterase (Geneser-Jensen and Blackstad, 1971); 3) myelin (Gallyas, 1979); or 4) staining for Nissl substance with thionin. In most brains, three additional series were reserved for additional reactions that were not included in the present analyses. Sections were mounted on glass slides and coverslipped. Architectonic analyses were conducted in sections stained for Nissl substance (N), myelinated fibers (MF), and acetylcholinesterase (AChE). The staining quality of the N, MF, and AChE was not noticeably degraded by postmortem delays to fixation of up to 23 hours. An exception was case Hu2, which was immersed in formalin for an unknown period of less than 24 hours (Table 1). Compared IDENTIFICATION OF THE AUDITORY CORE with cases Hu1 and Hu3, which were fixed at 6 and 23 hours postmortem, respectively, Hu2 exhibited weakened neuropil staining of AChE. AChE staining of cell soma was comparable in the three cases. The normal appearance of AChE expression in case Hu3 suggests that histological factors account for the weak neuropil staining in case Hu2. Data analysis Individual sections were studied at magnifications ranging from 1.25 to 200⫻ on three microscopes: Nikon E800S, Zeiss Axioscope 20, and Olympus BH-2. Borders between the core and belt were independently identified and marked on individual slides for matched sets of sections stained for N, MF, and AChE. Sections in which a border(s) could not be identified were not marked. For stack reconstructions (Figs. 11–13), grayscale images of individual AChE sections (1.25⫻, 300 dpi) were obtained with a Leaf Systems Lumina scanning digital camera (Southborough, MA) mounted on a Nikon E800M microscope and Adobe Photoshop 5.0 software (Adobe Systems, San Jose, CA). The images were adjusted uniformly for brightness (10%) and contrast (5%) and then printed. Borders in adjacent sections were marked on the printed AChE sections with reference to blood vessels, lesions, and surface landmarks by using a drawing tube affixed to the microscope. In most sections, the deviations in border location assessed independently using N, MF, and AChE were within 100 to 400 m. Deviations exceeding 400 m were uncommon, but in some sections ambiguous features or histological imperfections prohibited precise border identification. These sections were excluded from the analysis. The location of final borders represented a visual average of the three preparations. The marked AChE images were imported into Adobe Illustrator 7.0 and arranged in stacks, roughly perpendicular to the long axis of the core region. An outline of the core was made by a dashed line connecting the border markers (Figs. 11–13). Densitometric measurements of AChE expression Densitometric analyses were conducted on subgroups of sections processed for AChE from each brain. Grayscale images of individual sections were adjusted uniformly for brightness (10%) and contrast (5%) and then imported into NIH Image 1.61 for Macintosh for densitometric measurements and subsequent manipulations. Two analyses were used to support qualitative judgments about border locations. Both analyses were based on radial density profiles (Rivier and Clarke, 1997; Schleicher et al., 1999), obtained by measuring gray level density in rectangular samples (3 pixels in width) aligned parallel to radial fiber columns spanning layers I–VI (Fig. 3C). When possible, the open profiles of blood vessels, lesions, tissue tears, and other imperfections were circumvented to avoid an artifactual modulation of optical density. The raw gray level density values (0 –255), averaged across the 3-pixel width, were converted to relative values by dividing by the mean gray level density of the white matter underlying the region of interest (Fig. 3D). Based on these mean density values, the border between regions was estimated by the following procedures. The mean of 5 adjacent profiles (e.g., 1–5) was subtracted from the mean of the next 5 profiles (e.g., 6 –10). The starting profile was then incremented by 1 so that the mean of profiles 2– 6 was subtracted from the 201 mean of profiles 7–11. This procedure was repeated until the last profile was reached (e.g., Fig 3D, profile 41). The two arithmetic differences with the greatest absolute value were considered to represent the lateral and medial boundaries of the most intensely stained region, corresponding to the core. In Figure 3D, this simple procedure identified borders between profiles 12 and 13 (lateral), and 29 and 30 (medial). The data were then imported into a MATLAB routine (MathWorks, Natick, MA) for subsequent analyses. Relative gray level densities of each profile were plotted as a function of percentage distance from the pial surface for inspection of laminar trends (Fig. 3E–H). Compared with the actual distance (e.g., pixels or micron-equivalents), plotting the density values as a function of percentage distance from the surface resulted in better alignment of laminar peaks between samples because variability in cortical thickness altered absolute laminar relationships. Individual profiles were detrended by a linear regression, and then a cross-correlation matrix was computed from these values among all profiles in the section. Correlation coefficients were grouped into clusters of high and low coefficients by an arbitrary criterion (e.g., 0.65). Groups of profiles were related to predefined architectonic borders (Fig. 3C, arrows). RESULTS Gross anatomical features of the superior temporal plane In each of our macaque, chimpanzee, and human cases the auditory core region was confined to the supratemporal plane on the dorsal surface of the temporal lobe, hidden from view by the overlying frontoparietal operculum (Fig. 2A,B). In macaques there was no transverse temporal gyrus of Heschl (HG), and only gross anatomical features (e.g., vascular patterns, slight elevations or depression) sometimes coincided with the location of the core region. The core was elongated along the rostrocaudal axis of the temporal lobe in both species of macaque monkeys (M. mulatta, M. nemestrina). The core was at its widest caudally, narrowing rostrally as the supratemporal plane also diminished in width. One result of this narrowing was that the rostral portion of the core draped over the medial edge of the supratemporal plane in some cases to occupy the outer bank of the ventral circular sulcus. This feature is not always obvious in flattened sections of cortex (e.g., Hackett et al., 1998a). Among the four chimpanzee brains, three variants were noted. In one chimpanzee (Fig. 2C, case Ch1), the core region was located on a rudimentary transverse gyrus elongated rostrocaudally along the medial edge of the supratemporal plane and outer bank of the circular sulcus, similar to that of M. fuscata (e.g., Jones et al., 1995). The planum temporale and planum polare were located posterior and anterior to the core, respectively. In a second chimpanzee (see Fig. 12A) there was no clear evidence of a transverse gyrus. Instead, the surface of the supratemporal plane appeared relatively flat, as it does in the macaque. In the two other animals there was a single prominent HG oriented from posteromedial to anterolateral across the supratemporal plane (e.g., Fig. 2D, case Ch4; Fig. 12B). In these brains, HG was bounded anteromedially by the anterior transverse sulcus of Heschl (HSa) extending from the circular sulcus, and caudolaterally by the posterior transverse sulcus of Heschl (HSp). The ar- Figure 3 IDENTIFICATION OF THE AUDITORY CORE chitectonic analyses detailed below indicated that the core region was roughly coextensive with the single HG. Posterior to the HSp was a broad, mostly flat triangular region corresponding in location to the human planum temporale. Anterior to the HSa was a large region tentatively defined as the planum polare. In our human specimens, the left hemisphere contained either one or two HGs, the long axes of which were oriented from posteromedial to anterolateral (Fig. 2E,F). The HG variants were bounded by the HSa and HSp, as described above and by previous investigators (von Economo and Horn, 1930; Campaign and Minkler, 1976; Steinmetz et al., 1989; Musiek and Reeves, 1990; Rademacher et al., 1993; Penhune et al., 1996; Leonard et al., 1998; Kim et al., 2000). The double, or bifid, HG variant was bifurcated by the sulcus intermedius (SI). The anteromedial HG was labeled HG1, and the posterolateral HG was labeled HG2 (Fig. 2E, case Hu1). The core was confined to HG when one gyrus was present. For the double HG variants, the core occupied portions of HG1 and HG2. Note that because there is currently no consensus on the nomenclature for description of HG variants, we have adopted a nomenclature for labeling the HG based on anatomical location (i.e., HG1, most anterior; HG2, posterior to HG1; HG3, posterior to HG2, etc.). The sulci bounding single or multiple gyri of Heschl were named based on anatomical location relative to the HG complex, regardless of number; thus, HSa is always anterior to HG1, separating it from the planum polare region, and HSp is always posterior to the most posterior HG, dividing it from the planum temporale region. In several locations throughout the remaining text, we refer to the parabelt region of auditory cortex. This region was previously defined in macaque monkeys on the bases of architectonic features and connections (Hackett et al., 1998a). In macaques, the parabelt region lies on the exposed surface of the superior temporal gyrus, lateral to the lateral belt fields bordering the core. In this report, the term parabelt was used to denote that specific region only in the macaque preparations. Cytoarchitecture We found the cytoarchitecture of the cortex corresponding to core and belt regions to be generally consistent with earlier descriptions in the literature. A centrally located core region, with koniocortical cytoarchitecture, was surrounded by a number of belt fields with features typical of para- or pro-koniocortex. The koniocortical appearance of 203 the core derived from the predominance of small cells in all layers. The dense concentration of small cells in layers II through IV and VI contrasted with lower cell density in layer V (Figs. 4A– 6A). The inner and outer granular layers (IV and II) were prominent and densely populated by very small cells. Layer III was populated by small to mid-sized pyramidal cells from IIIa to IIIc. Large pyramidal cells were rare in layer III but were found more often at the border with the lateral belt region. In sections cut perpendicular to the radial orientation of the apical dendrites in layer III, the small pyramidal cells were arranged in short radial columns, extending partially into layers II and IV. This feature appears to correspond to the “rainshower formation” described by von Economo and Koskinas (1925). The core was not homogenous with respect to the cytoarchitectonic features described above. For example, the granular construction and radial orientation of small pyramidal cells in layer III were more prominent features in the medial and caudal (posterior) portions of the core. In the lateral and rostral (anterior) domains of the core, especially near the core/belt border, pyramidal cells were slightly larger in IIIc, and the “rainshower formation” was a less dominant feature. Thus, a range of minor cytoarchitectonic variants was observed within the “koniocortical” boundaries of the core. These variations were most obvious in the chimpanzee and human specimens. These structural variants sometimes contributed to ambiguity in border identification, although the cytoarchitecture of the core contrasted with the belt (Figs. 4B– 6B) in key ways that served as the primary criteria for border identification. First, cell packing density and columnar spacing was lower in the belt, a feature most noticeable in layers II–IV. Second, pyramidal cells in layer III of the belt areas were clearly larger and more numerous than in the core. This feature was most obvious in layer IIIc, where the largest layer III pyramidal cells were concentrated. Such cells were rarely found within the core but were sometimes found at the border between the core and lateral belt areas. With respect to columnar organization in the belt, layer III pyramidal cells in belt areas lateral to the core were arranged in well-organized vertical columns, referred to by von Economo and Koskinas (1925) as the “organ pipe formation.” The thin radial lines formed by strings of small pyramidal cells in the core were exaggerated in the lateral belt where pyramidal cell size increased from layer IIIa to IIIc (Figs. 4B– 6B). The columnar arrangement of pyramidal cells in layer III was less orderly in the belt fields medial and caudal (posterior) to the core. Myeloarchitecture Fig. 3. Densitometric measurements of auditory cortex. A: Coronal section from macaque monkey stained for AChE. Arrows indicate lateral (left) and medial (right) borders of the auditory core. B: Same image as in A after filtering and thresholding (see text). The dense band in layer IIIc/IV corresponds to dense AChE staining in the core. C: Same image as A showing placement of rectangular radial density samples used to obtain profiles in E–H. D: Mean relative gray level density values for each radial profile in C. The white horizontal line at 4.25 represents the grand mean. Arrows indicate lateral (left) and medial (right) borders of the core region. E–H: Radial density profiles sorted by cortical region. Within a panel, each curve represents the gray level density of a single rectangular sample plotted as a function of the percentage distance from the pial surface. Profiles were grouped into core, lateral belt, medial belt, and parabelt regions on the basis of architectonic criteria, as denoted by the arrows in A–C. For abbreviations, see list. Scale bar ⫽ 1 mm in A–C. In coronal sections stained for myelin, the auditory cortex of all three primates had a densely stained central core (Figs. 4E– 6E), flanked laterally and medially by belt regions of less dense myelination (Figs. 4F– 6F) in which prominent radial fiber bundles could be followed from the white matter to low/mid layer III. Following the typology of Pandya and Sanides (1973), the characteristic pattern of myelination in the core was astriate (i.e., no horizontal stria visible in layers IV or Vb due to uniformly dense fibrillarity from IV through VIb) to unistriate (i.e., only the outer stria in layer IV was visible due to relatively weaker myelination in layer Va). The density of myelination in the core was highest caudally, in presumptive AI, with a gradual reduction rostrally. In all three primates, it was pos- Fig. 4. Architecture of macaque monkey core and lateral belt regions. A,B: Thionin stain for Nissl substance. C,D: Acetylcholinesterase histochemistry. E,F: Myelin stain. A,C,E are from the primary auditory core region. B,D,F are from the lateral belt. Scale bar ⫽ 250 m. IDENTIFICATION OF THE AUDITORY CORE Fig. 5. Architecture of chimpanzee core and lateral belt regions. A,B: Thionin stain for Nissl substance. C,D: Acetylcholinesterase histochemistry. E,F: Myelin stain. A,C,E are from the primary auditory core region. B,D,F are from the lateral belt. Scale bar ⫽ 250 m. 205 206 T. HACKETT ET AL Fig. 6. Architecture of human core and lateral belt regions. A,B: Thionin stain for Nissl substance. C,D: Acetylcholinesterase histochemistry. E,F: Myelin stain. A,C,E are from the primary auditory core region. B,D,F are from the lateral belt. Scale bar ⫽ 250 m. IDENTIFICATION OF THE AUDITORY CORE sible to distinguish between medial and lateral domains within the core on the basis of subtle differences in myelination. The medial domain tended to be astriate, whereas fibrillar density was weaker in layer Va of the lateral domain, which was, therefore, unistriate. This distinction between medial and lateral domains of the core was maintained through most of the length of the core and was more apparent in sections stained for myelin than those stained for Nissl substance. In the belt areas myelin density was lower compared with the core, but particularly in the interstriate layers, VIa and Va. The resulting bistriate pattern of myelination resulted from prominent horizontal fiber bands in layers Vb and IV. The transition to this pattern was the principal criterion for identification of the core/belt border in myelin preparations. Acetylcholinesterase expression Throughout most of the superior temporal cortex, AChE was distributed in laminar-specific bands that varied in density between and within architectonic areas. AChEreactive elements included subpopulations of cell soma, proximal dendrites, and axons. The expression of AChE was greatest in layers I–IV and weaker in layers V and VI, except for minor bands in Vb and VIb (Figs. 4C,D– 6C,D). Reactivity in layer I was consistently high across architectonic subdivisions, whereas AChE expression in other layers varied by region. These areal differences served as the primary criteria for the localization of borders between fields. The borders identified in adjacent sections stained for myelin and Nissl substance matched closely those determined independently on the basis of AChE expression. Reactivity for AChE was higher in the core than in surrounding cortex (Figs. 4C– 6C). AChE expression increased markedly from layer IIIa to IIIc, reaching peak density in a band involving layers IIIc and IV. The density and radial extent (thickness) of the IIIc/IV band diminished abruptly at the borders with most of the belt areas. This rapid transition was one of the primary criteria for localization of the borders between auditory cortical regions in AChE preparations. In layers V and VI, AChE expression was much lower than in layer IV. A modest increase in the concentration of AChE⫹ elements formed a minor band in layer Vb. A second criterion for border identification was based on the significant increase in the number of moderate and large AChE⫹ pyramidal cells in layer IIIc in the belt (Figs. 4D– 6D). In the medial belt areas, these cells were loosely arranged, often in clusters. In the lateral belt areas, cellular arrangement was more orderly, as columns of AChE⫹ cells could be found extending from IIIc into IIIb. Recall that the increase in the number of moderate and large pyramidal cells in the belt was also observed in thionin-stained sections, but our present observations suggest that only a subpopulation of these cells were AChE⫹ in the lower part of layer III. One notable exception to the typical patterns of AChE expression described above was found in an adjacent belt field situated caudal (posterior) and medial to the core. In macaques this field is known as the caudomedial area (CM; Fig. 1). For convenience, we will use the term CM to denote the homologous field in chimpanzees and humans. In all three species the density of AChE expression in the IIIc/IV band of this field was high, comparable to that found in the core (Fig. 7A). Based on that criterion alone, CM could be considered part of the core. However, layers IIIb and IIIc were also populated by numerous AChE⫹ 207 pyramidal cells, medium to large in size (Fig. 7C). This feature was found only in fields outside of the core. Thus, with respect to the expression of AChE, CM shares features of core and belt cortex. In contrast, the cytoarchitecture and myeloarchitecture of CM were more typical of the belt. Thionin staining revealed that CM was highly granular, but layers IIIb and IIIc were populated by numerous medium and large pyramidal cells (Fig. 7B), and columnar organization in layer IV was somewhat irregular. In myelin, the staining pattern was bistriate, with a denser inner stria (layer Vb) and visible Kaes-Bechterew strip in layer IIIa (Fig. 7D). The combined architectonic picture, therefore, places CM outside of the core in the caudal (posterior) and medial domain of the belt region. This conclusion is consistent with connection patterns and electrophysiological recordings in macaque monkeys (see Discussion). Density measurements In most sections, the location of the core in AChE preparations could be estimated with great precision by thresholding the Gaussian smoothed grayscale image at one standard deviation above the mean density of the white matter (see Materials and Methods). Thresholding at this level preserved prominent suprathreshold regions corresponding to the dense bands of AChE expression in layers I and IIIc/IV of the core (Fig. 3A,B). In this example, only thin interrupted bands in layer IV extended from the borders of the core (arrows) into lateral and medial belt fields. Except for layer I, AChE expression outside of the core was strongly reduced or eliminated by the thresholding procedure, revealing the position of the core. Thresholding was of limited usefulness for border estimation in two conditions. First, in weakly stained sections, even mild thresholding degraded AChE-dense laminae in layers I and IIIc/IV (i.e., human case 2). Second, in the CM belt area, the layer IIIc/IV band was not degraded by thresholding; thus, the border between the core and CM was not revealed. Higher thresholds (e.g., 1.5 or 2 standard deviations above the mean) tended to erode even the dense bands corresponding to layers I and the IIIc/IV band in the core. The border between the core and most belt fields could be objectively identified by the two analyses based on radial density profiles (see Materials and Methods). In the example illustrated in Figure 3C,D, the lateral and medial borders of the core (arrows) were identified as profiles 13 and 29 by the sliding window analysis of mean profile densities. Post hoc comparisons supported these findings, indicating that mean relative gray level densities for profiles judged to be in the core (13–29) were significantly greater than profiles judged to be in the parabelt (1–7), lateral belt (8 –12), or medial belt (30 – 41), as determined by a two-tailed t-test (P ⬍ 0.001). Mean densities of parabelt and lateral belt profiles were not significantly different (P ⫽ 0.33). In Figure 3E–H, radial density profiles were plotted as a function of the percentage distance from the pial surface. Profiles in this example, and the majority of other cases, were typically characterized by two prominent peaks corresponding to dense AChE expression in layers I and IIIc/IV, respectively (Figs. 3E–H). Across cortical regions, layer I density was constant, whereas the density of the IIIc/IV band was weaker in the belt regions outside of the core. The reduction of the second peak in the belt and 208 T. HACKETT ET AL parabelt contributed to lower mean relative gray level densities in these regions, as described above (Fig. 3D). Across cases, cross-correlation coefficients among profiles within a region tended to be high (range 0.70 – 0.96), whereas between regions (e.g., core vs. belt), they were much lower (range 0.1– 0.55). These results indicate that profiles within an architectonic field were comparable, whereas profiles between regions were dissimilar. Border identification, achieved by grouping of correlation coefficients according to an arbitrary fixed criterion (e.g., 0.65) was generally in good agreement with borders identified by qualitative criteria. As with the thresholding approach to border estimation, the radial profile analyses yielded variable results under two conditions: 1) at borders located in or near deep sulci; and 2) at the border of the core and CM, which exhibited dense AChE expression in the IIIc/IV band. The density-based analyses were not sensitive to subtle differences between fields under these conditions. In the present study, most of the errors involved the medial portion of the lateral sulcus. Oblique planes of section and complex cortical folding were frequent there, resulting in distortions of cortical thickness and laminar relationships. The morphology of radial density profiles reflected these distortions. The most common error was that the border between the core and medial belt was not identified. By comparison, the border between the core and lateral belt was usually located on the surface of the superior temporal plane or HG. Here, the border was rarely misidentified. Identification of borders between core and belt regions Ambiguity in border identification was reduced when adjacent sets of sections (i.e., Nissl, myelin, AChE) were studied and compared; thus, the use of multiple architectonic techniques enabled greater precision in border determination compared with reliance on a single approach. The images in Figures 8 –10 are centered on the core/belt border (arrowheads) identified independently in thionin, AChE, and myelin stains. The panels in the top row are centered on the border between the medial belt and core. The panels in the bottom row are centered on the border between the lateral belt and core. In all panels the core is on the left. The borders between the core and adjacent belt fields were most easily identified in AChE preparations, but the architectonic details visible in the thionin- and myelin-stained sections are also sufficient to define the borders at this magnification. The transition from the core to the belt was abrupt in many sections but sometimes appeared to occur more gradually (over 300 –500 m), confounding identification of a precise border on this basis. Gradual transitions were observed more often at the border of the core and lateral belt than at the border of the core and medial belt. Fig. 7. Acetylcholinesterase (AchE) expression in the human caudomedial belt area (CM). A: Off-coronal section (perpendicular to HG1) caudal and medial to the core. Note the dense AChE expression in the layer IIIc/IV band in CM compared with the adjacent lateral field. Arrowheads indicate medial (left) and lateral (right) borders of CM. B–D: Architecture of CM. A: Thionin stain for Nissl substance. B: Acetylcholinesterase histochemistry. C: Myelin stain. Note the presence of AchE⫹ pyramidal cells in the IIIc/IV band. Scale bar ⫽ 1 mm in A, 250 m in B–D. IDENTIFICATION OF THE AUDITORY CORE 209 Fig. 8. Architecture of macaque auditory cortex showing borders (arrowheads) between the core and belt regions. Top row: Core is to the left of the arrowhead, and medial belt is to the right. Bottom row: Core is to the left of the arrowhead, and lateral belt is to the right. A,D: Thionin stain for Nissl substance. B,E: Acetylcholinesterase histochemistry. C,F: Myelin stain. Scale bar ⫽ 1 mm. Species differences In the cytoarchitecture and myeloarchitecture, laminar divisions and structural variants were easiest to resolve in the human tissue and most difficult in macaques. These observations may be related to differences in the relative concentration of the structural elements. In macaques, cellular and fibrillar density was relatively high, making laminar relationships more difficult to resolve. In the chimpanzee and human specimens, more generous spacing improved the resolution of such details (see also Buxhoeveden et al., 1996). Consequently, differences between the medial and lateral domains of the core were more obvious in humans and chimpanzees than in macaques (compare top and bottom panels in Figs. 8 –10). This may explain why subdivision of the core into lateral and medial zones is reported more often in studies of human tissue than monkeys, and why published parcellations of the auditory cortex in humans tend to be more variable (see Discussion). A second observation concerns myelination in layer III. As Figures 4 – 6 and 8 –10 reveal, the density and complexity of fibrillar organization in layer III differed between primates. The network of small-diameter horizontal and tangential fibers was most elaborate (or highly developed) in humans, intermediate in chimpanzees, and the least intricate in macaques. Similar differences may also characterize layers IV–VI, but these could not be resolved consistently due to heavy myelination in these layers. AChE⫹ pyramidal cells in layers III and V of the belt region were present in greater numbers in the chimpanzee and human than in the macaque material (refer to Figs. 4 – 6). Cell somata in the human specimens were darkly stained, and profiles were sharp. In the chimpanzee tissue, cell staining was not quite as intense, and somatic 210 T. HACKETT ET AL Fig. 9. Architecture of chimpanzee auditory cortex showing borders (arrowheads) between the core and belt regions. Top row: Core is to the left of the arrowhead, and and medial belt is to the right. Bottom row: Core is to the left of the arrowhead, and lateral belt is to the right. A,D: Thionin stain for Nissl substance. B,E: Acetylcholinesterase histochemistry. C,F: Myelin stain. Scale bar ⫽ 1 mm. profiles were somewhat less sharp, but were clearly more distinct than in macaques. In macaques, AChE⫹ somatic profiles were typically indistinct or absent. In contrast, the staining of fibers and dendritic processes did not seem to vary between species. Inspection of adjacent sections stained with thionin indicated that an increase in the number of middle- and large-size pyramidal cells characterized the belt in all three taxa; thus the disparity appeared to be related to a phyletic difference in AChE expression by a particular class of cells. This issue awaits further investigation. Reconstruction of serial sections and localization of the core The identification of the borders between the core and surrounding belt regions in individual sections allowed a fairly precise outline of the core region to be made in serial reconstructions. In Figures 11–13 we show reconstructions for two cases of each species. Stacks of sections stained for AChE were aligned approximately along the long axis of the core. Spacing between sections was somewhat arbitrary to allow better visualization of architectonic details. Sections were 50 m thick, and every 12th section was illustrated, on average (i.e., approximate distance of 600 m between sections); thus, the shape of the core (black dashed outlines) was slightly elongated compared with its shape in the whole brain (Fig. 14). Some borders were not visible due to complex folding of the cortex. The overlying parietal cortex was graphically deleted. Macaque There were few cues in the gross anatomy that would contribute to postmortem localization of the core. The superior temporal plane was relatively flat, and elevations IDENTIFICATION OF THE AUDITORY CORE 211 Fig. 10. Architecture of human auditory cortex showing borders (arrowheads) between the core and belt regions. Top row: Core is to the left of the arrowhead, and medial belt is to the right. Bottom row: Core is to the left of the arrowhead, and lateral belt is to the right. A,D: Thionin stain for Nissl substance. B,E: Acetylcholinesterase histochemistry. C,F: Myelin stain. Scale bar ⫽ 1 mm. or depressions were not consistent markers of architectonic boundaries. The position of the auditory fields in macaque monkeys (Fig. 11) varied only slightly across individual macaques. The core was positioned between the lateral and medial banks of the supratemporal plane; thus, it was completely contained within the lower bank of the lateral sulcus. In rare instances, the core region was shifted medially so that even the medial edge of AI dropped over the steep bank of the circular sulcus. In other cases, the width of the core relative to the width of the superior temporal plane was either greater or extended further laterally, shifting part of the lateral belt field over the edge of the lateral sulcus onto the exposed surface of the superior temporal gyrus. The magnitude of this variability in the mediolateral dimension was on the order of 1–2 mm. The shape of the core approximated an elongated oval, widest caudally in AI, and narrowing ros- trally in R. The boundaries of the core encompass the region of most intense AChE expression in layer IIIc/IV, except in CM, where density remained high in that band (e.g., Fig. 11A). Rostral to the core was a smaller region with core-like architecture that we tentatively identified as RT. This small field exhibited higher density AChE and myelin staining than adjacent fields; however, these features were less robust than in AI and R. Furthermore, this field had cytoarchitectonic properties of belt cortex (e.g., reduced cell packing density, larger pyramidal cells in layer III). At present, RT remains the least certain member of the core in macaques. Chimpanzee Among the four chimpanzee cases, substantial differences were found in the position of the core with respect to the gross anatomy of the superior temporal plane. In case 212 T. HACKETT ET AL Fig. 11. Reconstructions of AChE sections showing location of the core region (black dashed ovoid) on the dorsal surface of the superior temporal plane for two macaque monkeys (A, case M1; B, case M3). Small white dashed ovoid shows putative location of the CM and RT fields. The images were aligned on the long axis of the elongated core region and lateral edge of the lateral sulcus. C, caudal; L, lateral. Scale bar ⫽ 4 mm. Ch3 (Fig, 12A; not shown in Fig. 2), the planar surface was relatively flat and the bulk of the core region was found medially on the surface of the lateral sulcus. Rostrally, the medial edge of the core was shifted ventrally as a small rudimentary gyrus began to form. The medial border of the rostral core region was positioned in the depths of the inferior limiting (circular) sulcus. Note also that the position of the core was at a greater distance from the lateral edge of the superior temporal plane than in macaques. In case Ch1 (Figs. 2C, 12B), a transverse gyrus was more IDENTIFICATION OF THE AUDITORY CORE Fig. 12. Reconstructions of AChE sections showing location of the core region (black dashed ovoid) on the dorsal surface of the superior temporal plane for two chimpanzees (A, case Ch3; B, case Ch1). White dashed ovoid in A marks the estimated position of the caudomedial belt region (CM). The small white ovoid in B indicates location of 213 putative RT. The images were aligned on the long axis of the elongated core region and lateral edge of the lateral sulcus. PM, posteromedial; AM, anteromedial. For other abbreviations, see list. Scale bar ⫽ 4 mm. Fig. 13. Reconstructions of AChE sections showing location of the core region (black dashed ovoid) on the dorsal surface of the superior temporal plane for two human cases (A, case Hu1; B, case Hu2). White dashes indicate estimated position of the caudomedial belt region. The images were aligned on the long axis of the elongated core region and lateral edge of the lateral sulcus. PM, posteromedial; AM, anteromedial. For other abbreviations, see list. Scale bar ⫽ 4 mm. IDENTIFICATION OF THE AUDITORY CORE 215 Fig. 14. Dorsolateral views of the left superior temporal plane. A,B: Macaque cases M1 and M3. C,D: Chimpanzee cases Ch1 and Ch4. E,F: Human cases Hu1 and Hu2. Larger white dashed ovoids in all panels indicate approximate boundaries of the core. Smaller ovoids in A–D denote location of putative RT. Dashed straight white lines designate sulcal landmarks. a, anterior, m, medial. For other abbreviations, see list. Scale bars ⫽ 5 mm. evident but was still rudimentary compared with the prominent HG in case Ch4 (Fig. 2D). Except for its caudal portion, the core region was confined to this transverse gyrus. In all four cases the general shape of the core region was similar to that found in macaques, but variability between the chimpanzee cases was higher. The patchy staining in some sections (e.g., rostral to the core region in case 2) was histological. In case Ch3 (Fig. 12A), the approximate location of putative CM is outlined in white, as described above for macaques. The medial border of the field extended slightly onto the dorsal bank of the lateral sulcus, but in both cases the remaining portion of the field was removed during dissection of the tissue into blocks; thus the size and extent of this field could not be determined. Note also the outline of a field resembling RT in case Ch1 (Fig. 12B). This field was not obvious in case Ch3, but it may be included in the narrow anteromedial extension of the outlined core region in Figure 12A. Human Similar variations in gross anatomy complicate descriptions of the core in humans (Fig 13). In case Hu1 (Fig. 13A), there was a double/bifid HG, separated by an intermediate sulcus of Heschl. Along most of its length, the elongated core region straddles the intermediate sulcus, while its borders lie laterally and medially near the dorsal surface of each gyrus (HG1, HG2). The lateral border of the core shifted medially in more rostral sections, and eventually the entire core became confined to HG1 rostrally. Caudally, the intermediate sulcus became shallow, and then ended, but the core continued for a few sections. 216 T. HACKETT ET AL The darkly stained CM region was most evident in this case. In case 2 (Fig. 13B), a single HG was present. In this case, the core region was confined to the single HG along most of its length. In a third case (Hu3, not illustrated), a double HG was also present. Here, the core also straddled the intermediate sulcus, but the bulk of the core was shifted medially, compared with case Hu1, so that the lateral border of the core was often in the depths of the intermediate sulcus instead of on the dorsal surface of the gyrus. Thus, in addition to variability in the gross anatomy of the superior temporal region, there was some variability in the relative position of the core with respect to these landmarks. AChE neuropil staining in case Hu2 was less intense compared with case Hu1, so that the borders of the core region at low magnification were less obvious. It appears that the longer postmortem period in this case may have contributed to a reduction in the intensity of AChE neuropil staining. Interestingly, AChE expression in cells was not affected, nor was staining for myelin or Nissl. Accordingly, density-based measurements of AChE neuropil expression were not reliable for border identification for case Hu2. DISCUSSION The core region of auditory cortex was identified in macaque monkeys, chimpanzees, and humans by a combined analysis of cytoarchitecture, myeloarchitecture, and expression of acetylcholinesterase (AChE). In all three primates, the core was found to occupy an elongated region of cortex on the superior temporal plane, hidden from view by the overlying parietal cortex (Fig. 14). In macaques, the long axis of the core was oriented in the rostrocaudal plane. In chimpanzees and humans, the core was largely coextensive with the first transverse temporal gyrus, although notable variants were found. The core exhibited primary-like architectonic features that distinguished it from the adjacent belt fields flanking it on all sides. Systematic architectonic variations were also noted within the core itself and appear to be present in all three species. The combined architectonic approach using multiple markers was found to be more useful in border identification than reliance on a single method. The results indicate that the core region can be identified in humans and non-human primates by using the same anatomical criteria, suggesting that these criteria delineate homologous cortical regions in the taxa we examined. What is the auditory core? Most descriptions of the auditory cortex in mammals identify a single primary field known as AI. In the auditory cortex of monkeys more than one primary or primarylike field can be identified, and the local aggregation of these fields is referred to as the core. Current models (e.g., Fig. 1) include two or three distinct fields (AI, R, RT) arranged from caudal to rostral along the long axis of the core region at the initial stage of auditory cortical processing (Kaas and Hackett, 1998, 2000; Rauschecker, 1998, Rauschecker and Tian, 2000). The identity of a core field depends on a profile derived from its architecture, connections, and neuron response properties. Architectonic features include koniocortical cytoarchitecture, dense astriate/unistriate myelination, and dense expression of AChE, cytochrome oxidase (CO), and parvalbumin in the neuropil of layer IV. Major subcortical connections favor the ventral (principal) division of the medial geniculate complex (MGv), and cortical projections are primarily directed to the belt fields surrounding the core (Akert et al., 1959; Mesulam and Pandya, 1973; Pandya and Sanides, 1973; Forbes and Moskowitz, 1974; Burton and Jones, 1976; Casseday et al., 1976; Fitzpatrick and Imig, 1978; Oliver and Hall, 1978; Galaburda and Pandya, 1983; Aitkin et al., 1988; Cipolloni and Pandya, 1989; Luethke et al., 1989; Morel and Kaas, 1992; Morel et al., 1993; Pandya et al., 1994; Jones et al., 1995; Molinari et al., 1995; Rauschecker et al., 1997). Connections with the parabelt region and more distant cortical fields are at most minor (Hackett et al., 1998a), and subdivisions within the core are thought to process information in parallel (Rauschecker et al., 1997). Neurons in the core respond to a variable range of frequencies centered around a single characteristic frequency (CF). Neurons with a similar CF are arranged in rows, known as isofrequency contours. The organization of isofrequency contours is cochleotopic (e.g., high CF caudomedial, low CF rostrolateral) and roughly orthogonal to the isofrequency dimension (Licklider and Kryter, 1942; Walzl, 1947; Merzenich and Brugge, 1973; Imig et al., 1977; Pfingst and O’Connor, 1981; Aitkin et al., 1986; Luethke et al., 1989; Morel and Kaas, 1992; Morel et al., 1993; Kosaki et al., 1997; Rauschecker et al., 1997; Recanzone et al., 1999, 2000). The topography of CF maps in adjacent core fields (e.g., AI/R; R/RT) appears to be reversed in that AI and R share a common low-CF border. R and RT may share a high-CF border, but physiological evidence of a reversal in the CF gradient in RT is inconclusive. Consequently, of the areas considered, the status of RT as a member of the core is least certain (Morel et al., 1993; Hackett et al., 1998a). In apes and humans, anatomical identification of the core region primarily depends on the analysis of architectonic features in postmortem tissue. Most of the parcellations of auditory cortex were derived from analyses of cytoarchitecture and/or myeloarchitecture (chimpanzee: Beck, 1929; human: Campbell, 1905; Brodmann, 1909; Vogt and Vogt, 1919; Flechsig, 1920; von Economo and Koskinas, 1925; Beck, 1928; von Economo, 1929; von Economo and Horn, 1930; Poljak, 1932; Hopf, 1954; Pandya and Sanides, 1973; Galaburda and Sanides, 1980; Seldon 1981a,b, 1982; Morosan et al., 2001). A few studies in humans have also included additional markers (e.g., AChE, CO, NADPH-diaphorase) to identify or characterize auditory related cortical fields (Ong and Garey, 1991; Hutsler and Gazzaniga, 1996; Rivier and Clarke, 1997; Clarke and Rivier, 1998). Despite substantial variations in conclusions and nomenclature across studies, a common finding has been the identification of a central region with primary, or primary-like, architectonic features surrounded by several nonprimary fields. Functional evidence of a core-like region in apes and humans can be derived from a number of studies. Surface recordings in the chimpanzee have revealed the presence of an acoustically responsive region on the superior temporal plane with an orderly representation of stimulus frequency similar to that found in monkeys (Bailey et al., 1943; Woolsey, 1971). In humans, patterns of cochleotopic organization resembling those found in macaques and chimpanzees have been found along the HG by using a variety of techniques (electrophysiology/evoked potentials: IDENTIFICATION OF THE AUDITORY CORE 217 Gross anatomical relationships of the core often confined to the most anterior gyrus (HG1). In one case, area 41 “continued for a short distance onto the intrasulcal portion of the second, more caudal transverse gyrus” (HG2). In the present study, the two cases presenting with a bifid HG were similar to this in that core extended posterolaterally across the intermediate transverse sulcus and toward the crown of HG2. However, the anteromedial boundary of the core was near the crown of HG1; thus, the core did not encompass HG1 and extend onto HG2, but was shifted posterolaterally to involve about two-thirds of HG1 and one-third of HG2. The findings of both studies illustrate the variable relationship of the primary auditory fields to the surface landmarks. The diversity in the morphology and location of the HG in humans (Leonard et al., 1998) is especially problematic for functional studies in which the location of the auditory core is in question. Although a small number of brains have been sampled across studies, the best estimate of the position and areal extent of the core appears to be related to the boundaries of HG1, particularly when there is only one HG. For other configurations of HG, the position of HG1 is a less reliable guide to the location of the core. One of the most impressive findings in this study was the high level of variability in the gross anatomical features of the auditory cortical region between individuals. The greatest differences were found in chimpanzee and human brains, where the number, size, shape, and extent of the transverse temporal gyri varied between individuals. In all three species, the position of the core relative to sulcal and gyral landmarks was also variable; thus, these gross anatomical features were used only to approximate the location of the field. Precise localization depended on the architectonic analyses. In macaques, we found that the shape and orientation of the core (Fig. 14A,B) was consistent with previous anatomical and/or physiological descriptions of this region (for reviews, see Morel et al., 1993; Hackett et al., 1998a). Robust surface features marking the location of the core were generally lacking in macaques, although a slight elevation on the cortical surface seemed to correspond to the location of the core in some species (Poljak, 1932; Jones et al., 1995). In chimpanzees lacking a definitive HG (Figs. 12A, 14C), the orientation of the core on the superior temporal plane was situated deep in the lateral sulcus and elongated along the medial edge of the superior temporal plane. In chimpanzees with a prominent HG (Fig. 14D), the orientation and appearance of the core was more similar to that found in humans. The core in these cases was confined to the HG regardless of its orientation. In humans, the elongated shape of the core resembled that found in macaques and chimpanzees. The location of the core with respect to gross anatomical features, however, was more variable (Fig. 14E,F). When a single HG was present, the core occupied most of its surface and was constrained by its sulcal boundaries. When the HG was divided by an intermediate transverse sulcus (bifid HG, posterior duplication), the core region was found to occupy variable portions of both (i.e., HG1 and HG2), spanning the intermediate sulcus of Heschl. In their evaluation of the relationship between topographic landmarks and cytoarchitectonic fields, Rademacher et al. (1993) compared the areal extent of area 41 (auditory koniocortex) with variations in the topography of the HG. When the HG was bifurcated by an intermediate transverse sulcus, they found that area 41 was most Architectonic variations within a region commonly referred to as primary auditory cortex are well known (e.g., Beck, 1928, 1929; von Economo and Horn, 1930; Pandya and Sanides, 1973; Galaburda and Sanides, 1980; Morosan et al., 2001). In the cytoarchitectonic studies of von Economo and Horn (1930), for example, up to 11 distinct “types” of granular cortex were identified within a region (TC) that corresponds closely to our conception of the core. However, there is a relative uniformity with respect to the most consistent features that contributes to the profile of the core as a distinct region (Brodmann, 1909; Vogt and Vogt, 1919). Similarly, the fields comprising the belt region surrounding the core are architectonically distinct from those within the core, but within the belt region, individual areas share a number of features typical of the belt. To the extent that these sets of architectonic features are unique to the core and belt, they can provide the basis for identification of the borders between regions. In the present study, the architectonic features within the region defined as the core were not uniform. Subtle and sometimes systematic variations were observed, particularly in sections stained for Nissl substance and myelin. One of the more robust divided the core into medial and lateral domains along the long axis of the region. Myelination in the medial domain was more often astriate, whereas in the lateral portion, a reduction in fiber density allowed better delineation of layer IV, contributing to a unistriate profile. This particular feature was matched by trends in the cytoarchitecture. Granularity and columnar organization in layer III were more prominent in the medial domain. The identification of two koniocortical domains, splitting the region lengthwise, has been suggested previously for humans (Hopf, 1954; Sarkissow et al., 1955; Galaburda and Sanides, 1980). This feature was also identified in macaque monkeys by Pandya and Sanides (1973); however, it was not preserved in subsequent adaptations of their schema (e.g., Galaburda and Pandya, 1983). Pandya and Sanides (1973) concluded that the denser myelination in Kam was related to a proposed greater concentration of callosal fibers medially. As yet, additional support for a distinction between Verkindt et al., 1995; Howard et al., 1996; magnetoencephalography: Elberling et al., 1982; Pantev et al., 1988, 1995; Bertrand et al., 1991; Yamamoto et al., 1992; Romani et al., 1992; Tiitinen et al., 1993; Huotilainen et al., 1995; Langner et al., 1997; Hoke et al., 1998; Lutkenhoner and Steinstrater, 1998; Rosburg et al., 1998; positron emission tomography: Lauter et al., 1985; de Rossi et al., 1996; Ottaviani et al., 1997; Lockwood et al., 1999; functional magnetic resonance imaging: Strainer et al., 1997; Wessinger et al., 1997; Bilecen et al., 1998; Talavage et al., 2000; Di Salle et al., 2001). The anatomical and physiological findings reviewed above, coupled with those of the present study, strongly support the existence of a core-like region in chimpanzees and humans that should be considered homologous to the auditory core identified in monkeys (Walker, 1937). Detailed anatomical studies and the refinement of noninvasive functional assays will allow us to evaluate this hypothesis further and extend our inquiries to fields surrounding the core, such as CM. Architectonic variation within the core 218 T. HACKETT ET AL the medial and lateral domains has not been reported in either the connections or physiological profiles of neurons in the core. Architectonic variations also distinguished the caudal (posteromedial) and rostral (anterolateral) domains within the core. Myelination and AChE expression gradually increased caudally, where the cytoarchitecture was more typical of koniocortex. Previous architectonic studies in chimpanzees and humans have also identified regional variations along this dimension of granular cortex (Beck, 1928, 1929; von Economo and Horn, 1930; Morosan et al., 2001). The transverse prima interna and externa of Beck (1928, 1929), for example, mirror the relative positions of AI and R in the macaque. Most recently, Morosan et al. (2001) identified three adjacent zones (Te1.1, Te1.0, Te1.2) distributed along the long axis of HG in humans. These anatomical gradients may relate to functional gradients (e.g., cochleotopy), as identified within the core region of macaques (e.g., AI, R). Based on comparison of the findings in the present study with qualitative and quantitative descriptions of temporal cortex in previous studies, we conclude that the core corresponds most closely to the following fields: area 41 (Brodmann, 1909); TC (von Economo and Koskinas, 1925); ttrIi/e (Beck, 1928, 1929); koniosus supratemporalis (Bailey and von Bonin, 1951); Kam/Kalt (Pandya and Sanides, 1973; Galaburda and Sanides, 1980; Galaburda and Pandya, 1983); temporal granulous core (Braak, 1978); AI (Rivier and Clarke, 1997); and Te1.0 (Morosan et al., 2001). Architectonic variation within the belt Although we made no attempt to define subdivisions within the belt region in this study, our results indicate that the belt is not anatomically homogeneous in monkeys, chimpanzees, or humans. Systematic differences were evident within and between the medial, lateral, and caudal domains of the belt. However, these fields also share a basic architectonic profile that distinguishes them from the core and more distant regions, such as the parabelt in macaques (Hackett et al., 1998a). Additional support for these differences can be found in microelectrode studies of macaque monkeys. Functional subdivisions of the belt correlate well with those identified anatomically (e.g., Rauschecker et al., 1995, 1997; Kosaki et al., 1997; Romanski et al., 2000; Tian et al., 2001). In the present study, we briefly described the architectonic features of the belt field, CM, compared with the adjacent part of the core (i.e., AI). In macaque monkeys CM is considered to be a belt field on the basis of its architecture, connections, and neuron response properties. Major inputs to CM originate in the dorsal (MGd) and magnocellular (MGm) divisions of the medial geniculate complex (Rauschecker et al., 1997), and AI in the core (Galaburda and Pandya, 1983; Morel et al., 1993). Cortical projections of CM include the parabelt auditory cortex (Hackett et al., 1998a) and posterior parietal cortex (Lewis and van Essen, 2000). Many neurons in CM are broadly tuned, are more responsive to temporally and spectrally complex acoustic stimuli, and appear to be dependent on intact inputs from AI for responses to pure tones (Merzenich and Brugge, 1973; Rauschecker et al., 1997; Recanzone et al., 2000). The results of the present study indicate that the architectonic profile of CM is most consistent with its inclusion in the caudal portion of the belt region of macaques. The present findings also emphasize architectonic similarities between macaque CM and a field caudal (posterior) and medial to the core in chimpanzees and humans. Based on architectonic descriptions in previous studies, the CM field in humans appears to correspond most closely to the following areas: the medial portion of the koniocortical TD sector of the Regio acustica (von Economo and Koskinas, 1925; von Economo and Horn, 1930); ttrIin2 of the Pars intima of chimpanzees and humans (Beck, 1928, 1929); medial PaAc (Galaburda and Sanides, 1980); and medial Te1.1 (Morosan et al., 2001). Our preliminary results suggest that this field may be homologous in macaques, chimpanzees, and humans. Sources of variability in border identification Borders between adjacent cortical fields are not always characterized by an abrupt transition in the architecture. There is no a priori reason why margins must be sharp, but this is commonly reported in studies of sensory cortex. Indeed, conclusions sometimes depend on the identification of clean boundaries between fields. Transitional architecture may, in fact, separate some fields in cortex, but the blurring of clean borders is often related to technical variables. One important factor is the plane of section. Distortion results from cutting across and/or between the radial axes of cell columns, thereby altering the threedimensional relationships between the neural structures. An oblique plane of section alters the point of view, altering the appearance of the architecture. Depending on the nature of the distortion, the distinction between the two fields may be obvious only at some variable distance from the border. In brains with greater gyrification, the cortex exhibits complex folding in several directions (e.g., the HG in chimpanzees and humans). Thus, a coronal plane of section results in radially aligned sections through some areas and oblique sections through others. In the present study, we found no single plane of section to be ideal for all fields of interest. For macaques, a modified coronal plane, perpendicular to the surface of the superior temporal plane, resulted in minimal distortion and was roughly in line with the long axis of the elongated core. For chimpanzees and humans with a HG, a modified parasagittal plane, perpendicular to the long axis of the transverse gyrus (gyri) and its dorsal surface, resulted in minimal distortion of fields on and immediately adjacent to the HG. A second variant is the relationship of borders to the gross anatomy. The presence of deep sulci at and between architectonic borders substantially alters anatomical features, particularly laminar relationships. In our macaque, chimpanzee, and human specimens, for example, the border between the core and the medial belt was frequently located at or near the sharp turn of the inferior limiting (circular) sulcus. Another troublesome location was the intermediate sulcus of Heschl in those human specimens with a double, or bifid, HG. The compression of cortex in these sulci distorted laminar relationships, compromising the integrity of calculations based on radial density profiles. In severely distorted sections, even the identity of the fields at a distance from the estimated border were in question, so that judgements were based on modified criteria using identifiable features, as well as comparisons of the intact architecture on either side of the sulcus. A third source of variability is histological. Aberrant staining patterns can result from variations in postmor- IDENTIFICATION OF THE AUDITORY CORE tem delay, fixation, sectioning, buffer solutions, or minor details in the histological protocol. Although severe distortions are obvious and easily excluded from analysis, subtle variations may not be detected. A gradual dissipation of AChE expression at a border, for example, may be an inherent property of the architecture, but this does not explain why abrupt transitions are found in some sections, but not others. In this study, staining quality for Nissl substance and myelinated fibers was highly consistent across cases, regardless of species, perfusion, fixation, or postmortem delay. Staining for AChE was more variable (see Materials and Methods) but was not consistently related to any identifiable histological factor. As a partial control, densitometric analyses in the present study were limited to measurements within individual sections. No attempt was made to compare density measurements between cases or between sections. Given the many factors that contribute to histological variability, one of the merits of the multifaceted architectonic analysis is that the redundancy increases confidence in border identification. The intense expression of AChE in the core, for example, was an invaluable clue in approximating the position of the core, but the identification of borders was most precise when direct comparisons were made with adjacent thionin- and myelin-stained sections. When the borders were near or within sulci or when fields were sectioned tangential to the cortical surface (e.g., medial belt region), important features of the cytoarchitecture and myeloarchitecture were severely distorted, limiting border identification to differences in the density of cellular or fibrillar elements. In such cases, the rapid change in layer IIIc/IV expression of AChE was often visible and served as the most valid estimate of the border. The combined architectonic approach was also useful in case Hu2, in which AChE neuropil expression was relatively weak. In this case, border identification was reinforced by cytoarchitecture, myeloarchitecture, and AChE⫹ cell distribution. Observer-independent border identification One limitation of most architectonic studies is that decisions rely on the qualitative judgments of the observer. As discussed above, the use of multiple architectonic markers greatly improves the consistency and precision of border identification. The quantitative analyses employed herein were accurate under certain conditions (e.g., wellstained tissue, mild structural distortions imposed by cortical folding). The analyses did not, however, produce reliable border identification when radial density profiles were distorted by cortical folding, an oblique plane of section, low contrast staining, or gradual architectonic transitions. Similar problems have been reported by others using different techniques (Schleicher et al., 1999; Morosan et al., 2001). To account for such architectonic variability, the ideal reliable observer-independent method must be sensitive only to relevant features. Otherwise, actual borders may be missed or false borders identified, depending on the criteria chosen. We are evaluating ways of improving the sensitivity of such methods to key criteria, without introducing a bias toward the identification of false borders. The present findings suggest that the concurrent use of multiple histological markers could be used to reduce qualitative and quantitative errors in border identification. A combined approach may 219 be particularly useful in the validation of borders identified by a strictly quantitative approach. Directions for future research Functional studies in humans suggest that certain elements of the monkey model may be applicable to humans. In addition to evidence of tonotopic organization in the putative core region along the HG (see above), there is also evidence of hierarchical processing in human auditory cortex involving the core and surrounding regions. Numerous studies in humans indicate that auditory related activity in cortical fields outside of the core region can be dissociated from activity within by using stimuli of varied acoustic complexity or linguistic significance (Petersen et al., 1988; Liegeois-Chauvel et al., 1991, 1994; Price et al., 1992; Demonet et al., 1992; Zatorre et al., 1992; Binder et al., 1994, 2000; Berry et al., 1995; Hickock et al., 1997; Scheich et al., 1998; Nishimura et al., 1999; Belin et al., 1999, 2000; Celsis et al., 1999; Jancke et al., 1999; Howard et al., 2000; Scott et al., 2000; Talavage et al., 2000; Di Salle et al., 2001). These results are consistent with findings in monkeys that describe serial and parallel processing among subdivisions of the core and belt regions (e.g., Rauschecker et al., 1997). Because the homology of the core in monkeys and humans appears to be well established, and homologies among belt areas such as CM seem likely, further comparative studies will be needed to identify other similarities and differences in the organization of auditory cortex across taxonomic groups. For architectonic studies, progress will depend on the establishment of anatomical profiles to distinguish cortical fields. The sensitivity of observer-independent techniques could be improved by incorporating these features into the analyses. CONCLUSIONS In macaque monkeys the auditory core region represents the first stage of processing in auditory cortex. A homologous region can be identified in chimpanzees and humans by using architectonic criteria combining cytoarchitecture, myeloarchitecture, and acetylcholinesterase histochemistry. The combined architectonic approach using multiple markers provides a more reliable estimate of the boundaries of the core region than detailed analysis of a single preparation. In all three species, the core region is surrounded by a belt of areas with distinctive architectonic features that vary by location. The position of the core with respect to surface landmarks is most variable in humans and chimpanzees, but it appears to be largely confined to the first transverse temporal gyrus of Heschl, even when more than one such gyrus is present. Although we consider the region identified as the core to be homologous in macaques, chimpanzees, and humans, species differences were noted in patterns of myelination and acetylcholinesterase expression. These data establish a foundation for subsequent anatomical studies of auditory fields outside of the core and for functional studies of auditory cortex in these species. ACKNOWLEDGMENTS The authors thank the veterinary staff of the New Iberia Research Center for their assistance in obtaining chimpanzee material. We also thank Dr. William O. Whet- 220 T. HACKETT ET AL sell of the Vanderbilt University School of Medicine, Dr. Bruce Quinn and Dr. John Smiley of the Northwestern University Alzheimer’s Disease Center, and the Clinical Core of the Northwestern University Alzheimer’s Disease Center, Chicago for assistance in obtaining human material. The authors also recognize Thomas Dinsenbacher for assistance with data analysis, as well as Judy Ives and Laura Trice for histological assistance. T.A.H. was the recipient of grants DC00249 and DC04318 from the National Institutes of Health. T.M.P. was the recipient of grant JSMF 98-45 from the McDonnell-Pew Program in Cognitive Neuroscience and grant JSMF 20002029 from the James S. McDonnell Foundation. J.H.K. was the recipient of grant NS16446 from NINDS. Northwestern University was the recipient of grant NS1P30 AG13854-01 from the National Institute on Aging. LITERATURE CITED Ades HW, Felder R. 1942. The acoustic area of the monkey (Macaca mulatta). J Neurophysiol 5:49-59. Aitkin LM. 1990. The auditory cortex, structural and functional bases of auditory perception. London: Chapman and Hall. Aitkin LM, Merzenich MM, Irvine DRF, Clarey JC, Nelson JE. 1986. Frequency representation in auditory cortex of the common marmoset (Callithrix jacchus jacchus). J Comp Neurol 252:175–185. Aitkin LM, Kudo M, Irvine DRF. 1988. Connections of the primary auditory cortex in the common marmoset (Callithrix jacchus jacchus). J Comp Neurol 269:235–248. Akert K, Woolsey CN, Diamond IT, Neff WT. 1959. The cortical projection area of the posterior pole of the medial geniculate body in Macaca mulatta. Anat Rec 133:242. Bailey P, von Bonin G. 1951. The isocortex of man. Urbana: University of Illinois Press. Bailey P, von Bonin G, Garol HW, McCulloch WS. 1943. The functional organization of temporal lobe of the monkey (Macaca mulatta) and chimpanzee (Pan satyrus). J Neurophysiol 6:121–128. Bailey P, von Bonin G, McCulloch WS. 1950. The isocortex of the chimpanzee. Urbana: University of Illinois Press. Beck E. 1928. Die myeloarchitektonische Felderung es in der Sylvischen Furche gelegenen Teiles des menschlichen Schläfenlappens. J Psychol Neurol 36:1–21. Beck E. 1929. Der myeloarchitektonische Bau des in der Sylvischen Furche gelegenen Teiles des Schläfenlappens beim Schimpansen (Troglodytes niger). J Psychol Neurol 38:309 – 428. Belin P, Zatorre RJ, Hoge R, Evans AC, Pike B. 1999. Event-related fMRI of the auditory cortex. Neuroimage 10:417– 429. Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. 2000. Voice-selective areas in human auditory cortex. Nature 403:309 –312. Berry I, Demonet J-F, Warach S, Viallard G, Boulanouar K, Franconi JM, Marc-Vergnes J-P, Edelman R, Manelfe C. 1995. Activation of association auditory cortex demonstrated with functional MRI. Neuroimage 2:215–219. Bertrand O, Perrin F, Pernier J. 1991. Evidence for a tonotopic organization of the auditory cortex observed with electrical evoked potentials. Acta Otolaryngol Suppl 491:116 –123. Bilecen D, Scheffler K, Schmid N, Tschopp K, Seelig J. 1998. Tonotopic organization of the human auditory cortex as detected by BOLD-FMRI. Hear Res 126:19 –27. Binder JR, Rao SM, Hammeke TA, Yetkin FZ, Jewmanowicz A, Bandettini PA, Wong EC, Estkowski LD, Goldstein MD, Haughton VM, Hyde JS. 1994. Functional magnetic resonance imaging of human auditory cortex. Ann Neurol 35:662– 672. Binder JR, Frost JA, Hammeke TA, Bellgowan PSF, Springer JA, Kaufman JN, Possing ET. 2000. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex 10:512–528. Blinkov SM. 1955. The temporal region. In: Sarkissow SA, Filimonoff IN, Kononowa EP, Preobraschenskaja IS, Kukuew LA, editors. Atlas of the cytoarchitectonics of the human cerebral cortex. Moscow: Medgiz. p 168 –193. Braak H. 1978. The pigment architecture of the human temporal lobe. Anat Embryol 154:43–240. Brodmann K. 1909. Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig: Barth. Brugge JF, Reale RA. 1985. Auditory cortex. In: Peters A, Jones EG, editors. Cerebral cortex, vol 4, Association and auditory cortices. New York: Plenum Press. p 229 –271. Burton H, Jones EG. 1976. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol 168:249 –302. Buxhoeveden D, Lefkowitz W, Loats P, Armstrong E. 1996. The linear organization of cell columns in human and nonhuman anthropoid Tpt cortex. Anat Embryol 194:23–36. Campain R, Minckler J. 1976. A note on the gross configurations of the human auditory cortex. Brain Lang 3:318 –323. Campbell AW. 1905. Histological studies on the localization of cerebral function. Cambridge: Cambridge University Press. Casseday JH, Harting JK, Diamond IT. 1976. Auditory pathways to the cortex in Tupaia glis. J Comp Neurol 166:303–340. Celsis P, Boulanouar K, Doyon B, Ranjeva JP, Berry I, Nespoulous JL, Chollet, F. 1999. Differential fMRI responses in the left posterior superior temporal gyrus and left supramarginal gyrus to habituation and change detection in syllables and tones. Neuroimage 9:135–144. Cipolloni PB, Pandya DN. 1989. Connectional analysis of the ipsilateral and contralateral afferent neurons of the superio temporal region in the rhesus monkey. J Comp Neurol 281:567–585. Clarke S, Rivier F. 1998. Compartments within human primary auditory cortex: evidence from cytochrome oxidase and acetylcholinesterase staining. Eur J Neurosci 10:741–745. Demonet JF, Chollet F, Ramsay S, Cardebat D, Nespoulous JL, Wise R, Rascol A, Frackowiak R. 1992. The anatomy of phonological and semantic processing in normal subjects. Brain 115:1753–1768. de Ribaupierre F. 1997. Acoustical information processing in the auditory thalamus and cerebral cortex. In: Ehret G, Romand R, editors. The central auditory system. New York: Oxford University Press. p 317– 397. de Rossi G, Paludetti G, di Nardo W, Calcagni ML, di Guida D, Almadori G, Galli J. 1996. SPET monitoring of perfusion changes in auditory cortex following mono- and multi-frequency stimuli. Nuklearmedizin 35:112–115. Di Salle F, Formisano E, Seifritz E, Linden DEJ, Scheffler K, Saulino C, Tedeschi G, Zanella FE, Pepino A, Goebel R, Marciano E. 2001. Functional fields in human auditory cortex revealed by time-resolved fMRI without interference of EPI noise. Neuroimage 13:328 –338. Edeline J-M. 1999. Learning-induced physiological plasticity in the thalamo-cortical sensory systems: a critical evaluation of receptive field plasticity, map changes and their potential mechanisms. Prog Neurobiol 57:165–224. Ehret G. 1997. The auditory cortex. J Comp Physiol A 181:547–557. Elberling C, Bak C, Kofoed B, Lebeck J, Saermark K. 1982. Auditory magnetic fields: source location and ’tonotopical organization’ in the right hemisphere of the human brain. Scand Audiol 11:61– 65. Fitzpatrick KA, Imig TJ. 1978. Projections of auditory cortex upon the thalamus and midbrain in the owl monkey. J Comp Neurol 177:537– 556. Fitzpatrick KA, Imig TJ. 1980. Auditory cortico-cortical connections in the owl monkey. J Comp Neurol 192:589 – 610. Flechsig P. 1920. Anatomie des menschlichen Gehirns und Ruckenmarks auf myelogenetischer Grundlage. Leipzig: George Thieme. p 121. Forbes BF, Moskowitz N. 1974. Projections of auditory responsive cortex in the squirrel monkey. Brain Res 67:239 –254. Galaburda AM, Pandya DN. 1983. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. J Comp Neurol , 221:169 –184. Galaburda AM, Sanides F. 1980. Cytoarchitectonic organization of the human auditory cortex. J Comp Neurol 190:597– 610. Gallyas F. 1979. Silver staining of myelin by means of physical development. Neurol Res 1:203–209. Geneser-Jensen FA, Blackstad TW. 1971. Distribution of acetylcholinesterase in the hippocampal region of the guinea-pig: I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat 114: 460 – 481. Hackett TA, Stepniewska I, Kaas JH. 1998a. Subdivisions of auditory cortex and ipsilateral cortical connections of the parabelt auditory cortex in macaque monkeys. J Comp Neurol 394:475– 495. Hackett TA, Stepniewska I, Kaas JH. 1998b. Thalamocortical connections IDENTIFICATION OF THE AUDITORY CORE of the parabelt auditory cortex in macaque monkeys. J Comp Neurol 400:271–286. Hackett TA, Preuss TM, Kaas JH. 1998c. Architectonic identification of core and belt auditory cortex in macaque monkeys, chimpanzees, and humans. Soc Neurosci Abstr 24:1880. Hickock G, Love T, Swinney D, Wong EC, Buxton RB. 1997. Functional MR imaging during auditory word perception: a single-trial presentation paradigm. Brain Lang 58:197–201. Hoke ES, Ross B, Hoke M. 1998. Auditory afterimage: tonotopic representation in the auditory cortex. Neuroreport 9:3065–3068. Hopf P. 1954. Die myeloarchitektonic des isocortex temporalis beim menschen. J Hirnforsch 1:208 –279. Howard MA, Volkov IO, Abbas PJ, Damasio H, Ollendieck MC, Granner MA. 1996. A chronic microelectrode investigation of the tonotopic organization of human auditory cortex. Brain Res 724:260 –264. Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE. 2000. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol 416:79 –92. Huotilainen M, Tiitinen H, Lavikainen J, Ilmoniemi RJ, Pekkonen E, Sinkkonen J, Laine P, Naatanen R. 1995. Sustained fields of tones and glides reflect tonotopy of the auditory cortex. Neuroreport 6:841– 844. Hutsler JJ, Gazzaniga MS. 1996. Acetylcholinesterase staining in human auditory and language cortices: regional variation of structural features. Cereb Cortex 6:260 –270. Imig TJ, Ruggero MA, Kitzes LF, Javel E, Brugge JF. 1977. Organization of auditory cortex in the owl monkey (Aotus trivirgatus). J Comp Neurol 171:111–128. Jancke L, Mirzazade S, Shah NJ. 1999. Attention modulates activity in the primary and the secondary auditory cortex: a functional magnetic resonance imaging study in human subjects. Neurosci Lett 266:125– 128. Jones EG, Burton H. 1976. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal, and temporal regions of primates. J Comp Neurol 168:197–248. Jones EG, Dell’Anna ME, Molinari M, Rausell E, Hashikawa T. 1995. Subdivisions of macaque monkey auditory cortex revealed by calciumbinding protein immunoreactivity. J Comp Neurol 362:153–170. Kaas JH, Hackett TA. 1998. Subdivisions and levels of processing in primate auditory cortex. Audiol Neurootol 3:73– 85. Kaas JH, Hackett TA. 2000. Subdivisions of auditory cortex and processing streams in primates. Proc Natl Acad Sci USA 97:11793–11799. Kaas JH, Hackett TA, Tramo MJ. 1999. Auditory processing in primate cerebral cortex. Curr Opin Neurobiol 9:164 –170. Kim J-J, Crespo-Facorro B, Andreasen NC, O’Leary DS, Zhang B, Harris G, Magnotta VA. 2000. An MRI-based parcellation method for the temporal lobe. Neuroimage 11:271–288. Kosaki H, Hashikawa T, He J, Jones EG. 1997. Tonotopic organization of auditory cortical fields delineated by parvalbumin immunoreactivity in macaque monkeys. J Comp Neurol 386:304 –316. Langner G, Sams M, Heil P, Schultze H. 1997. Frequency and periodicity are represented in orthogonal maps in the human auditory cortex: evidence from magnetoencephalography. J Comp Physiol A 181:665– 676. Lauter JL, Herscovitch P, Formby C, Raichle ME. 1985. Tonotopic organization in human auditory cortex revealed by positron emission tomography. Hear Res 2:199 –205. Leonard CM, Puranik C, Kuldau JM, Lombardino LJ. 1998. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: where is it? Cereb Cortex 8:397– 406. Lewis JW, Van Essen DC. 2000. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol 428:112–137. Licklider JCR, Kryter KD. 1942. Frequency localization in the auditory cortex of the monkey. Fed Proc 1:51. Liegeois-Chauvel C, Peretz I, Babai M, Laguitton V, Chauvel P. 1998. Contribution of different cortical areas in the temporal lobes to music processing. Brain 121:1853–1867. Liegeois-Chauvel C, Musolino A, Badier J, Marquis P, Chauvel P. 1994. Evoked potentials recorded from the auditory cortex in man: evaluation and topography of the middle latency components. EEG Clin Neurophysiol 92:204 –214. Lockwood AH, Salvi RJ, Coad ML, Arnold SA, Wack DS, Murphy BW, Burkard RF. 1999. The functional anatomy of the normal human 221 auditory system: responses to 0.5 and 4.0 kHz tones at varied intensities. Cereb Cortex 9:65–76. Luethke LE, Krubitzer LA, Kaas JH. 1989. Connections of primary auditory cortex in the New World monkey, Saguinus. J Comp Neurol 285:487–513. Lutkenhoner B, Steinstrater O. 1998. High-precision neuromagnetic study of the functional organization of the human auditory cortex. Audiol Neurootol 3:191–213. Merzenich MM, Brugge JF. 1973. Representation of the cochlear partition on the superior temporal plane of the macaque monkey. Brain Res 50:275–296. Mesulam M, Geula C. 1992. Overlap between acetylcholinesterase-rich and choline acetyltransferase-positive (cholinergic) axons in human cerebral cortex. Brain Res 577:112–120. Mesulam MM, Pandya DN. 1973. The projections of the medial geniculate complex within the sylvian fissure of the rhesus monkey. Brain Res 60:315–333. Molinari M, Dell’Anna ME, Rausell E, Leggio MG, Hashikawa T, Jones EG. 1995. Auditory thalamocortical pathways defined in monkeys by calcium-binding protein immunoreactivity. J Comp Neurol 362:171– 194. Morel A, Kaas JH. 1992. Subdivisions and connections of auditory cortex in owl monkeys. J Comp Neurol 318:27– 63. Morel A, Garraghty PE, Kaas JH. 1993. Tonotopic organization, architectonic fields, and connections of auditory cortex in macaque monkeys. J Comp Neurol 335: 437– 459. Morosan P, Rademacher J, Schleicher A, Amunts K, Schormann T, Zilles Z. 2001. Human primary auditory cortex: cytoarchitectonic subdivisions and mapping into a spatial reference system. Neuroimage 13:684 –701. Musiek FE, Reeves AG. 1990. Asymmetries of the auditory areas of the cerebrum. J Am Acad Audiol 1(4):240-245. Nishimura H, Hashikawa K, Doi K, Iwaki T, Watanabe Y, Kusuoka H, Nishimura T, Kubo T. 1999. Sign language ’heard’ in the auditory cortex. Nature 397:116. Oliver DL, Hall WC. 1978. The medial geniculate body of the tree shrew Tupaia glis. II. Connections with the neocortex. J Comp Neurol 182: 459 – 494. Ong WY, Garey LJ. 1991. Distribution of GABA and neuropeptides in the human cerebral cortex. A light and electron microscopic study. Anat Embryol (Berl) 183:397– 413. Ottaviani F, Di Girolamo S, Briglia G, De Rossi G, di Giuda D, di Nardo W. 1997. Tonotopic organization of human auditory cortex analyzed by SPET. Audiology 36:241–248. Pandya DN, Sanides F. 1973. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Z Anat Entwickl-Gesch 139:127–161. Pandya DN, Rosene DL, Doolittle AM. 1994. Corticothalmic connections of auditory-related areas of the temporal lobe in the rhesus monkey. J Comp Neurol 345:447– 471. Pantev C, Hoke M, Lehnertz K, Lutkenhoner B, Anogianakis G, Wittkowski W. 1988. Tonotopic organization of the human auditory cortex revealed by transient auditory evoked magnetic fields. EEG Clin Neurophysiol 69:160 –170. Pantev C, Bertrand O, Eulitz C, Verkindt C, Hampson S, Schuierer G, Elbert T. 1995. Specific tonotopic organization of different areas of the human auditory cortex revealed by simultaneous magnetic and electric recordings. EEG Clin Neurophysiol 94:26 – 40. Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. 1996. Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex 6:661– 672. Petersen SE, Fox PT, Posner MI, Mintum M, Raichle ME. 1988. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature 231:585–589. Pfingst BE, O’Connor TA. 1981. Characteristics of neurons in auditory cortex of monkeys performing a simple auditory task. J Neurophysiol 45:16 –34. Poljak S. 1932. Origin, course, termination and internal organization of the auditory radiation. In: Evans HM, Thompson IM, editors. The main afferent fiber systems of the cerebral cortex in primates. Berkeley: University of California Press. p 81–104. Price C, Wise R, Ramsay S, Friston K, Howard D, Patterson K, Frackoiak R. 1992. Regional response differences within the human auditory cortex when listening to words. Neurosci Lett 146:179 –182. Rademacher J, Caviness V, Steinmetz H, Galaburda A. 1993. Topograph- 222 ical variation of the human primary cortices: implications for neuroimaging, brain mapping and neurobiology. Cereb Cortex 3:313–329. Rauschecker JP. 1998. Parallel processing in the auditory cortex of primates. Audiol Neurootol 3:86 –103. Rauschecker JP, Tian B. 2000. Mechanisms and streams for processing of “what” and “where” in auditory cortex. Proc Natl Acad Sci USA 97: 11800 –11806. Rauschecker JP, Tian B, Hauser M. 1995. Processing of complex sounds in the macaque nonprimary auditory cortex. Science 268:111–114. Rauschecker JP, Tian B, Pons T, Mishkin M. 1997. Serial and parallel processing in rhesus monkey auditory cortex. J Comp Neurol 382:89 – 103. Recanzone GH, Schreiner CE, Sutter ML, Beitel RE, Merzenich MM. 1999. Functional organization of spectral receptive fields in the primary auditory cortex of the owl monkey. J Comp Neurol 415:460 – 481. Recanzone GH, Guard DC, Phan ML. 2000. Frequency and intensity response properties of single neurons in the auditory cortex of the behaving macaque monkey. J Neurophysiol 83:2315–2331. Rivier F, Clarke S. 1997. Cytochrome oxidase, acetylcholinesterase, and NADPH-diaphorase staining in human supratemporal and insular cortex: evidence for multiple auditory areas. Neuroimage 6:288 –304. Romani GL, Williamson SJ, Kaufman L. 1982. Tonotopic organization of the human auditory cortex. Science 216:1339 –1340. Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP. 1999. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nature Neurosci 2:1131– 1136. Rosburg T, Kreitschmann-Andermahr I, Emmerich E, Nowak H, Sauer H. 1998. Hemispheric differences in frequency dependent dipole orientation of the human auditory evoked field component N100m. Neurosci Lett 258:105–108. Rouiller EM. 1997. Functional organization of the auditory pathways. In Ehret G, Romand R, editors. The central auditory system. New York: Oxford University Press. p 3–96. Sarkissow SA, Filimonoff IN, Kononowa EP, Preobraschenskaja IS, Kukuew LA. 1955. Atlas of the cytoarchitectonics of the human cerebral cortex. Moscow: Medgiz. Scheich H, Baumgart F, Gaschler-Markefski B, Tegeler C, Tempelmann C, Heinze HJ, Schindler F, Stiller D. 1998. Functional magnetic resonance imaging of a human auditory cortex area involved in foregroundbackground decomposition. Eur J Neurosci 10:803– 809. Schleicher A, Amunts K, Geyer S, Morosan P, Zilles K. 1999. Observerindependent method for microstructural parcellation of cerebral cortex: a quantitative approach to cytoarchitectonics. Neuroimage 9:165–177. Schreiner CE. 1992. Functional organization of the auditory cortex: maps and mechanisms. Curr Opin Neurobiol 2:516 –521. Schreiner CE. 1998. Spatial distribution of responses to simple and complex sounds in the primary auditory cortex. Audiol Neurootol 3:104 – 122. Scott SK, Blank CC, Rosen S, Wise RJS. 2000. Identification of a pathway for intelligible speech in the left temporal lobe. Brain 123:2400 –2406. Seldon HL. 1981a. Structure of human auditory cortex. I. Cytoarchitecture and dendritic distributions. Brain Res 229:277–294. Seldon HL. 1981b. Structure of human auditory cortex. II. Axon distributions and morphological correlates of speech perception. Brain Res 229:295–310. Seldon HL. 1982. Structure of human auditory cortex. III. Statistical analysis of dendritic trees. Brain Res 249:211–221. Strainer JC, Ulmer JL, Yetkin FZ, Haughton VM, Daniels DL, Millen SJ. 1997. Functional MR of the primary auditory cortex: an analysis of pure tone activation and tone discrimination. Am J Neuroradiol 18: 601– 610. Steinmetz H, Rademacher J, Huang Y, Hefter H, Zilles K, Thron A, Freund T. HACKETT ET AL HJ. 1989. Cerebral asymmetry: MR planimetry of the human planum temporale. J Comput Assist Tomogr 13:996 –1005. Talavage TM, Ledden PJ, Benson RR, Rosen BR, Melcher JR. 2000. Frequency-dependent responses exhibited by multiple regions in human auditory cortex. Hear Res 150:225–244. Tian B, Reser D, Durham A, Kustov A, Rauschecker JP. 2001. Functional specialization in rhesus monkey auditory cortex. Science 292:290 –294. Tiitinen H, Alho K, Huotilainen R, Ilmoniemi RJ, Simola J, Naatanen R. 1993. Tonotopic auditory cortex and the magnetoencephalographic (MEG) equivalent of the mismatch negativity. Psychophysiology 30: 537–540. van Brederode JF, Mulligan KA, Hendrickson AE. 1990. Calcium-binding proteins as markers for subpopulations of GABAergic neurons in monkey striate cortex. J Comp Neurol 298:1–22. Verkindt C, Bertrand O, Perrin F, Echallier JF, Pernier J. 1995. Tonotopic organization of the human auditory cortex: N100 topography and multiple dipole model analysis. EEG Clin Neurophysiol 96:143–156. Vogt C, Vogt O. 1919. Allgemeinere Ergebnisse unserer Hirnforschung. J Psych Neurol 24:279 – 462. von Bonin G. 1938. The cerebral cortex of the cebus monkey. J Comp Neurol 69:181–227. von Bonin G, Bailey P. 1947. The neocortex of Macaca mulatta. In: Allen RB, Kampmeier OF, Schour I, Serles ER, editors. Illinois Monographs in the Medical Sciences, vol 5. Urbana: University of Illinois Press. p 1–163. von Economo C. 1929. The cytoarchitectonics of the human cerebral cortex. London: Oxford University Press. p 110 –128. von Economo C, Horn L. 1930. Über Windungsrelief, Mae und Rindenarchitektonik der Supratemporalfläche, ihre individuellen und ihre Seitenunterschiede. Z Neurol Psychiatr 130:678 –757. von Economo C, Koskinas GN. 1925. Die Cytoarchitectonik der Hirnrinde des erwachsenen menschen. Berlin: Julius Springer. Walker AE. 1937. The projection of the medial geniculate body to the cerebral cortex in the macaque monkey. J Anat (Lond) 71:319 –331. Wallace MN, Kitzes LM, Jones EG. 1991. Chemoarchitectonic organization of the cat primary auditory cortex, Exp Brain Res 86:527–544. Walzl EM. 1947. Representation of the cochlea in the cerebral cortex. Laryngoscope, 57:778 –787. Walzl EM, Woolsey CN. 1943. Cortical auditory areas of the monkey as determined by electrical excitation of nerve fibers in osseous spiral lamina and by click stimulation. Fed Proc 2:52. Wessinger CM, Buonocore MH, Kussmaul CL, Mangun GR. 1997. Tonotopy in human auditory cortex examined with functional magenetic resonance imaging. Hum Brain Mapp 5:18 –25. Wobst P, Wenzel R, Kohl M, Obrig H, Villringer A. 2001. Linear aspects of changes in deoxygenated hemoglobin concentration and cytochrome oxidase oxidation during brain activation. Neuroimage 13:520 –530. Wong-Riley MT. 1989. Cytochrome oxidase: an endogenous metabolic marker for neuronal activity. Trends Neurosci 12:94 –101. Woolsey CN. 1947. Patterns of sensory representation in the cerebral cortex. Fed Proc 6:437– 441. Woolsey CN. 1971. Tonotopic organization of the auditory cortex. In: MB Sachs, editor. Physiology of the auditory system. Baltimore: Nat. Ed. Consults. p 271–282. Woolsey CN, Walzl EM. 1982. Cortical auditory area of Macaca mulatta and its relation to the second somatic sensory area (Sm II). In Woolsey CN, editor. Cortical sensory organization. Multiple auditory areas. Totowa, NJ: Humana Press. p 231–256. Yamamoto T, Uemura Y, Llinas R. 1992. Tonotopic organization of human auditory cortex revealed by multi channel SQUID system. Acta Otolaryngol (Stockh) 112:201–204. Zatorre RJ, Evans AC, Meyer E, Gjedde A. 1992. Lateralization of phonetic and pitch discrimination in speech processing. Science 256:846 – 849.